Abstract
The active form of vitamin D, 1α,25-dihydroxyvitamin D3 [1,25(OH)2D3], suppresses disease development in the experimental autoimmune encephalomyelitis (EAE) model of multiple sclerosis (MS). However, complete disease prevention only occurs with doses that dramatically elevate serum calcium levels, thus limiting the usefulness of 1,25(OH)2D3 as a potential MS therapeutic agent. Because calcitonin (CT) is believed to be released by hypercalcemia and has been shown to be anti-inflammatory, we examined whether suppression of EAE by 1,25(OH)2D3 could be mediated either in part or entirely by CT. Continuous administration of pharmacological doses of CT did not prevent EAE. However, a combination of CT and a subtherapeutic dose of 1,25(OH)2D3 additively suppressed EAE without causing hypercalcemia. Moreover, CT decreased the dose of 1,25(OH)2D3 required for disease suppression. Our results suggest that CT may be a significant factor but cannot account entirely for 1,25(OH)2D3-mediated suppression of EAE.
Keywords: calcium, calcium homeostasis, immune system, multiple sclerosis, vitamin D
Multiple sclerosis (MS) is a chronic, debilitating disease of the central nervous system characterized by inflammatory cell infiltration and subsequent demyelination of axonal tracts in the brain and spinal cord. Demyelination interferes with normal signal conduction along neuronal axons, ultimately resulting in a number of clinical symptoms including fatigue, pain, muscle weakness, and visual disturbances (1). Although the exact cause of MS remains elusive, a number of genetic and environmental factors are believed to contribute to MS susceptibility. Epidemiological studies have revealed an unusual geographical gradient for MS, in which MS incidence increases with latitude in both hemispheres (2). One potential explanation for this observation is that MS susceptibility is dependent on exposure to sunlight and the subsequent production of vitamin D (3). Consistent with this hypothesis are the findings that vitamin D supplementation and higher circulating vitamin D levels are associated with a decreased risk for MS (4, 5).
Vitamin D is obtained either from production in the skin upon exposure to sunlight or through ingestion of foods containing vitamin D. Vitamin D undergoes 2 successive hydroxylation steps to form the active hormone 1α,25-dihydroxyvitamin D3 [1,25(OH)2D3], which is critical for maintaining a serum calcium level of 9–10 mg/dL for proper bone mineralization and neuromuscular function (6). A decrease in serum calcium stimulates parathyroid hormone (PTH) secretion from the parathyroid gland, leading to increased production of 1,25(OH)2D3 in the kidney. PTH and 1,25(OH)2D3 act in concert to effect both calcium release from bone and reabsorption of calcium in the kidney. 1,25(OH)2D3 also increases intestinal calcium absorption independent of PTH, thus elevating serum calcium back to normal levels.
In addition to its role in regulating serum calcium levels, vitamin D acts as an important immune system modulator. The vitamin D receptor is present in a number of different immune cell types, including monocytes, macrophages, dendritic cells, and activated T cells (7–9). In vitro studies have demonstrated that 1,25(OH)2D3 inhibits T-cell proliferation and decreases the expression of inflammatory cytokines such as IL-2 and IFN-γ (10, 11). A number of in vivo studies have demonstrated that 1,25(OH)2D3 can suppress inflammation and autoimmune pathology in a variety of animal models of autoimmune disease, including models for insulin-dependent diabetes mellitus, rheumatoid arthritis, and MS (12–14). In the experimental autoimmune encephalomyelitis (EAE) model of MS, 1,25(OH)2D3 has been shown to prevent clinical signs of disease as well as to suppress disease progression (14, 15). The suppressive effects of 1,25(OH)2D3 appear to be closely linked to calcium. Mice on a higher calcium diet require much lower doses of 1,25(OH)2D3 for disease prevention than those on a low-calcium diet (16). Moreover, complete prevention of EAE only occurs using doses of 1,25(OH)2D3 that elevate serum calcium concentrations to harmful levels, thus limiting its clinical utility (16). Studies from our laboratory demonstrate that hypercalcemia independent of 1,25(OH)2D3, prevents EAE in female mice. These results suggest that hypercalcemia, or some factor released under hypercalcemic conditions, may be partially responsible for EAE disease protection (17).
Hypercalcemia stimulates the release of the peptide hormone calcitonin (CT) from parafollicular cells in the thyroid gland. CT causes a rapid decrease in serum calcium levels by interacting directly with osteoclasts, thereby inhibiting osteoclast-mediated bone resorption. This action has led to the use of CT for the treatment of bone disorders such as osteoporosis and Paget's disease (18). CT exerts its biological effects by binding to a G protein-coupled receptor and modulating a number of downstream secondary messenger pathways. Although the hypocalcemic effects of CT have been well characterized, the importance of CT in calcium homeostasis under normal conditions has been questioned. For example, mice deficient in CT have normal serum calcium levels (19). Likewise, postthyroidectomy patients who are deficient in CT do not develop hypercalcemia (20). However, CT levels during pregnancy and lactation are elevated (21). Thus, it is likely that CT plays a role in skeletal conservation during times of calcium stress (22, 23). The CT receptor has been identified on circulating lymphocytes and shows differential expression in response to various cytokines. These observations suggest that CT may play a role in immune system modulation (24, 25). IL-6, which has been identified in MS brain lesions, causes a marked decrease in CT binding to lymphocytes (25). Conversely, the anti-inflammatory cytokine TGF-β causes a significant increase in CT binding by blood monocytes (26). A number of in vivo animal models of inflammation have demonstrated that CT treatment has an anti-inflammatory effect (27, 28). Additional studies in human subjects indicate that CT treatment significantly improves the clinical symptoms of the autoimmune disease rheumatoid arthritis, partially through inhibition of the inflammatory cytokine IL-1 (29, 30). However, the exact role of CT in the immune system remains unclear.
