Abstract
It is now recognized that extensive maturational changes take place in the human brain during adolescence, and that the trajectories of these changes are best studied longitudinally. We report the first longitudinal study of the adolescent decline in non-rapid eye movement (NREM) delta (1–4 Hz) and theta (4–8 Hz) EEG. Delta and theta are the homeostatic frequencies of human sleep. We recorded sleep EEG in 9- and 12-year-old cohorts twice yearly over a 5-year period. Delta power density (PD) was unchanged between age 9 and 11 years and then fell precipitously, decreasing by 66% between age 11 and 16.5 years (P < .000001). The decline in theta PD began significantly earlier than that in delta PD and also was very steep (by 60%) between age 11 and 16.5 years (P < .000001). These data suggest that age 11–16.5 years is a critically important maturational period for the brain processes that underlie homeostatic NREM EEG, a finding not suggested in previous cross-sectional data. We hypothesize that these EEG changes reflect synaptic pruning. Comparing our data with published longitudinal declines in MRI-estimated cortical thickness suggests the theta age curve parallels the earlier maturational thinning in 3-layer cortex, whereas the delta curve tracks the later changes in 5-layer cortex. This comparison also reveals that adolescent declines in NREM delta and theta are substantially larger than decreases in cortical thickness (>60% vs. <20%). The magnitude, interindividual difference, and tight link to age of these EEG changes indicate that they provide excellent noninvasive tools for investigating neurobehavioral correlates of adolescent brain maturation.
Keywords: adolescence, brain development, EEG, sleep
The human brain undergoes pervasive maturational changes during the second decade of life. Cross-sectional data show dramatic and parallel declines of about 50% in cortical synaptic density, cortical metabolic rate, and non-rapid eye movement (NREM) delta wave amplitude between age 10 and 20 years (1). We have hypothesized (1, 2) that the synaptic pruning of adolescence (3) drives the metabolic and EEG changes. We speculate that this late brain change is the final ontogenetic manifestation of the recurrent motif of overproduction and regression that sculpts vertebrate nervous systems. The potential clinical importance of adolescent brain reorganization is suggested by the frequent onset of schizophrenia and other psychiatric disorders in the second decade of life (2, 4).
Longitudinal studies can discern the timing and trajectories of maturational development more efficiently than cross-sectional studies. For this reason, longitudinal studies of adolescent brain maturation are now being pursued in several laboratories, many using structural and functional MRI (5, 6). For example, the National Institutes of Health has initiated a multi-institutional longitudinal study of developmental changes using structural MRI (7), and a recent National Institute of Mental Health position paper emphasized the importance of using longitudinal designs to determine the trajectories and correlates of adolescent brain changes. Profound alterations in sleep and sleep EEG are known to occur over the course of adolescence, but these changes have not yet been studied longitudinally. Here we present the first longitudinal sleep EEG data documenting the maturational trajectories of NREM delta and theta EEG across adolescence.
NREM delta (1–4 Hz) and theta (4–8 Hz) EEG are of special interest in human sleep research, because they behave homeostatically and are strongly linked to age. Cross-sectional data show that delta power is high in children and declines across adolescence (8, 9). Delta behaves homeostatically, with its intensity increasing (to a limit) as prior waking duration increases (10, 11) and declining across sleep as though some substrate or neuronal change produced by waking brain activity is being metabolized or reversed (10). NREM theta EEG behaves similarly to delta EEG with respect to both age (12) and waking duration (13).
We investigated the adolescent changes in NREM delta and theta in 2 cohorts of normal children. All-night sleep EEG was recorded twice yearly for 4 consecutive nights in the children's homes with ambulatory recorders. EEG was analyzed visually and by computer using fast Fourier transform (FFT). The younger cohort (designated C9) was studied between age 9 and 14 years, and the older cohort (designated C12) was studied between age 12 and 17 years. Thus, the cohorts overlapped at age 12–14 years, and the study spanned age 9–17 years. Of the 70 subjects enrolled in the study, 59 completed all 10 recording periods; their data are presented here. In addition to these longitudinal data, we used the first recording of a 6-year-old cohort (designated C6) to investigate cross-sectionally whether delta and theta power density (PD) change between age 6 and 9 years; see Materials and Methods for more details.
