Abstract
Despite the longstanding appreciation of communication between the nervous and the immune systems, the nature and significance of these interactions to immunity remain enigmatic. Here, we show that 6-hydroxydopamine-mediated ablation of the mouse peripheral sympathetic nervous system increases primary CD8+ T cell responses to viral and cellular antigens presented by direct priming or cross-priming. The sympathetic nervous system also suppresses antiviral CD4+ T cell responses, but this is not required for suppressing CD8+ T cell responses. Adoptive transfer experiments indicate that enhanced CD8+ responses do not result from permanent alterations in CD8+ T cell function in sympathectomized mice. Rather, additional findings suggest that the sympathetic nervous system tempers the capacity of antigen-presenting cells to activate naïve CD8+ T cells. We also show that antiviral CD8+ T cell responses are enhanced by administration of a β2 (but not β1 or α) adrenergic antagonist. These findings demonstrate a critical role for the sympathetic nervous system in limiting CD8+ T cell responses and indicate that CD8+ T cell responses may be altered in patients using β-blockers, one of the most widely prescribed classes of drugs.
Keywords: antigen presentation, vaccine, cross-priming, direct-priming, dendritic cell
Viral infections activate innate and adaptive immune mechanisms, whose integrated functions ultimately limit viral replication, pathogenesis, and transmission. Because of its medical and veterinary importance, the interaction of viruses with the immune system has been intensively studied. Remarkably little attention, however, has been paid to the influence of the nervous system on antiviral immunity.
It is well-established that the nervous and immune systems communicate through soluble mediators, such as cytokines, hormones, and neurotransmitters (1, 2). Indeed, the definition of molecules as “immune” vs. “neuronal” mediators is frequently an arbitrarily designation based on the chronology of discovery. Many biological mediators have been unwittingly investigated as distinct entities by immunologists and neuroscientists until their molecular identity was revealed by cloning or protein sequencing.
The sympathetic nervous system (SNS), one arm of the autonomic nervous system, is responsible for the “fight or flight” response. The SNS consists of adrenergic nerve fibers that exit the spinal cord to form para-spinal ganglia that innervate peripheral organs, including all primary and secondary lymphoid tissues (3–5). Studies of splenic architecture identified adrenergic nerve fibers in the capsule, traberculae, and white pulp (6). The close proximity of adrenergic fibers to immune cells in lymphoid organs suggests that the SNS may regulate immune responses. Electron microscopy reveals synapse-like interactions can exist between sympathetic nerves and splenocytes (4). Communication between the SNS and the immune system most likely occurs through the release of neurotransmitters by adrenergic nerves. Stimulation of sympathetic nerves results in the release of preformed granules containing norepinephrine, neuropeptide Y, and ATP. Granule release appears to occur nonsynaptically, so neurotransmitters probably diffuse throughout the tissue (7). Because the utilization half-life of norepinephrine in rats spleen is ≈7 h (8), it is likely that norepinephrine can act at considerable distance from its source neurons. Importantly, most cell types in spleen express adrenergic receptors (ARs) and therefore would be able to receive signals from the SNS (9–11).
Numerous studies have reported SNS-mediated immunomodulation, particularly the TH2 polarization of responses to protein immunogens. Interestingly, TH2 CD4+ T cells have been reported to lack ARs. It has been proposed that the SNS reduces secretion of TH1 cytokines, therefore skewing a response toward TH2 (12). Studies by Maestroni (13, 14) suggest that catelcholamine-induced TH1 inhibition induced may occur at the level of the dendritic cell (DC).
SNS function is frequently studied by ablating SNS peripheral nerves by i.p. administration of 6-hydroxydopamine (6-OHDA). Peritoneal 6-OHDA is rapidly distributed by the circulation into tissues, where it destroys sympathetic fibers based on internalization into recycling synaptic vesicles, where it is oxidized to generate neurotoxic free radicals (15). Because 6-OHDA is excluded by the blood–brain barrier, this treatment results in a peripheral “chemical sympathectomy.”
