Abstract
Background
Nuclear magnetic resonance (NMR) spectroscopy measures the number and size of lipoprotein particles, instead of their cholesterol or triglyceride content, but its clinical utility is uncertain.
Methods and Results
Baseline lipoproteins were measured by NMR in 27,673 initially healthy women followed for incident cardiovascular disease (CVD, N=1,015) over 11 years. Adjusting for non-lipid risk factors, hazard ratios (HRs) and 95% confidence intervals (CIs) for top vs bottom quintile of NMR-measured lipoprotein particle concentration (particles/L) were, for low-density lipoprotein (LDLNMR) 2.51 (1.91−3.30), high-density lipoprotein (HDLNMR) 0.91 (0.75−1.12), very-low-density lipoprotein (VLDLNMR) 1.71 (1.38−2.12), and LDLNMR/HDLNMR ratio 2.25 (1.80−2.81). Similarly-adjusted results for NMR-measured lipoprotein particle size (nanometers) were, for LDLNMR size 0.64 (0.52−0.79), HDLNMR size 0.65 (0.51−0.81), and VLDLNMR size 1.37 (1.10−1.70). Hazard ratios for NMR measures were comparable but not superior to standard lipids: total cholesterol 2.08 (1.63−2.67), LDL cholesterol 1.74 (1.40−2.16), HDL cholesterol 0.52 (0.42−0.64), triglycerides 2.58 (1.95−3.41), non-HDL cholesterol 2.52 (1.95−3.25), total/HDL cholesterol ratio 2.82 (2.23−3.58); and apolipoproteins: B100 2.57 (1.98−3.33), A-1 0.63 (0.52−0.77), B100/A-1 ratio 2.79 (2.21−3.54). There was essentially no reclassification improvement with adding LDLNMR particle concentration or apolipoprotein B100 to a model that already included the total/HDL cholesterol ratio and non-lipid risk factors (net reclassification index [NRI], 0% and 1.9%, respectively), nor did the addition of either variable result in a statistically significant improvement in the c-index.
Conclusions
In this prospective study of healthy women, CVD risk prediction associated with lipoprotein profiles evaluated by NMR was comparable but not superior to standard lipids or apolipoproteins.
Keywords: Lipoproteins, lipids, apolipoproteins
While current prevention guidelines recommend measurement of standard lipids to assess risk of cardiovascular disease (CVD),1-3 it has been suggested that alternative lipoprotein measures may improve risk prediction. However, it remains uncertain how well such measures predict CVD when compared with the standard lipids that are routinely obtained in clinical practice.
One method of alternative lipid testing is proton nuclear magnetic resonance (NMR) spectroscopy. This technique simultaneously quantifies the number and size of very low-density lipoprotein (VLDLNMR), low-density lipoprotein (LDLNMR), and high-density lipoprotein (HDLNMR) particles, expressed each as a lipoprotein particle concentration (particles/L), or as an average particle size (nanometers).4 By contrast, standard lipid tests quantify the cholesterol or triglyceride content of lipoproteins, expressed as mg/dL of cholesterol or triglyceride. The cholesterol content of lipoprotein particles varies between individuals because of heterogeneity in particle size and in the relative content of cholesterol ester and triglycerides contained in the particle core.5 6
Whether information about lipoprotein particle concentration or size obtained from NMR predicts CVD risk in asymptomatic individuals is uncertain. In addition, direct comparison data with apolipoproteins are scant. Each particle of LDL and VLDL carries 1 molecule of apolipoprotein B100 on its surface regardless of its cholesterol or triglyceride content,7 hence apolipoprotein B100 is another measure of atherogenic lipoprotein particle number, obtained by immunoassay, and high levels have been associated with higher CVD risk.8 Apolipoprotein A-1 is the major molecule that is carried on HDL particles, but because it is not carried in a 1-to-1 fashion, it is not a measure of HDL particle number, although low levels have been associated with higher CVD risk.9 We conducted this study to evaluate prospectively whether NMR lipoprotein particles predict CVD in initially healthy women, and how they compare with directly-measured standard lipids and immunoassay-measured apolipoproteins.
METHODS
Study Population
Study participants were drawn from the Women's Health Study (WHS), a recently completed randomized, double-blinded, placebo-controlled trial of low-dose aspirin and vitamin E in the primary prevention of CVD and cancer in women.10-12 WHS participants were apparently healthy female health care professionals, ages 45 years or older, who were free of self-reported CVD and cancer at study entry (1992−1995). Women gave written informed consent and completed questionnaires at the time of enrollment on demographics, anthropometrics, medical history, and lifestyle factors. They were also asked to provide a baseline blood sample; 28,345 women did so, and of these, 98.5% (N=27,909) had NMR measurements. For this study, we excluded women with missing data on baseline lipids or apolipoproteins (N=236), leaving 27,673 women for analysis. The study was approved by the institutional review board of the Brigham and Women's Hospital (Boston, Mass). Drs. Mora and Ridker had full access to the data and take full responsibility for its integrity. All authors have read and agree to the manuscript as written.
Laboratory Measurements
EDTA blood samples were obtained at the time of enrollment into the WHS and stored in vapor phase liquid nitrogen (−170° C). Samples for lipoprotein particle analysis by proton NMR spectroscopy were thawed, aliquoted (200 ul), refrozen, and shipped on dry ice to LipoScience, Inc. (Raleigh, NC). Particle concentrations of lipoproteins of different sizes were calculated from the measured amplitudes of their spectroscopically distinct lipid methyl group NMR signals. Weighted-average lipoprotein particle sizes are derived from the sum of the diameter of each subclass multiplied by its relative mass percentage based on the amplitude of its methyl NMR signal.5 Particle diameters and coefficients of variation (CVs) are shown in Supplementary Table 1. The NMR lipoprotein variables that we examined are those that are provided when ordering an NMR lipoprotein profile for clinical use.
In a laboratory (N. Rifai, Children's Hospital, Boston, MA) certified by the National Heart, Lung, and Blood Institute/Centers for Disease Control and Prevention Lipid Standardization program, baseline samples were thawed and analyzed for standard lipids and apolipoproteins. Standard lipids were directly measured using reagents from Roche Diagnostics (Indianapolis, IN), with CVs <3%. Apolipoproteins B100 and A-1 were measured using immunoturbidimetric assays (DiaSorin, Stillwater, Minn), with CVs of 5% and 3%, respectively.
