Abstract
Accumulating evidence suggests that protein tyrosine phosphorylation-based signaling pathways are under the regulation of reactive oxygen species. Although protein tyrosine phosphatases are directly regulated by reversible oxidation, it is not clear whether protein tyrosine kinases (PTKs) are also directly regulated by reduction/oxidation (redox). In this study we report a mechanism of direct oxidative inactivation specific for the PTKs in the Src and fibroblast growth factor receptor (FGFR) families, key enzymes in mammalian signal transduction. Src is fully active when reduced and retains 8–25% of the full activity toward various substrates when oxidized. This inactivation is caused by oxidation of a specific cysteine residue (Cys-277), which results in homodimerization of Src linked by a disulfide bridge. Cys-277 is located in the Gly loop in the catalytic domain. This cysteine residue is conserved only in 8 of the >90 PTKs in the human kinome, including 3 of the 10 Src family kinases and all 4 kinases of the FGFR family. FGFR1 is also reversibly regulated by redox because of this cysteine residue, whereas Csk, a PTK that lacks a cysteine residue at the corresponding position, is not similarly regulated. These results demonstrate a mechanism of direct redox regulation conserved in certain specific PTKs.
Reactive oxygen species (ROS), such as hydrogen peroxide and superoxide, can alter the function of proteins by oxidizing free sulfhydryl groups to sulfenic, sulfinic, or sulfonic acids (1, 2). Cellular responses to ROS are historically considered a damage-control mechanism to certain pathological situations that lead to oxidative stress. However, recent studies indicate that certain growth factors and cell adhesion also stimulate the production of ROS, which serve as secondary messengers to regulate downstream signaling pathways (3, 4). Numerous protein phosphorylation pathways respond to ROS (5–9), and identifying the proteins and the residues sensitive to oxidation will help elucidate the mechanism of cross-talk between redox and protein tyrosine phosphorylation.
The level of protein tyrosine phosphorylation is the function of opposing actions of protein tyrosine kinases (PTKs) and protein tyrosine phosphatases (PTPs). All PTPs contain a catalytic Cys residue in the active site, and oxidation of this residue leads to the inactivation of the PTP activity (10). This response is recognized as a major mechanism by which ROS regulate the level of protein tyrosine phosphorylation. However, whether PTKs also directly respond to ROS is not established. PTK Src is a key regulator of cell survival, cytoskeleton reorganization, DNA synthesis, and cell division (11, 12). A number of studies suggest that Src also plays an important role in cellular response to ROS, because Src specific inhibitors and dominant-negative Src mutants strongly attenuate cellular response to ROS (13–16). However, how ROS regulate Src activity has been controversial, likely reflecting the complexity of Src regulation. Src contains regulatory structures such as a myristoylation motif, a unique region, a Src homology 3 (SH3) domain, an SH2 domain, and a regulatory tail in addition to the catalytic domain (11). Src activity is regulated directly by reversible phosphorylation on multiple Tyr and Ser residues (11, 17, 18), interaction with cell surface receptors through its SH2 domain (19), Pro-rich proteins through its SH3 domain (20), and activated α-subunit of trimeric G proteins (21). Thus, in cell-based studies it is often difficult to differentiate the direct effect of redox regulation on Src activity from the indirect effects through Src regulators. For example, both activation (22) and inactivation (23, 24) of Src in response to oxidative stress have been reported. This inconsistency is likely because the activation or inactivation is accompanied by an increase in Src phosphorylation level on Tyr-416 or Tyr-527. Because phosphorylation of Tyr-416 and Tyr-527, respectively, activates (17) and inactivates (18) Src family kinases, it is not clear whether ROS directly regulate Src kinases or simply inactivate the PTPs that dephosphorylate these Tyr phosphorylation sites.
In the current study, we use purified Src enzyme and mutants to demonstrate a direct and specific Src response to ROS and identify the underlying mechanistic basis for the response. Src is fully active when reduced and inactivated by oxidation. When oxidized, Src forms a disulfide-linked dimer between 2 Cys-277 residues. Further, fibroblast growth factor receptor type 1 (FGFR1), as one of the few PTKs with a corresponding Cys residue, is also inactivated by oxidation because of this Cys residue, whereas Csk, which lacks a corresponding Cys residue, is not similarly regulated. These results reveal a distinct mechanism for redox regulation of specific PTKs.
Results
Sensitivity of Src Kinase Activity to Reducing/Oxidizing Conditions.
