Abstract
Defective genome maintenance mechanisms, involving DNA repair and cell-cycle checkpoint pathways, initiate genetic instability in many sporadic and hereditary cancers. The DNA damage effector Checkpoint kinase 1 (Chk1) is a critical component of DNA replication, intra-S phase, and G2/M phase checkpoints and a recently reported mitotic spindle-assembly checkpoint. Here, we report for the first time that haploinsufficiency of Chk1 in mice resulted in multiple mitotic defects and enhanced binucleation. We observed that Aurora B, a critical cytokinetic regulator and a recently identified Chk1 substrate, was mislocalized in mitotic Chk1+/− mammary epithelia. Chk1 also exhibited distinct mitotic localization patterns and was active during unperturbed mitosis and cytokinesis in mammalian cells. Active Chk1 expression was not dependent on treatment with spindle poisons such as colcemid during mitosis and cytokinesis. Furthermore, two different complementary approaches demonstrated that abrogation of Chk1 in mitotic mammalian cells resulted in cytokinetic regression and binucleation, increased chromosome lagging and/or nondisjunction, and abnormal localization of Aurora B at late mitotic structures. Thus, Chk1 is a multifunctional kinase that serves as a nexus between the DNA damage response and the mitotic exit pathways during cell-cycle progression to prevent genomic instability and cancer.
Keywords: Aurora B, binucleation, Chk1, genomic instability, nondisjunction
One of the hallmarks of cancer cells is the accumulation of chromosomal aberrations caused by defective genome maintenance mechanisms, involving cell-cycle checkpoints and DNA repair pathways. Chk1 is an evolutionarily conserved serine/threonine protein kinase essential for normal cell-cycle progression and maintaining genomic integrity during cell division (1, 2). In the presence of DNA damage, the key role of Chk1 is to relay checkpoint signals from upstream PI3-related kinases, such as ATR and ATM, to induce cell-cycle arrest for DNA repair (3). Primary breast carcinomas that lack PTEN expression and display elevated AKT phosphorylation show decreased expression of nuclear Chk1 and are aneuploid (4). Thus, frequent alterations in the PI3-kinase pathway observed in breast cancer may result in nuclear-to-cytoplasmic translocation of Chk1 and decreased Chk1 function, as opposed to more conventional mechanisms involving epigenetic silencing or loss of heterozygosity. Using the MMTV-Wnt-1 mouse model, the tumor suppression function of Chk1 has also been investigated. In these studies, Wnt-1/Chk1 heterozygous mice exhibited a significantly decreased tumor latency as compared with wild-type Chk1 littermates (1). Thus, studies with both mouse models and human breast tumors demonstrate that Chk1 activity is essential for signal transduction and candidate tumor suppression and for preserving genomic integrity. In mammalian cells undergoing mitosis, a transient interaction between Chk1 and the inhibitor of apoptosis protein (IAP) family member XIAP on condensed chromosomes during metaphase was reported earlier (5), but the mechanism remained unknown. Another study by Tang et al. (6) using siRNA in mammalian cells demonstrated that Chk1 deficiency leads to mitotic defects and that Chk1 acts as a negative regulator of polo-like-kinase 1 (Plk1). Recently, Zachos et al. (7) used siRNA in human carcinoma cell lines and gene targeting in DT-40 avian B-lymphoma cells to show that Chk1 colocalizes with BubR1 during prometaphase and its deficiency causes mitotic defects and activates spindle checkpoint signaling, required for regulation of Aurora B and BubR1 to delay anaphase after exposure to taxol, but not nocodozale. Although this study demonstrated that Chk1 localizes to the midbody during cytokinesis, the functional significance of Chk1 in the completion of cytokinesis was not elucidated (8).
Here, we report that Chk1+/− mammary epithelia exhibit misaligned chromosomes with multipolar spindle formation, chromosome missegregation, and enhanced binucleation. Moreover, Chk1 has unique mitotic localization patterns and is active during both unperturbed mitosis and cytokinesis in mammalian cells, independent of colcemid and UV damage. Furthermore, we show that abrogation of Chk1 specifically during mitosis causes increased chromosome lagging and/or nondisjunction and abnormal localization of cytokinetic regulator Aurora B from late mitotic structures. Finally, these Chk1-deficient mitotic cells undergo cytokinesis failure and multinucleation. Thus, our findings demonstrate that Chk1 is not only essential during S and G2 phases of cell cycle, but also during mitosis and cytokinesis to complete cell division.
Results and Discussion
Chk1+/− Mice Display Multiple Mitotic Defects and Enhanced Binucleation.