Ascertainment of CVD Events
The primary endpoint of interest was a composite endpoint of incident CVD (nonfatal myocardial infarction, percutaneous coronary intervention, coronary artery bypass grafting, nonfatal ischemic stroke, or cardiovascular death). During the 11 year follow-up period, women reported the endpoints of interest on follow-up questionnaires every 6 or 12 months, and medical records were obtained to confirm events by a blinded end-points committee of physicians as previously described.12
Statistical Analysis
Statistical analyses were performed using STATA version 8.2 (STATA Corporation, College Station, Texas). We calculated Spearman rank correlation coefficients to evaluate the interrelations between the measured lipid biomarkers. Following guidelines from the Department of Health and Human Services,13 lipid biomarkers were divided into quintiles based on the distribution among women not taking hormone replacement. Cox proportional hazard regression models were used to calculate the hazard ratios (HRs) and 95% confidence intervals (CIs) according to these quintiles. The proportional hazard assumption was satisfied using Schoenfeld residuals and the natural logarithm of follow-up time.
To examine the extent to which each lipid biomarker was associated with incident events, we initially considered each lipid variable in a separate model that adjusted for non-lipid risk factors (age, randomized treatment assignment, smoking status, menopausal status, postmenopausal hormone use, blood pressure, diabetes, and body mass index). Removing body mass index and diabetes from the multivariable analyses did not substantially affect the findings, nor did the addition of physical activity or alcohol use. Excluding the 883 women who were on baseline lipid lowering therapy did not change the results, and hence these women were included in the analyses. Analyses were also stratified according to fasting/nonfasting status based on our prior work in this cohort.14 P value for linear trend was obtained using the median value for each quintile. All P-values were two-tailed. Since lipoprotein particles are metabolically interrelated and their concentrations are not independent,4,15 NMR lipoproteins were also analyzed in a model that included the 9 NMR lipoprotein subclasses (large and small LDLNMR, IDLNMR, 3 HDLNMR and 3 VLDLNMR lipoprotein subclasses). We also analyzed LDLNMR lipoprotein concentration in multivariate Cox models that adjusted for other lipids.
The likelihood ratio χ2 statistic was used to evaluate the goodness-of-fit of predictive models. Model discrimination was examined using the c-index,16a generalization of the area under the receiver operator characteristic curve. Model calibration was assessed using the Hosmer-Lemeshow goodness-of-fit test.17 Risk reclassification was assessed by categorizing the predicted 10-year risk for each model into categories of less than 5%, 5% to less than 10%, 10% to less than 20%, and 20% or higher. We calculated the proportion of participants who were reclassified by the comparison model as compared to the reference model. We computed the Net Reclassification Improvement (NRI),18 which compares the shifts in reclassified categories by observed outcome, and the Integrated Discrimination Improvement (IDI),18 which compares the integrals of sensitivity and specificity under two models.
RESULTS
During a mean follow-up of 11 years (302,399 person-years), a total of 1,015 first CVD events occurred, with “hard” events comprising 74% of these events (155 CVD deaths, 265 myocardial infarctions, and 334 strokes). In comparison to the standard lipid measurements which reflect the cholesterol or triglyceride content of lipoprotein particles, the NMR-measured lipoprotein particle concentrations of total LDLNMR and VLDLNMR were higher in the women who developed CVD (Table 1), but no difference in total HDLNMR was found. Women with CVD had significantly smaller LDLNMR and HDLNMR particle sizes and larger VLDLNMR particle size.
Table 1.
Baseline characteristics of participants according to incident cardiovascular disease
| No CVD N=26,658 | CVD N=1,015 | P Value | |
|---|---|---|---|
| Age, mean (SD), y | 54.5 (7.0) | 59.8 (8.1) | <0.001 |
| Current smoking, % | 11.2 | 22.4 | <0.001 |
| Hypertension, % | 24.2 | 48.5 | <0.001 |
| Diabetes, % | 2.3 | 15.2 | <0.001 |
| Postmenopausal status, % | 53.6 | 72.0 | <0.001 |
| Postmenopausal hormone use, % | 43.7 | 41.1 | 0.11 |
| Body mass index, mean (SD), kg/m2 | 25.9 (4.9) | 27.3 (5.4) | <0.001 |
| NMR lipoprotein particle concentrations, nmol/L | |||
| Low-density lipoprotein particles (LDLNMR) | |||
| Total | 1277 (1033−1600) | 1557 (1251−1943) | <0.001 |
| Large | 541 (401−684) | 518 (315−690) | <0.001 |
| Small | 655 (396−1020) | 955 (577−1461) | <0.001 |
| Intermediate-density lipoprotein particles (IDLNMR) | 33 (11−68) | 50 (20−91) | <0.001 |
| High-density lipoprotein particles (HDLNMR) | |||
| Total | 35000 (31100−39400) | 34800 (31000−39200) | 0.59 |
| Large | 7500 (5000−10400) | 5900 (3400−9000) | <0.001 |
| Medium | 2800 (800−6000) | 2600 (700−6100) | 0.77 |
| Small | 23700 (20000−27300) | 24700 (21300−28400) | <0.001 |
| Very low-density lipoprotein particles (VLDLNMR) | |||
| Total | 68.3 (49.1−90.2) | 80.3 (58.8−102.6) | <0.001 |
| Large | 1.4 (0.4−3.7) | 2.8 (0.9−5.4) | <0.001 |
| Medium | 20.7 (11.2−31.9) | 24.2 (14.3−36.7) | <0.001 |
| Small | 44.6 (32.4−57.8) | 50.7 (37.9−64.3) | <0.001 |
| NMR average particle size, nm | |||
| LDLNMR size | 21.4 (20.8−21.8) | 20.9 (20.3−21.6) | <0.001 |
| HDLNMR size | 9.0 (8.6−9.4) | 8.8 (8.5−9.1) | <0.001 |
| VLDLNMR size | 46.7 (42.2−52.1) | 48.6 (43.4−55.1) | <0.001 |
| Standard chemical lipids, mg/dL | |||
| Total cholesterol | 208 (183−235) | 223 (199−252) | <0.001 |
| Low-density lipoprotein cholesterol (LDL-C) | 121 (100−144) | 133 (113−156) | <0.001 |
| High-density lipoprotein cholesterol (HDL-C) | 52 (43−63) | 46 (39−56) | <0.001 |
| Triglycerides (TG) | 118 (83−173) | 158 (109−239) | <0.001 |
| Apolipoproteins, mg/dL | |||
| Apolipoprotein B100 | 100 (84−120) | 118 (97−137) | <0.001 |
| Apolipoprotein A-1 | 149 (133−168) | 143 (127−164) | <0.001 |
| Combined measures | |||
| LDLNMR/HDLNMR ratio | 36.2 (28.9−46.2) | 44.0 (34.6−57.7) | <0.001 |
| Non-high-density lipoprotein cholesterol (non-HDL-C) | 154 (129−181) | 174 (150−202) | <0.001 |
| Total/high-density lipoprotein cholesterol ratio (TC/HDL-C) | 3.9 (3.2−4.9) | 4.7 (3.8−5.9) | <0.001 |
| Apolipoprotein B100/A-1 ratio | 0.67 (0.54−0.84) | 0.82 (0.65−1.02) | <0.001 |
Values are median (25th−75th percentile) unless otherwise indicated. P values for age and body mass index were obtained from t tests. P values for categorical variables were obtained from chi-square tests. P values for the lipid biomarkers were obtained from the Wilcoxon rank-sum test.