Results
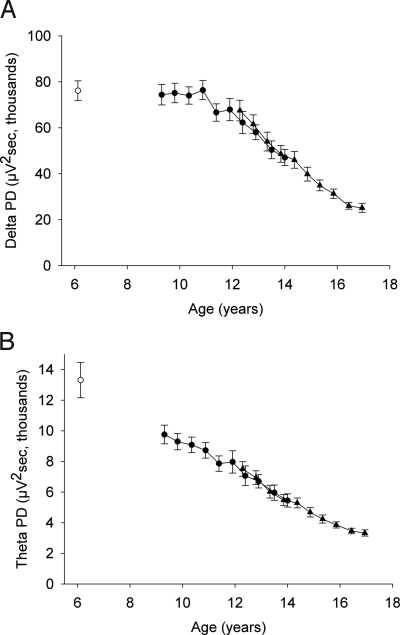
As shown in Fig. 1, both delta and theta PD dropped steeply across adolescence. From the first recording in C9 (mean age, 9.3 years) to the tenth recording in C12 (mean age, 16.9 years), the average delta PD declined by 66% (F1,529 = 229; P < .000001), as did the average theta PD (F1,529 = 259; P < .000001). The delta and theta PD declines differed in terms of age of onset, however. Between age 9.3 and 10.9 years, delta PD did not change (F1,74 = 0.29; P = .59), whereas theta PD declined by 11% (F1,74 = 12.87; P = .0006). In the period of greatest decline, between age 10.9 and 16.4 years, delta declined by 66%, and theta declined by 60%. For both theta and delta, the rate of decline appeared to slow after age 16.4 years. This apparent inflection point will be evaluated with additional longitudinal data from these subjects.
Fig. 1.
PD (mean ± SE) of C9 (filled circles) and C12 (triangles) plotted against mean cohort age for each semiannual recording period. Mean delta and theta data from the first recording of a new 6-year-old cohort (C6) are plotted as open circles. (A) In the longitudinal data, NREM delta (1–4 Hz) PD did not change between age 9.3 and 10.9 years but declined steeply to age 16.4 years, after which the decline slowed. A cross-sectional comparison revealed no significant difference in delta PD between age 6 and 9.3 years. (B) In the longitudinal data, NREM theta (4–8 Hz) PD declined significantly between age 9.3 and 10.9 years, then decreased steadily to age 16.4 years, after which the decline slowed. A cross-sectional comparison with C6 showed a significant theta decline between age 6 and 9 years. The data indicate that delta PD remained at a plateau level until it began to decline between age 11 and 12 years. In contrast, theta PD declined from early childhood. Both delta and theta PD dropped by >60% between age 10.9 and 16.4 years. Both curves show highly significant curvature (see text). PD values were virtually identical in the 2 cohorts where they overlapped in age.
We compared the data available from C6 (mean age, 6.1 years) with the first recording from C9 to explore whether theta PD and/or delta PD change between age 6 and 9 years. This cross-sectional comparison demonstrated a 26% lower theta PD at age 9 years (t51 = 2.67; P = .010). In contrast, delta PD at age 9 did not differ significantly from that at age 6 (t51 = 0.28; P = .78). These findings suggest that delta PD remains at a plateau between age 6 and 11 years, whereas theta PD declines starting in early childhood. As a result, our longitudinal C9 data captured the onset of the delta decline (between age 11 and 12 years) but not the onset of the theta decline, which begins at or before age 6 years.
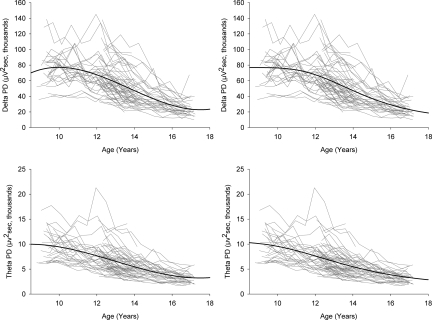
Fig. 2 plots delta and theta PD by age for all 59 subjects in C9 and C12. Statistical analysis demonstrated that these age curves are better fit by cubic functions than by linear or quadratic functions. The delta and theta data also were fit by a Gompertz function, an equation long used to describe tumor growth (14). A negative coefficient was used, because our data decline rather than grow across time. The coefficients for the cubic and Gompertz functions are given in Table S1. Both the cubic and Gompertz functions confirmed the significantly earlier theta decline compared with the delta decline. The delta cubic function peaked at age 10.0 years, whereas the theta cubic function peaked at age 8.4 years. The Gompertz equation also showed an earlier peak rate of decline for theta compared with delta (12.3 ± 0.3 years vs. 13.5 ± 0.2 years). The 95% CIs for these ages (11.75–12.91 years and 13.19–13.90 years, respectively) did not overlap.