Investigators have found both enhanced and repressed immune responses by using 6-OHDA-treated mice, depending on the experimental conditions. Chemical sympathectomy decreases bacterial loads and increases innate immune responses to Listeria monocytogenes (16). However, 6-OHDA-treated mice have also exhibited decreased antibody responses to sheep red blood cells (17), although the extent of inhibition varies between inbred mouse strains (6). Of note, immune responses to antigen and mitogen are dramatically different in chemically sympathectomized mice. The immune response to mitogens seems to be depressed, whereas the response to antigen is enhanced in 6-OHDA-treated mice (6, 18). The seemingly divergent results are most likely due to variations in mouse strain and type of immune stimuli used. However, all of the reports point to the conclusion that the SNS can regulate the magnitude and quality of immune responses.
Influenza A virus (IAV) and other viruses are known to activate both the hypothalamic–pituitary axis (HPA) and the SNS (19). Previous studies by Sheridan et al. have clearly demonstrated that the nervous system can have an impact on IAV responses (reviewed in ref. 20). In the present study, we address the role of the SNS in the generation of CD8+ T cell (TCD8+) responses to viruses by investigating the effects of 6-OHDA treatment on well-defined mouse TCD8+ responses to viral and tumor antigens. Our findings have important basic implications for understanding antipathogen immunity and practical implications for clinical medicine, because SNS agonists and antagonists are routinely used in patients to control physiological disorders, such as blood pressure and asthma.
Results
Chemical Sympathectomy Increases Antiviral T Cell Responses in Vivo.
We next examined the influence of the SNS on TCD8+ responses to influenza A virus (IAV) in C57/Bl6 (B6) mice. Mice were treated with 6-OHDA 3 times over a 1-week interval and infected via i.p. or intranasal (i.n.) route with A/Puerto Rico/8/34 (H1N1) (PR8) 3 days after the last 6-OHDA treatment. Antiviral TCD8+ responses were measured in the spleen by intracellular cytokine staining (ICS) for IFN-γ production ex vivo at the peak of primary responses (days 7 and 10, respectively for i.p. vs. i.n. infection). TCD8+ responses were measured against the 3 IAV determinants that top the immunodominance hierarchy in B6 mice (PA224–233, NP366–374, and PB1-F262–70) (21), and also against PR8-infected cells as an approximate measure of overall anti-PR8 response.
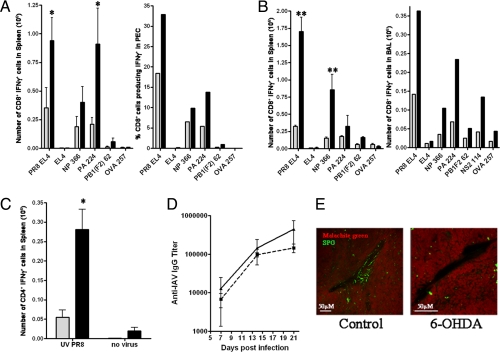
Remarkably, by using mice infected i.p., 6-OHDA treatment resulted in a 2- to 4-fold increase in the absolute numbers (or percent) of anti-IAV TCD8+ recovered either from spleen or from the peritoneum, the local site of infection (Fig. 1A). The SNS remained depleted in the spleen of 6-OHDA-treated mice at the time of T cell analysis (Fig. 1E). An increase of similar magnitude in splenic and local TCD8+ responses was observed following i.n. infection of 6-OHDA-treated mice. Local responses were monitored by quantitating antigen-specific TCD8+ present in the bronchiolar lavage (BAL) fluid collected 7 days after infection (Fig. 1B). 6-OHDA treatment did not greatly modify the immunodominance hierarchy following i.p. or i.n. infection in splenic or BAL TCD8+. The number of virus-induced inflammatory cells in the BAL was not increased by 6-OHDA treatment.
Fig. 1.