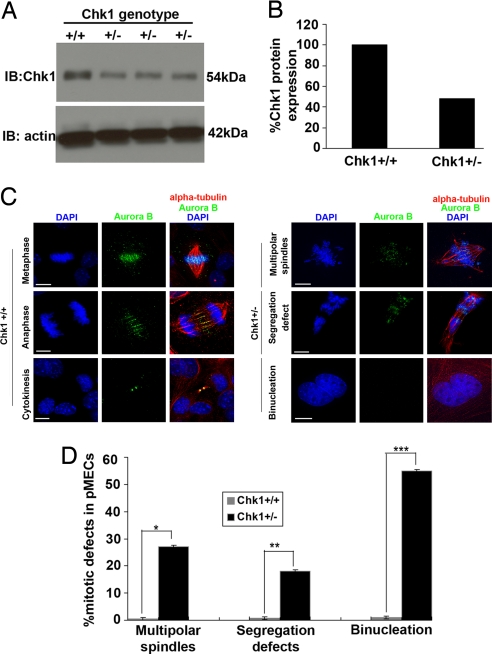
Previous studies from our laboratory (9) showed that conditional deletion of Chk1 from the mammary gland of parous mice results in the formation of spontaneous DNA damage foci. Chk1+/− epithelia also exhibit miscoordinated cell-cycle events in which S-phase cells display an early mitotic phenotype. To further analyze these phenomena, we characterized primary mammary epithelial cells (pMECs) isolated from Chk1+/− mice (1). First we performed immunoblotting, using mammary-gland extracts from Chk1 female littermates, to confirm that Chk1 heterozygous mice express approximately half the level of Chk1 (≈48%) as compared with wild-type mice (Fig. 1 A and B). Next, we isolated pMECs from the mammary glands of Chk1+/− or Chk1+/+ female mice and cultured them for 96 h. They were immunostained with mitotic markers AIM-1/Aurora B and alpha-tubulin to ascertain whether there were any mitotic defects associated with Chk1 haploinsufficiency. AIM-1/Aurora B is a serine/threonine kinase (related to Drosophila Aurora and Saccharomyces cerevisiae Ipl1) and belongs to a key group of mitotic passenger proteins that localize to distinct mitotic structures during cell division and are required for proper chromosome segregation and cytokinesis (10–12). Previous studies in mammalian cells have shown that aberrant quantity or localization of AIM-1/Aurora B from midzone and midbody induces abortive cytokinesis and multinucleation (10, 13). Moreover, as discussed earlier, Aurora B has also been identified as a mitotic substrate for Chk1 necessary to mitotic spindle checkpoint by Zachos et al. (7). The asynchronous Chk1+/− pMEC cultures exhibited misaligned chromosomes with multipolar spindles, chromosome missegregation, and enhanced binucleation compared with Chk1+/+ pMECs (Fig. 1C). Interestingly, Aurora B was mislocalized from the mitotic structures in cultured Chk1+/− pMECs compared with Chk1+/+ pMECs (Fig. 1C). Furthermore, quantitation of immunostained mitotic pMECs (n = 88) revealed misaligned chromosomes with multipolar spindles and missegregation in 18% of Chk1+/− pMECs, whereas 55% of Chk1+/− pMECs were binucleated, as compared with Chk1+/+ mitotic pMECs (Fig. 1D). Therefore, these interesting observations demonstrate that Chk1 haploinsufficiency during somatic cell division induces multiple mitotic defects, thereby impeding the separation of 2 daughter cells.
Fig. 1.
Chk1+/− mice display multiple mitotic defects and increased binucleation. (A and B) Western blot analysis of whole mammary-gland extract from Chk1+/− (n = 4) mice shows 48% of Chk1(anti-Chk1(G4)) expression relative to Chk1+/+ mice. β-actin was used as loading control. (C) Merged images of Chk1+/+ pMECs undergoing mitosis and cytokinesis or Chk1+/− pMECs undergoing various mitotic defects were stained for Aurora B (green), alpha-tubulin (red), and DNA (DAPI blue). (D) Bar graph illustrates that 18% of Chk1+/− pMECs (n = 88) displayed multiple mitotic defects and 55% of Chk1+/− pMECs were binucleated as compared with Chk1+/+ pMECs. ∗, P = 0.001; ∗∗, P = 0.001; and ∗∗∗, P = 0.03. All images have 15 micron scale bars at 63× magnification.
Chk1 Has Distinct Localization Patterns and Is Active in Both Unperturbed Mitosis and Cytokinesis.
Because decreased Chk1 levels in proliferating Chk1+/− mammary epithelia induced various mitotic defects and increased binucleation, the mitotic distribution of total Chk1 was examined in conjunction with Aurora B in asynchronous murine HC11 mammary epithelial cells by using immunocytochemistry. During prometaphase, Chk1 not only outlined chromosome arms but also partially colocalized with Aurora B at the kinetochores. During anaphase, Chk1 foci decorated the spindle midzone and partially colocalized with Aurora B. During cytokinesis, Chk1 accumulated at the midbody and partly colocalized with Aurora B(supporting information (SI) Fig. S1a). Similar mitotic localization patterns for Chk1 in association with Aurora B were observed in human HeLa cervical cancer cell lines (Fig. S1b), suggesting a significant role for Chk1 in completing the final stages of cell division.