Fig. 2.
NREM EEG PD in delta (Top) and theta (Bottom) for each of the 59 subjects in C9 and C12, plotted against age at each recording (gray lines). Black lines represent the cubic (Left) and Gompertz (Right) curves fit to the longitudinal data. Both functions show an earlier decline for theta compared with delta. The cubic function for theta peaks at a younger age. Also, the age of peak rate of decline estimated by the Gompertz function is significantly younger for theta. Mixed effects analysis shows that the individual differences in starting level and slope are highly significant for both delta and theta. In addition, higher initial values are correlated with steeper slopes.
The significance of the marked individual differences in patterns of maturational decline shown in Fig. 2 was calculated by linear mixed effect analyses as “random effects.” For both delta and theta, individual intercepts (starting levels) and the rates (slopes) of decline varied significantly (P < .0001). The intercepts and slopes also covaried significantly (P < .0001), such that subjects who started at higher levels manifested steeper rates of decline.
Discussion
The data from this longitudinal study of sleep EEG across late childhood and adolescence establish age 11–16.5 years as a critical period for late brain maturation. This finding was not apparent in previous cross-sectional studies (9). Both theta and delta PD showed high rates of change, each declining by >60%, over the 5.5-year period. These significant and rapid changes in brain electrophysiology indicate that age 11–16.5 years should be an important focus for biological and behavioral studies of adolescent brain maturation. Although one might plausibly assume that these changes must be linked to ongoing pubertal development, this does not appear to be the case. We have statistically established that the delta EEG decline is related to age rather than to pubertal development (15). We note, however, that our measure of pubertal development, Tanner stage, is a physical manifestation far downstream from the brain changes of puberty; thus, it remains possible that the hormonal or neural events that trigger sexual maturation also trigger the delta EEG decline.
Our longitudinal data reveal different maturational trajectories for delta and theta PD that were not apparent in previous cross-sectional studies. The cross-sectional data demonstrate a steady linear decline in NREM delta between age 5 and 22 years (9). In contrast, our longitudinal data demonstrate no change in delta PD between age 9 and 11 years, followed by a steep decline beginning at about age 11 years. Moreover, our data from a newly initiated 6 year-old cohort indicate that delta PD is at the same level at age 6 years and age 9 years. Taken together, these longitudinal and cross-sectional data sets demonstrate that delta PD remains at a plateau between age 6 and 11 years and then declines.
The trajectory of the theta PD decline is different. Our longitudinal data show that theta PD declined significantly between age 9 and 11 years, and then declined even more significantly (by 60%) to age 16.5 years. Moreover, our cross-sectional sample revealed that theta PD was significantly lower at age 9 than at age 6. These findings indicate that theta PD declines starting in early childhood, with a steep decline occurring between age 11 and 16.5 years. These differing age trajectories of the 2 homeostatic frequencies of NREM sleep were previously unknown.
Recently published structural MRI data (16) describing the trajectories of the decline in cortical thickness across adolescence suggest anatomic correlates for the different age trajectories of delta and theta EEG. Allocortical (3-layer cortex) structures show an early and mostly linear decline in thickness beginning in early childhood. The theta PD decline that begins in early childhood and extends through adolescence appears to parallel the trajectories of thinning in some of the allocortical structures. Thinning in isocortical (5-layer cortex) structures, such as the frontal cortex, begins later and has more complex, nonlinear trajectories. The trajectory of the delta PD decline has relatively late onset and is markedly nonlinear, appearing to parallel the trajectories of MRI thinning in isocortical structures such as the frontal lobe. In this regard, it is interesting that delta power is maximal over frontal areas.