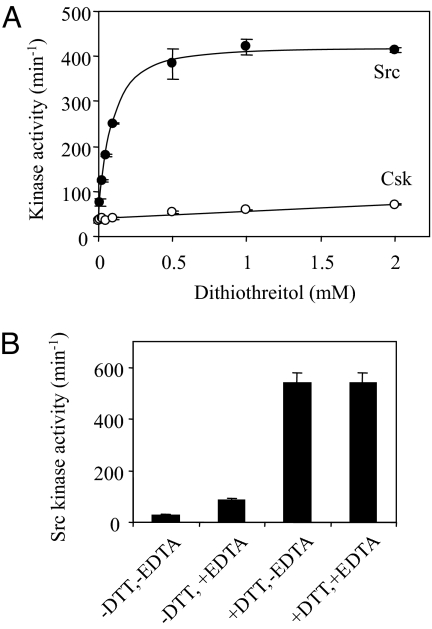
Given the inconsistency in the literature regarding Src response to ROS, we sought to address this issue directly by using purified Src enzyme. To determine whether Src kinase activity directly responds to redox modulation, the kinase activity of purified Src was determined in the presence of varying concentrations of DTT. The Src protein is purified by Ni2+-affinity chromatography in the absence of a reducing agent. As shown in Fig. 1A, Src has a turnover number of 30 min−1 in the absence of DTT, and the activity increases to ≈400 min−1 in the presence of 1 mM or higher concentrations of DTT. This activation of 13-fold is not caused by DTT-chelating metal cation inhibitors that might be present in the assay medium, because the addition of 0.2 mM ethylene diamine tetraacetic acid only slightly stimulates the kinase activity (Fig. 1B). This strong activation by the reducing condition is not observed for another PTK, Csk, which is only mildly activated by DTT (Fig. 1A). The modest activation of Csk by DTT is caused by the reduction of a disulfide bond between Cys-122 and Cys-164 located in the SH2 domain (25). We also tested whether Src activity responds to the presence of H2O2. The presence of up to 20 mM H2O2 has no effect on Src kinase activity directly, but it can reverse the activation by DTT over time. These observations indicate that the absence of a reducing agent alone is sufficiently oxidizing to cause Src inactivation. These results indicate that Src kinase activity is specifically sensitive to redox modulation and suggest that there is a specific redox-sensing mechanism built into the structure of Src.
Fig. 1.
Responses of Src and Csk kinase activity to DTT. (A) The kinase activities of Src and Csk at different concentrations of DTT were determined as described. The results are based on 3 independent assays, with standard errors represented by the error bars. (B) Effects of 1 mM DTT and 1 mM EDTA individually or in combination on Src kinase activity.
Cys-277 Is Responsible for Src Oxidation.
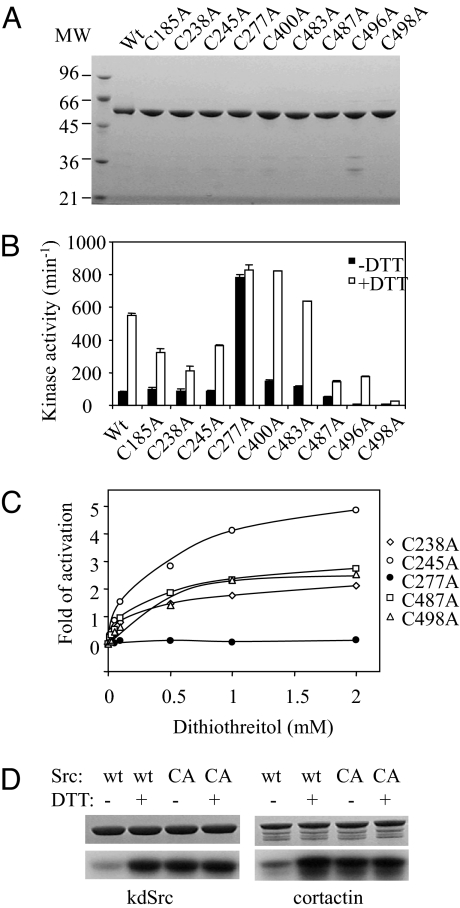
The sensitivity of Src activity to oxidation suggests that certain Cys residues in Src are prone to reversible oxidation that inactivates Src. The Src enzyme contains 9 Cys residues. To determine which Cys residue is responsible for oxidation, each Cys residue was individually mutated to Ala and the mutant enzymes were purified (Fig. 2A). The kinase activities of these mutant enzymes in the presence and absence of 1 mM DTT were determined (Fig. 2B). Although the mutations have varying effects on the kinase activity, all except 1 mutant retain a clear activation by DTT. The lone exception is Src-Cys-277–Ala (Src-C277A), which displays approximately the same kinase activity in the presence and absence of DTT (Fig. 2B), indicating that Cys-277 alone is responsible for the redox sensitivity. Indeed, Src-C277A completely loses its dose-dependent activation by DTT (Fig. 2C) and remains fully active even in the absence of any reducing agent, whereas all other mutants display a dose-dependent activation by DTT (Fig. 2C). It is noted that some mutants are less sensitive to activation by DTT compared with WT Src, but they still respond to activation by DTT, indicating that the redox-sensitive Cys residue is still present in these mutants.
Fig. 2.
Src Cys-to-Ala mutants and their responses to DTT. (A) WT Src and 9 Cys-Ala mutants were analyzed by SDS/PAGE under reducing conditions. Two micrograms of protein was loaded in each lane, and the gel was stained by Coomassie brilliant blue. (B) The kinase activity of WT Src and Cys-Ala mutants in the presence of absence of 1 mM DTT. (C) Dose-dependent activation of selected Cys-Ala mutants of Src. The activity of each enzyme in the absence of the reducing agent is used as the base, and the fold of activation is the fold of increase at a given DTT concentration over that base level. (D) Phosphorylation of kdSrc and cortactin by Src (wt) and Src-C277A (CA) in the presence or absence of 2 mM DTT. The Src and DTT combinations are indicated on the top, and the substrates are indicated at the bottom. (Upper) Coomassie blue-stained gels. (Lower) Autoradiograms.