The anti-inflammatory effects of CT, coupled with the data suggesting that 1,25(OH)2D3 is only effective at preventing EAE under conditions favoring high circulating CT levels, led us to explore the potential therapeutic usage of CT in the EAE model of MS. Our results indicate that mice treated with continuously administered pharmacological doses of CT exhibit a modest decrease in clinical scores. However, when CT is used in combination with 1,25(OH)2D3, the dual-hormone therapy dramatically suppresses EAE without significantly altering serum calcium levels. These results suggest a potential role for this combination as an MS therapeutic intervention.
Results
CT Treatment Causes a Mild Suppression of EAE.
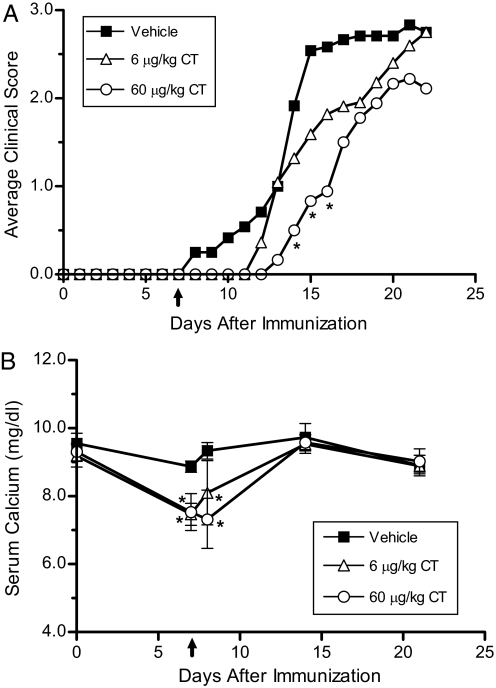
Treatment of female C57BL/6J mice with 6 μg/kg CT per day had no significant effect on any of the EAE clinical parameters tested (Fig. 1A and Table 1). Treatment with 60 μg/kg CT caused a significant delay in the onset of disease and a slight reduction in disease severity. CT caused a significant drop in serum calcium levels at 6 and 18 h after pump implantation (Fig. 1B). Serum calcium levels returned to normal within 1 week after pump implantation and remained normal for the duration of the experiment.
Fig. 1.
Treatment with calcitonin causes a mild suppression of EAE. (A) Average clinical EAE scores were determined in vehicle- and CT-treated mice (n = 9–12). Ten-week-old female C57BL/6J mice were fed a regular chow diet and were immunized with MOG35–55. Seven days after immunization, pumps containing vehicle, 6, or 60 μg/kg per day of salmon CT were implanted s.c. (B) Serum calcium levels (± SD) were determined at selected time points, including 6 and 18 h after pump implantation. CT caused a transient drop in serum calcium levels. ↑, CT treatment initiated and continued for the duration of the experiment. *, P < 0.05 compared with vehicle group.
Table 1.
Calcitonin treatment delayed the onset of EAE in female mice
| Treatment | Incidence | Day of onset | Peak severity | CDI |
|---|---|---|---|---|
| Vehicle | 75% (9/12) | 13 ± 3 | 4.2 ± 1.1 | 27 ± 20 |
| 6 μg/kg CT | 82% (9/11) | 14 ± 3 | 3.8 ± 0.6 | 19 ± 12 |
| 60 μg/kg CT | 67% (6/9) | 16 ± 2* | 4.6 ± 2.4 | 13 ± 13 |
Experiments were performed as described in Fig. 1. The clinical data demonstrate the mean ± SD from one representative of 3 individual experiments. The cumulative disease index (CDI) was calculated by summing the daily clinical scores and dividing by the number of mice in each group as described in Materials and Methods. *, P < 0.05 compared to the vehicle group.
CT and 1,25(OH)2D3 Suppress EAE Without Elevating Serum Calcium Levels.