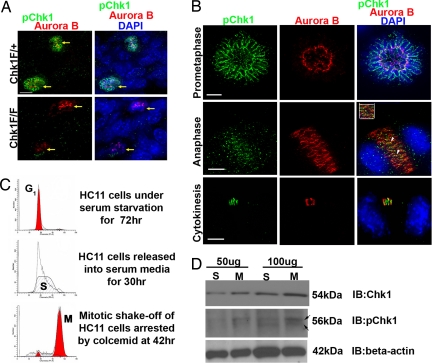
Next we wanted to confirm whether Chk1 was active during mitosis and cytokinesis in unperturbed mammalian cells. To address this question, we used an antibody against a previously well-characterized phospho-site, pChk1Ser317 (pChk1) for immunostaining. Previous studies (3, 14) have shown that Chk1 is phosphorylated on Serine 317 by ATR in response to DNA replication stress and that this modification regulates Chk1 activity during cell-cycle progression to induce cell-cycle arrest required for DNA repair. Once again the chromosomal passenger protein and Chk1 mitotic substrate Aurora B was used as a marker to characterize various localization patterns of pChk1 during mitosis. Unexpectedly, during prometaphase and metaphase, pChk1 was seen specifically decorating the chromosome arms and forming an organized structure called the peri-chromosomal layer (PCL), whereas Aurora B decorated the kinetochores in mammary-gland tissue sections from conditional Chk1 heterozygous (Chk1Flox/+) (Fig. 2A and Fig. S2d ) and wild-type (Fig. S2d) mice. However, the unique PCL staining pattern disappeared in mammary-gland tissue sections derived from conditional Chk1-null (Chk1Flox/Flox) mice, thus confirming the specificity of pChk1 antibody (Fig. 2A). Typically, PCL is a fibrillo-granular layer formed after nuclear-envelope breakdown during mitotic entry and consists of hyperphosphorylated nuclear and nucleolar proteins (15, 16). The functional significance of PCL during mitosis remains unclear. Moreover, to further confirm the PCL staining pattern seen in mice, a well-known nuclear/nucleolar PCL marker, Ki67, was used for immunostaining with anti-Chk1(G4) in HC11 cells. Because the Ki67 antibody and pChk1pChk1 antibodies were both polyclonal in origin, an antibody recognizing total Chk1 was used instead to confirm PCL localization during prometaphase. A major fraction of total Chk1 foci were observed to colocalize with Ki67 around the PCL layer during prometaphase, confirming the localization of Chk1 to PCL in mammary epithelial cells (Fig. S2e).
Fig. 2.
Active Chk1 has distinct localization patterns and expression during both unperturbed mitosis and cytokinesis. (A) Merged images of mammary-gland tissue sections from conditional Chk1Flox/+ (F/+) and Chk1Flox/Flox (F/F) female mice (9) stained for pChk1 (green) and Aurora B (red) and DNA stained with DAPI (blue) demonstrate the presence of active Chk1 during mitosis and confirm the specificity of anti-pChk1 antibody (yellow arrows). (B) Merged images of HC11 cells stained for pChk1 (green), Aurora B (red), and DNA (DAPI blue) showing distinct localization patterns during mitosis and cytokinesis. Inset shows partial colocalization of distinct pChk1 foci with Aurora B during anaphase. (C) FACS cell-cycle profile of Propidium Iodide-stained synchronized HC11 cells at various time points (G1, S, and M). (D) Western blot showing pChk1 (56 kDa) and total Chk1 (54 kDa) expression in synchronized HC11 S-phase and mitotic (M) cells at 50-μg and 100-μg total protein concentrations. β-actin was used as a loading control. A slight shift in pChk1 expression in M phase (black arrows). All images have 15 micron scale bars at 63× magnification.
In addition, the distinct localization patterns for pChk1 during unperturbed mitosis and cytokinesis were also examined in conjunction with Aurora B at a high-resolution single-cell level in both asynchronous HC11 cells. The observed in vivo localization patterns were recapitulated in both cell lines, wherein pChk1 localized to the PCL during prometaphase, whereas Aurora B marked the kinetochores. At the onset of sister-chromatid separation during anaphase, pChk1 was translocated to the spindle midzone and pChk1 foci partially colocalized with Aurora B. Finally, during cytokinesis a small fraction of pChk1 colocalized with Aurora B at the midbody (Fig. 2B). This finding signifies that active Chk1 may be required for conducting normal mitotic progression and completion of cytokinesis to prevent accumulation of mitotic errors during cell division.
Active Chk1 Expression Is Not Dependent on Colcemid Treatment and DNA Damage During Mitosis and Cytokinesis.
Next, we examined the relative levels of pChk1 expression during unperturbed S phase and mitosis by immunoblotting using synchronized HC11 cell lysates to further confirm the unexpected results of immunostaining. For these studies, a synchronization protocol for collecting mitotic HC11 cells was established and validated by using flow cytometry. First, asynchronous HC11 cells were serum-starved for 72 h and then released into serum-rich media for collecting S- and M-phase cells at various time points. After 30 h, the purity of the S-phase HC11 population (≈100%) was confirmed by using flow cytometry. To collect synchronized mitotic HC11 cells, a mitotic blocker colcemid that depolymerizes microtubules and arrests cells in prometaphase was added for 10–12 h to cells undergoing synchronized S phase. Following mitotic shake-off after colcemid treatment, flow cytometry confirmed that ≈80% of HC11 cells were in M phase (Fig. 2C). Two different total protein concentrations of 50 μg and 100 μg of S- and M-phase HC11 cell lysates were resolved by 10% SDS/PAGE gel electrophoresis and subjected to Western blotting. Interestingly, total Chk1 and pChk1 expression were detected not only in synchronized S-phase lysates, but also in M phase. A small increase in pChk1 expression was also observed in M phase compared with S phase (Fig. 2D).