The above analysis used polyE4Y, a copolymer of Glu and Tyr in the ratio of 4:1, as a substrate. To confirm the redox effect on Src kinase activity with physiological Src substrates, we determined the effect of 2 mM DTT of Src phosphorylation of 2 physiological substrates Src (kinase-defective Src) and cortactin (Fig. 2D). DTT (2 mM) stimulates Src activity 4-fold toward either substrate according to scintillation counting of the gel slices. Mutation of C277A abolished the sensitivity to DTT. These results indicate that oxidative inactivation of Src also applies to physiological substrates.
WT Src Forms a Cys-277-Dependent Disulfide Dimer When Oxidized.
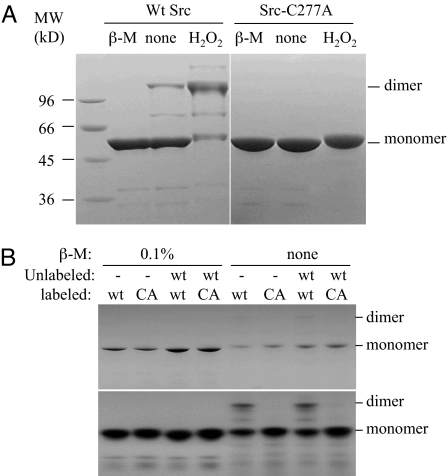
A Cys residue can be oxidized to 3 different states, sulfenic (−SOH), sulfinic (−SO2H), or sulfonic (−SO3H) acid. Two Cys-sulfenic groups can form a disulfide bond, which can be reversed by reduction. Alternatively, a Cys-sulfenic group can also form a sulfenyl-amide intermediate with a neighboring backbone amine, which is also reversible (26, 27). Both mechanisms would prevent the Cys-sulfenic group from further oxidation to a sulfonic group, which cannot be reduced back to the free sulfhydryl group (1). In the tertiary structure of Src (28), Cys-277 is not near any other Cys residue. For it to form an intramolecular disulfide bond with any other Cys residue would require gross alterations to the tertiary structure. We tested whether Src forms a dimer linked by an intermolecular disulfide bond. When WT Src is fractionated in the presence of βmercaptoethanol, it runs as a 55-kDa protein, the expected size of the monomeric Src (Fig. 3A Left). In the absence of the reducing agent, while a majority of Src runs as the monomer, a significant portion runs as a 110-kDa protein, the apparent molecular mass of a dimer. Incubation of Src with H2O2 results in a significant increase in the 110-kDa band and a simultaneous decrease in the monomer. These observations indicate that when Src is purified in the absence of a reducing agent a significant portion is present as a dimer, and the dimer can be reduced into monomers by a reducing agent.
Fig. 3.
Analysis of Src dimerization caused by oxidation. (A) WT Src (Left) and Src-C277A mutant (Right) were fractionated in the absence or presence of 2% β-mercaptoethanol or H2O2. The positions of the expected monomer and dimer of Src are indicated on the right. (B) Detection of dimer between Src-C277A and WT Src. WT Src and Src-C277A (CA) were radioactively labeled by autophosphorylation. The dimer formation of different combinations of labeled and unlabeled Src as indicated is analyzed by SDS/PAGE and visualized by Coomassie staining (Upper) and autoradiography (Lower). The positions of the expected monomer and dimer are indicated.
If oxidized Src indeed forms a disulfide dimer, and Cys-277 is the residue that is oxidized, then Src-C277A mutant would be expected not to form such a disulfide dimer. Indeed, no dimer is detectable under any of the conditions that lead to significant dimer formation for WT Src (Fig. 3A Right). This result demonstrates that dimer formation by oxidized Src depends on Cys-277.
Although the above results compellingly demonstrate that the disulfide dimerization of Src depends on Cys-277, they do not exclude the possibility that the disulfide bond is between Cys-277 of one Src molecule and a different Cys residue in another Src molecule. To test this possibility, we examined whether radioactively-labeled Src-C277A could form a disulfide dimer with unphosphorylated WT Src. If the disulfide bond is between Cys-277 of one molecule to a different Cys residue in another molecule, Src-C277A would be able to form a disulfide dimer with WT Src. Radioactively-labeled WT Src or Src-C277A was mixed with equal amounts of unlabeled WT Src, and the mixtures were fractionated by SDS/PAGE (Fig. 3B). Under reducing condition, WT Src or C277A does not dimerize either alone or in the presence of unlabeled WT Src. Under oxidizing condition, labeled WT Src dimerizes either alone or when mixed with unlabeled WT Src. However, oxidizing condition does not lead to dimer formation between Src-C277A and WT Src (Fig. 3B). This result indicates that no other Cys residue in Src-C277A is capable of forming disulfide bond with a Cys-277 from the WT Src. Thus, the intermolecular disulfide bond is formed between 2 Cys-277 residues in 2 Src molecules when oxidized. This result is consistent with the fact that no other Cys residue is found to be required for the redox sensitivity by the mutagenic analysis presented earlier.
FGFR1 Responds to Redox Regulation in a Similar Manner.
