Abstract
Although p27 and p57 are structurally related cyclin-dependent kinase inhibitors (CKIs), and are thought to perform similar functions, p27 knockout (p27KO) and p57KO mice show distinct phenotypes. To elucidate the in vivo functions of these CKIs, we have now generated a knock-in mouse model (p57p27KI), in which the p57 gene has been replaced with the p27 gene. The p57p27KI mice are viable and appear healthy, with most of the developmental defects characteristic of p57KO mice having been corrected by p27 knock-in. Such developmental defects of p57KO mice were also ameliorated in mice deficient in both p57 and the transcription factor E2F1, suggesting that loss of p57 promotes E2F1-dependent apoptosis. The developmental defects apparent in a few tissues of p57KO mice were unaffected or only partially corrected by knock-in expression of p27. Thus, these observations indicate that p57 and p27 share many characteristics in vivo, but that p57 also performs specific functions not amenable to substitution with p27.
Keywords: development, cell cycle, genetics
The regulation of cyclin-dependent kinase (CDK) activities is pivotal for the precisely ordered progression of the cell cycle. Such regulation is achieved by various mechanisms, including changes in the concentration, phosphorylation, and subcellular localization of cyclin–CDK complexes as well as in the interaction of CDKs with CDK inhibitors (CKIs) (1, 2). Two families of CKIs have been identified to date. The Cip-Kip family consists of p21 (Cip1), p27 (Kip1), and p57 (Kip2), and inhibits many types of cyclin-CDK complex, whereas the INK4 family includes p15, p16, p18, and p19, and specifically inhibits CDK4 or CDK6.
The CKIs p27 and p57 are structurally related proteins that share a conserved CDK binding-inhibitory domain and a QT domain in their NH2- and COOH-terminal regions, respectively, and exhibit similar biochemical characteristics (3, 4). Also, several aspects of their cellular functions have suggested that these 2 CKIs are effectively “twin” molecules. For example, their expression levels are high in G0 and G1 phases of the cell cycle and decrease during progression from G1 to S phase (5–10), in association with the activation of cyclin-CDK complexes. Such activation results in phosphorylation of Rb family proteins and consequent activation of transcription factors of the E2F family, which target genes required for entry into and progression through S phase. Consistent with this scenario, overexpression of either p27 or p57 in cultured cells induces G1 arrest (3, 4, 6, 11).
Despite the similarities between p27 and p57, the phenotypes of p27 knockout (p27KO) and p57KO mice differ substantially. Whereas p27KO mice are viable and show a hyperproliferative phenotype characterized by organ hyperplasia and tumorigenesis (12–14), consistent with the expected function of p27 as an inhibitor of cell proliferation, p57KO mice manifest neonatal death, as well as developmental defects in multiple tissues (15–17). These differences are possibly attributable to differences in the spatiotemporal patterns of p27 and p57 expression (18), to structural features of p57 not shared by p27 (Fig. 1A), or to differences in the interactions of the 2 CKIs with proteins other than CDKs (19–27). However, given that previous studies have not evaluated the intrinsic differences between p27 and p57 under physiological conditions, the differences in their in vivo roles have remained unclear.
Fig. 1.
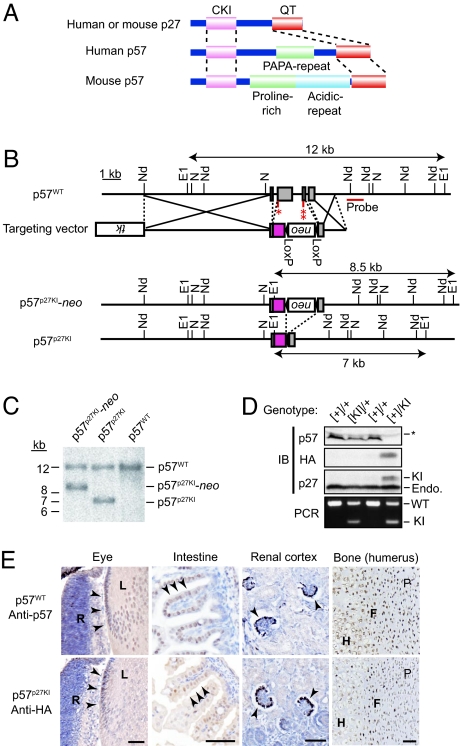
Generation of p57p27KI mice. (A) Structures of p27 and p57. The 2 proteins share conserved CKI and QT domains in their NH2- and COOH-terminal regions, respectively. Mouse p57 possesses a unique central domain that is not conserved in p27 or in human p57. (B) Schematic representations of the wild-type mouse p57 allele (p57WT), the targeting vector, the knocked-in p27 allele with a LoxP-neo cassette (p57p27KI-neo), and the knocked-in p27 allele after removal of the neo cassette by Cre recombinase. The expected sizes of DNA fragments that hybridize with the indicated probe in Southern blot analysis are shown. The ORF for mouse p27 tagged at its NH2 terminus with HA is indicated by the red box. All introns of the p57 gene were removed in the resulting targeted allele to avoid splicing defects. E1, EcoRI; N, NotI; Nd, NdeI. Indicated are the initiation (1 asterisk) and stop (2 asterisks) codons of the p57 gene. (C) Southern blot analysis of EcoRI-digested DNA from ES cells harboring the indicated alleles. The sizes of the hybridizing fragments correspond to those indicated in B. (D) Immunoblot and PCR analyses of the expression and imprinting of the knocked-in p27 allele. Kidneys of newborn mice [postnatal day (P)0] were lysed and subjected to immunoblot analysis with antibodies to p57, to HA, and to p27. The asterisk indicates nonspecific bands, and the band positions for endogenous (Endo.) and knocked-in (KI) p27 are shown. Genomic DNA of the corresponding animals was subjected to PCR analysis to determine their genotypes. Brackets indicate a paternal imprinted allele. Both p57 and HA-p27 were expressed only from the maternal allele. WT and KI indicate the wild-type and knocked-in alleles, respectively. (E) Immunohistochemical analysis of the indicated tissues of wild-type (p57WT) or p57p27KI neonates performed with antibodies to p57 or to HA. Sections were counterstained with hematoxylin. Arrowheads indicate the same types of cells expressing either p57 or HA-p27 in p57WT and p57p27KI mice, respectively. R, retina; L, lens; P, zone of proliferative cells; F, zone of flattened cells; H, zone of hypertrophic cells. (Scale bar, 50 μm.)