We have hypothesized that the decline in NREM EEG power across adolescence is driven by the decrease in synaptic density reported by Huttenlocher (3). EEG potentials are produced by synchronous changes in the membrane potentials in large populations of cortical neurons. Declining synaptic density would decrease the amplitude of these summed potentials (and thus PD) by decreasing the number of interconnected neurons that oscillate synchronously in response to subcortical or intracortical pacemakers (17, 18). Synaptic pruning also could decrease PD in homeostatic frequencies by reducing the intensity of waking brain activity. This would reduce homeostatic need. PET studies (19) have demonstrated that waking brain metabolic rate, the most direct measure of the intensity of waking brain activity, drops by about 50% across adolescence, roughly paralleling the declines in synaptic density and in delta and theta EEG (1).
Comparing our longitudinal EEG data with the longitudinal MRI data of Shaw et al. (16) illustrates the different magnitudes of these developmental changes. We found that theta and delta EEG PD decline by >60% between age 11 and 16.5 years. In contrast, Shaw et al. found a <20% decline in MRI-measured cortical thickness. The declining EEG power in homeostatic frequencies clearly results from an age-linked diminuendo of cortical membrane potentials that could be directly produced by decreasing synaptic density. The MRI-measured trajectories of cortical thinning presumably reflect neuropil changes produced by synaptic, neuronal, vascular, glial, and myelin change (5, 17). Because the synaptic changes are ultramicroscopic, they may not be directly measurable by MRI and are thus unlikely to determine variations in MRI-measured cortical thickness.
We propose a hypothesis on MRI-measured cortical thinning that integrates several known brain changes. We start with the highly plausible view that high synaptic density produces the high rate of cortical metabolism in childhood (19). This high metabolic rate results in increased blood flow. As synaptic density and brain metabolism decline across adolescence, cerebral blood flow decreases. A persistent decrease, as would be the case with declining synaptic density, leads to diminished vascularity (density of capillaries) in the cortex. This decreased vascularity could contribute significantly to the MRI-measured cortical thinning. From this viewpoint, cortical thinning would still reflect synaptic elimination, but indirectly and with a time lag. Obviously, the anatomic basis of cortical thinning measured by structural MRI ultimately must be determined ultrastructurally. The numerous developmental brain MRI studies in progress and planned (7) make this determination an important task.
The magnitude of the maturational changes in NREM delta and theta and their strong between-subject variation make these frequencies outstanding candidates in the search for behavioral correlates of adolescent brain maturation. We have begun to exploit these properties of our data. In our adolescent subjects, we have demonstrated that the increase in daytime sleepiness across adolescence is related to declining delta and theta PD, even when changes in sleep schedule are statistically controlled (20).
Delta and theta EEG have been linked to plasticity and learning. In addition, these frequencies might be useful markers for investigating neurodevelopmental psychopathology. Schizophrenia, which often has its onset in late adolescence or early adulthood, could result from errors in late synaptic pruning (2) or from other abnormalities in adolescent brain reorganization. Investigations into the relationship between adolescent brain changes and the emergence of psychopathology are more likely to be fruitful when the developmental change is substantial, has significant interindividual variation, and can be reliably measured. We have demonstrated that the NREM homeostatic EEG frequencies have these characteristics, and other studies have shown that these sleep EEG measures have exceptionally high within-subject reliability (21, 22). EEG studies have the further advantages of being inexpensive, noninvasive, and easily quantifiable.
Materials and Methods
Subjects.
Our data are from an ongoing longitudinal study of sleep and EEG changes across adolescence. A total of 70 subjects were enrolled at the start of the study. Here we analyze data from only the 59 subjects who completed the first 5 years of the study. We studied 2 cohorts, 1 with an initial age of 9 years (C9: n = 27; 14 girls) and the other with an initial age of 12 years (C12: n = 32; 18 girls) Both groups were studied for 5 years, spanning age ranges 9–14 and 12–17 years, overlapping at 12–14 years. All subjects were screened to rule out developmental abnormalities, head injury, and psychiatric or sleep disorders. No subject was using any medication that alters EEG or sleep. Urine testing ruled out abused drugs. A 6-year-old cohort (C6: n = 26; 11 girls) was initiated in 2007. We plan to follow this cohort with semiannual recordings until the subjects are 10 years old, providing a 1-year overlap with the C9 cohort. In this work, we cross-sectionally compared data from the first recording from C6 with those of the first recording from C9 to test for earlier theta or delta PD decline. Subjects were paid to participate, and the University of California Davis Institutional Review Board approved the study design.