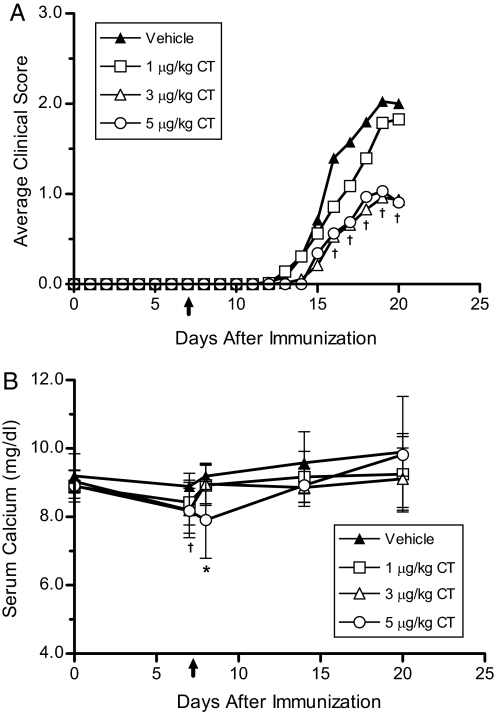
Female C57BL/6J mice were treated with a subtherapeutic dose (1 ng/d) of 1,25(OH)2D3 in combination with either 1, 3, or 5 μg/kg CT per day. The maximum dose of CT was reduced from 60 to 5 μg/kg per day, because mice maintained on the purified diet were more sensitive to hypocalcemic toxicity than mice given a regular chow diet (unpublished observation). Interestingly, treatment with the combination of 1 ng of 1,25(OH)2D3 and 3 μg/kg CT per day caused a significant reduction (P < 0.01) in disease incidence from 78% in the vehicle group to 47% in the group receiving 3 μg/kg CT per day (Table 2). Additionally, treatment with 3 μg/kg CT per day caused a significant reduction in the average clinical scores, peak disease severity, and cumulative disease index (CDI) compared with vehicle (Fig. 2A and Table 2). Increasing the CT dose to 5 μg/kg per day also caused a significant suppression of clinical EAE but did not increase the efficacy of treatment. Decreasing the dose of CT to 1 μg/kg per day eliminated the protective effect of the combination therapy. Treatment with CT caused a transient reduction of 0.7 mg/dL in serum calcium levels 6 hours after pump implantation, which returned to baseline levels within a week of treatment (Fig. 2B). Furthermore, serum calcium levels remained in the normal range (9–10 mg/dL) for the duration of the experiment.
Table 2.
Combination therapy using calcitonin and 1,25(OH)2D3 suppressed clinical signs of EAE
| Treatment | Incidence | Day of onset | Peak severity | CDI |
|---|---|---|---|---|
| Vehicle | 78% (31/40) | 16 ± 2 | 2.9 ± 1.0 | 11 ± 8 |
| 1 μg/kg CT | 68% (26/38) | 16 ± 2 | 3.0 ± 0.78 | 9 ± 8 |
| 3 μg/kg CT | 47% (18/38)* | 17 ± 2 | 2.1 ± 0.9* | 5 ± 6* |
| 5 μg/kg CT | 56% (9/16) | 17 ± 2 | 1.9 ± 1.0* | 5 ± 5* |
Experiments were performed as described in Fig. 2. The clinical data represent the pooled mean ± SD from 2 identical experiments. *, P < 0.05 compared to vehicle.
Fig. 2.
Treatment with a combination of 1,25(OH)2D3 and calcitonin results in an increased suppression of EAE. (A) Average clinical scores were determined in mice treated with varying doses of CT and 1 ng of 1,25(OH)2D3. Data points represent the pooled averages of 2 separate experiments (n = 18–40). Seven-week-old female C57BL/6J mice were fed a 0.87%-calcium-purified diet containing 1 ng of 1,25(OH)2D3 per day. Two weeks after initiation of vitamin D therapy, the mice were immunized with MOG35–55. Pumps delivering vehicle, 1, 3, or 5 μg/kg per day of salmon CT were implanted s.c. 7 days after immunization. (B) Serum calcium levels (± SD) were determined at selected time points, including 6 h after pump implantation (day 7). ↑, CT treatment initiated and continued for the duration of the experiment. *, P < 0.05 compared with vehicle group. †, P < 0.05 for 3 μg/kg and 5μg/kg CT groups compared with vehicle.
CT Lowers the Effective Dose of 1,25(OH)2D3 Required to Suppress EAE.
We next sought to determine if the well-known hypocalcemic effect of CT could prevent hypercalcemia in mice treated with higher, more immunosuppressive 1,25(OH)2D3 doses. Pilot studies indicated that 1,25(OH)2D3 doses greater than 1 ng/d caused hypercalcemia (data not shown). Female C57BL/6J mice were treated with either 0, 1, or 2 ng of 1,25(OH)2D3 per day in combination with vehicle or 3 μg/kg CT per day. Treatment with either 1 or 2 ng of 1,25(OH)2D3 had little effect on EAE progression without coadministration of CT (Fig. 3A and Table 3). As before, treatment with the combination of 1 ng of 1,25(OH)2D3 and CT per day caused a significant reduction in clinical EAE. Although increasing the 1,25(OH)2D3 dose to 2 ng/d also suppressed clinical EAE when delivered in combination with CT, it did not improve the efficacy of the combination treatment. Furthermore, although CT treatment lowered serum calcium levels of the group receiving 2 ng of 1,25(OH)2D3 + CT to within the normal range 24 h after pump implantation, the effect was transient and the mice were hypercalcemic at the end of the study (Fig. 3B). Thus, CT treatment lowers the effective dose of 1,25(OH)2D3 required to suppress EAE but does not prevent hypercalcemia caused by higher doses of 1,25(OH)2D3.
Fig. 3.