To overcome such limitations of previous studies, and to compare directly the physiological roles of p27 and p57, we have now generated a knock-in mouse model in which the p57 gene has been replaced with the p27 gene, and in which p27 is, therefore, expressed instead of p57. Our genetic model has uncovered both common and unique features of p27 and p57 that manifest in a tissue-specific manner.
Results
Generation of p57p27KI Mice.
Both p27 and p57 possess a CKI domain in their NH2-terminal regions and a QT domain that contributes to regulation of their stability at their COOH-termini, whereas mouse p57 contains a unique central domain that is not conserved in p27 or in human p57 (Fig. 1A). These structural characteristics led us to hypothesize that mouse p57 might have a specific role in development that is mediated through its central domain. Indeed, mouse p57 binds to LIM domain kinase (LIMK) through its central domain; thus, it regulates actin formation in chondrocytes (19). To investigate the functional equivalence and specificity of p27 and p57 in vivo, we developed a knock-in mouse model in which the endogenous p57 gene is replaced by a construct encoding hemagglutinin epitope (HA)-tagged mouse p27 (Fig. 1B). We showed that the HA tag did not impair the interaction of p27 with CDK2 or CDK4 in vivo (Fig. S1). Recombination events and removal of the neo cassette were confirmed by Southern blot analysis (Fig. 1C). The HA-p27 protein was expressed at the expected molecular size in embryonic tissues of, and in mouse embryonic fibroblasts (MEFs) derived from, the resulting p57p27KI mice (Fig. 1D; see also Fig. 4A). We confirmed that expression of HA-p27 under the control of the p57 gene promoter did not affect that of endogenous p27 (Fig. 1D). Also, the paternal knocked-in HA-p27 allele was not expressed in heterozygotes (Fig. 1D), suggesting that genomic imprinting, which normally suppresses expression of the paternal p57 allele, was not disturbed by the genetic manipulation. Thus, we crossed wild-type males and p57+/− or p57+/p27KI females, with the resulting heterozygous offspring designated as p57KO or p57p27KI mice, respectively. Spatial expression patterns of HA-p27 in p57p27KI mice were almost identical to those of endogenous p57 in wild-type mice, with no substantial differences apparent in equatorial epithelial cells of the lens, gut epithelial cells, podocytes in the kidney, or cells of the proliferative to hypertrophic zones of epiphysial cartilage (Fig. 1E). Therefore, we concluded that the knocked-in HA-p27 allele was correctly expressed in place of the endogenous p57 allele with only a few exceptions apparent in some tissues (see below).
Fig. 4.
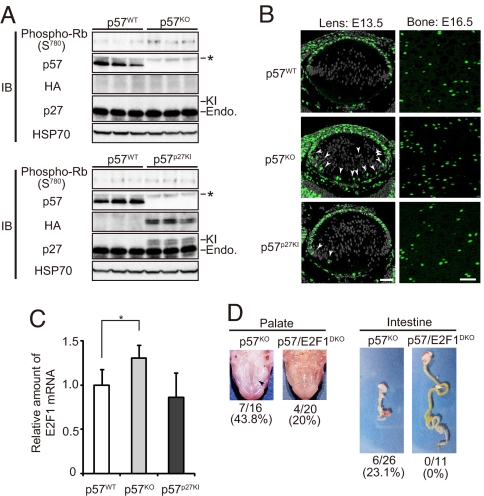
Replacement of the CKI activity of p57 by knocked-in p27. (A) MEFs derived from p57WT, p57KO, or p57p27KI mice were deprived of serum (cultured in the presence of 1% FBS) for 48 h, lysed, and subjected to IP with antibodies to Rb followed by immunoblot analysis with antibodies to phosphorylated Rb (Ser780). Lysates were also subjected directly to immunoblot analysis with antibodies to p57, to HA, to p27, and to HSP70 (loading control). Asterisk indicates nonspecific bands. The band positions for endogenous (Endo.) and knocked-in (KI) p27 are shown. Quantitative data for Rb phosphorylation are shown in Fig. S3B. (B) In situ BrdU incorporation was evaluated for embryos at E13.5 (lens) or E16.5 (zones of proliferative and flattened cells in the humerus). BrdU-positive cells, as well as nuclei stained with Hoechst 33258 (lens only) are green and gray, respectively. Arrowheads indicate ectopic BrdU-labeled cells in the posterior chamber of the lens. (Scale bar, 50 μm.) (C) Quantitative RT-PCR analysis of E2F1 expression in the intestine of E18.5 embryos. Normalized data for E2F1 mRNA are expressed relative to the corresponding value for wild-type mice, and are means ± SD (n = 7 or 8). *, P < 0.05 (ANOVA followed by Tukey–Kramer test). (D) Amelioration of cleft palate (arrowhead), and the intestinal defect of p57KO mice in p57/E2F1DKO mice. Penetrance of the defects at E17.5 (palate) and P0 (intestine) is indicated.