EEG Recording.
Actigraphy watches were used to confirm that subjects maintained their habitual weekday sleep schedule for 5 days before EEG recording. All-night EEG was recorded from each subject in his or her own home using a Grass Technologies H2O or Aura ambulatory EEG recorder. Recordings were carried out for 4 consecutive nights at 6-month intervals. For the first 2 nights, the subjects maintained their habitual weekday sleep schedule. On nights 3 and 4, the subjects retired at their weekday bedtime but slept as long as possible (up to 12 h in bed). EEG was performed with electrodes applied at Fz, Cz, C3, C4, O1, and either Pz or O2, with mastoid electrodes at A1 and A2. The EEG recordings for this study were C3 or C4 versus the contralateral mastoid. Electro-oculograms were recorded from right and left outer canthus electrodes against a forehead reference. Signals were recorded versus a reference, and electrode pairs were obtained via subtraction. The H2O EEG amplifier digitizes at 200 Hz; the Aura, at 400 Hz. The low-frequency hardware filter on the H2O and Aura devices is a single-pole filter with a −3-dB point at 0.5 Hz and a 6-dB/octave rolloff.
EEG Analysis.
Two experienced raters scored each 20-s epoch as wake, NREM, REM, stage 1, or movement on a computer monitor using PASS PLUS (Delta Software), applying the criteria of Rechtschaffen and Kales (24) modified by combining stages 2, 3, and 4 into a single NREM stage. Artifacts were marked independently of sleep stage. A senior lab scientist reconciled differences in the scoring. PASS PLUS analyzed the digitized EEG with FFT in 5.12-s Welch tapered windows with a 2.62-s overlap. The data for each 20-s epoch comprised the sum of 8 windows. We calculated delta and theta power per min of NREM (PD) for all artifact-free epochs in the first 5 h of NREM sleep. We used the first 5 h of NREM rather than all-night NREM because sleep duration changes across adolescence (24). The data point for each subject at each recording period is the mean of nights 1, 2, and 3. Although night 3 is an extended night, the first 5 h of NREM occur before sleep extension. Night 4 could not be used because of the homeostatic and circadian effects of prolonged sleep on night 3.
Statistical Analysis.
Although the figures plot mean data at each recording, age was a continuous variable in all statistical analyses. Mixed effects analysis is widely used for longitudinal data because it takes into account the correlated nature of repeated measurements on the same subject and can accommodate missing data points (25, 26). The close correspondence of the C9 and C12 mean PD values where the cohorts overlap in age between 12 and 14 years indicates that they are drawn from the same population. This overlap justifies combining the data for these 2 cohorts for statistical analysis of age-related EEG changes between age 9 and 17 years. SAS Proc Mixed was used for linear, quadratic, and cubic equations of the relationship between the EEG PD decline and age. SAS Proc NLMixed was used for Gompertz equations. A t test for independent samples was used to examine (cross-sectional) differences in delta and theta PD between age 6 and 9 years.
Supplementary Material
Acknowledgments.
We thank Rahman Azari for assistance with the statistical analyses and J. D. March for helpful comments and suggestions. We also thank our laboratory staff, undergraduate assistants, and the subjects and families who participated in this demanding study. This work was supported by U.S. Public Health Services grant 1R01 MH62521.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0812947106/DCSupplemental.