As stated earlier, Zachos et al. demonstrated that spindle poisons such as taxol and nocodozole can activate the mitotic checkpoint and induce Chk1 phosphorylation during mitosis. To determine whether the pChk1 and Chk1 expression observed in mitotic HC11 cells was due to colcemid treatment, an alternative non-colcemid-based mitotic shake-off technique was designed and validated to collect mitotic HC11 cells after release into serum-rich media as described in SI Methods. After shake-off, mitotic HC11 cells were stained with mitotic markers Aurora B and pH3S10 to confirm the purity of this population. Examples of merged images demonstrate an increased expression of Aurora B and pH3S10 within the mitotic HC11 cells (Fig. S2a). As controls for immunoblotting, asynchronous HC11 cell lysates were prepared with or without DNA damage by UV (+/−UV). In addition to non-colcemid-treated mitotic HC11 cells, colcemid-treated mitotic HC11 cells subjected to +/−UV damage were also analyzed in the immunoblotting experiment. All of the lysates were resolved by using a 4%–12% gradient SDS/PAGE gel, which gives a better separation and confirms the expression of pChk1 and Chk1 during mitosis without colcemid treatment. Interestingly, pChk1 expression was observed in all of the mitotic lysates but remained essentially unchanged in mitotic HC11 cells with or without colcemid treatment and/or UV damage as confirmed by densitometry analysis (Fig. S2 b and c). As expected, a major increase in the expression of pChk1 was observed in control asynchronous HC11 cells after UV damage, as compared with nondamaged asynchronous HC11 cells (Fig. S2 b and c). Collectively, these results demonstrate that active Chk1 is required during mitosis and cytokinesis both in the presence and absence of spindle poison colcemid and DNA damage. A recent study by Zachos et al. concluded that a caffeine-sensitive kinase, possibly other than ATR or ATM, may be the candidate kinase phosphorylating Chk1 at multiple canonical or noncanonical sites during mitosis (7, 8). However, which mitotic kinase is responsible for Chk1 phosphorylation and how this process is regulated during mitosis and cytokinesis remains to be explored and is beyond the scope of this present study.
Abrogation of Chk1 in Mitotic Mammalian Cells Displaces Aurora B at the Midbody and Induces Cytokinetic Regression and Binucleation.
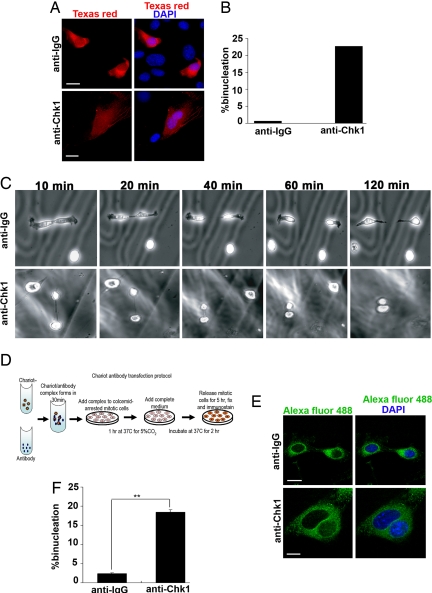
Based on our intriguing observations, we hypothesized that Chk1 is active and may be required to conduct mitosis and cytokinesis during unperturbed cell division. Accordingly, we designed experiments to directly test this hypothesis. Numerous studies performed using various human tumor cell lines have shown that RNA interference (RNAi) knockdown of Chk1 during interphase results in the increased formation of nonviable DNA replication forks, activation of S or G2/M checkpoint arrest, and induction of apoptosis (17). Therefore, RNAi knockdown was not a feasible approach to dissect the mitosis-specific functions of Chk1. To overcome this problem, Chk1 function was abrogated at the single-cell level by microinjecting 2 different anti-Chk1 antibodies, monoclonal anti-Chk1(G4) and polyclonal anti-Chk1(Fl476) into mitotic NIH 3T3 cells. Because of the small size of HC11 cells, it was technically nonfeasible to use them for microinjection studies. Instead, mouse NIH 3T3 or human HeLa cell lines were used for antibody-microinjection experiments. Numerous studies have used and confirmed the specificity of these 2 different anti-Chk1 antibodies in identifying total Chk1 in various model systems (1, 14, 18). In addition, immunostaining of mouse and human cell lines with the anti-Chk1(G4) antibody used in this study demonstrated that this antibody recognizes total Chk1 during mitosis and cytokinesis (Fig. S1 a and b). Furthermore, immunoprecipitation (IP) with anti-Chk1(G4) from mitotic HeLa cell lysates and immunoblotting for anti-Chk1(Fl476) specifically recognizes a single band of Chk1 corresponding to total Chk1 in the 10% input lane, further confirming the specificity of the antibodies used in the microinjection experiments. A low-intensity heavy-chain signal is seen in both lanes used for IP with anti-Chk1(G4) and its respective control IgG, caused by cross-reactivity with secondary antibody used in this procedure (Fig. S1c). As a control for microinjection experiments, a random polyclonal or monoclonal IgG was injected into mitotic cells at 10-fold the concentration of that used for the anti-Chk1 antibody-injected samples. To mark injected cells, the antibodies were mixed with a Texas red dextran marker before microinjecting prometaphase or metaphase cells from an asynchronous NIH 3T3 culture. After injection, the cells were incubated for ≈5 h to allow for the completion of mitosis and cytokinesis, followed by immunostaining (Fig. 3A). Strikingly, 23% of monoclonal anti-Chk1 (G4) antibody-injected mitotic cells analyzed from multiple independent experiments (n = 185) displayed a binucleated phenotype in comparison with <1% of IgG-injected control cells (Fig. 3B). Similarly, ≈20% of polyclonal anti-Chk1 (Fl476) antibody-injected mitotic cells from multiple experiments (n = 76) showed binucleation as compared with IgG-injected control cells (Fig. S3b).