Calcitonin lowers the effective dose required to suppress EAE. (A) Average daily clinical scores were determined in mice treated with selected doses of 1,25(OH)2D3 and 3 μg/kg CT per day (n = 13–15). Eight-week-old female C57BL/6J mice were fed a 0.87%-calcium-purified diet containing 0, 1, or 2 ng of 1,25(OH)2D3 per day. Two weeks after initiating vitamin D therapy, the mice were immunized with MOG35–55. Ten days after immunization, pumps delivering either vehicle or 3 μg/kg per day of salmon CT were implanted s.c. (B) Serum calcium levels (± SD) were measured at selected time points, including 24 h after pump implantation (day 11). ↑, CT treatment initiated and continued for the duration of the experiment. *, P < 0.05 compared with 0-ng 1,25(OH)2D3 + vehicle and 1-ng 1,25(OH)2D3 + vehicle groups. †, P < 0.05 compared with 0-ng 1,25(OH)2D3 + vehicle group.
Table 3.
Treatment with calcitonin reduced the 1,25(OH)2D3 dose required to suppress EAE
| Treatment | Incidence | Day of onset | Peak severity | CDI |
|---|---|---|---|---|
| 0 ng 1,25D3 + vehicle | 79% (11/14) | 14 ± 1 | 3.0 ± 0.5 | 22 ± 12 |
| 1 ng 1,25D3 + vehicle | 80% (12/15) | 14 ± 2 | 2.8 ± 0.9 | 20 ± 12 |
| 1 ng 1,25D3 + CT | 40% (6/15) | 17 ± 3 | 2.3 ± 1.0 | 7 ± 10** |
| 2 ng 1,25D3 + vehicle | 80% (12/15) | 16 ± 3 | 3.0 ± 0.8 | 17 ± 12 |
| 2 ng 1,25D3 + CT | 60% (9/15) | 18 ± 4* | 2.4 ± 0.8 | 9 ± 11** |
Experiments were performed as described in Fig. 3. The clinical data demonstrate the mean ± SD from one representative of 2 individual experiments. *, P < 0.05 compared to 0 ng 1,25D3 + vehicle.
**, P < 0.05 compared to all three vehicle groups.
Dietary Calcium Regulates the Suppression of EAE by the Combination of 1,25(OH)2D3 and CT.
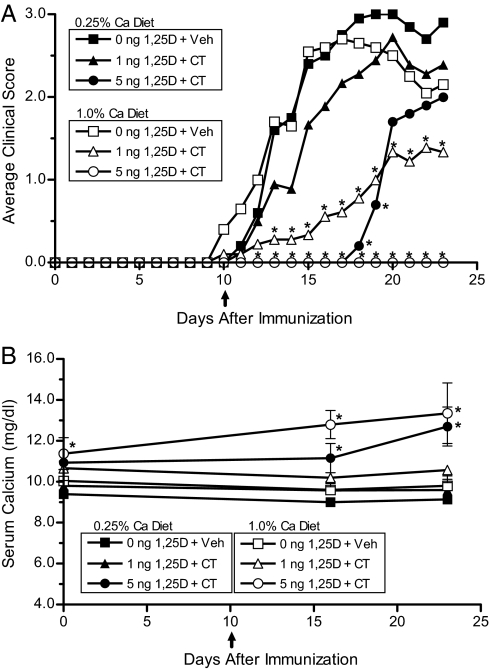
Dietary calcium has been shown to be an important factor regulating the protective effect of 1,25(OH)2D3 on EAE (16). To examine the dependence of CT and 1,25(OH)2D3 combination therapy on dietary calcium, female C57BL/6J mice were treated with either 0, 1, or 5 ng of 1,25(OH)2D3 delivered in a low-calcium (0.25%) or high-calcium (1.0%) diet in combination with vehicle or 3 μg/kg CT per day. There were no significant differences in any of the disease parameters measured between the 2 vehicle-treated groups maintained on different levels of dietary calcium (Fig. 4A and Table 4). Mice maintained on the 1.0% calcium diet treated with 1 ng of 1,25(OH)2D3 + CT showed a dramatic suppression of clinical EAE signs compared with the vehicle-treated groups and the 0.25% dietary calcium group that received the same treatment. Furthermore, complete disease prevention occurred in mice maintained on the 1.0% calcium diet and treated with 5 ng of 1,25(OH)2D3 + CT compared with a 60% disease incidence rate in the 0.25% calcium diet group receiving the same treatment (Fig. 4B). Although the animals treated with 5 ng of 1,25(OH)2D3 animals were clearly hypercalcemic, their weights were only slightly lower than the heaviest group (1 ng of 1,25(OH)2D3 + CT), having an average weight of 17.5 g per animal vs. 19.2 g per animal in the 1% calcium/1-ng 1,25(OH)2D3 + CT group, and the control group had a final weight of 18.5 g per animal. Food consumption was not significantly different in any group. These experiments demonstrate that the efficacy of the combination therapy using 1,25(OH)2D3 and CT is dependent on dietary calcium.
Fig. 4.