References
- 1.Feinberg I, Thode HC, Chugani HT, March JD. Gamma distribution model describes maturational curves for delta wave amplitude, cortical metabolic rate and synaptic density. J Theor Biol. 1990;142:149–161. doi: 10.1016/s0022-5193(05)80218-8. [DOI] [PubMed] [Google Scholar]
- 2.Feinberg I. Schizophrenia: Caused by a fault in programmed synaptic elimination during adolescence? J Psychiatr Res. 1982;17:319–334. doi: 10.1016/0022-3956(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 3.Huttenlocher PR. Synaptic density in human frontal cortex: Developmental changes and effects of aging. Brain Res. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- 4.Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9:947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jernigan TL, Trauner DA, Hesselink JR, Tallal P. Maturation of human cerebrum observed in vivo during adolesence. Brain. 1991;114:2037–2049. doi: 10.1093/brain/114.5.2037. [DOI] [PubMed] [Google Scholar]
- 6.Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann N Y Acad Sci. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- 7.Almli CR, Rivkin MJ, McKinstry RC. The NIH MRI study of normal brain development (Objective-2): Newborns, infants, toddlers, and preschoolers. Neuroimage. 2007;35:308–325. doi: 10.1016/j.neuroimage.2006.08.058. [DOI] [PubMed] [Google Scholar]
- 8.Feinberg I, Carlson VR. Sleep variables as a function of age in man. Arch Gen Psychiatry. 1968;18:239–250. [Google Scholar]
- 9.Feinberg I, et al. Maturational changes in amplitude, incidence and cyclic pattern of the 0 to 3 Hz (Delta) electroencephalogram of human sleep. Brain Dysfunction. 1990;3:183–192. [Google Scholar]
- 10.Feinberg I. Changes in sleep cycle patterns with age. J Psychiatr Res. 1974;10:283–306. doi: 10.1016/0022-3956(74)90011-9. [DOI] [PubMed] [Google Scholar]
- 11.Borbely AA. A two-process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 12.Gaudreau H, Carrier J, Montplaisir J. Age-related modifications of NREM sleep EEG: From childhood to middle age. J Sleep Res. 2001;10:165–172. doi: 10.1046/j.1365-2869.2001.00252.x. [DOI] [PubMed] [Google Scholar]
- 13.Borbely A, Baumann F, Brandeis D, Strauch I, Lehmann D. Sleep deprivation: Effect on sleep stages and EEG power density in man. Electroencephalogr Clin Neurophysiol. 1981;51:483–493. doi: 10.1016/0013-4694(81)90225-x. [DOI] [PubMed] [Google Scholar]
- 14.Laird AK. Dynamics of tumor growth. Br J Cancer. 1964;13:490–502. doi: 10.1038/bjc.1964.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feinberg I, Higgins LM, Khaw WY, Campbell IG. The adolescent decline of NREM delta, an indicator of brain maturation, is linked to age and sex but not to pubertal stage. Am J Physiol. 2006;291:R1724–R1729. doi: 10.1152/ajpregu.00293.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaw P, et al. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 2008;28:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- 18.Steriade M, Nunez A, Amzica F. Intracellular analysis of relations between the slow (<1 Hz) neocortical oscillation and other sleep rhythms of the electroencephalogram. J Neurosci. 1993;13:3266–3283. doi: 10.1523/JNEUROSCI.13-08-03266.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chugani HT, Phelps ME, Mazziotta JC. Positron emission tomography study of human brain functional development. Ann Neurol. 1987;22:487–497. doi: 10.1002/ana.410220408. [DOI] [PubMed] [Google Scholar]
- 20.Campbell IG, Higgins LM, Trinidad JM, Richardson P, Feinberg I. The increase in longitudinally measured sleepiness across adolescence is related to the maturational decline in low-frequency EEG power. Sleep. 2007;30:1677–1687. doi: 10.1093/sleep/30.12.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan X, Campbell IG, Feinberg I. Internight reliability and benchmark values for computer analyses of non-rapid eye movement (NREM) and REM EEG in normal young adult and elderly subjects. Clin Neurophysiol. 2001;112:1540–1552. doi: 10.1016/s1388-2457(01)00570-3. [DOI] [PubMed] [Google Scholar]
- 22.Van Dongen HP, Vitellaro KM, Dinges DF. Individual differences in adult human sleep and wakefulness: Leitmotif for a research agenda. Sleep. 2005;28:479–496. doi: 10.1093/sleep/28.4.479. [DOI] [PubMed] [Google Scholar]
- 23.Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring Systems for Sleep Stages of Human Subjects. Washington, DC: U.S. Public Health Service; 1968. [Google Scholar]
- 24.Wolfson AR, Carskadon MA. Sleep schedules and daytime functioning in adolescents. Child Dev. 1998;69:875–887. [PubMed] [Google Scholar]
- 25.Singer JD. Using SAS PROC MIXED to fit multilevel models, hierarchical models, and individual growth models. J Educ Behav Stat. 1998;24:323–355. [Google Scholar]
- 26.Twisk J. Applied Longitudinal Data Analysis for Epidemiology. Cambridge, UK: Cambridge Univ Press; 2003. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.