IAV-specific CD8+ T cell responses but not antibody responses are increased in 6-OHDA-treated mice. Black bars represent 6-OHDA-treated mice, and gray bars represent saline-treated mice. CD8+ T cell responses were measured against PR8-infected cells (PR8 EL4) and viral peptides [NP 366, PA 224, NS2 114, and PB1(F2) 62]. Uninfected EL4 and OVA 257 peptides were used as controls. Experiments were performed independently 3 times for each assay. *, P < 0.05; **, P < 0.005. (A) Mice were infected i.p. with PR8, and anti-IAV TCD8+ responses were measured on day 7 after infection. Spleens were analyzed individually from 3 mice per group. PECs were pooled from 3 mice per group for analysis. (B) Mice were infected i.n. with PR8 (0.1 LD50). Ten days after infection, spleens were harvested and analyzed for flu-specific TCD8+. Spleens were analyzed individually from 3 mice per group. The T cell response in the BAL was measured on day 7 after infection, and BAL fluid was pooled from 5 mice per group. (C) Mice were infected i.p. with PR8, and the anti-IAV TCD4+ response to UV-inactivated PR8 was measured 7 days after infection. Spleens were analyzed individually from 3 mice per group. (D) Mice were bled before and at days 7, 14, and 21 after infection i.p. with PR8, and antibody titers were determined by ELISA. Solid line with triangles represents control mice, and dashed line with squares represents 6-OHDA-treated mice. (E) Spleens from control or 6-OHDA-treated mice were harvested 1 week after infection with PR8 i.p. and were analyzed for sympathectomy by SPG fluorescence. Representative pictures of blood vessels (seen as dark areas) from each group are shown. Green staining represents the sympathetic nerves, and the red stain is malachite green counterstain to view the surrounding tissue.
We also examined the CD4+ T cell (TCD4+) responses in the spleens of control and 6-OHDA-treated mice 7 days following i.p. infection. UV-inactivated PR8 virus was used to restimulate TCD4+ ex vivo, and the response was quantified by ICS for IFN-γ. Much like what was observed for the TCD8+ response, the TCD4+ response was enhanced by the 6-OHDA treatment (Fig. 1C). Enhanced TCD4+ responses were not, however, required for 6-OHDA-mediated enhancement of TCD8+ responses, because mice lacking CD4+ cells (due to targeted deletion of the CD4 gene or depletion with anti-CD4 antibody) exhibited enhanced 6-OHDA-mediated TCD8+ responses to IAV (Fig. S1 A and B).
Flow cytometric analysis of spleens from uninfected 6-OHDA-treated vs. saline-treated mice did not reveal significant differences in the fractions of TCD8+, TCD4+, CD4+CD25+ regulatory T cells, B cells, macrophages, or DCs. 6-OHDA treatment increased total splenocyte numbers following i.p. (but not i.n.) infection by ≈10%. However, the percent of T cells responding, along with the absolute number of IFN-γ+ T cells, was increased in 6-OHDA-treated animals compared with control animals. The increase in cell number was proportionally distributed among the cell types listed above. There also were no significant changes in the expression of T cell activation markers CD69, CD62L, CD44, or CD25. Of note, depletion of CD4+CD25+ regulatory T cells did not eliminate the enhanced TCD8+ response in 6-OHDA-treated mice, strongly suggesting that the increased response is not mediated by regulatory T cells (Fig. S1 C and D).
6-OHDA treatment did not enhance serum IgG responses to IAV (Fig. 1D), demonstrating that 6-OHDA does not generally increase adaptive immune responses or lymphocyte proliferation due to potential global alterations in physiology; e.g., blood flow to immune organs. 6-OHDA enhancement of anti-IAV responses cannot be attributed to increased viral replication, because pulmonary viral titers were not increased by 6-OHDA treatment (Fig. S2). Further, as shown below, 6-OHDA also enhances TCD8+ responses to noninfectious antigens. Taken together, these data imply that the SNS functions to restrain TCD8+ responses.
6-OHDA Treatment of Host but Not TCD8+ Donor Enhances Antiviral TCD8+ Response.
We next examined whether the effect of 6-OHDA on TCD8+ responses is due to intrinsic persistent alterations in responding TCD8+ or alterations in the milieu that supports TCD8+ activation. For this, we turned to OT-I TCR transgenic mice, which generate TCD8+ specific for Kb-Ova257–264 complexes. OT-I mice, expressing serologically distinct congenic CD45 alleles CD45.1 or CD45.2, were treated, respectively, with 6-OHDA or saline. CD8+ T cells were purified from the 2 groups of mice, mixed in a 1:1 ratio, labeled with carboxyfluorescein succinimidyl ester, and transferred into normal mice. Mice were infected i.p. with an IAV genetically engineered to express Ova257–264 in the stalk of the NA glycoprotein, (WSN-OVA), and division of the 2 splenic OT-I populations was analyzed ex vivo by flow cytometry. On day 2 after infection, OT-I T cells from 6-OHDA-treated mice and the OT-I T cells from the control mice had divided to a similar extent (Fig. S3B). By contrast, 6-OHDA treatment of recipients greatly enhanced the division of OT-Is transferred from untreated mice (Fig. S3A). This experiment demonstrates first, that OT-I responses to IAV are enhanced by 6-OHDA treatment, and second, that this is due to alterations in the responding environment and not to permanent intrinsic alterations in the TCD8+ themselves.