Suppression of EAE by the combination of 1,25(OH)2D3 and calcitonin is dependent on dietary calcium. (A) Average clinical scores were determined in mice placed on either a low 0.25% (closed symbols) or a high 1.0% (open symbols) calcium diet treated with selected doses of 1,25(OH)2D3 and CT (n = 5–10). Eight-week-old female C57BL/6J mice were fed 0.25%-calcium- or 1.0%-calcium-purified diet containing 0, 1, or 5 ng of 1,25(OH)2D3 per day. Two weeks after changing the diet, the mice were immunized with MOG35–55. Ten days after immunization, pumps delivering either vehicle or 3 μg/kg per day of salmon CT were implanted s.c. (B) Serum calcium levels (± SD) were measured by atomic absorption spectroscopy at selected time points. ↑, CT treatment initiated and continued for the duration of the experiment. *, P < 0.05 compared with both vehicle-treated groups.
Table 4.
Dietary calcium regulates suppression of EAE by 1,25(OH)2D3 and calcitonin
| Dietary Ca | Treatment | Incidence | Day of onset | Peak severity | CDI |
|---|---|---|---|---|---|
| 0.25% | 0 ng 1,25D3 + Veh | 100% (10/10) | 14 ± 3 | 3.5 ± 0.7 | 29 ± 10 |
| 1.0% | 0 ng 1,25D3 + Veh | 90% (9/10) | 13 ± 2 | 3.4 ± 0.9 | 28 ± 16 |
| 0.25% | 1 ng 1,25D3 + CT | 78% (7/9) | 14 ± 2 | 3.7 ± 0.8 | 23 ± 16 |
| 1.0% | 1 ng 1,25D3 + CT | 67% (6/9) | 17 ± 4 | 2.4 ± 0.7† | 10 ± 10** |
| 0.25% | 5 ng 1,25D3 + CT | 60% (3/5) | 19 ± 1* | 3.3 ± 0.6 | 8 ± 9** |
| 1.0% | 5 ng 1,25D3 + CT | 0% (0/5)* | NA | NA | 0** |
Experiments were performed as described in Fig. 4. The clinical data demonstrate the mean ± SD from 1 representative of 2 individual experiments. *, P < 0.05 compared to both vehicle groups.
**, P < 0.05 compared to both vehicle groups and the 0.25% Ca 1-ng 1,25(OH)2D3 + CT group. NA, not applicable.
Discussion
Our results demonstrate that treatment with pharmacological doses of CT cause a modest suppression of EAE. Comparing the slight suppressive effect exerted by CT with the complete prevention of disease in hypercalcemic mice from previous studies clearly indicates that CT is not the sole factor responsible for prevention of EAE under hypercalcemic conditions. Consistent with previous reports, CT treatment caused a transient decrease in serum calcium levels, which returned to baseline levels within 48 h (31). The transient nature of the hypocalcemic effect by CT has been attributed to down-regulation of the CT receptor by CT itself (32).
Surprisingly, when CT was delivered in combination with a subtherapeutic dose of 1,25(OH)2D3, it significantly enhanced the suppression of EAE by 1,25(OH)2D3. Importantly, after the transient decrease at the start of CT treatment, serum calcium levels remained in the normal range for the duration of the experiment. Therefore, with CT present, dramatic suppression of EAE was achieved without hypercalcemia and the therapeutic dose of 1,25(OH)2D3 was reduced. Further research will be needed to elucidate the mechanism through which CT is acting synergistically with 1,25(OH)2D3 to suppress EAE. One potential explanation for this synergism is that CT is causing increased production of endogenous 1,25(OH)2D3. CT has been shown to increase expression of the 1α-hydroxylase enzyme that is responsible for converting 25(OH)D3 to the active hormone 1,25(OH)2D3 (33, 34). Elevated 1α-hydroxylase expression by CT, leading to increased endogenous 1,25(OH)2D3 production, could be responsible for the synergistic effect on EAE. Another potential explanation is that CT has the ability to enhance the suppressive effect of certain immunomodulatory agents. A recent study demonstrated a similar synergistic effect by CT and a corticosteroid in an animal model of rheumatoid arthritis. Mice treated with CT and a subtherapeutic dose of prednisolone showed a dramatic reduction in inflammation and attenuation of disease (35). This suggests that the synergistic effect by CT may be more general, and CT could potentially enhance the ability of several immunosuppressive agents to suppress a number of autoimmune diseases.
Although the combination therapy significantly suppressed clinical signs of EAE beyond subtherapeutic doses of 1,25(OH)2D3 alone, it did not completely prevent EAE unless the animals were hypercalcemic. Furthermore, similar to 1,25(OH)2D3 treatment alone, the combination therapy was much more effective at suppressing disease when the animals were maintained on a high-calcium diet. These findings again underscore the importance of calcium in 1,25(OH)2D3-mediated prevention of EAE. In addition, our results demonstrate that CT does not prevent hypercalcemia associated with 1,25(OH)2D3 treatment. A number of alternate strategies have been used to enhance suppression of EAE while eliminating the hypercalcemia associated with 1,25(OH)2D3 treatment, including the development of less calcemic analogues, decreasing dietary calcium intake, and the use of bone-resorption inhibitors such as bisphosphonates (36, 37). An alternative approach is to identify immunomodulatory agents that potentiate the protective effect of vitamin D analogues without elevating calcium levels. Supporting this strategy are the findings that cyclosporine and sirolimus enhance the suppressive effect of subtherapeutic doses of 1,25(OH)2D3 (38, 39).