The identification of Cys-277 of Src as the redox sensor raises the question of whether this regulation is unique to Src or is also present in other PTKs. Cys-277 in Src is located in the small N-terminal lobe of the catalytic domain in the sequence context of GQGCFG, referred to as the Gly loop. The 3 Gly residues are a universally-conserved signature motif among all protein kinases, even though the intervening residues are variable. Kinetic and structural studies of both protein Ser/Thr kinase, cAMP-dependent protein kinases (29), and a PTK, FPS (30), indicate that this loop is directly involved in catalysis. An examination of the amino acid sequences of all human PTKs indicates that only a small number of PTKs contain a Cys residue at the position equivalent to Cys-277 in Src (Table S1). Among the 10 members of the Src kinase family, only 3 (Src, Yes, and Fgr) contain a Cys residue, whereas most other members contain a Gln at the corresponding position. The receptor kinases in the FGFR family contain 4 members, FGFR1 through FGFR4, and all contain a Cys residue (Cys-488 in FGFR1) at the equivalent position. Only 1 additional PTK, Tnk1 in the Ack family, in the human tyrosine kinome contains a Cys at the equivalent position, which raises the possibility that the identified mechanism of redox regulation is conserved in and unique to these PTKs. As a representative of the FGFR family, FGFR1 was next tested for its sensitivity to redox modulation.
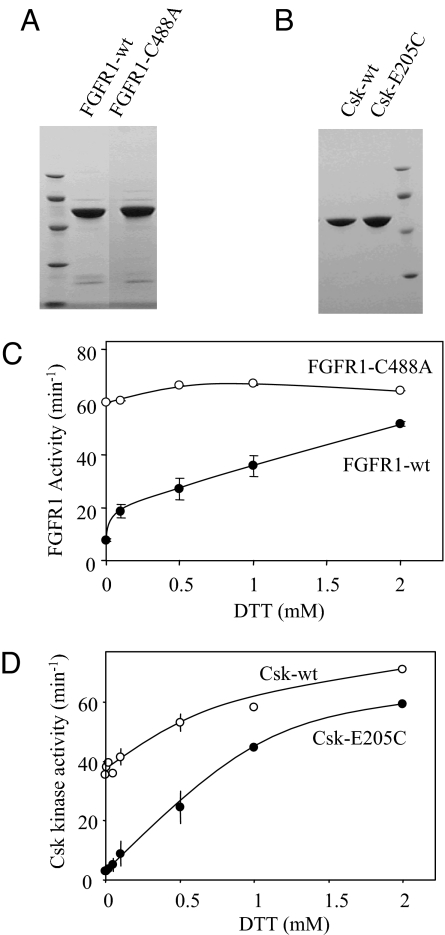
Both FGFR1-WT and FGFR1–Cys-488–Ala were expressed and purified (Fig. 4A). The kinase activity of FGFR1-WT has a strong dependence on the presence of DTT. It has a kinase activity of ≈8 min−1 in the absence of a reducing agent and is activated to an activity of 51 min−1 by DTT in a dose-dependent manner. The mutation of Cys-488–Ala completely abolishes the sensitivity of FGFR1 activity to DTT and renders FGFR1 fully active regardless of the redox condition (Fig. 4C). This result demonstrates that FGFR1 is also subject to redox regulation because of the Cys residue in the Gly loop. FGFR1 also forms a disulfide-linked dimer under oxidizing condition. However, mutation of Cys-488–Ala did not completely abolish the dimerization, indicating that oxidation of other Cys residues may also result in dimerization.
Fig. 4.
Sensitivity of FGFR1 and Csk to redox regulation. (A) WT FGFR1 catalytic domain and a Cys-488–Ala mutant were purified. (B) WT Csk and a Glu-205–Ala mutant were purified. (C) The kinase activity (turnover number) of FGFR1 and FGFR1–Cys-488–Ala as a function of DTT concentration in the kinase assay. (D) The kinase activity (turnover number) of Csk and Csk–Glu-205–Ala as a function of DTT concentration in the kinase assay.
We then determined whether a Cys residue at the equivalent position is sufficient to confer redox sensitivity to a kinase that is otherwise insensitive to redox. As shown earlier, Csk is marginally sensitive to redox modulation because of the formation of an intramolecular disulfide bond in the SH2 domain (25). Csk contains a Glu residue, Glu-205, at the position equivalent to Cys-277 of Src. A Csk mutant (Csk–Glu-205–Cys) was purified and tested for its response to redox (Fig. 4 B and D). Although WT Csk is activated by DTT only marginally, Csk–Glu-205–Cys is activated ≈20-fold by 2 mM DTT, from 2.8 min−1 to 59 min−1 (Fig. 4D). This result demonstrates that Csk and potentially other PTKs could be made redox-sensitive by the introduction of a Cys residue at this position.
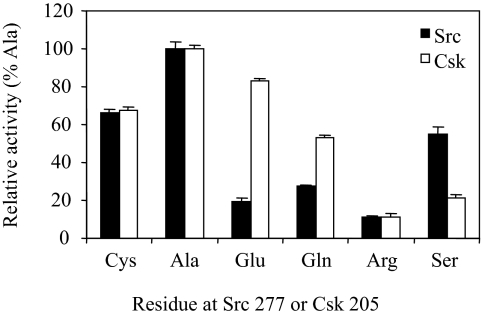
Residue Identity at the Cys-277 Position in Src and the Equivalent Position in Csk Dictate the Kinase Activity.