6-OHDA Augments TCD8+ Response to Both Direct and Cross-Primed Antigens.
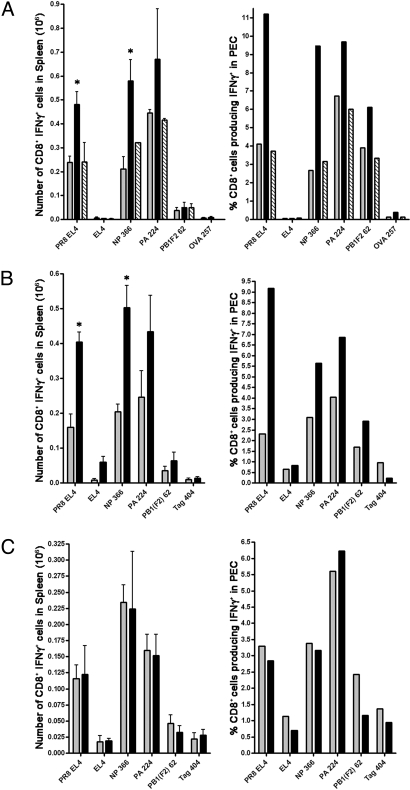
During an antiviral response, antigens can be presented to TCD8+ by infected professional antigen-presenting cells (pAPCs; “direct priming”) or by pAPCs that acquire exogenous viral antigens (“cross-priming”). To directly examine SNS involvement in cross-priming, we injected KD2SV cells [H-2d cells expressing SV40 large T antigen (Tag)] into C57BL/6 (H-2b) mice and measured primary TCD8+ responses ex vivo to 4 defined determinants (22). 6-OHDA-treated mice demonstrated greatly enhanced local (PEC) and splenic responses to each of the determinants, which maintained their relative status in the well-defined Tag immunodominance hierarchy (Fig. 2A). Overall, Tag-specific TCD8+ responses measured against H-2b cells expressing Tag (C57SV) demonstrated a similar increase.
Fig. 2.
6-OHDA treatment enhances CD8+ T cell responses to both direct and crossprimed antigens by enhancing the stimulatory capacity of the pAPC. Black bars represent 6-OHDA-treated mice, and gray bars represent saline-treated mice. *, P < 0.05; **, P < 0.005; ***, P < 0.0005. (A) Mice challenged i.p. with KD2SV cells. Seven days later, SV40 CD8+ T cell responses against SV40 (Tag 206, Tag 404, Tag 289, and Tag 223) and control (OVA 257) peptides and C57SV cells were measured. Responses in the spleen were analyzed from 3 individual mice, and PECs were pooled from 3 mice per group. Experiments were performed independently 3 times. (B) Mice were infected i.p. with recombinant VV-VFP-Ub-OVA257 minigene, and the CD8+ T cell responses against VV (B8R 20), Minigene (OVA 257), and control (NP366) peptides were measured. Responses were analyzed from 3 individual mice per group for the spleen and PEC. Experiments were performed independently 3 times. (C) Mice were infected i.v. with VV-encoding minigenes, and spleens were stained with 25D-1.16. Solid gray represents uninfected mice, dotted line represents mice infected with irrelevant virus (VV-VFP-Ub-NP366), solid line represents control mice infected with VV-VFP-Ub-OVA257, and the dashed line represents 6-OHDA-treated mice infected with VV-VFP-Ub-OVA257. The number of Venus-positive cells is inside the gate, and the median fluorescence intensity (MFI) of the gated population is shown above the gate. This experiment was repeated independently 2 times. (D) pAPC preparations from infected (control: gray bars; 6-OHDA: black bars) and uninfected (control: white bars; 6-OHDA: hatched bars) mice were used to stimulate OT-I T cells ex vivo in a proliferation assay. The pAPC populations were stained with 25D-1.16. Solid gray represents uninfected control mice, dotted line represents uninfected 6-OHDA-treated mice, solid line represents control mice infected with PR8-OVA, and the dashed line represents 6-OHDA-treated mice infected with PR8-OVA. Spleens from 3 mice per group were pooled to generate pAPC preparations. (E) Sorted DC populations from infected mice were used as stimulators in proliferation assay similar to D and plated at a 1:1 ratio with OT-I cells. Spleens from 5 mice per group were pooled to generate the DC populations. Experiments in D and E were repeated independently 3 times.