Table 2 shows the Spearman correlation coefficients for NMR lipoproteins with each other and with standard lipids and apolipoproteins. Total LDLNMR particle concentration correlated positively with LDL cholesterol (r = 0.62) but correlated more closely with apolipoprotein B100 (r = 0.83), non-HDL cholesterol (r = 0.74), total/HDL cholesterol ratio (r = 0.80), and apolipoprotein B100/A-1 ratio (r = 0.80), all P<0.001.
Table 2.
Spearman correlation coefficients for NMR lipoproteins with lipids and apolipoproteins
| Total Chol | LDL-C | HDL-C | Trigs | Apo B100 | Apo A-1 | Non HDL-C | Total Chol/HDL-C | Apo B100/A-1 | |
|---|---|---|---|---|---|---|---|---|---|
| Low-density lipoprotein particles (LDLNMR) | |||||||||
| Total | 0.57 | 0.62 | −0.48 | 0.62 | 0.84 | −0.23 | 0.74 | 0.80 | 0.80 |
| Large | 0.35 | 0.34 | 0.46 | −0.27 | 0.17 | 0.37 | 0.19 | −0.19 | −0.05 |
| Small | 0.28 | 0.33 | −0.62 | 0.61 | 0.58 | −0.39 | 0.50 | 0.74 | 0.68 |
| IDLNMR | 0.33 | 0.34 | −0.20 | 0.37 | 0.44 | −0.02 | 0.40 | 0.39 | 0.37 |
| High-density lipoprotein particles (HDLNMR) | |||||||||
| Total | 0.23 | 0.00 | 0.41 | 0.25 | 0.10 | 0.69 | 0.09 | −0.22 | −0.26 |
| Large | −0.03 | −0.22 | 0.82 | −0.41 | −0.37 | 0.73 | −0.32 | −0.76 | −0.67 |
| Medium | −0.06 | −0.16 | −0.07 | 0.26 | −0.03 | 0.08 | −0.04 | 0.02 | −0.07 |
| Small | 0.29 | 0.26 | −0.07 | 0.34 | 0.36 | 0.18 | 0.32 | 0.25 | 0.20 |
| Very-low-density lipoprotein particles (VLDLNMR) | |||||||||
| Total | 0.46 | 0.50 | −0.40 | 0.64 | 0.61 | −0.20 | 0.61 | 0.66 | 0.60 |
| Large | 0.24 | 0.14 | −0.40 | 0.85 | 0.41 | −0.07 | 0.38 | 0.51 | 0.37 |
| Medium | 0.28 | 0.22 | −0.30 | 0.68 | 0.39 | −0.09 | 0.39 | 0.45 | 0.36 |
| Small | 0.48 | 0.60 | −0.34 | 0.38 | 0.60 | −0.24 | 0.62 | 0.62 | 0.61 |
| LDLNMR size | −0.06 | −0.12 | 0.64 | −0.55 | −0.36 | 0.43 | −0.29 | −0.62 | −0.52 |
| HDLNMR size | −0.14 | −0.31 | 0.75 | −0.54 | −0.47 | 0.57 | −0.42 | −0.77 | −0.68 |
| VLDLNMR size | 0.01 | −0.14 | −0.20 | 0.59 | 0.11 | 0.05 | 0.07 | 0.18 | 0.06 |
| LDLNMR/HDLNMR | 0.41 | 0.57 | −0.64 | 0.45 | 0.72 | −0.55 | 0.64 | 0.85 | 0.88 |
Abbreviations: Trigs: triglycerides; IDLNMR intermediate-density lipoprotein particles.
All P values for the correlation coefficients were <0.01, except for VLDLNMR size with total cholesterol (P=0.22).
Association of NMR Lipoproteins, Lipids, and Apolipoproteins with CVD
Table 3 shows the association of each of the NMR lipoproteins, standard lipids, and apolipoproteins with CVD examined in separate Cox regression models that adjusted for non-lipid risk factors. Of the NMR measures, total LDLNMR particle concentration had the largest hazard ratio and best goodness-of-fit likelihood ratio χ2. The concentration of small LDLNMR particles was associated with higher CVD, but large LDLNMR was not. However, when small and large LDLNMR were examined in a model that included all 9 NMR-measured lipoprotein particle concentrations (data not shown), both large and small LDLNMR were significantly associated with CVD to a similar degree.
Table 3.