Circumvention of Neonatal Death and Developmental Anomalies of p57KO Mice by Knock-In of p27.
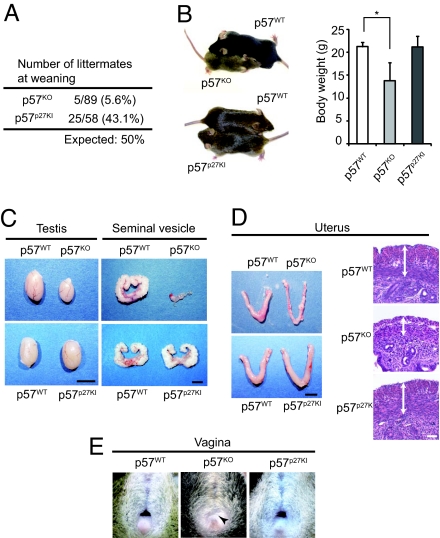
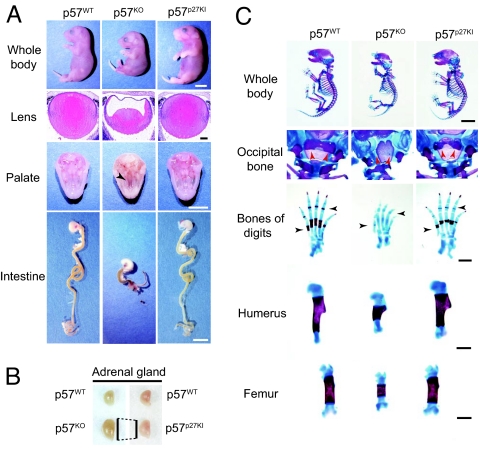
Most p57KO mice die soon after birth, whereas some of them survive until weaning, but show marked growth retardation (Fig. 2 A and B; Table S1) (16). In contrast, p57p27KI mice were born in approximately the expected ratio, and grew at the same rate as wild-type littermates. Surviving p57KO mice manifest atrophy of various tissues or organs, including the seminal vesicle, testis, and uterus, as well as vaginal atresia (16), whereas such developmental anomalies were not apparent or were greatly ameliorated in p57p27KI mice (Fig. 2 C–E; Table S1). We further investigated whether specific defects apparent at birth in p57KO mice were corrected by the knock-in of p27. The growth retardation in utero, as well as developmental defects of the lens, palate, and intestine apparent with p57KO mice were absent or markedly diminished in p57p27KI mice (Fig. 3A; Table S1 and Fig. S2). Also, the enlargement of the adrenal gland apparent in p57KO mice was less pronounced in p57p27KI mice (Fig. 3B; Table S1). The deformities of the vertebrae and ribs, delayed ossification of the occipital bone, digits, and long bones, and shortening of the long bones apparent in p57KO mice were also substantially corrected in p57p27KI mice (Fig. 3C; Table S1). These observations suggested that p27 is able to substitute for p57 in most tissues.
Fig. 2.
Correction of adult p57KO mouse phenotypes in p57p27KI mice. (A) Number of littermates remaining at weaning from crosses between p57WT (p57[+]/+) males and females harboring a paternal mutant allele (p57[−]/+ or p57[KI]/+). Thus, half of the littermates were expected to have a maternal mutant allele (p57[+]/− or p57[+]/KI). (B) Representative animals and mean body weight for the indicated genotypes at 7 weeks of age. The animals of each pair shown are littermates. Quantitative data are means ± SD (n = 3 to 7). *, P < 0.05 (ANOVA followed by Tukey–Kramer test). (C and D) Representative testes, seminal vesicles, and uteri from mice of each genotype at 7 weeks of age. Tissues of each pair were from littermates. Sections of the uterus were also subjected to hematoxylin-eosin staining; the muscle layer of the p57KO uterus was thinner than that of the p57WT or p57p27KI uteri (arrows). (Scale bars: macroscopic comparisons, 5 mm; uterine sections, 50 μm.) (E) Vaginal atresia (arrowhead) of a p57KO mouse at 7 weeks of age, and its correction in a p57p27KI mouse.
Fig. 3.
Correction of embryonic p57KO mouse phenotypes in p57p27KI mice. (A) Representative examples of the whole body, lens, and palate at E18.5, as well as of the intestine at P0. The growth retardation, cataracts in the lens (stained with hematoxylin–eosin), cleft palate (arrowhead), and short intestine of p57KO mice were corrected in p57p27KI mice. (Scale bars: macroscopic comparisons, 5 mm; lens sections, 100 μm.) (B) Representative examples of the adrenal gland at P0. Vertical lines indicate comparison of the major axis between the glands from p57KO and p57p27KI mice. (C) Representative examples of skeletal staining of E18.5 embryos. Red and black arrowheads indicate the ossification of occipital bone and digits, respectively. (Scale bars: whole body, 5 mm; digits, humerus, and femur, 1 mm.)
Abnormal Inactivation of Rb in p57KO Mice Is Suppressed by Knock-In of p27.