Our results demonstrate that CT enhances the suppressive effect of 1,25(OH)2D3 in the EAE model of MS, suggesting a potential role for CT in 1,25(OH)2D3-mediated prevention of EAE. Furthermore, the combination therapy did not significantly elevate calcium levels, thus eliminating a major drawback in the use of 1,25(OH)2D3 as a therapeutic agent in MS.
Materials and Methods
Animals and Diet.
Six-week-old female C57BL/6J mice were purchased from Jackson Laboratory. All mice were housed at the University of Wisconsin–Madison Department of Biochemistry animal facility under specific pathogen-free conditions and exposed to 12-h light-dark cycles. Before administration of experimental diets, mice were fed standard rodent Labdiet 5008 chow (Purina Mills International). For the indicated experiments, 7–8-week-old mice were switched to a purified diet containing all the essential nutrients for normal growth (40). 1,25(OH)2D3 (Sigma-Aldrich Fine Chemicals) was added to the purified diet at doses ranging from 0–5 ng/d based on the average daily consumption of 4 g per mouse. The diet was delivered in solidified agar form 3 times per week beginning 2 weeks before immunization and continued until the termination of the experiment. Animal protocols were approved by the Institutional Animal Care and Use Committee.
Induction of EAE.
Myelin oligodendrocyte glycoprotein peptide (MOG35–55) (MEVGWYRSPFSRVVHLYRNGK) was synthesized at the University of Wisconsin–Madison Biotechnology Center using standard Fmoc chemistry and purified to ≥95% by RP-HPLC. The MOG35–55 peptide was resuspended in sterile PBS to a concentration of 4 mg/mL and then emulsified with an equivalent volume of complete Freund's adjuvant (CFA) supplemented with 5 mg/mL Mycobacterium tuberculosis H37Ra (DIFCO Laboratories). EAE was induced in 9–10-week-old female C57BL/6J mice by s.c. injection of 100 μL of MOG35–55/CFA homogenate delivering 200 μg of MOG35–55 peptide. On the day of immunization and 48 h later, mice were injected i.p. with 200 ng of pertussis toxin (List Biological Laboratories) diluted in PBS. Mice were scored daily for clinical signs of EAE using the following scale: 0 = no clinical disease, 1 = loss of tail tone, 2 = unsteady gait, 3 = hind limb paralysis, 4 = forelimb paralysis, 5 = death.
Delivery of CT.
Salmon CT was purchased from Bachem and resuspended to a concentration of 1 mg/mL in a vehicle containing 150 mM NaCl, 1 mM HCl, and 2% (vol/vol) heat-inactivated sera from female C57BL/6J mice. Model 1002 micro-osmotic pumps (Durect Corporation) were used to deliver CT continuously at an average rate of 0.24 μL/h for a total of 14 days. One day before pump implantation, mice were weighed to determine the proper CT dose. Pharmacological doses ranged from 0–60 μg/kg per day depending on the experiment, and the pump reservoirs were filled with either vehicle or CT. The pumps were placed in sterile PBS and primed overnight at 37 °C. Seven to 10 days after immunization, pumps were implanted s.c. in the upper back of mice anesthetized with isoflurane. Successful delivery of CT was determined by measuring the liquid volume remaining in the pump reservoir at the termination of the experiment.
Serum Calcium Analysis.
Blood was collected at selected time points during the experiments. Blood samples were spun at 2,938 g for 15 min, followed by a second spin at 16,883 g for 1 min. Serum calcium levels were determined by diluting 25 μL of serum in 975 μL of 0.1% LaCl3 and analyzed with a model 3110 Perkin-Elmer atomic absorption spectrometer.
Data Analysis.
Individual mice were scored daily, and the mean clinical score ± SD was calculated for each group. Average onset and severity were calculated in affected mice displaying a clinical score of ≥1.0 for a minimum of 2 consecutive days. Onset was calculated by averaging the first day when clinical signs appeared. Severity was determined by averaging the maximum disease score reached during the entire experiment. The CDI was calculated by summing the daily clinical scores for each group for all time points divided by the number of mice per group. Because the duration of each experiment was different, the CDIs from different experiments cannot be directly compared. Statistical analysis was performed using the two-tailed Fisher exact probability test for incidence rates and the unpaired Student's t test for all other measurements. A value of P < 0.05 was considered statistically significant.
Acknowledgments.
We thank Terry Meehan, Souriya Vang, Wendy Hellwig, and James Kim for technical assistance and Pat Mings for helping with the manuscript preparation. This work was supported in part from a fund from the Wisconsin Alumni Research Foundation.
Footnotes
The authors declare no conflict of interest.