Associations of lipoprotein and lipid measures with incident cardiovascular disease, adjusted for non-lipid risk factors
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | LR χ2 | P for Linear Trend | |
|---|---|---|---|---|---|---|---|
| NMR Lipoprotein Particle Concentrations | |||||||
| LDL particles (LDLNMR) | |||||||
| Total | |||||||
| Range, nmol/L | <963 | 963−1165 | 1166−1387 | 1388−1703 | ≥1704 | ||
| HR (95% CI) | 1.00 | 1.37 (1.01−1.85) | 1.35 (1.01−1.81) | 1.80 (1.36−2.38) | 2.51 (1.91−3.30) | 1107.6 | <0.001 |
| Large | |||||||
| Range, nmol/L | <354 | 354−471 | 472−574 | 575−695 | ≥696 | ||
| HR (95% CI) | 1.00 | 0.69 (0.56−0.84) | 0.67 (0.54−0.82) | 0.75 (0.61−0.91) | 0.86 (0.72−1.03) | 1050.4 | 0.21 |
| Small | |||||||
| Range, nmol/L | <352 | 352−564 | 565−794 | 795−1172 | ≥1173 | ||
| HR (95% CI) | 1.00 | 0.89 (0.69−1.15) | 0.96 (0.75−1.22) | 1.24 (0.99−1.56) | 1.76 (1.41−2.18) | 1089.8 | <0.001 |
| IDL particles (IDLNMR) | |||||||
| Range, nmol/L | <6 | 6−20 | 21−40 | 41−74 | ≥75 | ||
| HR (95% CI) | 1.00 | 1.16 (0.91−1.49) | 1.06 (0.83−1.35) | 1.26 (1.00−1.58) | 1.48 (1.19−1.84) | 1047.0 | <0.001 |
| HDL particles (HDLNMR) | |||||||
| Total | |||||||
| Range, nmol/L | <29200 | 29200−31900 | 32000−34400 | 34500−37400 | ≥37500 | ||
| HR (95% CI) | 1.00 | 1.01 (0.80−1.26) | 0.83 (0.66−1.05) | 0.91 (0.73−1.13) | 0.91 (0.75−1.12) | 1031.8 | 0.34 |
| Large | |||||||
| Range, nmol/L | <3900 | 3900−5800 | 5900−7600 | 7700−9900 | ≥10000 | ||
| HR (95% CI) | 1.00 | 0.74 (0.61−0.89) | 0.62 (0.51−0.75) | 0.56 (0.45−0.69) | 0.58 (0.48−0.71) | 1070.6 | <0.001 |
| Medium | |||||||
| Range, nmol/L | <400 | 400−1400 | 1500−3000 | 3100−5600 | ≥5700 | ||
| HR (95% CI) | 1.00 | 0.90 (0.73−1.11) | 0.89 (0.72−1.10) | 0.82 (0.66−1.02) | 1.00 (0.82−1.21) | 1033.6 | 0.60 |
| Small | |||||||
| Range, nmol/L | <18900 | 18900−21900 | 22000−24500 | 24600−27400 | ≥27500 | ||
| HR (95% CI) | 1.00 | 1.24 (0.98−1.56) | 1.26 (1.01−1.59) | 1.16 (0.93−1.46) | 1.22 (0.99−1.51) | 1033.3 | 0.19 |
| VLDL particles (VLDLNMR) | |||||||
| Total | |||||||
| Range, nmol/L | <46.0 | 46.0−62.1 | 62.2−77.6 | 77.7−97.5 | ≥97.6 | ||
| HR (95% CI) | 1.00 | 1.18 (0.93−1.50) | 1.22 (0.97−1.55) | 1.53 (1.23−1.92) | 1.71 (1.38−2.12) | 1062.2 | <0.001 |
| Large | |||||||
| Range, nmol/L | <0.2 | 0.2−0.7 | 0.8−1.9 | 2.0−4.0 | ≥4.1 | ||
| HR (95% CI) | 1.00 | 1.15 (0.86−1.54) | 1.42 (1.06−1.90) | 1.48 (1.11−1.97) | 1.77 (1.34−2.33) | 1056.0 | <0.001 |
| Medium | |||||||
| Range, nmol/L | <8.4 | 8.4−16.1 | 16.2−24.1 | 24.2−34.4 | ≥34.5 | ||
| HR (95% CI) | 1.00 | 1.13 (0.89−1.44) | 1.30 (1.03−1.64) | 1.29 (1.02−1.62) | 1.46 (1.17−1.82) | 1042.7 | <0.001 |
| Small | |||||||
| Range, nmol/L | <31.8 | 31.8−42.2 | 42.3−51.6 | 51.7−63.3 | ≥63.4 | ||
| HR (95% CI) | 1.00 | 1.01 (0.81−1.27) | 1.31 (1.06−1.62) | 1.34 (1.08−1.65) | 1.56 (1.27−1.91) | 1054.9 | <0.001 |
| NMR Lipoprotein Particle Size | |||||||
| LDLNMR size | |||||||
| Range, nm | <20.6 | 20.6−21.0 | 21.2−21.5 | 21.6−21.9 | 22.0−23.0 | ||
| HR (95% CI) | 1.00 | 0.68 (0.56−0.82) | 0.57 (0.47−0.69) | 0.56 (0.45−0.69) | 0.64 (0.52−0.79) | 1022.7 | <0.001 |
| HDLNMR size | |||||||
| Range, nm | <8.6 | 8.6−8.7 | 8.8−9.0 | 9.1−9.4 | 9.5−10.8 | ||
| HR (95% CI) | 1.00 | 1.02 (0.85−1.23) | 0.81 (0.67−0.97) | 0.65 (0.53−0.79) | 0.65 (0.51−0.81) | 1059.1 | <0.001 |
| VLDLNMR size | |||||||
| Range, nm | <40.7 | 40.7−44.1 | 44.2−47.5 | 47.6−52.3 | 52.4−131.2 | ||
| HR (95% CI) | 1.00 | 1.14 (0.90−1.44) | 0.97 (0.76−1.23) | 0.98 (0.77−1.23) | 1.37 (1.10−1.70) | 1048.1 | 0.002 |
| Standard Chemical Lipid Concentrations | |||||||
| Total cholesterol | |||||||
| Range, mg/dL | <176 | 176−197 | 198−216 | 217−242 | ≥243 | ||
| HR (95% CI) | 1.00 | 1.35 (1.03−1.78) | 1.57 (1.21−2.04) | 1.84 (1.43−2.36) | 2.08 (1.63−2.67) | 1075.8 | <0.001 |
| LDL cholesterol | |||||||
| Range, mg/dL | <97.7 | 97.7−115.4 | 115.5−132.1 | 132.2−153.9 | ≥154 | ||
| HR (95% CI) | 1.00 | 1.12 (0.88−1.42) | 1.31 (1.04−1.64) | 1.56 (1.25−1.94) | 1.74 (1.40−2.16) | 1064.7 | <0.001 |
| HDL cholesterol | <39.5 | 39.5−45.9 | 46.0−52.7 | 52.8−61.5 | ≥61.6 | ||
| Range, mg/dL | |||||||
| HR (95% CI) | 1.00 | 0.85 (0.71−1.03) | 0.70 (0.58−0.86) | 0.59 (0.48−0.73) | 0.52 (0.42−0.64) | 1075.8 | <0.001 |
| Triglycerides | |||||||
| Range, mg/dL | <72 | 72−96 | 97−128 | 129−182 | ≥183 | ||
| HR (95% CI) | 1.00 | 1.34 (0.98−1.83) | 1.87 (1.39−2.50) | 1.89 (1.42−2.52) | 2.58 (1.95−3.41) | 1097.4 | <0.001 |
| Apolipoprotein Concentrations | |||||||
| Apolipoprotein B100 | |||||||