Rb and related proteins have an important role in exit of cells from the cell cycle and in cell differentiation. The observation that p57KO mice show characteristics similar to those of mice deficient in Rb or Rb-related proteins, such as defects in lens and bone (28, 29), suggests that p57 regulates Rb-dependent pathways to ensure timely exit from the cell cycle and cell differentiation during development (2). To confirm that the knocked-in p27 in p57p27KI mice actually functions as a CKI, we prepared MEFs from wild-type, p57KO, and p57p27KI mice, and evaluated endogenous cyclin-CDK activities. The extent of phosphorylation of Rb in serum-deprived p57KO MEFs was greater than that in wild-type MEFs (Fig. 4A; Fig. S3), although the difference was not pronounced, probably as a result of the higher level of expression of p27 than of p57 in MEFs, and the consequent limited impact of p57 loss. Knock-in of p27 significantly reduced the extent of Rb phosphorylation to a level similar to that apparent in the wild-type cells, suggesting that the ectopic p27 inhibited cyclin-CDK activities in place of p57 in arrested cells. To assess the CKI function of knocked-in p27 in vivo, we examined the extent of cell proliferation in lens and bone by measuring incorporation of BrdU at embryonic day (E)13.5 or E16.5, respectively. The numbers of BrdU-positive cells in lens (equatorial zone and posterior chamber) and bone (zones of proliferative and flattened cells) were increased in p57KO mice compared with those in wild-type mice, and these increases were largely abolished in p57p27KI mice (Fig. 4B). Thus, these results suggested that aberrant proliferation of cells in lens and bone of p57KO mice was inhibited by knock-in of p27, resulting in restoration of normal development of these tissues (Fig. 3 A and C).
Some developmental anomalies, such as the intestinal defect, of p57KO mice are likely attributable to aberrant apoptosis triggered by the lack of p57. Loss of p57 may result in inactivation of Rb by cyclin–CDK complexes and consequent induction of E2F1-dependent apoptosis, given that E2F1 contributes not only to proliferation but also, in some settings, to programmed cell death (30). We monitored E2F1 activity by determining the abundance of E2F1 mRNA, given that E2F1 activates transcription of its own gene in a positive feedback loop (31, 32). Indeed, the level of E2F1 mRNA was greater in the intestine of p57KO mice than in that of wild-type mice (Fig. 4C). In contrast, the amount of E2F1 mRNA in the intestine of p57p27KI mice was similar to that in wild-type animals. A role for E2F1-induced apoptosis in the developmental defects of p57KO mice was also confirmed by the substantial amelioration of the cleft palate and intestinal defect of p57KO mice that was apparent in p57/E2F1DKO mice (Fig. 4D), which were generated by crossing p57KO mice with E2F1KO mice (33). These observations suggest that p57 functions as a CKI in certain tissues during mouse development, and that it is replaceable in these tissues by p27.
Phenotypes of p57KO Mice Not Corrected by Knock-In of p27.
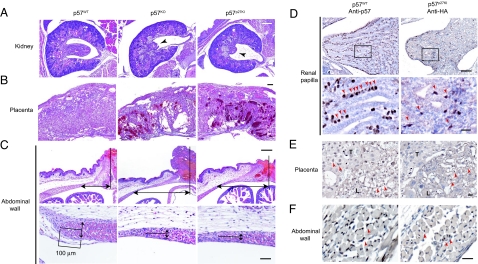
Although most abnormalities of p57KO mice were found to be corrected in p57p27KI mice, there were some exceptions. Dysplasia of the renal papilla, placental dysplasia, and thinning of the abdominal wall or omphalocele, thus, remained in p57p27KI mice (Fig. 5 A–C; Table S1 and Fig. S4). These observations suggested that such phenotypes of the kidney, placenta, and abdominal wall are not responsible for the early death of p57KO mice. We examined the spatial expression patterns of p57 and HA-p27 in these tissues by immunohistochemical analysis. In the renal papilla of wild-type mice, p57 was found to be expressed predominantly in interstitial cells adjacent to renal tubular cells (Fig. 5D). Although the expression level of knocked-in p27 in p57p27KI mice appeared similar to that of p57 in wild-type mice, HA-p27 was detected in only a subset of interstitial cells, and the array of these cells was unorganized in p57p27KI mice (Fig. 5D). In the placenta, p57 was expressed both in trophoblasts of the basal and labyrinth zones, as well as in epithelial cells of the labyrinth zone in wild-type mice, whereas HA-p27 was virtually undetectable in trophoblasts of p57p27KI mice (Fig. 5E). Thus, persistent abnormalities observed in p57p27KI mice were associated with changes in the expression pattern of knocked-in p27, suggesting that regulation of the expression of p57 differs from that of HA-p27 in a cell type-dependent manner. RT-PCR analysis revealed that the expression of HA-p27 mRNA in the kidney or placenta of p57p27KI mice was similar to that of endogenous p57 mRNA in wild-type mice (Fig. S5A). Also, immunohistofluorescence analysis showed that KPC (34) and Pirh2 (35; T. Hattori and M. Kitagawa, personal communication), both of which are p27-specific E3 ubiquitin ligases, were expressed prominently in the trophoblast layer, but to a lesser extent in the labyrinth layer of the placenta (Fig. S5B), suggesting that HA-p27, but not p57, may undergo ubiquitin-dependent degradation in trophoblasts. In contrast, the expression pattern of HA-p27 in epithelial cells of the labyrinth zone, as well as in skeletal muscle cells of the abdominal wall appeared similar to that of p57 (Fig. 5 E and F), despite the remaining mutant phenotypes in p57p27KI mice (Fig. 5 B and C), suggesting that p57 has a specific role in these tissues. Together, these various observations suggest that p57 and p27 share many roles in vivo, but that p57 also performs specific functions not amenable to substitution with p27.
Fig. 5.