The fact that oxidation of the Cys residue in the Gly loop can inactivate a PTK suggests that the Gly loop is a motif sensitive to regulation. Thus, the identity of the residue at this position would be expected to dictate the kinase activity. To test this idea, Cys-277 in Src and Glu-205 in Csk were mutated to several representative residues, Ala, Gln, Arg, and Ser, in addition to Cys and Glu. In both Csk and Src, the most active residue is Ala, and the least active is Arg, rendering ≈10% of that of Ala (Fig. 5). A reduced Cys gives both kinases ≈65% of the activity of the Ala mutants. There is significant variation regarding Gln, Ser, and especially Glu. Although the activity of Csk with a Glu at this position is close to that of Ala, Src with a Glu at this position displays only 20% of the activity of the Ala mutant, which provides an explanation for the selection of Glu by Csk. Overall, these results support the idea that the identity of the residue at this position dictates the catalytic activity of a kinase.
Fig. 5.
The effect on kinase activity of Src and Csk by the residue identity at positions 277 and 205, respectively. A group of Src mutants, where Cys-277 was mutated to Ala, Glu, Gln, Arg, or Ser, was analyzed for their kinase activity under reducing condition. Similarly, a group of Csk mutants, where Glu-205 was mutated to Cys, Ala, Gln, Arg, or Ser was similarly analyzed.
Discussion
Several studies have demonstrated an important role of Src in cell response to ROS (13–16), but how Src responds to ROS has not been determined. This current study, using purified Src enzyme and mutants, elucidates a mechanism by which Src is directly inactivated by oxidation. The inactivation is caused by oxidation of Cys-277, which leads to the formation of a disulfide homodimer. Furthermore, this mechanism is uniquely conserved in part of the Src family and the FGFR family. These findings provide a distinct mechanism for ROS to directly regulate signaling by Src and FGFR kinases. Our study defines a mechanism by which a group of specific kinases are directly regulated by ROS. A recent study demonstrates that Src is directly oxidized in cells under oxidative stress (22), but the Cys residue responsible for oxidation has not been identified.
The approach in this current study has one clear advantage over cell-based studies in defining the effect of oxidation on Src activity and the underlying mechanism for the effect. The effects of oxidation on Src have been inconsistent in the literature. Some reports suggest that Src is activated by ROS (22), whereas others indicate that Src is inactivated (23, 24). A survey of the literature identifies 2 potential reasons for this inconsistency. First, Src is regulated by numerous mechanisms, such as activation by autophosphorylation on Tyr-416, ligands to the SH3 and SH2 domains, and activated G proteins, and inactivation by phosphorylation on Tyr-527. It is difficult to distinguish whether the effect of ROS is caused by direct oxidation of Src or indirectly through other regulatory mechanisms. Indeed, reported Src activation by ROS is accompanied by increased phosphorylation on Tyr-416 (22), whereas inactivation is invariably accompanied by increased phosphorylation of Tyr-527 (23, 24). Second, cell-based studies rely on immunoprecipitation to isolate Src. The redox state of a given Cys residue can be readily altered during cell lysis and immunoprecipitation, thus the final redox state of immunoprecipitated Src may not correlate to the original treatment to the cells. This reversibility precludes any reliable conclusion about a relationship between the final Src activity level and the original treatment. In contrast, using purified enzymes and mutants, the direct effects of oxidation can be determined without the interference by other regulatory mechanisms, and the Cys residue responsible for the oxidation can be identified.
How the oxidative inactivation of Src fits in the overall regulation of Src kinases remains to be determined. Because Src is under the regulation of numerous mechanisms, redox regulation could cross-talk with any of these mechanisms in addition to directly affecting Src kinase activity. The cross-talk could occur on 2 levels. First, ROS may regulate any of the upstream regulatory kinases, phosphatases and G proteins, which would indirectly regulate Src activity. For example, the SH2 domain of Csk contains a novel disulfide bond, which modulates the Csk activity (25). By regulating Csk activity, ROS could indirectly alter Src activity. Second, oxidation of Cys-277 of Src may alter how Src responds to any of these other regulatory mechanisms. For example, a recent structural study (31) reveals a disulfide bond between Src Cys-277 and Csk Cys-290, linking Src from one Csk–Src kinase–substrate complex to Csk from another Csk–Src complex. We demonstrate (Fig. S1) that oxidized Src actually prefers Csk as a partner for forming disulfide dimer. It is possible that redox regulation could modulate Src phosphorylation and/or the subsequent inactivation by Csk. It is worth noting that only Src, Yes, and Fgr contain a Cys residue in the Gly loop. This selectivity could provide a mechanism for specifically regulating some Src kinases. The physiological implications of these observations remain to be examined.
The finding of Cys-277 being responsible for Src oxidation raises the questions of why a Cys residue is sensitive to oxidation whereas others are not. Cys-277 is located in the Gly loop of the Src catalytic domain. In the crystal structure of Src (28), the side chain of Cys-277 is readily accessible, which may explain its sensitivity to oxidation. Furthermore, kinase activity appears particularly sensitive to modification to the Gly (29, 30), as demonstrated for Src and Csk. All of these observations provide a mechanistic basis for the redox regulation at this residue. Disulfide dimer formation has not been previously reported for Src or FGFR. However, a symmetric dimer of FGFR2 has been recently reported (ref. 32; Protein Data Bank ID code 2PSQ). Even though a disulfide bond is not assigned in this structure, the 2 sulfur atoms from the equivalent Cys residues are only 3 Å apart, leaving the distinct possibility that a disulfide bridge can be formed between the 2 molecules of FGFR2.