| Range, mg/dL | <79.1 | 79.1−93.3 | 93.4−108.9 | 109.0−126.2 | ≥126.3 | ||
| HR (95% CI) | 1.00 | 1.29 (0.96−1.73) | 1.48 (1.12−1.96) | 1.65 (1.26−2.17) | 2.57 (1.98−3.33) | 1117.2 | <0.001 |
| Apolipoprotein A-1 | |||||||
| Range, mg/dL | <124.1 | 124.1−135.4 | 135.5−146.2 | 146.3−159.8 | ≥159.9 | ||
| HR (95% CI) | 1.00 | 0.93 (0.76−1.13) | 0.69 (0.55−0.85) | 0.64 (0.52−0.80) | 0.63 (0.52−0.77) | 1059.2 | <0.001 |
| Combined Measures | |||||||
| LDLNMR/HDLNMR ratio | |||||||
| Range, mg/dL | <29.0 | 29.0−34.9 | 35.0−41.5 | 41.6−52.0 | ≥52.1 | ||
| HR (95% CI) | 1.00 | 1.23 (0.96−1.57) | 1.29 (1.01−1.64) | 1.61 (1.28−2.03) | 2.25 (1.80−2.81) | 1098.2 | <0.001 |
| Non-HDL cholesterol | |||||||
| Range, mg/dL | <123.3 | 123.3−144.9 | 145.0−165.6 | 165.7−191.1 | ≥191.2 | ||
| HR (95% CI) | 1.00 | 1.19 (0.89−1.59) | 1.87 (1.43−2.43) | 1.94 (1.49−2.52) | 2.52 (1.95−3.25) | 1115.9 | <0.001 |
| Total/HDL cholesterol ratio | |||||||
| Range | <3.20 | 3.20−3.81 | 3.82−4.49 | 4.50−5.44 | ≥5.45 | ||
| HR (95% CI) | 1.00 | 1.42 (1.10−1.84) | 1.54 (1.20−1.98) | 1.87 (1.46−2.39) | 2.82 (2.23−3.58) | 1125.5 | <0.001 |
| Apolipoprotein B100/A-1 ratio | |||||||
| Range | <0.54 | 0.54−0.64 | 0.65−0.77 | 0.78−0.94 | ≥0.95 | ||
| HR (95% CI) | 1.00 | 1.38 (1.07−1.79) | 1.63 (1.28−2.09) | 1.78 (1.40−2.27) | 2.79 (2.21−3.54) | 1122.0 | <0.001 |
*Adjusted for age, randomized treatment assignment, smoking status, menopausal status, postmenopausal hormone use, blood pressure, diabetes, and body mass index. P value for linear trend was obtained using the median value for each quintile. LR χ2 is the likelihood ratio chi-square statistic, which evaluates goodness of fit of predictive models associated with each lipid markers separately, with higher values consistent with better goodness of fit.
Of the HDLNMR measures, the total concentration of HDLNMR particles was not significantly associated with CVD. Large HDLNMR particles were significantly and inversely associated with CVD, while medium and small HDLNMR particles had no significant associations. All VLDLNMR particles were associated with higher CVD. Associations of NMR lipoproteins with CVD, analyzed according to self-reported fasting/nonfasting status (< or ≥ 8 hours to last meal) resulted in stronger associations for large and medium VLDLNMR particles with CVD in the nonfasting state.
LDLNMR and HDLNMR particle size were inversely associated, and VLDLNMR particle size directly associated, with CVD. After adjusting for LDLNMR particle concentration, there was no additional contribution of LDLNMR size to CVD risk (P for trend=0.25), while HDLNMR and VLDLNMR particle size remained significantly associated with CVD after adjustment for the respective concentrations.
When we removed body mass index and diabetes from the adjusted models, the adjusted HRs for top vs bottom quintiles were: total LDLNMR particle concentration 2.92 (2.24−3.81), apolipoprotein B100 2.89 (2.24−3.72), non-HDL cholesterol 2.61 (2.04−3.35), and the total/HDL cholesterol ratio 3.19 (2.54−3.99).
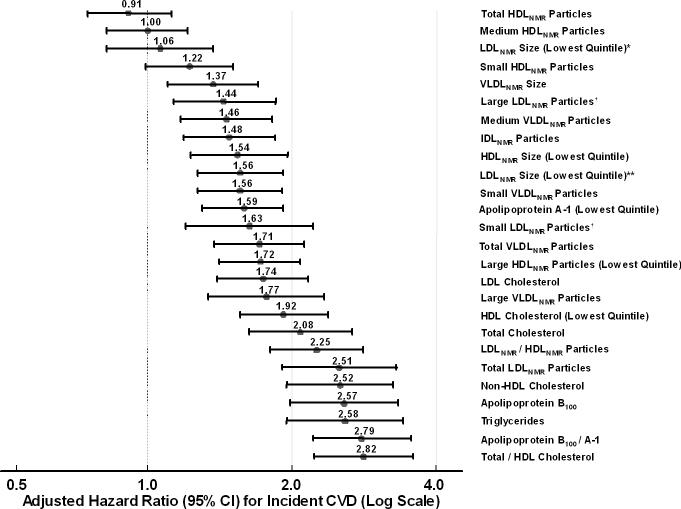
As shown in Table 3 and summarized in the Figure, hazard ratios for NMR measures were of approximately similar magnitude as those for standard lipids and apolipoproteins, although the total/HDL cholesterol ratio had the largest hazard ratio for any lipid or lipoprotein measure with CVD and the best goodness-of-fit likelihood ratio χ2.
Hazard ratios and 95% CIs for the top vs bottom quintile, unless otherwise noted, adjusted for non-lipid risk factors (age, randomized treatment assignment, smoking status, menopausal status, postmenopausal hormone use, blood pressure, diabetes, and body mass index).
*LDLNMR size adjusted for non-lipid risk factors and additionally for total LDLNMR particle concentration.
†Large and small LDLNMR particles were adjusted for non-lipid risk factors and additionally for the other NMR lipoproteins.
**LDLNMR size adjusted for non-lipid risk factors but not for total LDLNMR particle concentration.
Multivariate Lipid Models
In models that included non-lipid risk factors plus other lipids, the association of LDLNMR particle concentration with CVD was attenuated (Supplementary Table 2). In particular, after adjustment for the total/HDL cholesterol ratio, the association of LDLNMR examined as quintiles was attenuated but remained significant (top quintile HR 1.63; 95% CI 1.18−2.25). However, when LDLNMR was examined as a continuous variable there was no significant association after including the total/HDL cholesterol ratio.