Tissue-specific residual phenotypes and corresponding expression patterns of knocked-in p27 in p57p27KI mice. (A and B) Representative histopathology of the renal papilla and placenta, respectively, of E18.5 embryos of the indicated genotypes. Hematoxylin–eosin staining shows developmental defects in the renal papilla (arrowheads), and necrosis in the placenta of p57KO and p57p27KI mice. (Scale bar, 200 μm.) (C) Representative histopathology of the umbilical region (Upper), and the tip of the rectus abdominis muscle (Lower) of P0 embryos. Arrows indicate the distance between the umbilicus and the tip of the rectus abdominis muscle (U-M distance) (Upper), and the thickness of the muscle layer at a position 100 μm from the tip (Lower). Hematoxylin–eosin staining reveals an increased U-M distance and thinner muscle layer in both p57KO and p57p27KI mice. Quantitative data are shown in Fig. S4. (Scale bars: for Upper, 200 μm; for Lower, 50 μm.) (D–F) Immunohistochemical analysis of the renal papilla (E18.5), placenta (E18.5), and abdominal wall (P0), respectively, of p57WT or p57p27KI mice performed with antibodies to p57 or to HA, respectively. Sections were counterstained with hematoxylin. Arrowheads indicate corresponding cells expressing either p57 or knocked-in p27. The boxed regions in D Upper are shown at higher magnification in the D Lower. T, spongiotrophoblast zone; L, labyrinth zone. (Scale bars: for D Upper, 100 μm; for D Lower, E, and F, 20 μm.)
Discussion
We have examined whether the in vivo functions of p27 and p57 are identical by generating a knock-in mouse model in which the p57 gene is replaced with the p27 gene. The p57p27KI mice were born in the expected numbers and survived without gross abnormalities, whereas most p57KO mice manifest multiple developmental defects, and die shortly after birth. The inactivation of the Rb pathway and increased cell proliferation, as well as the associated developmental defects that result from p57 deficiency, were corrected by knock-in of p27. Thus, our data show, in a physiological setting, that p27 and p57 are functionally similar, with p27 being able to substitute for p57, and that the CKI activity, rather than other potential functions, of p57 is critical for development of most tissues. Our observations support the notion that differences between the phenotypes of p27KO and p57KO mice are mainly attributable to differences in the spatial and temporal expression patterns of p27 and p57 (18), as well as to differences in the sensitivities of tissues to insufficient inhibition of cell proliferation, rather than to differences in the intrinsic molecular activities of the 2 proteins. The abundance of p27 is greater than that of p57 in some tissues, whereas that of p57 is greater than that of p27 in others (18). Thus, the former tissues may be less sensitive than the latter to the ablation of p57. For example, the amount of p27 is greater than that of p57 in MEFs, consistent with the observation that the ablation of p57 in these cells results in only a moderate change in CKI activity (this study), and a minimal biological effect (16).
In humans, the p57 gene maps to chromosomal region 11p15.5, which is implicated in Beckwith–Wiedemann syndrome (BWS) (36, 37). BWS is characterized by various growth abnormalities, some of which are recapitulated in p57KO mice (17, 38, 39). Given that the central domains of human and mouse p57 differ, the well-conserved CKI domain may be responsible for the abnormalities shared by p57KO mice and BWS patients. Knock-in of p27 corrected many of these abnormalities, including cleft palate, enlargement of the adrenal gland, as well as intestinal, skeletal, and lens defects, supporting the idea that the conserved CKI domain has a key role in organ development not only in mice but also in humans.
The defects in some tissues of p57KO mice remained apparent in p57p27KI mice. Also, slight defects sometimes remained even in tissues that showed recovery. These findings might be explained by several possible scenarios. First, there might be differences in the activity or specificity of p27 and p57 as CKIs. Indeed, phosphorylation of p27 at tyrosine residues 74, 88, and 89 affects its binding preferences and CKI activity (40–43). Thus, it is possible that cell type-specific kinases phosphorylate CKIs and, thus, regulate their CDK-inhibitory activity. Second, non-CKI functions of p27 and p57 may be important for developmental processes. Both p27 and p57 bind various molecules specifically (20–27, 44), and contribute to certain developmental processes in a manner independent of their CKI activity (22, 25, 45–48). For example, stabilization of MyoD and inhibition of JNK by p57 promote myoblast differentiation (23, 26, 49), consistent with our finding that knocked-in p27 did not correct the abdominal muscle defect of p57KO mice, despite its expression pattern being apparently identical to that of p57. Also, given that mouse p57 binds to LIMK through its unique central domain to regulate actin formation (19), and that LIMK2 knockout mice manifest abnormalities in the kidney (50), defective cell migration might be responsible for the kidney defect of p57KO mice. Third, the stability of p27 and p57 proteins may also differ in a cell type-dependent manner. The expression level of knocked-in p27 in placental trophoblasts of p57p27KI mice was greatly reduced compared with that of p57 in wild-type mice, suggesting the existence of a posttranscriptional regulatory mechanism specific for p27 in these cells. Indeed, there appear to be specific pathways for p27 or p57 degradation. Only p27 (not p57) is ubiquitylated by the KPC-dependent pathway (34), or by Pirh2-dependent pathway (35; T. Hattori and M. Kitagawa, personal communication), whereas the F-box protein FBL12 was recently shown to contribute to the degradation of p57 (51). Thus, various mechanisms may determine the specific features of p27 and p57.
In conclusion, p27 and p57 possess similar CKI activities, but each of the 2 proteins shows specific features in certain cellular contexts. The extent of correction of the developmental defects of p57KO mice by p27 knock-in varied in a tissue-dependent manner, presumably reflecting the extent to which p27 and p57 are molecularly equivalent.
Materials and Methods
For more details, see SI Materials and Methods.
Generation of p57p27KI Mice.