The fact that only a small number of PTKs have the corresponding Cys residue suggests specificity to this potential regulation. Of 94 human PTKs, only 8 contain a Cys residue in the Gly loop: 4 members of the FGFR family, 3 in the Src family, and TNK1 of the Ack family. A thorough literature search identified no report of redox regulation of FGFRs, TNK1, Yes, or Fgr. Further investigation is required to determine how redox regulation fits into signaling by these kinases. The fact that the redox regulation is limited to select PTKs contrasts the situation in PTPs, all of which are inactivated by oxidation (10). This, of course, does not exclude other potential mechanisms by which a PTK may respond to redox regulation. Janus kinase 2 is also reversibly inactivated by oxidation (9) although it does not contain a Cys residue in the Gly loop.
Materials and Methods
Reagents and Chemicals.
All reagents used for bacterial culture and protein expression were purchased from Fisher. The chromatographic resin, iminodiacetic acid-Agarose, was purchased from Sigma. DNA primers were synthesized by Integrated DNA Technologies. [γ-32P]ATP (6,000 Ci·mol−1) was purchased from PerkinElmer.
Plasmid Construction.
The plasmid pRSET-Src was constructed as described (33). Src mutants were generated by introducing specific residue substitutions by using QuikChange (Stratagene). The pRSET-Src and mutant plasmids were introduced into Escherichia coli BL21 (DE3) cells harboring the pREP4groESL plasmid and pCDF-PTP1B by electroporation. The plasmid pREP4groESL contains the genes for the GroES/EL chaperone, which helps the correct folding of Src (34). The pCDF-PTP1B directs the expression of PTP1B, which keeps Src dephosphorylated and reduces the toxicity of Src expression to bacterial cells (35). The catalytic domain of FGFR1, Csk, and their respective mutants were expressed by the same system.
Protein Expression and Purification.
Src, Csk, FGFR1, and their mutants were expressed and purified as described below. In a 2-L flask, a single bacterial colony containing recombinant plasmids was inoculated into 400 mL of LB culture medium containing appropriate antibiotics. The cultures were grown to an OD600 of 2.5 and diluted with 400 mL of fresh LB containing the antibiotics. The cultures were air-cooled to ≈25 °C, and 0.4 mM isopropyl-β-d-thiogalactopyranoside was added to induce the production of the fusion protein. Cultures were allowed to induce for 4 h at 25 °C during shaking at 250 rpm × g. Bacterial cultures were harvested by centrifugation at 4,500 × g and stored at −20 °C.
Bacterial cell pellets were resuspended with ice-cold lysis buffer [50 mM Hepes (pH 8.0), 200 mM NaCl, 5 mM imidazole, and 0.1% Triton-X]. Cells were lysed by sonication and clarified by centrifugation at 47,800 × g for 30 min at 4 °C. The supernatant was added to 1.2 mL of iminodiacetic acid-Agarose beads charged with NiCl2 and gently mixed by rotation at 4 °C for 30 min. The beads were loaded into a column and then washed with wash buffer 1 (50 mM Hepes, pH 8.0, and 10 mM imidazole), wash buffer 2 (50 mM Hepes, pH 8.0, and 20 mM imidazole), and wash buffer 3 (50 mM Hepes, pH 8.0, and 30 mM imidazole). Proteins were eluted by using 200–500 mM imidazole in 50 mM Hepes (pH 8.0). Enzymes were desalted and stored in 50 mM Hepes (pH 8.0) at −20 °C in 40% glycerol. Protein concentration was determined by Bradford Reagent (BioRad) standardized with BSA (0.2–1.0 mg·mL−1). Purity of protein fractions was determined by 12% SDS/PAGE (BioRad) and stained with Coomassie blue.
Kinase Assays.
For quantifying PTK activities, we measured the phosphorylation of polyE4Y by using the acid precipitation assay (36). Standard assay reactions of 50 μL contained 75 mM 4-(2-Hydroxyethyl)-1-piperazinepropanesulfonic acid (EPPS) (pH 8.0), 1 mg·mL−1 polyE4Y, 200 μM [32]ATP (≈1,000 dpm/pmol), 12 mM MgCl2, 5% glycerol, and 0.005% Triton X-100. To determine the effect of reducing or oxidizing agents, DTT or H2O2, at the indicated concentrations were included in the reaction mixture. All assays were performed at least 3 times, and the reported activities are the average with standard errors.
To determine the effect of DTT on Src activity toward kdSrc or cortactin, 1 nM Src or Src-C277A was incubated with 10 μM kdSrc or cortactin in the presence or absence of 2 mM DTT under otherwise standard phosphorylation conditions for 30 min. (His)6-tagged cortactin was expressed and purified from bacteria. The reaction was terminated by the addition of SDS/PAGE sample buffer containing 2% β-mercaptoethanol. Samples were boiled for 5 min and fractionated by 12% SDS/PAGE, and the gels were stained with Coomassie blue. The level of phosphorylation was determined by autoradiography and scintillation counting of the excised bands.