Model Discrimination, Calibration, and Reclassification
Finally, we compared measures of model discrimination, calibration, and reclassification (Table 4). The referent model was comprised of the total/HDL cholesterol ratio and non-lipid risk factors, and compared to two other models: one that additionally incorporated LDLNMR particle concentration, and the other additionally incorporated apolipoprotein B100. All three models were well-calibrated.19 There was no statistically significant difference in the c-index for the models that added LDLNMR or apolipoprotein B100 to the referent model. There was essentially no reclassification improvement with adding LDLNMR particle concentration or apolipoprotein B100 to the referent model (NRI 0% and 1.9%, respectively).
Table 4.
Comparison of models based on discrimination, calibration, and reclassification measures
| Models | Discrimination C-Indexa | Goodness-of-Fit, Likelihood Ratio X2 (p value)b | Calibration Cox X2c | NRId (p value) | IDIe (p value) | Percent reclassifiedf |
|---|---|---|---|---|---|---|
| Non-lipid covariates and total/HDL cholesterol ratio (referent model) | 0.784 | Referent | 16.7 | Referent | Referent | Referent |
| Referent model covariates plus LDLNMR | 0.785 | 2.01 (0.16) |
17.2 | 0% (0.52) |
0.0% (0.65) |
1.1 |
| Referent model covariates plus apolipoprotein B100 | 0.786 | 10.5 (0.001) |
14.3 | 1.9% (0.02) |
0.1% (0.12) |
2.6 |
All statistical measures were calculated at 10-years of follow-up.
The c-index for the referent model (non-lipid covariates and total/HDL cholesterol ratio) was not statistically significantly different from the models that additionally included LDLNMR or apolipoprotein B100.
Values are likelihood ratio X2 and p values obtained from the Cox proportional hazards regression comparing models that added either LDLNMR or apolipoprotein B100 to the referent model (non-lipid covariates and total/HDL cholesterol ratio). A higher X2 value indicates a better model fit.
Values are modified Hosmer-Lemeshow X2, comparing differences between the predicted and actual event rates (X2 values greater than 20 indicate poor calibration).19
NRI is the net reclassification index, which compares the proportions moving up or down in clinical categories in cases versus controls, comparing models that added either LDLNMR or apolipoprotein B100 to the referent model.
IDI is the integrated discrimination improvement, comparing the integrals of sensitivity and specificity under two models (referent model compared with the model that added either LDLNMR or apolipoprotein B100).
The proportion of individuals that move up or down a risk category using the model that incorporates either LDLNMR or apolipoprotein B100 compared with the referent model.
DISCUSSION
In this prospective cohort of 27,673 initially healthy women, we found that NMR-measured lipoproteins were significantly associated with incident CVD after adjusting for non-lipid risk factors, with a magnitude of risk comparable but not superior to standard lipids or immunoassay-measured apolipoproteins. Even though NMR-measured LDLNMR particle concentration performed well for CVD risk prediction in this study, and was similar in risk to apolipoprotein B100, neither measurement was better than the total/HDL cholesterol ratio which is readily obtained from a standard lipid panel. These data support current guidelines that recommend the use of a standard lipid panel, in particular the total/HDL cholesterol ratio, for CVD risk assessment in clinical practice.
Our findings have direct clinical relevance on several fronts. First, major European and North American guidelines have endorsed the use of standard lipids for CVD risk prediction in asymptomatic individuals.1-3 By contrast, a recent statement involving an international panel of lipid experts proposed that CVD risk may be more closely related to atherogenic lipoprotein particle number than to LDL cholesterol.8 Atherogenic particle concentration may be measured by NMR, which provides the number per unit volume of lipoprotein particles of varying size, or by immunoassay measurement of apolipoprotein B100, since each VLDL, IDL, and LDL particle carries on its surface only one molecule of apolipoprotein B100.8
Previous studies, predominantly cross-sectional or case-control studies found that NMR-measured LDLNMR particle concentration may predict atherosclerotic diseases better than LDL cholesterol levels.15 20-24 Data from INTERHEART25 and other studies26-29 have found that apolipoprotein B100 or the apolipoprotein B100/A-1 ratio predict CVD. However, direct prospective comparison data for NMR measurements with apolipoproteins and standard lipid ratios are scarce.
To our knowledge, this study is the first large prospective comparison of associations of NMR-measured lipoproteins with both standard lipids and immunoassay-measured apolipoproteins for predicting incident CVD. We found that NMR-measured total LDLNMR particle concentration was similar in CVD risk prediction to apolipoprotein B100 and both measurements performed better than LDL cholesterol. However, the differences compared with triglycerides, non-HDL cholesterol and the total/HDL cholesterol ratio were small and do not support the routine measurement of NMR lipoproteins or immunoassay apolipoproteins when a standard lipid panel is available. The data from this study, along with our prior findings14,29 and recent data from the Framingham Study,30 provide evidence-based confirmation for guidelines that are based on the use of standard lipid measurements, particularly the total/HDL cholesterol ratio.
Second, our study provides new data regarding the potential atherogenicity of the various HDL particles, which are heterogeneous in size and composition, carrying variable amounts of cholesterol and apolipoprotein A-1 molecules.31 In this population of women, only large HDLNMR particles were associated with lower CVD risk. The magnitude of the inverse association of large HDLNMR particles with CVD was similar to that of apolipoprotein A-1 or HDL cholesterol, suggesting that the potentially protective effects of HDL cholesterol may be due to the large HDLNMR particles. Prior studies have demonstrated strong inverse relationships between insulin resistance and the large HDLNMR subclass as measured by NMR,32 or the corresponding HDL2 (sometimes referred to as “buoyant” HDL) as measured by ultracentrifugation.33 This observation of the potential cardioprotective role of large HDLNMR but not smaller HDLNMR particles may have clinical implications for developing therapeutic agents that target HDL metabolism, such as CETP inhibitor drugs.31 CETP inhibitors, such as torcetrapib, increase HDL cholesterol, predominantly altering the large HDL subclass, but there is controversy as to whether this results in reduced or enhanced cholesterol efflux from macrophages.34,35
While our study addresses primary prediction of CVD with NMR-based lipoprotein testing, our data should not be construed to exclude possible utility in this setting for alternative lipid or lipoprotein testing assessed by other measurement methods. Since our study is largely limited to Caucasian women, these data may not be generalizable to men or other patient groups. In particular, since we studied an apparently healthy cohort at low overall risk for CVD, our data do not address the question of whether or not lipoprotein testing with NMR has clinical utility for risk assessment and treatment strategies for higher risk patients, such as those with known cardiovascular disease, diabetes/insulin resistance, dyslipidemia, or for the monitoring of patients taking lipid altering therapy. Such studies need to be performed in the appropriate patient settings, preferably within the context of randomized trials of primary or secondary prevention.