Fig. 3.
Abrogation of Chk1 in mitotic mammalian cells results in cytokinetic regression and binucleation. (A) Examples of mitotic NIH 3T3 cells microinjected with control IgG or anti-Chk1(G4) antibody mixed with Texas red dextran marker (red) or DNA (DAPI blue). (B) Bar graph illustrates ≈23% binucleation after anti-Chk1(G4) antibody injection (n = 185) as compared with control IgG-antibody injection pooled from 7 independent experiments for each group. (C) A series of phase contrast time-lapse images captured at 10-min intervals of NIH 3T3 cells after anti-IgG or anti-Chk1(G4) antibody-microinjected cells undergoing cytokinesis. anti-Chk1(G4) antibody-injected mitotic cells undergo cytokinetic regression to form binucleated cells, and anti-IgG-injected mitotic cells complete cytokinesis. (D) A Chariot-based transfection protocol to deliver antibodies into mitotic cells. (E) Examples of mitotic NIH 3T3 cells transfected with control IgG or anti-Chk1(G4) antibody (n = 348) and stained with secondary antibody Alexa fluor 488 (green) and DNA (DAPI blue). (F) Bar graph illustrates ≈18% binucleation after anti-Chk1(G4) antibody transfection as compared with ≈2% after control anti-IgG transfection. ∗∗, P = 0.01. All images have 15 micron scale bars at 63× magnification.
To determine the reason behind the formation of these binucleated cells, microinjected mitotic cells were imaged by using time-lapse microscopy with phase-contrast optics at 10-min intervals. Real-time analysis of anti-Chk1(G4) antibody-injected cells revealed an inter-cytoplasmic bridge between 2 separating daughter cells during cytokinesis. Instead of undergoing abscission, the bridge regressed, giving rise to binucleated cells within an hour, and the cells appeared to remain polyploid (Fig. 3C and Movie S1). In contrast, the IgG antibody-injected cells completed cytokinesis, forming 2 separate daughter cells (Fig. 3C and Movie S2). Previous studies have shown that chromosome nondisjunction during anaphase and/or chromatin trapping in the cytokinetic cleavage furrow can contribute to furrow regression and tetraploidization (19, 20). Moreover, as seen from numerous studies, aberrant localization of key cytokinetic regulators (10–13) may also contribute to cytokinetic regression and binucleation in anti-Chk1 antibody-injected cells.
Therefore, to directly test this hypothesis and to confirm our results in a human cell line, anti-Chk1(G4) or anti-Chk1(Fl476) antibodies were mixed with either Dextran 488 or Texas red markers and microinjected into mitotic HeLa cells from asynchronous cultures. After microinjection, mitotic HeLa cells were stained with cytokinetic regulator and recently identified Chk1 mitotic substrate AIM-1/Aurora B. Interestingly, anti-Chk1(G4) antibody-injected HeLa cells undergoing cytokinesis exhibited abnormal localization or displaced Aurora B at the midbody, compared with the control IgG-injected cells (Fig. S3a). Similar results were obtained in anti-Chk1(Fl476) antibody-injected HeLa cells undergoing cytokinesis (Fig. S3a). As discussed in Zachos et al. (7), since Aurora B is a known mitotic substrate for Chk1 and a fraction of Chk1 is seen colocalizing with Aurora B at the midzone and midbody, it is possible that disruption of Chk1 function may have in part influenced the proper recruitment of Aurora B at the midbody. Thus, a small amount of Aurora B is still observed at the midbody in anti-Chk1 antibody-injected HeLa cells during cytokinesis. Because an aberrant quantity or localization of Aurora B can cause abortive cytokinesis (10, 13), insufficient Aurora B at the midbody in the anti-Chk1 antibody-injected mitotic HeLa cells could have contributed to cytokinetic regression and binucleation. Thus, our results suggest that Chk1–Aurora B interplay may be critical during last stages of cell division to facilitate the completion of cytokinesis.
Mitotic Mammalian Cells After Abrogation of Chk1 via Chariot Exhibit Increased Chromosome Lagging and/or Nondisjunction and Abnormal Aurora B Localization, Resulting in Cytokinesis Failure and Multinucleation.
To confirm the phenotype from microinjection experiments and to further dissect the preceding events leading to cytokinesis failure, a complementary approach was used to efficiently deliver antibodies into a large number of colcemid-synchronized mitotic cells. The Chariot transfection reagent has been used previously to transport biologically active proteins, peptides, and antibodies directly into both in vitro and in vivo model systems (21). Because the nuclear membrane disassembles during mitosis, the Chariot reagent should allow targeting of antibodies against nuclear proteins such as Chk1. Employing this approach, we designed and validated a protocol for successfully delivering the same anti-Chk1 antibodies used in microinjection experiments into colcemid-arrested mitotic NIH 3T3 cells (Fig. 3D). After antibody transfection, mitotic cells were released for ≈5 h and processed by immunostaining with corresponding fluorescently conjugated secondary antibodies (Fig. 3E), allowing the identification of positive transfectants. Approximately 18% of anti-Chk1(G4) antibody-transfected cells were bi- or trinucleated as compared with only ≈2% of the IgG-transfected controls from multiple experiments (n = 348) (Fig. 3F). Because similar results were observed after anti-Chk1 antibody transfection and microinjection techniques, we confirmed that Chk1 is required for the successful completion of cytokinesis to prevent binucleation. Importantly, the antibody transfection provides a new method to inhibit key mitotic proteins, which can be examined in a much greater number of cells than feasible by conventional microinjection.