The knock-in mouse was generated as described previously (14, 16). The targeting vector was constructed by replacement of exons 2 and 3, which contain the ORF, as well as intron 2 of the p57 gene with a cDNA encoding HA-tagged mouse p27 as well as with a neomycin-resistance gene (neo) cassette flanked by LoxP sequences. The 5′ region of homology in the targeting vector consisted of a 1.6-kb fragment generated by PCR with appropriate primers to remove intron 3 of the p57 gene; the 3′ region of homology comprised a 7-kb fragment generated by PCR with appropriate primers to remove intron 1, and connected an NdeI-NotI fragment spanning the promoter region and 5′ untranslated region of the p57 gene. The ES cell clones that manifested homologous recombination were subjected to an additional electroporation to introduce the Cre-Pac vector (52), and puromycin-resistant, G418-sensitive colonies were isolated. Examination of embryonic and neonatal phenotypes was performed with mice of the C57BL/6J background or the C57BL/6J × 129/Sv background, whereas adult phenotypes were examined on the C57BL/6J × 129/Sv background, because most p57KO mice die at birth on the C57BL/6J background.
Immunoprecipitation (IP) and Immunoblot Analysis (IB).
Kidneys of newborn mice or E13.5 MEFs were lysed in radioimmunoprecipitation assay (RIPA) buffer supplemented with phosphatase and protease inhibitor (PPI) mix (10 mM sodium pyrophosphate/10 mM NaF/2 mM sodium orthovanadate/1 mM phenylmethylsulfonyl fluoride/10 μg/mL aprotinin/20 μg/mL leupeptin). MEF lysates were subjected to IP with antibodies to Rb (BD PharMingen) in IP buffer (0.5% Triton X-100/150 mM NaCl/PPI mix). Primary antibodies for IB included those to p57 (P-0357, Sigma), to HA (HA-11, Covance), to p27 (BD TDL), to Rb phosphorylated on Ser780 (Cell Signaling), and to HSP70 (BD TDL).
Histology, Immunostaining, and Skeletal Staining.
Hematoxylin–eosin staining was performed according to the standard protocol. Immunohistochemical analysis was performed as described previously (18), with the use of antibodies to p57 (H-91, Santa Cruz Biotechnology) or to HA (Y-11, Santa Cruz Biotechnology). E18.5 embryos were subjected to skeletal staining as described previously (16).
BrdU Incorporation.
Embryos were labeled for 2 h with BrdU by i.p. injection of dams (100 μg of BrdU per gram of body weight). Paraffin-embedded tissue was sectioned at a thickness of 3 μm (lens, E13.5) or 4 μm (humerus, E16.5), and was immunostained with biotinylated antibodies to BrdU (BD PharMingen) and Alexa 488-conjugated streptavidin (Molecular Probes). Nuclei were stained with Hoechst 33258.
Quantitative RT-PCR Analysis.
Total RNA was extracted and purified from the intestine of E18.5 embryos and subjected to reverse transcription with a QuantiTect kit (Qiagen). The resulting cDNA was subjected to real-time PCR analysis as described previously (53), with 200 nM primers specific for E2F1 (54) or GAPDH (53). The abundance of E2F1 mRNA was normalized relative to that of GAPDH mRNA.
Supplementary Material
Acknowledgments.
We thank M. Kitagawa for discussion; N. Kitajima, Y. Yamada, and K. Takeda for technical assistance; members of our laboratories for comments on the manuscript; and A. Ohta and M. Kimura for help in preparation of the manuscript. This work was supported in part by a grant from the Ministry of Education, Science, Sports, and Culture of Japan, and by a research grant from the Takeda Science Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0811712106/DCSupplemental.
References
- 1.Sherr CJ, Roberts JM. CDK inhibitors: Positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 2.Nakayama KI, Nakayama K. Cip/Kip cyclin-dependent kinase inhibitors: Brakes of the cell cycle engine during development. BioEssays. 1998;20:1020–1029. doi: 10.1002/(SICI)1521-1878(199812)20:12<1020::AID-BIES8>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 3.Lee M-H, Reynisdottir I, Massague J. Cloning of p57KIP2, a cyclin-dependent kinase inhibitor with unique domain structure and tissue distribution. Genes Dev. 1995;9:639–649. doi: 10.1101/gad.9.6.639. [DOI] [PubMed] [Google Scholar]
- 4.Matsuoka S, et al. p57KIP2, a structurally distinct member of the p21CIP1 Cdk inhibitor family, is a candidate tumor suppressor gene. Genes Dev. 1995;9:650–662. doi: 10.1101/gad.9.6.650. [DOI] [PubMed] [Google Scholar]
- 5.Polyak K, et al. p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-β and contact inhibition to cell cycle arrest. Genes Dev. 1994;8:9–22. doi: 10.1101/gad.8.1.9. [DOI] [PubMed] [Google Scholar]
- 6.Toyoshima H, Hunter T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell. 1994;78:67–74. doi: 10.1016/0092-8674(94)90573-8. [DOI] [PubMed] [Google Scholar]
- 7.Hengst L, Dulic V, Slingerland JM, Lees E, Reed SI. A cell cycle-regulated inhibitor of cyclin-dependent kinases. Proc Natl Acad Sci USA. 1994;91:5291–5295. doi: 10.1073/pnas.91.12.5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nourse J, et al. Interleukin-2 mediated elimination of the p27(Kip1) cyclin-dependent kinase inhibitor prevented by rapamycin. Nature. 1994;372:570–573. doi: 10.1038/372570a0. [DOI] [PubMed] [Google Scholar]