In sum, CVD risk prediction associated with NMR lipoprotein profiles in this large prospective cohort of women was comparable but not superior to standard lipids or immunoassay-measured apolipoproteins. Thus, our data support the use of standard lipids, in particular the total/HDL cholesterol ratio, which are highly effective and readily available, for routine CVD risk assessment.
Supplementary Material
Sources of Funding
The research for this article was supported by a grant to Dr. Mora from the American Heart Association (0670007N). Dr. Mora is also supported by the National Heart, Lung, and Blood Institute (K08 HL094375), the Sandra A. Daugherty Foundation and the Lerner Research Young Investigator Award. The Women's Health Study is supported by grants HL 43851, HL 080467 and CA 47988 from the National Heart, Lung, and Blood Institute and the National Cancer Insitute, the Donald W. Reynolds Foundation (Las Vegas, NV), and Leducq Foundation (Paris, France). The funding agencies played no role in the design, conduct, data management, analysis, or manuscript preparation related to this manuscript.
Footnotes
Disclosures
Dr Otvos is employed by, is a stockholder of, and serves on the board of directors of LipoScience, Inc., a diagnostic laboratory company that performed the lipoprotein subclass analyses described in the manuscript. Dr Rosenson is a stockholder of LipoScience, Inc., and he serves as a member of its Scientific Advisory Board.
References
- 1.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 2.De Backer G, Ambrosioni E, Borch-Johnsen K, Brotons C, Cifkova R, Dallongeville J, Ebrahim S, Faergeman O, Graham I, Mancia G, Cats VM, Orth-Gomer K, Perk J, Pyorala K, Rodicio JL, Sans S, Sansoy V, Sechtem U, Silber S, Thomsen T, Wood D. European guidelines on cardiovascular disease and prevention in clinical practice. Atherosclerosis. 2003;171:145–155. doi: 10.1016/j.atherosclerosis.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 3.McPherson R, Frohlich J, Fodor G, Genest J, Canadian Cardiovascular S. Canadian Cardiovascular Society position statement--recommendations for the diagnosis and treatment of dyslipidemia and prevention of cardiovascular disease. Can J Cardiol. 2006;22:913–927. doi: 10.1016/s0828-282x(06)70310-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeyarajah EJ, Cromwell WC, Otvos JD. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin Lab Med. 2006;26:847–870. doi: 10.1016/j.cll.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 5.Otvos JD, Jeyarajah EJ, Cromwell WC. Measurement issues related to lipoprotein heterogeneity. Am J Cardiol. 2002;90:22i–29i. doi: 10.1016/s0002-9149(02)02632-2. [DOI] [PubMed] [Google Scholar]
- 6.Sacks FM, Campos H. Clinical review 163: Cardiovascular endocrinology: Low-density lipoprotein size and cardiovascular disease: a reappraisal. J Clin Endocrinol Metab. 2003;88:4525–4532. doi: 10.1210/jc.2003-030636. [DOI] [PubMed] [Google Scholar]
- 7.Mudd JO, Borlaug BA, Johnston PV, Kral BG, Rouf R, Blumenthal RS, Kwiterovich PO., Jr. Beyond low-density lipoprotein cholesterol: defining the role of low-density lipoprotein heterogeneity in coronary artery disease. J Am Coll Cardiol. 2007;50:1735–1741. doi: 10.1016/j.jacc.2007.07.045. [DOI] [PubMed] [Google Scholar]
- 8.Barter PJ, Ballantyne CM, Carmena R, Castro Cabezas M, Chapman MJ, Couture P, de Graaf J, Durrington PN, Faergeman O, Frohlich J, Furberg CD, Gagne C, Haffner SM, Humphries SE, Jungner I, Krauss RM, Kwiterovich P, Marcovina S, Packard CJ, Pearson TA, Reddy KS, Rosenson R, Sarrafzadegan N, Sniderman AD, Stalenhoef AF, Stein E, Talmud PJ, Tonkin AM, Walldius G, Williams KM. Apo B versus cholesterol in estimating cardiovascular risk and in guiding therapy: report of the thirty-person/ten-country panel. J Intern Med. 2006;259:247–258. doi: 10.1111/j.1365-2796.2006.01616.x. [DOI] [PubMed] [Google Scholar]
- 9.Walldius G, Jungner I. The apoB/apoA-I ratio: a strong, new risk factor for cardiovascular disease and a target for lipid-lowering therapy--a review of the evidence. J Intern Med. 2006;259:493–519. doi: 10.1111/j.1365-2796.2006.01643.x. [DOI] [PubMed] [Google Scholar]
- 10.Cook NR, Lee IM, Gaziano JM, Gordon D, Ridker PM, Manson JE, Hennekens CH, Buring JE. Low-dose aspirin in the primary prevention of cancer: the Women's Health Study: a randomized controlled trial. JAMA. 2005;294:47–55. doi: 10.1001/jama.294.1.47. [DOI] [PubMed] [Google Scholar]
- 11.Lee IM, Cook NR, Gaziano JM, Gordon D, Ridker PM, Manson JE, Hennekens CH, Buring JE. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women's Health Study: a randomized controlled trial. JAMA. 2005;294:56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 12.Ridker PM, Cook NR, Lee IM, Gordon D, Gaziano JM, Manson JE, Hennekens CH, Buring JE. A Randomized Trial of Low-Dose Aspirin in the Primary Prevention of Cardiovascular Disease in Women. N Engl J Med. 2005;352:1293–1304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 13.Hainline A, Karon J, Lippel K. Manual of Laboratory Operations: Lipid Research Clinics Program and Lipid and Lipoprotein Analysis. 2nd ed. Md. Dept of Health and Human Services; Bethesda: 1982. [Google Scholar]
- 14.Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker PM. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA. 2007;298:309–316. doi: 10.1001/jama.298.3.309. [DOI] [PubMed] [Google Scholar]