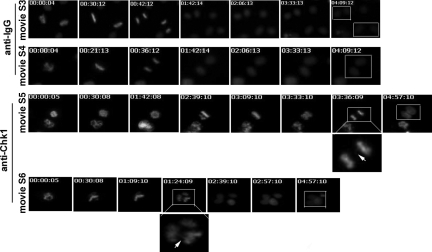
As seen previously from the microinjection studies, we predicted that mitotic mammalian cells after abrogation of Chk1 underwent abortive cytokinesis not only because of displaced Aurora B from the midbody, but also because of chromosome lagging and/or nondisjunction during anaphase. To test this hypothesis, we extended our Chariot transfection protocol to inhibit Chk1 function in a previously published H2B-GFP HeLa cell line (22). Additionally, the presence of a histone-GFP fusion protein enabled the visualization of chromatin-associated defects such as lagging strands, nondisjunction, and chromatin trapping occurring during mitotic progression in real-time. After antibody transfection, mitotic H2B-GFP HeLa cells were released from colcemid arrest and monitored by using time-lapse microscopy to capture both brightfield and fluorescent images at 3-min intervals for ≈5 h. Using this approach, after anti-Chk1 (G4) antibody transfection, mitotic H2B-GFP HeLa cells showed chromosome lagging and nondisjunction leading to cytokinesis failure and bi- or trinucleation. As observed earlier in Chk1+/− pMECs, misaligned chromosomes were also observed during metaphase after anti-Chk1 antibody transfection in mitotic H2B-GFP HeLa cells. These misaligned chromosomes took more time to align on the metaphase plate, before forming a nearly normal metaphase. Surprisingly, the nearly normal metaphase cells underwent defective sister-chromatid separation with lagging strands during anaphase, leading to cytokinesis failure and the formation of bi- and trinucleated cells (Fig. 4). In contrast, after control IgG antibody transfection, mitotic H2B-GFP HeLa cells completed metaphase, anaphase, and cytokinesis and gave rise to 2 separate daughter cells in <5 h (Fig. 4). Thus, these results confirm that Chk1 deficiency increases chromosome lagging and/or nondisjunction during anaphase, which can contribute to cytokinesis failure and multinulceation. Moreover, these results also explain one of the reasons behind increased binucleation observed in Chk1+/− mammary epithelia.
Fig. 4.
Mitotic mammalian cells after abrogation of Chk1 via Chariot exhibit increased chromosome lagging and/or nondisjunction, resulting in cytokinesis failure and multinucleation. A series of grayscale images of GFP fluorescent time-lapse movies after Chariot-based control IgG- or anti-Chk1(G4) antibody transfection of mitotic H2B-GFP HeLa cells after release from colcemid arrest. Movie S3 and Movie S4 demonstrate that after control IgG antibody transfection, mitotic H2B-GFP HeLa cells complete metaphase, anaphase, and cytokinesis and form 2 separate daughter cells (white squares in 7th images of Movies S3 and S4). Movie S5 and Movie S6 demonstrate that after anti-Chk1 antibody transfection, mitotic H2B-GFP HeLa cells become misaligned during metaphase. But with time, they complete nearly normal metaphase and finally exhibit chromosome lagging and/or nondisjunction during anaphase (white squares and magnifications in 7th image of Movie S5 and 4th image of Movie S6), resulting in cytokinesis failure and multinucleated progeny (white square in 7th image of Movie S6).
Next, to directly test whether Aurora B was displaced from the late mitotic structures as seen in the microinjection experiments, anti-Chk1(G4) or anti-Chk1(Fl476) antibodies were transfected into colcemid-synchronized mitotic HeLa cells by using the Chariot reagent. The antibody-transfected mitotic cells were released from colcemid arrest at various time points and subjected to immunostaining with Aurora B. Similar to microinjection studies, after anti-Chk1(G4) antibody transfection, HeLa cells undergoing anaphase and cytokinesis have abnormal localization of Aurora B at the midzone and midbody, respectively, compared with the control IgG-injected cells (Fig. S3d). Similar results were obtained after anti-Chk1(Fl476) antibody transfection in HeLa cells undergoing anaphase and cytokinesis (Fig. S3e). Moreover, an increase in sister-chromatid lagging and/or nondisjunction was clearly observed during anaphase after anti-Chk1 antibody transfection in mitotic HeLa cells (Fig. S3 d and e) and can be clearly visualized from our multiple time-lapse movies after abrogation of Chk1 in mammalian cells. Therefore, our studies directly demonstrate that Chk1 is required for proper chromosome segregation and completion of cytokinesis using non-colcemid- and colcemid-based single-cell knockout techniques. Furthermore, these results are consistent with the multiple mitotic defects, mislocalized Aurora B, and increased binucleation seen in Chk1+/− pMECs isolated from the germ-line Chk1 mouse model.