Analysis of Dimer Formation.
Disulfide dimers were visualized by SDS/PAGE in the absence of 2% β-mercaptoethanol and Coomassie blue staining, or autoradiography when the proteins were radioactively labeled. WT Src and Src-C277A were labeled with 32P by incubating 1 mg of each protein with 2 mM DTT, 200 μM [32]ATP (≈2,500 cpm/pmol), and 12 mM MgCl2 in the kinase assay buffer for 60 min at 30 °C. The labeled proteins were desalted on Econo-Pac 10DG columns (BioRad) equilibrated with 50 mM Hepes, 1 mM EDTA, and 1 mM DTT (pH 8.0). Approximately 10 μg of each protein, labeled and/or nonlabeled, was combined in the presence of 1 mM DTT to maintain Src in the monomer form. Samples were then subjected to oxidizing conditions by the addition of 20 mM H2O2, boiled for 5 min in SDS/PAGE sample buffer in the presence or absence of 2% β-mercaptoethanol, and fractionated by 10% SDS/PAGE stained with Coomassie blue. Radioactively-labeled fractions were visualized by exposing gel to autoradiographic film (Sigma) for 2 h.
Supplementary Material
Acknowledgments.
DNA sequencing was performed at the University of Rhode Island Genomics and Sequencing Center. This work was supported by American Cancer Society Grant RSG-04-247-01-CDD and National Institutes of Health Grant 1RO1CA111687.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0806117106/DCSupplemental.
References
- 1.Meng TC, Lou YW, Chen YY, Hsu SF, Huang YF. Cys-oxidation of protein tyrosine phosphatases: Its role in regulation of signal transduction and its involvement in human cancers. J Cancer Mol. 2006;2:9–16. [Google Scholar]
- 2.Saurin AT, Neubert H, Brennan JP, Eaton P. Widespread sulfenic acid formation in tissues in response to hydrogen peroxide. Proc Natl Acad Sci USA. 2004;101:17982–17987. doi: 10.1073/pnas.0404762101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 4.Rhee SG. Cell signaling. H2O2, a necessary evil for cell signaling. Science. 2006;312:1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- 5.Pu M, et al. Evidence of a novel redox-linked activation mechanism for the Src kinase which is independent of tyrosine 527-mediated regulation. Oncogene. 1996;13:2615–2622. [PubMed] [Google Scholar]
- 6.Chowdhury AK, et al. Src-mediated tyrosine phosphorylation of p47phox in hyperoxia-induced activation of NADPH oxidase and generation of reactive oxygen species in lung endothelial cells. J Biol Chem. 2005;280:20700–20711. doi: 10.1074/jbc.M411722200. [DOI] [PubMed] [Google Scholar]
- 7.Sato M, et al. c-Src and hydrogen peroxide mediate transforming growth factor-β1-induced smooth muscle cell-gene expression in 10T1/2 cells. Arterioscler Thromb Vasc Biol. 2005;25:341–347. doi: 10.1161/01.ATV.0000152608.29351.8f. [DOI] [PubMed] [Google Scholar]
- 8.Chiarugi P, Buricchi F. Protein tyrosine phosphorylation and reversible oxidation: Two cross-talking posttranslation modifications. Antioxid Redox Signal. 2007;9:1–24. doi: 10.1089/ars.2007.9.1. [DOI] [PubMed] [Google Scholar]
- 9.Duhé RJ, et al. Nitric oxide and thiol redox regulation of Janus kinase activity. Proc Natl Acad Sci USA. 1998;95:126–131. doi: 10.1073/pnas.95.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tonks NK. Redox redux: Revisiting PTPs and the control of cell signaling. Cell. 2005;121:667–670. doi: 10.1016/j.cell.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 11.Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 12.Ma YC, Huang XY. Novel regulation and function of Src tyrosine kinase. Cell Mol Life Sci. 2002;59:456–462. doi: 10.1007/s00018-002-8438-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehdi MZ, Pandey NR, Pandey SK, Srivastava AK. H2O2-induced phosphorylation of ERK1/2 and PKB requires tyrosine kinase activity of insulin receptor and c-Src. Antioxid Redox Signal. 2005;7:1014–1020. doi: 10.1089/ars.2005.7.1014. [DOI] [PubMed] [Google Scholar]
- 14.Sato H, et al. Mitochondrial reactive oxygen species and c-Src play a critical role in hypoxic response in vascular smooth muscle cells. Cardiovasc Res. 2005;67:714–722. doi: 10.1016/j.cardiores.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 15.Saito S, et al. Ligand-independent transactivation of the platelet-derived growth factor receptor by reactive oxygen species requires protein kinase C-delta and c-Src. J Biol Chem. 2002;277:44695–44700. doi: 10.1074/jbc.M208332200. [DOI] [PubMed] [Google Scholar]
- 16.Basuroy S, et al. Expression of kinase-inactive c-Src delays oxidative stress-induced disassembly and accelerates calcium-mediated reassembly of tight junctions in the Caco-2 cell monolayer. J Biol Chem. 2003;278:11916–11924. doi: 10.1074/jbc.M211710200. [DOI] [PubMed] [Google Scholar]