- 15.Mora S, Szklo M, Otvos JD, Greenland P, Psaty BM, Goff DC, Jr., O'Leary DH, Saad MF, Tsai MY, Sharrett AR. LDL particle subclasses, LDL particle size, and carotid atherosclerosis in the Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis. 2007;192:211–217. doi: 10.1016/j.atherosclerosis.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 16.Harrell FE, Jr., Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 17.Lemeshow S, Hosmer DW., Jr. A review of goodness of fit statistics for use in the development of logistic regression models. Am J Epidemiol. 1982;115:92–106. doi: 10.1093/oxfordjournals.aje.a113284. [DOI] [PubMed] [Google Scholar]
- 18.Pencina MJ, D'Agostino RB, Sr., D'Agostino RB, Jr., Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 19.D'Agostino RB, Sr., Grundy S, Sullivan LM, Wilson P. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286:180–187. doi: 10.1001/jama.286.2.180. [DOI] [PubMed] [Google Scholar]
- 20.Kuller L, Arnold A, Tracy R, Otvos J, Burke G, Psaty B, Siscovick D, Freedman DS, Kronmal R. Nuclear magnetic resonance spectroscopy of lipoproteins and risk of coronary heart disease in the cardiovascular health study. Arterioscler Thromb Vasc Biol. 2002;22:1175–1180. doi: 10.1161/01.atv.0000022015.97341.3a. [DOI] [PubMed] [Google Scholar]
- 21.Blake GJ, Otvos JD, Rifai N, Ridker PM. Low-density lipoprotein particle concentration and size as determined by nuclear magnetic resonance spectroscopy as predictors of cardiovascular disease in women. Circulation. 2002;106:1930–1937. doi: 10.1161/01.cir.0000033222.75187.b9. [DOI] [PubMed] [Google Scholar]
- 22.Otvos J, Collins D, Freedman DS, Shalaurova I, Schaefer EJ, McNamara JR, Bloomfield HE, Robins SJ. LDL and HDL particle subclasses predict coronary events and are changed favorable by gemfibrozil therapy in the Veterans Affairs HDL Intervention Trial (VA-HIT). Circulation. 2006;113:1556–1563. doi: 10.1161/CIRCULATIONAHA.105.565135. [DOI] [PubMed] [Google Scholar]
- 23.El Harchaoui K, van der Steeg WA, Stroes ES, Kuivenhoven JA, Otvos JD, Wareham NJ, Hutten BA, Kastelein JJ, Khaw KT, Boekholdt SM. Value of low-density lipoprotein particle number and size as predictors of coronary artery disease in apparently healthy men and women: the EPIC-Norfolk Prospective Population Study. J Am Coll Cardiol. 2007;49:547–553. doi: 10.1016/j.jacc.2006.09.043. [DOI] [PubMed] [Google Scholar]
- 24.Cromwell WC, Otvos J, Keyes MJ, Pencina MJ, Sullivan D, Vasan RS, Wilson PWF, D'Agostino RB. LDL particle number and risk for future cardiovascular disease in the Framingham Offspring Study - implications for LDL management. J Clin Lipidol. 2007;1:583–592. doi: 10.1016/j.jacl.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 26.Lamarche B, Tchernof A, Moorjani S, Cantin B, Dagenais GR, Lupien PJ, Despres JP. Small, dense low-density lipoprotein particles as a predictor of the risk of ischemic heart disease in men. Prospective results from the Quebec Cardiovascular Study. Circulation. 1997;95:69–75. doi: 10.1161/01.cir.95.1.69. [DOI] [PubMed] [Google Scholar]
- 27.Walldius G, Jungner I, Holme I, Aastveit AH, Kolar W, Steiner E. High apolipoprotein B, low apolipoprotein A-I, and improvement in the prediction of fatal myocardial infarction (AMORIS study): a prospective study. Lancet. 2001;358:2026–2033. doi: 10.1016/S0140-6736(01)07098-2. [DOI] [PubMed] [Google Scholar]
- 28.Sharrett AR, Ballantyne CM, Coady SA, Heiss G, Sorlie PD, Catellier D, Patsch W. Coronary heart disease prediction from lipoprotein cholesterol levels, triglycerides, lipoprotein(a), apolipoproteins A-I and B, and HDL density subfractions: The Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2001;104:1108–1113. doi: 10.1161/hc3501.095214. [DOI] [PubMed] [Google Scholar]
- 29.Ridker PM, Rifai N, Cook NR, Bradwin G, Buring JE. Non-HDL cholesterol, apolipoproteins A-I and B100, standard lipid measures, lipid ratios, and CRP as risk factors for cardiovascular disease in women. JAMA. 2005;294:326–333. doi: 10.1001/jama.294.3.326. [DOI] [PubMed] [Google Scholar]
- 30.Ingelsson E, Schaefer EJ, Contois JH, McNamara JR, Sullivan L, Keyes MJ, Pencina MJ, Schoonmaker C, Wilson PW, D'Agostino RB, Vasan RS. Clinical utility of different lipid measures for prediction of coronary heart disease in men and women. JAMA. 2007;298:776–785. doi: 10.1001/jama.298.7.776. [DOI] [PubMed] [Google Scholar]
- 31.Singh IM, Shishehbor MH, Ansell BJ. High-density lipoprotein as a therapeutic target: a systematic review. JAMA. 2007;298:786–798. doi: 10.1001/jama.298.7.786. [DOI] [PubMed] [Google Scholar]
- 32.Festa A, Williams K, Hanley AJ, Otvos JD, Goff DC, Wagenknecht LE, Haffner SM. Nuclear magnetic resonance lipoprotein abnormalities in prediabetic subjects in the Insulin Resistance Atherosclerosis Study. Circulation. 2005;111:3465–3472. doi: 10.1161/CIRCULATIONAHA.104.512079. [DOI] [PubMed] [Google Scholar]
- 33.Tilly-Kiesi M, Knudsen P, Groop L, Taskinen MR. Hyperinsulinemia and insulin resistance are associated with multiple abnormalities of lipoprotein subclasses in glucose-tolerant relatives of NIDDM patients. Botnia Study Group. J Lipid Res. 1996;37:1569–1578. [PubMed] [Google Scholar]
- 34.Ishigami M, Yamashita S, Sakai N, Arai T, Hirano K, Hiraoka H, Kameda-Takemura K, Matsuzawa Y. Large and cholesteryl ester-rich high-density lipoproteins in cholesteryl ester transfer protein (CETP) deficiency can not protect macrophages from cholesterol accumulation induced by acetylated low-density lipoproteins. J Biochem (Tokyo) 1994;116:257–262. doi: 10.1093/oxfordjournals.jbchem.a124516. [DOI] [PubMed] [Google Scholar]
- 35.Yvan-Charvet L, Matsuura F, Wang N, Bamberger MJ, Nguyen T, Rinninger F, Jiang XC, Shear CL, Tall AR. Inhibition of cholesteryl ester transfer protein by torcetrapib modestly increases macrophage cholesterol efflux to HDL. Arterioscler Thromb Vasc Biol. 2007;27:1132–1138. doi: 10.1161/ATVBAHA.106.138347. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.