Similar studies have revealed potential mitotic roles of DNA repair and DNA damage-recognizing proteins such as Brca2 and TopBP1 (23, 24) to ensure normal mitotic progression. Levels of these multifunctional cell-cycle regulators are critical to prevent abnormal anaphase and cytokinesis, binucleation, and genomic instability. For example, epithelial cancer cell line Capan-1, isolated from a patient carrying nonfunctional BRCA2 6174delT mutation, displayed increasing cytokinetic abnormalities (25). The current study emphasizes the importance of precisely regulating Chk1 expression and activity during normal mitotic progression to prevent genomic instability because Chk1 is associated with multiple mitotic substrates such as Aurora B, BubR1, and Plk1. Cytokinesis failure and initiation of binucleation can be detrimental to the organism because they promote tetraploidy and tumorigenesis (26). Chk1 has been proposed as an attractive therapeutic target for cancer treatment especially because Chk1 inhibitors can sensitize p53-deficient human tumor cells to programmed cell death (27). A recent study has identified a “Chk1-suppressed pathway” that regulates caspase-2 apoptotic response to DNA damage and can bypass p53, BCL-2, and caspase-3 (28). In addition to tumors with alterations in p53, other types of p53-independent prosurvival signals may also respond positively to Chk1 inhibitors (28). Thus, an in-depth understanding of multiple functions of Chk1 during various stages of the cell division will be required to predict the selectivity and long-term effects of Chk1 inhibitors in clinical trials.
Materials and Methods
Mice and Mammary Glands.
Trigenic mice were generated by mating the mammary-specific WAP-Cre line obtained from Mouse Repository (NCI Frederick) with conditional floxed-allele Chk1 mice and Chk1 wild type as described in ref. 9. Inguinal mammary glands were harvested at day 1 of lactation from these mice for immunohistochemistry and microscopy as described in ref. 9. Murine pMECs were isolated from mammary glands of 12-week-old virgin Chk1+/− and Chk1+/+ female mice (3 animals per experiment) as described in Pullan et al. (29). After 96 h in culture, pMECs were fixed in 4% PFA for immunocytochemistry. Mice used for all these studies were euthanized according to Institutional Animal Care and Use Committee approved animal protocol guidelines.
Cell Culture and Mitotic Shake-Off.
Cell synchronization of HC11, NIH 3T3, and H2B-GFP HeLa cells using colcemid are described in SI Methods. Noncolcemid mitotic shake-off of HC11 cells is described in SI Methods.
Antibody Microinjection.
NIH 3T3 or HeLa cells were grown on photo-etched gridded polylysine-d-coated glass coverslips (Bellco), and mitotic prometaphase or metaphase cells were injected with anti-Chk1 antibodies as described in SI Methods.
Chariot Antibody Transfection.
Exponentially growing NIH 3T3 or H2B-GFP HeLa cells were transfected with Chariot–antibody complex as described in SI Methods and according to manufacturer's instructions.
Immunoblotting.
Asynchronous, S-phase, and/or mitotic HC11 cells were washed in ice-cold PBS 3 times and lysed using RIPA buffer (1 mL per 100-mm dish) for immunoblotting as described in SI Methods.
Statistical Analysis.
All of the results were confirmed in multiple independent experiments. Data quantification and analysis were performed by Student's t test and expressed as ± SEM. P values of less than 0.05 are considered statistically significant.
The high-resolution images of the figures and supplementary figures of this manuscript can be accessed at http://www.bcm.edu/rosenlab/.
Supplementary Material
Acknowledgments.
We thank Drs. William R. Brinkley, Sharon Plon, and Li Yuan-Yulee and Rosen laboratory members for helpful discussions in preparation of manuscript and Drs. Pumin Zhang, Thomas Zwaka, and Michael Mancini for allowing us to use time-lapse microscope and microinjection systems for this project. These studies were supported by National Institutes of Health Grant CA 16303 (to J.M.R.) and Department of Defense Breast Cancer Research Program Grant W81XWH-06–1-0305 (to S.P.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0806671106/DCSupplemental.
