Abstract
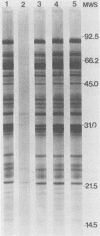
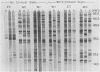
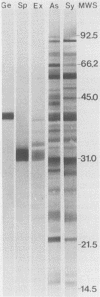
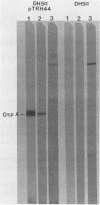
Immunoblots were used to study the immunoglobulin G response to Borrelia burgdorferi in experimentally and naturally exposed dogs. Adsorption studies confirmed that the antibodies were specific for B. burgdorferi. Experimentally exposed dogs were asymptomatic. Naturally exposed dogs included both asymptomatic animals and animals showing signs compatible with Lyme disease. Naturally exposed dogs were from four geographic regions of the country. No differences were detected between immunoblot patterns of naturally exposed symptomatic or asymptomatic dogs from different areas of the country. The immunoblot patterns obtained with sera from experimentally exposed dogs were different from those obtained with sera from naturally exposed dogs and were characterized by reactivity to fewer and different protein bands. Immunoblot analysis using an OspA-protein-producing Escherichia coli recombinant showed that experimentally exposed dogs produced antibodies to OspA, whereas naturally exposed dogs did not. Modifications of the immune response over time, different routes of antigen presentation, and strain variation are factors postulated to account for the observed differences.
Full text
PDF


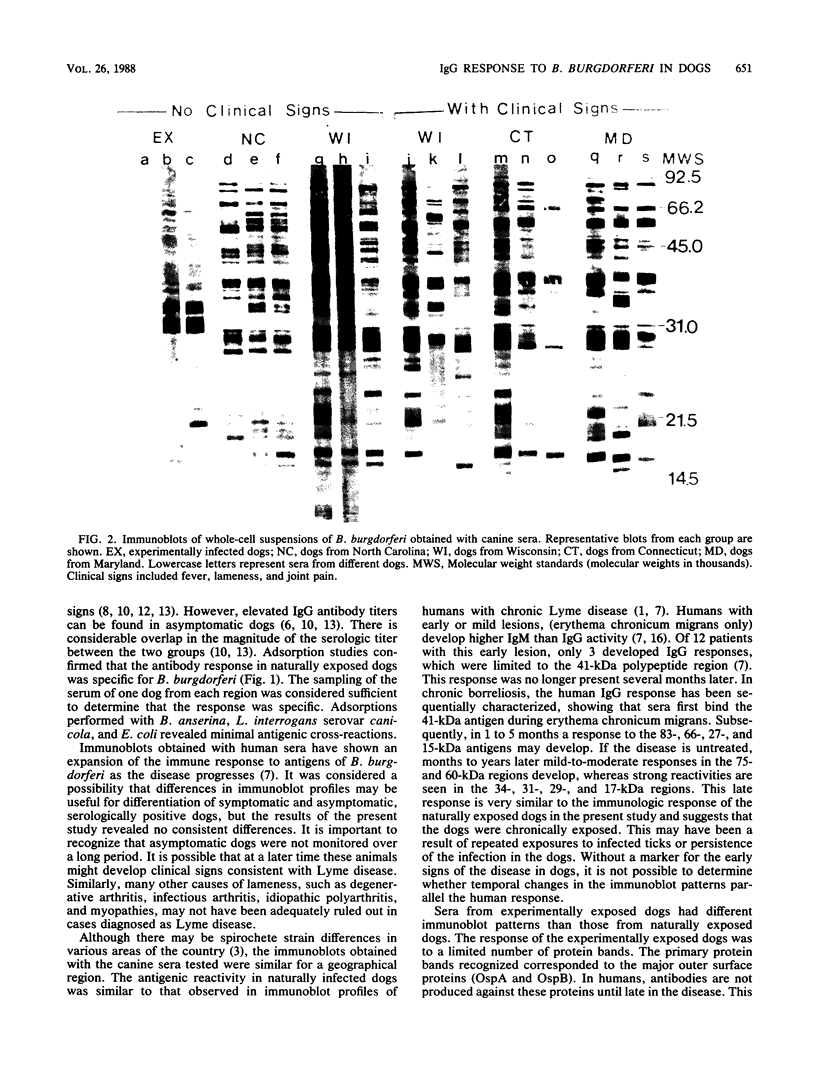


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbour A. G., Burgdorfer W., Grunwaldt E., Steere A. C. Antibodies of patients with Lyme disease to components of the Ixodes dammini spirochete. J Clin Invest. 1983 Aug;72(2):504–515. doi: 10.1172/JCI110998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Hayes S. F., Heiland R. A., Schrumpf M. E., Tessier S. L. A Borrelia-specific monoclonal antibody binds to a flagellar epitope. Infect Immun. 1986 May;52(2):549–554. doi: 10.1128/iai.52.2.549-554.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Tessier S. L., Hayes S. F. Variation in a major surface protein of Lyme disease spirochetes. Infect Immun. 1984 Jul;45(1):94–100. doi: 10.1128/iai.45.1.94-100.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Tessier S. L., Todd W. J. Lyme disease spirochetes and ixodid tick spirochetes share a common surface antigenic determinant defined by a monoclonal antibody. Infect Immun. 1983 Aug;41(2):795–804. doi: 10.1128/iai.41.2.795-804.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess E. C. Experimental inoculation of dogs with Borrelia burgdorferi. Zentralbl Bakteriol Mikrobiol Hyg A. 1986 Dec;263(1-2):49–54. doi: 10.1016/s0176-6724(86)80102-x. [DOI] [PubMed] [Google Scholar]