References
- 1.Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med. 2000;343:938–952. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- 2.Ebers GC. Environmental factors and multiple sclerosis. Lancet Neurol. 2008;7:268–277. doi: 10.1016/S1474-4422(08)70042-5. [DOI] [PubMed] [Google Scholar]
- 3.Goldberg P. Multiple sclerosis: Vitamin D and calcium as environmental determinants of prevalence (a viewpoint). Part 1: Sunlight, dietary factors, and epidemiology. Int J Environ Stud. 1974;6:19–27. [Google Scholar]
- 4.Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. J Am Med Assoc. 2006;296:2832–2838. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 5.Munger KL, et al. Vitamin D intake and incidence of multiple sclerosis. Neurology. 2004;62:60–65. doi: 10.1212/01.wnl.0000101723.79681.38. [DOI] [PubMed] [Google Scholar]
- 6.Jones G, Strugnell SA, DeLuca HF. Current understanding of the molecular actions of vitamin D. Physiol Rev. 1998;78:1193–1231. doi: 10.1152/physrev.1998.78.4.1193. [DOI] [PubMed] [Google Scholar]
- 7.Veldman CM, Cantorna MT, DeLuca HF. Expression of 1,25-dihydroxyvitamin D(3) receptor in the immune system. Arch Biochem Biophys. 2000;374:334–338. doi: 10.1006/abbi.1999.1605. [DOI] [PubMed] [Google Scholar]
- 8.Provvedini DM, Tsoukas CD, Deftos LJ, Manolagas SC. 1,25-dihydroxyvitamin D3 receptors in human leukocytes. Science. 1983;221:1181–1183. doi: 10.1126/science.6310748. [DOI] [PubMed] [Google Scholar]
- 9.Brennan A, et al. Dendritic cells from human tissues express receptors for the immunoregulatory vitamin D3 metabolite, dihydroxycholecalciferol. Immunology. 1987;61:457–461. [PMC free article] [PubMed] [Google Scholar]
- 10.Bhalla AK, Amento EP, Serog B, Glimcher LH. 1,25-Dihydroxyvitamin D3 inhibits antigen-induced T cell activation. J Immunol. 1984;133:1748–1754. [PubMed] [Google Scholar]
- 11.Rigby WF, Denome S, Fanger MW. Regulation of lymphokine production and human T lymphocyte activation by 1,25-dihydroxyvitamin D3. Specific inhibition at the level of messenger RNA. J Clin Invest. 1987;79:1659–1664. doi: 10.1172/JCI113004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zella JB, McCary LC, DeLuca HF. Oral administration of 1,25-dihydroxyvitamin D3 completely protects NOD mice from insulin-dependent diabetes mellitus. Arch Biochem Biophys. 2003;417:77–80. doi: 10.1016/s0003-9861(03)00338-2. [DOI] [PubMed] [Google Scholar]
- 13.Cantorna MT, Hayes CE, DeLuca HF. 1,25-Dihydroxycholecalciferol inhibits the progression of arthritis in murine models of human arthritis. J Nutr. 1998;128:68–72. doi: 10.1093/jn/128.1.68. [DOI] [PubMed] [Google Scholar]
- 14.Lemire JM, Archer DC. 1,25-dihydroxyvitamin D3 prevents the in vivo induction of murine experimental autoimmune encephalomyelitis. J Clin Invest. 1991;87:1103–1107. doi: 10.1172/JCI115072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cantorna MT, Hayes CE, DeLuca HF. 1,25-Dihydroxyvitamin D3 reversibly blocks the progression of relapsing encephalomyelitis, a model of multiple sclerosis. Proc Natl Acad Sci USA. 1996;93:7861–7864. doi: 10.1073/pnas.93.15.7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cantorna MT, Humpal-Winter J, DeLuca HF. Dietary calcium is a major factor in 1,25-dihydroxycholecalciferol suppression of experimental autoimmune encephalomyelitis in mice. J Nutr. 1999;129:1966–1971. doi: 10.1093/jn/129.11.1966. [DOI] [PubMed] [Google Scholar]
- 17.Meehan TF, Vanhooke J, Prahl J, Deluca HF. Hypercalcemia produced by parathyroid hormone suppresses experimental autoimmune encephalomyelitis in female but not male mice. Arch Biochem Biophys. 2005;442:214–221. doi: 10.1016/j.abb.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 18.Zaidi M, Inzerillo AM, Moonga BS, Bevis PJ, Huang CL. Forty years of calcitonin—where are we now? A tribute to the work of Iain Macintyre, FRS. Bone. 2002;30:655–663. doi: 10.1016/s8756-3282(02)00688-9. [DOI] [PubMed] [Google Scholar]
- 19.Hoff AO, et al. Increased bone mass is an unexpected phenotype associated with deletion of the calcitonin gene. J Clin Invest. 2002;110:1849–1857. doi: 10.1172/JCI200214218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilkin TJ, Isles TE, Paterson CR, Crooks J, Beck J. Post-thyroidectomy hypocalcaemia: A feature of the operation or the thyroid disorder? Lancet. 1977;1:621–623. doi: 10.1016/s0140-6736(77)92057-8. [DOI] [PubMed] [Google Scholar]
- 21.Body JJ. Calcitonin: From the determination of circulating levels in various physiological and pathological conditions to the demonstration of lymphocyte receptors. Horm Res. 1993;39:166–170. doi: 10.1159/000182719. [DOI] [PubMed] [Google Scholar]