To specifically examine the effect of 6-OHDA on direct priming, we infected mice with a recombinant vaccinia virus (rVV) encoding a chimeric protein consisting of carboxyl-terminal Venus fluorescent protein (VFP), ubiquitin (Ub), and Ova257–264. As described by van Endert and colleagues (23), peptide determinants are rapidly liberated from the fusion protein by Ub-hydrolases and behave like cytosolic minigene products. 6-OHDA treatment increased the primary TCD8+ response to Ova257–264 and to the immunodominant VV determinant B8R20–27 (Fig. 2B). Similar findings were made by using an rVV expressing Tag404–412 as a cytosolic determinant, which also is presented exclusively by direct priming. Together, these data strongly suggest that the SNS functions to regulate primary TCD8+ responses to direct and cross-presented antigens.
One potential explanation for 6-OHDA-enhanced immunogenicity of directly presented, rVV-encoded antigens is that the SNS limits VV infection of pAPCs or the amount of cognate peptide class I complexes expressed by infected pAPCs. To address this possibility, we infected mice with a rVV expressing VFP-Ub-Ova257–264 (or a control expressing VFP-Ub-NP366–374) and measured splenocyte expression of VFP and Kb-Ova257–264 complex surface expression by using the 25-D1.16 mAb. 6-OHDA treatment did not significantly affect expression of either VFP or Kb-Ova257–264 complexes (Fig. 2C).
6-OHDA Increases pAPC Stimulation of Naive TCD8+.
A previous study reported that peritoneal macrophages from chemically sympathectomized mice are able to activate TCD4+ responding to a protein antigen to a greater extent than control macrophages ex vivo (24). We therefore tested the capacity of pAPCs from infected 6-OHDA-treated and control animals to activate naive T cells ex vivo.
We tested the stimulatory capabilities of splenic pAPCs obtained 12 h after infecting mice with PR8-OVA i.p. We found that naïve OT-I division was enhanced 2- to 3-fold when stimulator pAPC preparations were derived from 6-OHDA-treated vs. control mice (Fig. 2D). This was not apparently due to increased expression of cognate antigen, because 6-OHDA did not enhance levels of cell surface Kb-Ova257–264 complexes as measured by 25-D1.16 antibody staining (Fig. 2D).
Splenic pAPCs include DCs, which are considered to be the major APCs involved in priming naive TCD8+ responses in vivo. We sorted DCs from infected mice into CD8α+ and CD11b+ DCs subsets. CD8α+ DCs were better stimulators than CD11b+ DCs. Importantly, 6-OHDA treatment of mice enhanced the capacity of both CD11c+ subsets to activate naïve OT-I compared with controls (Fig. 2E). Although it has been reported previously that exposure of bone marrow-derived DCs to norepinephrine reduces IL-12 secretion while increasing IL-10 secretion (34), this is unlikely to account for the effect of 6-OHDA on DC function we observed.
First, although norepinephrine reduced CD11b+ DC IL-12 secretion, it had little effect on CD8α+ DC IL-12 secretion (Fig. S4A). This is not due to differences in β2 AR expression, at least as determined at the level of mRNA expression (Fig. S4B). Second, direct measurement of splenic IL-10 and IL-12 from 1 to 7 days after infection did not support the hypothesis that 6-OHDA acts by decreasing IL-10 and increasing IL-12; indeed, IL-12 is actually reduced on D1 (Fig. S4C).
Taken together, these data demonstrate that 6-OHDA treatment enhances the ability of pAPCs, including DCs, to activate naïve T cells ex vivo, suggesting a potential mechanism for the enhancing effect of 6-OHDA on antiviral TCD8+ responses. It does not appear, however, that this effect is mediated by modulating IL-10 or IL-12.
β- but Not α-ARs Modulate TCD8+ Response in Vivo.