- Burgess E. C. Natural exposure of Wisconsin dogs to the Lyme disease spirochete (Borrelia burgdorferi). Lab Anim Sci. 1986 Jun;36(3):288–290. [PubMed] [Google Scholar]
- Craft J. E., Fischer D. K., Shimamoto G. T., Steere A. C. Antigens of Borrelia burgdorferi recognized during Lyme disease. Appearance of a new immunoglobulin M response and expansion of the immunoglobulin G response late in the illness. J Clin Invest. 1986 Oct;78(4):934–939. doi: 10.1172/JCI112683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe T. R., LaQuier F. W., Barbour A. G. Organization of genes encoding two outer membrane proteins of the Lyme disease agent Borrelia burgdorferi within a single transcriptional unit. Infect Immun. 1986 Oct;54(1):207–212. doi: 10.1128/iai.54.1.207-212.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornblatt A. N., Urband P. H., Steere A. C. Arthritis caused by Borrelia burgdorferi in dogs. J Am Vet Med Assoc. 1985 May 1;186(9):960–964. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lissman B. A., Bosler E. M., Camay H., Ormiston B. G., Benach J. L. Spirochete-associated arthritis (Lyme disease) in a dog. J Am Vet Med Assoc. 1984 Jul 15;185(2):219–220. [PubMed] [Google Scholar]
- Magnarelli L. A., Anderson J. F., Kaufmann A. F., Lieberman L. L., Whitney G. D. Borreliosis in dogs from southern Connecticut. J Am Vet Med Assoc. 1985 May 1;186(9):955–959. [PubMed] [Google Scholar]
- Schwan T. G., Burgdorfer W. Antigenic changes of Borrelia burgdorferi as a result of in vitro cultivation. J Infect Dis. 1987 Nov;156(5):852–853. doi: 10.1093/infdis/156.5.852-a. [DOI] [PubMed] [Google Scholar]
- Steere A. C., Grodzicki R. L., Kornblatt A. N., Craft J. E., Barbour A. G., Burgdorfer W., Schmid G. P., Johnson E., Malawista S. E. The spirochetal etiology of Lyme disease. N Engl J Med. 1983 Mar 31;308(13):733–740. doi: 10.1056/NEJM198303313081301. [DOI] [PubMed] [Google Scholar]
- Steere A. C., Malawista S. E., Bartenhagen N. H., Spieler P. N., Newman J. H., Rahn D. W., Hutchinson G. J., Green J., Snydman D. R., Taylor E. The clinical spectrum and treatment of Lyme disease. Yale J Biol Med. 1984 Jul-Aug;57(4):453–461. [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]