- 17.Sun G, et al. Effect of autophosphorylation on the catalytic and regulatory properties of protein tyrosine kinase Src. Arch Biochem Biophys. 2002;397:11–17. doi: 10.1006/abbi.2001.2627. [DOI] [PubMed] [Google Scholar]
- 18.Cooper JA, Gould KL, Cartwright CA, Hunter T. Tyr527 is phosphorylated in pp60c-src: Implications for regulation. Science. 1986;231:1431–1434. doi: 10.1126/science.2420005. [DOI] [PubMed] [Google Scholar]
- 19.Abram CL, Courtneidge SA. Src family tyrosine kinases and growth factor signaling. Exp Cell Res. 2000;254:1–13. doi: 10.1006/excr.1999.4732. [DOI] [PubMed] [Google Scholar]
- 20.Moarefi I, et al. Activation of the Src-family tyrosine kinase Hck by SH3 domain displacement. Nature. 1997;385:650–653. doi: 10.1038/385650a0. [DOI] [PubMed] [Google Scholar]
- 21.Ma YC, Huang J, Ali S, Lowry W, Huang XY. Src tyrosine kinase is a novel direct effector of G proteins. Cell. 2000;102:635–646. doi: 10.1016/s0092-8674(00)00086-6. [DOI] [PubMed] [Google Scholar]
- 22.Giannoni E, Buricchi F, Raugei G, Ramponi G, Chiarugi P. Intracellular reactive oxygen species activate Src tyrosine kinase during cell adhesion and anchorage-dependent cell growth. Mol Cell Biol. 2005;25:6391–6403. doi: 10.1128/MCB.25.15.6391-6403.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang H, Hao Q, Rutherford SA, Low B, Zhao ZJ. Inactivation of SRC family tyrosine kinases by reactive oxygen species in vivo. J Biol Chem. 2005;280:23918–23925. doi: 10.1074/jbc.M503498200. [DOI] [PubMed] [Google Scholar]
- 24.Cunnick JM, et al. Role of tyrosine kinase activity of epidermal growth factor receptor in the lysophosphatidic acid-stimulated mitogen-activated protein kinase pathway. J Biol Chem. 1998;273:14468–14475. doi: 10.1074/jbc.273.23.14468. [DOI] [PubMed] [Google Scholar]
- 25.Mills JE, et al. A novel disulfide bond in the SH2 domain of the C-terminal Src kinase controls catalytic activity. J Mol Biol. 2006;365:1460–1468. doi: 10.1016/j.jmb.2006.10.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salmeen A, et al. Redox regulation of protein tyrosine phosphatase 1B involves a sulphenyl-amide intermediate. Nature. 2003;423:769–773. doi: 10.1038/nature01680. [DOI] [PubMed] [Google Scholar]
- 27.van Montfort RL, Congreve M, Tisi D, Carr R, Jhoti H. Oxidation state of the active-site cysteine in protein tyrosine phosphatase 1B. Nature. 2003;423:773–777. doi: 10.1038/nature01681. [DOI] [PubMed] [Google Scholar]
- 28.Xu W, Harrison SC, Eck MJ. Three-dimensional structure of the tyrosine kinase c-Src. Nature. 1997;385:595–602. doi: 10.1038/385595a0. [DOI] [PubMed] [Google Scholar]
- 29.Grant BD, Hemmer W, Tsigelny I, Adams JA, Taylor SS. Kinetic analyses of mutations in the glycine-rich loop of cAMP-dependent protein kinase. Biochemistry. 1998;37:7708–7715. doi: 10.1021/bi972987w. [DOI] [PubMed] [Google Scholar]
- 30.Hirai TJ, Tsigelny I, Adams JA. Catalytic assessment of the glycine-rich loop of the v-Fps oncoprotein using site-directed mutagenesis. Biochemistry. 2000;39:13276–13284. doi: 10.1021/bi001216g. [DOI] [PubMed] [Google Scholar]
- 31.Levinson NM, Seeliger MA, Cole PA, Kuriyan J. Structural basis for the recognition of c-Src by its inactivator Csk. Cell. 2008;134:124–134. doi: 10.1016/j.cell.2008.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen H, et al. A molecular brake in the kinase hinge region regulates the activity of receptor tyrosine kinases. Mol Cell. 2007;27:717–730. doi: 10.1016/j.molcel.2007.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kemble DJ, Wang YH, Sun G. Bacterial expression and characterization of catalytic loop mutants of SRC protein tyrosine kinase. Biochemistry. 2006;45:14749–14754. doi: 10.1021/bi061664+. [DOI] [PubMed] [Google Scholar]
- 34.Wang D, Huang XY, Cole PA. Molecular determinants for Csk-catalyzed tyrosine phosphorylation of the Src tail. Biochemistry. 2001;40:2004–2010. doi: 10.1021/bi002342n. [DOI] [PubMed] [Google Scholar]
- 35.Seeliger MA, et al. High yield bacterial expression of active c-Abl and c-Src tyrosine kinases. Protein Sci. 2005;14:3135–3139. doi: 10.1110/ps.051750905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee S, Lin X, Nam NH, Parang K, Sun G. Determination of the substrate-docking site of protein tyrosine kinase C-terminal Src kinase. Proc Natl Acad Sci USA. 2003;100:14707–14712. doi: 10.1073/pnas.2534493100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.