Our findings strongly implicate an important role for the SNS in modulating antiviral TCD8+ response. This conclusion is based strictly on 6-OHDA ablation of the peripheral SNS. Although it has not been described, 6-OHDA could affect other cell types in mice that directly or indirectly affect immune responses. Further, we cannot rule out the possibility that the 6-OHDA ablation of the SNS results in alterations in the immune system unrelated or indirectly related to the real-time effect of the SNS on antiviral TCD8+ responses.
To address this serious concern, we acutely treated mice with drugs that selectively block α- or β-ARs. Nadolol, a β-blocker, and phentolamine, an α-blocker, were delivered by implanting an osmotic pump 1 day before infection with IAV. The TCD8+ response to IAV was measured 7 days after infection by intracellular cytokine staining for IFN-γ. Phentolamine had no significant affect on TCD8+ responses, indicating that α-adrenergic stimulation does not enhance or suppress TCD8+ responses to IAV. Strikingly, nadolol enhanced both splenic and peritoneal TCD8+ responses (Fig. 3). The magnitude was similar to that induced by 6-OHDA treatment, pointing to an important role in SNS activation of β-adrenergic receptors in limiting antiviral TCD8+ responses. It is important to note that nadolol is used in humans at a dose equivalent to 25 μg/g per day in mice, and that we observed effects at a 25-fold lower dose, suggesting that at clinical doses nadolol may modify human TCD8+ responses.
Fig. 3.
Blocking β-ARs but not α-ARs enhances anti-IAV CD8+ T cell responses. (A) Mice were treated with the β-adrenergic blocker nadolol (black bars), the α-blocker phentolamine (hatched bars), or saline (gray bars). Mice were infected i.p. with PR8, and anti-IAV TCD8+ responses were measured on day 7 after infection. TCD8+ responses were measured against PR8-infected cells (PR8 EL4) and viral peptides [NP 366, PA 224, and PB1(F2) 62]. Uninfected EL4 and OVA 257 peptides were used as controls. Spleens were analyzed individually from 3 mice per group. PEC samples were pooled from 3 mice per group for analysis. The experiment was performed independently 3 times. (B) Mice were treated with the β2-adrenergic blocker ICI118,551 (black bars) or vehicle (gray bars), and TCD8+ response was measured as in A. (C) Mice were treated with the β1-adrenergic blocker metoprolol (black bars) or vehicle (gray bars), and TCD8+ response was measured as in A. Experiments in both B and C were repeated independently 3 times. *, P < 0.05.
To further define the particular β-AR modulating the magnitude of the T cell response in vivo, we used drugs specific for either β1 (metoprolol) or β2 (ICI118,551) ARs. Drugs were again delivered by osmotic pump throughout the course of infection, and anti-IAV TCD8+ response was measured 7 days later by ICS. ICI118,551 treatment enhanced the TCD8+ response, much like nadolol, whereas the metoprolol had no significant effect. Based on these data, we conclude that the SNS dampens TCD8+ antiviral responses via stimulation of β2 adrenergic receptors.
Discussion
We have provided evidence that strongly supports a role for the SNS in limiting T cell responses to viral and cellular antigens. Although we focused on characterizing TCD8+ responses, the TCD4+ response to IAV was also enhanced in 6-OHDA-treated mice. By contrast, antibody responses to IAV were not detectably altered by 6-OHDA treatment. Increased antiviral TCD8+ responses cannot be attributed to alterations in viral replication, number of virus-infected cells in the spleen, or the amount of peptide class I complexes expressed by splenic pAPCs.
In contrast to our findings, Bonneau and colleagues (25) reported that 6-OHDA decreases TCD8+ responses to i.p. infections with herpes simplex virus (HSV). This discrepancy could be explained by numerous differences between the studies, particularly the timing of 6-OHDA treatment, because Bonneau ablated the SNS 24 h after viral infection (25). Because 6-OHDA treatment can greatly modify innate immune responses (13, 16), this could contribute to the differences between our findings and those of Bonneau and colleagues (25). Using a different HSV model system in mice, Carr and colleagues (26) reported that 6-OHDA treatment before ocular infection does not significantly modify TCD8+ responses in draining lymph nodes.