- 9.Reynisdottir I, Polyak K, Iavarone A, Massague J. Kip/Cip and Ink4 Cdk inhibitors cooperate to induce cell cycle arrest in response to TGF-β. Genes Dev. 1995;9:1831–1845. doi: 10.1101/gad.9.15.1831. [DOI] [PubMed] [Google Scholar]
- 10.Kamura T, et al. Degradation of p57Kip2 mediated by SCFSkp2-dependent ubiquitylation. Proc Natl Acad Sci USA. 2003;100:10231–10236. doi: 10.1073/pnas.1831009100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Polyak K, et al. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell. 1994;78:59–66. doi: 10.1016/0092-8674(94)90572-x. [DOI] [PubMed] [Google Scholar]
- 12.Fero ML, et al. A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27Kip1-deficient mice. Cell. 1996;85:733–744. doi: 10.1016/s0092-8674(00)81239-8. [DOI] [PubMed] [Google Scholar]
- 13.Kiyokawa H, et al. Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27Kip1. Cell. 1996;85:721–732. doi: 10.1016/s0092-8674(00)81238-6. [DOI] [PubMed] [Google Scholar]
- 14.Nakayama K, et al. Mice lacking p27Kip1 display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell. 1996;85:707–720. doi: 10.1016/s0092-8674(00)81237-4. [DOI] [PubMed] [Google Scholar]
- 15.Yan Y, Frisen J, Lee M-H, Massague J, Barbacid M. Ablation of the CDK inhibitor p57Kip2 results in increased apoptosis and delayed differentiation during mouse development. Genes Dev. 1997;11:973–983. doi: 10.1101/gad.11.8.973. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi K, Nakayama KI, Nakayama K. Mice lacking a CDK inhibitor, p57Kip2, exhibit skeletal abnormalities and growth retardation. J Biochem. 2000;127:73–83. doi: 10.1093/oxfordjournals.jbchem.a022586. [DOI] [PubMed] [Google Scholar]
- 17.Zhang P, et al. Altered cell differentiation and proliferation in mice lacking p57KIP2 indicates a role in Beckwith-Wiedemann syndrome. Nature. 1997;387:151–158. doi: 10.1038/387151a0. [DOI] [PubMed] [Google Scholar]
- 18.Nagahama H, et al. Spatial and temporal expression patterns of the cyclin-dependent kinase (CDK) inhibitors p27Kip1 and p57Kip2 during mouse development. Anat Embryol. 2001;203:77–87. doi: 10.1007/s004290000146. [DOI] [PubMed] [Google Scholar]
- 19.Yokoo T, et al. p57Kip2 regulates actin dynamics by binding and translocating LIM-kinase 1 to the nucleus. J Biol Chem. 2003;278:52919–52923. doi: 10.1074/jbc.M309334200. [DOI] [PubMed] [Google Scholar]
- 20.Laman H, et al. Transforming activity of Fbxo7 is mediated specifically through regulation of cyclin D/cdk6. EMBO J. 2005;24:3104–3116. doi: 10.1038/sj.emboj.7600775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Besson A, Gurian-West M, Schmidt A, Hall A, Roberts JM. p27Kip1 modulates cell migration through the regulation of RhoA activation. Genes Dev. 2004;18:862–876. doi: 10.1101/gad.1185504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen L, et al. p27kip1 independently promotes neuronal differentiation and migration in the cerebral cortex. Genes Dev. 2006;20:1511–1524. doi: 10.1101/gad.377106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang T-S, et al. p57KIP2 modulates stress-activated signaling by inhibiting c-Jun NH2-terminal kinase/stress-activated protein kinase. J Biol Chem. 2003;278:48092–48098. doi: 10.1074/jbc.M309421200. [DOI] [PubMed] [Google Scholar]
- 24.Joaquin M, Watson RJ. The cell cycle-regulated B-Myb transcription factor overcomes cyclin-dependent kinase inhibitory activity of p57KIP2 by interacting with its cyclin-binding domain. J Biol Chem. 2003;278:44255–44264. doi: 10.1074/jbc.M308953200. [DOI] [PubMed] [Google Scholar]
- 25.Joseph B, et al. p57Kip2 cooperates with Nurr1 in developing dopamine cells. Proc Natl Acad Sci USA. 2003;100:15619–15624. doi: 10.1073/pnas.2635658100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reynaud EG, et al. Stabilization of MyoD by direct binding to p57Kip2. J Biol Chem. 2000;275:18767–18776. doi: 10.1074/jbc.M907412199. [DOI] [PubMed] [Google Scholar]
- 27.Watanabe H, et al. Suppression of cell transformation by the cyclin-dependent kinase inhibitor p57KIP2 requires binding to proliferating cell nuclear antigen. Proc Natl Acad Sci USA. 1998;95:1392–1397. doi: 10.1073/pnas.95.4.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morgenbesser SD, Williams BO, Jacks T, DePinho RA. p53-dependent apoptosis produced by Rb-deficiency in the developing mouse lens. Nature. 1994;371:72–74. doi: 10.1038/371072a0. [DOI] [PubMed] [Google Scholar]
- 29.Cobrinik D, et al. Shared role of the pRB-related p130 and p107 proteins in limb development. Genes Dev. 1996;10:1633–1644. doi: 10.1101/gad.10.13.1633. [DOI] [PubMed] [Google Scholar]
- 30.Putzer BM. E2F1 death pathways as targets for cancer therapy. J Cell Mol Med. 2007;11:239–251. doi: 10.1111/j.1582-4934.2007.00030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsiao KM, McMahon SL, Farnham PJ. Multiple DNA elements are required for the growth regulation of the mouse E2F1 promoter. Genes Dev. 1994;8:1526–1537. doi: 10.1101/gad.8.13.1526. [DOI] [PubMed] [Google Scholar]