- 22.Lewis P, Rafferty B, Shelley M, Robinson CJ. A suggested physiological role of calcitonin: The protection of the skeleton during pregnancy and lactation. J Endocrinol. 1971;49:ix–x. [PubMed] [Google Scholar]
- 23.Woodrow JP, et al. Calcitonin plays a critical role in regulating skeletal mineral metabolism during lactation. Endocrinology. 2006;147:4010–4021. doi: 10.1210/en.2005-1616. [DOI] [PubMed] [Google Scholar]
- 24.Body JJ, et al. Calcitonin receptors on circulating normal human lymphocytes. J Clin Endocrinol Metab. 1990;71:675–681. doi: 10.1210/jcem-71-3-675. [DOI] [PubMed] [Google Scholar]
- 25.Body JJ, Fernandez G, Lacroix M, Vandenbussche P, Content J. Regulation of lymphocyte calcitonin receptors by interleukin-1 and interleukin-6. Calcif Tissue Int. 1994;55:109–113. doi: 10.1007/BF00297185. [DOI] [PubMed] [Google Scholar]
- 26.Mbalaviele G, Orcel P, Bouizar Z, Jullienne A, De Vernejoul MC. Transforming growth factor-beta enhances calcitonin-induced cyclic AMP production and the number of calcitonin receptors in long-term cultures of human umbilical cord blood monocytes in the presence of 1,25-dihydroxycholecalciferol. J Cell Physiol. 1992;152:486–493. doi: 10.1002/jcp.1041520307. [DOI] [PubMed] [Google Scholar]
- 27.Abdullahi SE, Martelli EA, Bramm E, Franco L, Velo GP. Effect of calcitonin on different inflammatory models. Agents Actions. 1977;7:533–538. doi: 10.1007/BF02111126. [DOI] [PubMed] [Google Scholar]
- 28.Strettle RJ, Bates RF, Buckley GA. Evidence for a direct anti-inflammatory action of calcitonin: Inhibition of histamine-induced mouse pinnal oedema by porcine calcitonin. J Pharm Pharmacol. 1980;32:192–195. doi: 10.1111/j.2042-7158.1980.tb12888.x. [DOI] [PubMed] [Google Scholar]
- 29.Aida S. Effects of eel calcitonin on rheumatoid arthritis. Ann Rheum Dis. 1991;50:202–203. doi: 10.1136/ard.50.3.202-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aida S, Okawa-Takatsuji M, Aotsuka S, Shimoji K, Yokohari R. Calcitonin inhibits production of immunoglobulins, rheumatoid factor and interleukin-1 by mononuclear cells from patients with rheumatoid arthritis. Ann Rheum Dis. 1994;53:247–249. doi: 10.1136/ard.53.4.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lausson S, et al. Regulation of plasma calcium and phosphate in calcitonin-infused rats. Am J Physiol. 1990;259(3 Pt 1):E370–E377. doi: 10.1152/ajpendo.1990.259.3.E370. [DOI] [PubMed] [Google Scholar]
- 32.Bouizar Z, Rostene WH, Milhaud G. Down-regulation of rat kidney calcitonin receptors by salmon calcitonin infusion evidenced by autoradiography. Proc Natl Acad Sci USA. 1987;84:5125–5128. doi: 10.1073/pnas.84.15.5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawashima H, Torikai S, Kurokawa K. Calcitonin selectively stimulates 25-hydroxyvitamin D3–1 alpha-hydroxylase in proximal straight tubule of rat kidney. Nature. 1981;291:327–329. doi: 10.1038/291327a0. [DOI] [PubMed] [Google Scholar]
- 34.Shinki T, Ueno Y, DeLuca HF, Suda T. Calcitonin is a major regulator for the expression of renal 25-hydroxyvitamin D3–1alpha-hydroxylase gene in normocalcemic rats. Proc Natl Acad Sci USA. 1999;96:8253–8258. doi: 10.1073/pnas.96.14.8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mancini L, et al. Calcitonin and prednisolone display antagonistic actions on bone and have synergistic effects in experimental arthritis. Am J Pathol. 2007;170:1018–1027. doi: 10.2353/ajpath.2007.060830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mattner F, et al. Inhibition of Th1 development and treatment of chronic-relapsing experimental allergic encephalomyelitis by a non-hypercalcemic analogue of 1,25-dihydroxyvitamin D(3) Eur J Immunol. 2000;30:498–508. doi: 10.1002/1521-4141(200002)30:2<498::AID-IMMU498>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 37.Van Etten E, et al. Combination of a 1,25-dihydroxyvitamin D3 analog and a bisphosphonate prevents experimental autoimmune encephalomyelitis and preserves bone. Bone. 2003;32:397–404. doi: 10.1016/s8756-3282(03)00030-9. [DOI] [PubMed] [Google Scholar]
- 38.Branisteanu DD, et al. Prevention of murine experimental allergic encephalomyelitis: Cooperative effects of cyclosporine and 1 alpha, 25-(OH)2D3. J Neuroimmunol. 1995;61:151–160. doi: 10.1016/0165-5728(95)00076-e. [DOI] [PubMed] [Google Scholar]
- 39.Branisteanu DD, Mathieu C, Bouillon R. Synergism between sirolimus and 1,25-dihydroxyvitamin D3 in vitro and in vivo. J Neuroimmunol. 1997;79:138–147. doi: 10.1016/s0165-5728(97)00116-1. [DOI] [PubMed] [Google Scholar]
- 40.Smith SM, Levy NS, Hayes CE. Impaired immunity in vitamin A-deficient mice. J Nutr. 1987;117:857–865. doi: 10.1093/jn/117.5.857. [DOI] [PubMed] [Google Scholar]