References
- 1.Liu Q, et al. Chk1 is an essential kinase that is regulated by Atr and required for the G(2)/M DNA damage checkpoint. Genes Dev. 2000;14:1448–1459. [PMC free article] [PubMed] [Google Scholar]
- 2.Takai H, et al. Aberrant cell cycle checkpoint function and early embryonic death in Chk1(−/−) mice. Genes Dev. 2000;14:1439–1447. [PMC free article] [PubMed] [Google Scholar]
- 3.Abraham RT. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 2001;15:2177–2196. doi: 10.1101/gad.914401. [DOI] [PubMed] [Google Scholar]
- 4.Puc J, et al. Lack of PTEN sequesters CHK1 and initiates genetic instability. Cancer Cell. 2005;7:193–204. doi: 10.1016/j.ccr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Galvan V, Kurakin AV, Bredesen DE. Interaction of checkpoint kinase 1 and the X-linked inhibitor of apoptosis during mitosis. FEBS Lett. 2004;558:57–62. doi: 10.1016/S0014-5793(03)01488-1. [DOI] [PubMed] [Google Scholar]
- 6.Tang J, Erikson RL, Liu X. Checkpoint kinase 1 (Chk1) is required for mitotic progression through negative regulation of polo-like kinase 1 (Plk1) Proc Natl Acad Sci USA. 2006;103:11964–11969. doi: 10.1073/pnas.0604987103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zachos G, et al. Chk1 is required for spindle checkpoint function. Dev Cell. 2007;12:247–260. doi: 10.1016/j.devcel.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zachos G, Gillespie DA. Exercising restraints: Role of Chk1 in regulating the onset and progression of unperturbed mitosis in vertebrate cells. Cell Cycle. 2007;6:810–813. doi: 10.4161/cc.6.7.4048. [DOI] [PubMed] [Google Scholar]
- 9.Lam MH, Liu Q, Elledge SJ, Rosen JM. Chk1 is haploinsufficient for multiple functions critical to tumor suppression. Cancer Cell. 2004;6:45–59. doi: 10.1016/j.ccr.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 10.Terada Y, et al. AIM-1: A mammalian midbody-associated protein required for cytokinesis. EMBO J. 1998;17:667–676. doi: 10.1093/emboj/17.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vagnarelli P, Earnshaw WC. Chromosomal passengers: The four-dimensional regulation of mitotic events. Chromosoma. 2004;113:211–222. doi: 10.1007/s00412-004-0307-3. [DOI] [PubMed] [Google Scholar]
- 12.Terada Y. Role of chromosomal passenger complex in chromosome segregation and cytokinesis. Cell Struct Funct. 2001;26:653–657. doi: 10.1247/csf.26.653. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, et al. Aberrant quantity and localization of Aurora-B/AIM-1 and survivin during megakaryocyte polyploidization and the consequences of Aurora-B/AIM-1-deregulated expression. Blood. 2004;103:3717–3726. doi: 10.1182/blood-2003-09-3365. [DOI] [PubMed] [Google Scholar]
- 14.Zhao H, Piwnica-Worms H. ATR-mediated checkpoint pathways regulate phosphorylation and activation of human Chk1. Mol Cell Biol. 2001;21:4129–4139. doi: 10.1128/MCB.21.13.4129-4139.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hernandez-Verdun D, Gautier T. The chromosome periphery during mitosis. Bioessays. 1994;16:179–185. doi: 10.1002/bies.950160308. [DOI] [PubMed] [Google Scholar]
- 16.Van Hooser AA, Yuh P, Heald R. The perichromosomal layer. Chromosoma. 2005;114:1–12. doi: 10.1007/s00412-005-0021-9. [DOI] [PubMed] [Google Scholar]
- 17.Syljuasen RG, et al. Inhibition of human Chk1 causes increased initiation of DNA replication, phosphorylation of ATR targets, and DNA breakage. Mol Cell Biol. 2005;25:3553–3562. doi: 10.1128/MCB.25.9.3553-3562.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zachos G, Rainey MD, Gillespie DA. Chk1-deficient tumour cells are viable but exhibit multiple checkpoint and survival defects. EMBO J. 2003;22:713–723. doi: 10.1093/emboj/cdg060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weaver BA, Silk AD, Cleveland DW. Cell biology: Nondisjunction, aneuploidy and tetraploidy. Nature. 2006;442:E9–E10. doi: 10.1038/nature05139. discussion E10. [DOI] [PubMed] [Google Scholar]
- 20.Shi Q, King RW. Chromosome nondisjunction yields tetraploid rather than aneuploid cells in human cell lines. Nature. 2005;437:1038–1042. doi: 10.1038/nature03958. [DOI] [PubMed] [Google Scholar]
- 21.Aoshiba K, Yokohori N, Nagai A. Alveolar wall apoptosis causes lung destruction and emphysematous changes. Am J Respir Cell Mol Biol. 2003;28:555–562. doi: 10.1165/rcmb.2002-0090OC. [DOI] [PubMed] [Google Scholar]
- 22.Kanda T, Sullivan KF, Wahl GM. Histone-GFP fusion protein enables sensitive analysis of chromosome dynamics in living mammalian cells. Curr Biol. 1998;8:377–385. doi: 10.1016/s0960-9822(98)70156-3. [DOI] [PubMed] [Google Scholar]
- 23.Reini K, et al. TopBP1 localises to centrosomes in mitosis and to chromosome cores in meiosis. Chromosoma. 2004;112:323–330. doi: 10.1007/s00412-004-0277-5. [DOI] [PubMed] [Google Scholar]
- 24.Daniels MJ, Wang Y, Lee M, Venkitaraman AR. Abnormal cytokinesis in cells deficient in the breast cancer susceptibility protein BRCA2. Science. 2004;306:876–879. doi: 10.1126/science.1102574. [DOI] [PubMed] [Google Scholar]
- 25.Goggins M, et al. Germline BRCA2 gene mutations in patients with apparently sporadic pancreatic carcinomas. Cancer Res. 1996;56:5360–5364. [PubMed] [Google Scholar]
- 26.Fujiwara T, et al. Cytokinesis failure generating tetraploids promotes tumorigenesis in p53-null cells. Nature. 2005;437:1043–1047. doi: 10.1038/nature04217. [DOI] [PubMed] [Google Scholar]
- 27.Bartek J, Lukas J. Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell. 2003;3:421–429. doi: 10.1016/s1535-6108(03)00110-7. [DOI] [PubMed] [Google Scholar]
- 28.Sidi S, et al. Chk1 suppresses a caspase-2 apoptotic response to DNA damage that bypasses p53, Bcl-2, and caspase-3. Cell. 2008;133:864–877. doi: 10.1016/j.cell.2008.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pullan S, et al. Requirement of basement membrane for the suppression of programmed cell death in mammary epithelium. J Cell Sci. 1996;109:631–642. doi: 10.1242/jcs.109.3.631. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.