- 32.Neuman E, Flemington EK, Sellers WR, Kaelin WG., Jr Transcription of the E2F-1 gene is rendered cell cycle dependent by E2F DNA-binding sites within its promoter. Mol Cell Biol. 1994;14:6607–6615. doi: 10.1128/mcb.14.10.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Degregori J, Leone G, Miron A, Jakoi L, Nevins JR. Distinct roles for E2F proteins in cell growth control and apoptosis. Proc Natl Acad Sci USA. 1997;94:7245–7250. doi: 10.1073/pnas.94.14.7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kamura T, et al. Cytoplasmic ubiquitin ligase KPC regulates proteolysis of p27Kip1 at G1 phase. Nat Cell Biol. 2004;6:1229–1235. doi: 10.1038/ncb1194. [DOI] [PubMed] [Google Scholar]
- 35.Hattori T, et al. Pirh2 promotes ubiquitin-dependent degradation of the cyclin-dependent kinase inhibitor p27Kip1. Cancer Res. 2007;67:10789–10795. doi: 10.1158/0008-5472.CAN-07-2033. [DOI] [PubMed] [Google Scholar]
- 36.Koufos A, et al. Familial Wiedemann-Beckwith syndrome and a second Wilms tumor locus both map to 11p15.5. Am J Hum Genet. 1989;44:711–719. [PMC free article] [PubMed] [Google Scholar]
- 37.Ping AJ, et al. Genetic linkage of Beckwith-Wiedemann syndrome to 11p15. Am J Hum Genet. 1989;44:720–723. [PMC free article] [PubMed] [Google Scholar]
- 38.Swanger WJ, Roberts JM. p57KIP2 targeted disruption and Beckwith-Wiedemann syndrome: Is the inhibitor just a contributor? BioEssays. 1997;19:839–842. doi: 10.1002/bies.950191002. [DOI] [PubMed] [Google Scholar]
- 39.Caspary T, et al. Oppositely imprinted genes p57Kip2 and Igf2 interact in a mouse model for Beckwith-Wiedemann syndrome. Genes Dev. 1999;13:3115–3124. doi: 10.1101/gad.13.23.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kardinal C, et al. Tyrosine phosphorylation modulates binding preference to cyclin-dependent kinases and subcellular localization of p27Kip1 in the acute promyelocytic leukemia cell line NB4. Blood. 2006;107:1133–1140. doi: 10.1182/blood-2005-05-1771. [DOI] [PubMed] [Google Scholar]
- 41.Grimmler M, et al. Cdk-inhibitory activity and stability of p27Kip1 are directly regulated by oncogenic tyrosine kinases. Cell. 2007;128:269–280. doi: 10.1016/j.cell.2006.11.047. [DOI] [PubMed] [Google Scholar]
- 42.Chu I, et al. p27 phosphorylation by src regulates inhibition of cyclin E-Cdk2. Cell. 2007;128:281–294. doi: 10.1016/j.cell.2006.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.James MK, Ray A, Leznova D, Blain SW. Differential modification of p27Kip1 controls its cyclin D-cdk4 inhibitory activity. Mol Cell Biol. 2008;28:498–510. doi: 10.1128/MCB.02171-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakanishi M, Kaneko Y, Matsushime H, Ikeda K. Direct interaction of p21 cyclin-dependent kinase inhibitor with the retinoblastoma tumor suppressor protein. Biochem Biophys Res Commun. 1999;263:35–40. doi: 10.1006/bbrc.1999.1296. [DOI] [PubMed] [Google Scholar]
- 45.Messina G, et al. p27Kip1 acts downstream of N-cadherin-mediated cell adhesion to promote myogenesis beyond cell cycle regulation. Mol Biol Cell. 2005;16:1469–1480. doi: 10.1091/mbc.E04-07-0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kawauchi T, Chihama K, Nabeshima Y-I, Hoshino M. Cdk5 phosphorylates and stabilizes p27kip1 contributing to actin organization and cortical neuronal migration. Nat Cell Biol. 2006;8:17–26. doi: 10.1038/ncb1338. [DOI] [PubMed] [Google Scholar]
- 47.Dugas JC, Ibrahim A, Barres BA. A crucial role for p57Kip2 in the intracellular timer that controls oligodendrocyte differentiation. J Neurosci. 2007;27:6185–6196. doi: 10.1523/JNEUROSCI.0628-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dyer MA, Cepko CL. P57Kip2 regulates progenitor cell proliferation and amacrine interneuron development in the mouse retina. Development. 2000;127:3593–3605. doi: 10.1242/dev.127.16.3593. [DOI] [PubMed] [Google Scholar]
- 49.Reynaud EG, Pelpel K, Guillier M, Leibovitch MP, Leibovitch SA. p57Kip2 stabilizes the MyoD protein by inhibiting cyclin E-Cdk2 kinase activity in growing myoblasts. Mol Cell Biol. 1999;19:7621–7629. doi: 10.1128/mcb.19.11.7621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takahashi H, Koshimizu U, Miyazaki J-I, Nakamura T. Impaired spermatogenic ability of testicular germ cells in mice deficient in the LIM-kinase 2 gene. Dev Biol. 2002;241:259–272. doi: 10.1006/dbio.2001.0512. [DOI] [PubMed] [Google Scholar]
- 51.Kim M, Nakamoto T, Nishimori S, Tanaka K, Chiba T. A new ubiquitin ligase involved in p57KIP2 proteolysis regulates osteoblast cell differentiation. EMBO Rep. 2008;9:878–884. doi: 10.1038/embor.2008.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taniguchi M, et al. Efficient production of Cre-mediated site-directed recombinants through the utilization of the puromycin resistance gene, pac: A transient gene-integration marker for ES cells. Nucleic Acids Res. 1998;26:679–680. doi: 10.1093/nar/26.2.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Onoyama I, et al. Conditional inactivation of Fbxw7 impairs cell-cycle exit during T cell differentiation and results in lymphomatogenesis. J Exp Med. 2007;204:2875–2888. doi: 10.1084/jem.20062299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.David G, et al. Specific requirement of the chromatin modifier mSin3B in cell cycle exit and cellular differentiation. Proc Natl Acad Sci USA. 2008;105:4168–4172. doi: 10.1073/pnas.0710285105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.