Based on adoptive transfer experiments, it appears that TCD8+ are not lastingly affected by the 6-OHDA treatment. This suggests that the SNS either directly acts on the TCD8+ during their activation, or it modulates the immediate environment of responding TCD8+ to dampen their responsiveness. This effect could be subtle. Assuming that 13 divisions occur before the peak response is attained (27), only a 15% decrease in division time is required to observe the 4-fold increase in TCD8+ we observed following 6-OHDA treatment.
DCs and macrophages are known to express β-ARs and to respond to norepinephrine (13), and we provide evidence that both CD11b+ CD8α+ DC subsets express β2 ARs. We show that splenic pAPCs, including DCs, isolated from 6-OHDA-treated, infected mice stimulate OT-I cells to a greater extent than the pAPCs from control mice. This appears to occur independently of the number of cells expressing Kb-Ova257–264 complexes or the number of complexes expressed per cell, suggesting that 6-OHDA treatment alters the stimulatory capacity of DCs. Because T cells are at least an order of magnitude more sensitive than 25-D1.16 antibody staining, however, we cannot rule out the alternative (but not mutually exclusive) possibility that 6-OHDA increases the number of TCD8+-activating pAPCs expressing a low number of cognate–peptide class I complexes.
We show that it is unlikely that the SNS modulates DC stimulatory capacity by altering IL-10 or IL-12 secretion in DCs or other splenic cells. Little is known about the effect of catecholamines on costimulatory molecule expression on DCs, which could also contribute to the magnitude of the T cell response. We failed, however, to find 6-OHDA-induced differences in splenic DC expression of defined costimulatory molecules 41BB, B7-1, CD80, CD86, CTL-A4, and OX40L. Our working hypothesis is that norepinephrine released from the SNS upon infection interacts with β2 ARs on DCs and modifies them to dampen their costimulatory capacity mediated by yet-to–be-defined surface molecules or secreted cytokines.
Sympathetic nerves release a number of characterized messenger molecules, including norepinephrine, neuropeptide Y, and adenosine. The findings that nadolol and ICI118,551, pan β- and β2-blockers, respectively, enhance antiviral TCD8+ responses suggest that SNS suppression of TCD8+ responses is largely due to the effects of released norepinephrine. Although we favor the idea that the β-blockers act directly on immune cells, we cannot eliminate the potential contribution of more global effects of norepinephrine on mouse physiology (e.g., blood flow to immune tissues).
β-Adrenergic agonists and antagonists are among the most widely used drugs in clinical practice, and they are used for a number of distinct diseases. Our findings raise the possible contribution of altered TCD8+ responses to the clinical effects of β-adrenergic modifiers in patients with ongoing TCD8+ responses to microbial and tumor antigens. Given the vagaries of the mechanisms of drug action in humans, it is also possible that the effects of β-adrenergic modifiers on cellular immune responses unknowingly contribute to their therapeutic or side effects in “nonimmune” diseases.
Materials and Methods
Mice.
Female 6- to 8-week-old C57BL/6 OT-I Rag−/−, OT-I CD45.1 Rag−/−, and CD4−/− mice were obtained from Taconic Farms. Mice were housed under specific pathogen-free conditions.
Viruses and Immunizations.
A/Puerto Rico/8/34 (PR8), PR8-OVA (28), and WSN-OVA (29) were used as infectious allantoic fluid. Virus titers were determined by tissue culture 50% infective dose (TCID50) in Madin Darby canine kidney (MDCK) cells and by LD50 in 8-week-old B6 mice. Mice were infected i.p. with PR8 at 2 × 106/mL TCID50 or 1 mL of PR8-OVA at 6 × 107/mL TCID50. Mice were i.n. infected with 0.1 LD50 PR8 in 25 μL of PBS. Recombinant vaccinia viruses were generated as described previously (30). Mice were infected i.p. with 5 × 106 pfu of VV. Mice were depleted of CD4+ cells by injecting 200 μg of GK1.5 i.p. on days −3, −2, −1, and +4. Mice were depleted of CD25+ cells by injecting 500 μg of PC61 i.p. on day −4.
Chemical Sympathectomy and Antagonists.
Mice were treated with 100 μg/kg 6-OHDA (Sigma) in 0.9% NaCl and 10−7 M ascorbic acid on day −7 and day −5 and 200 μg/kg on day −3. Control mice received injections of 0.9% NaCl and 10−7 M ascorbic acid. Sympathectomy was confirmed by staining frozen splenic sections with tyrosine hydroxylase Abs or the sucrose-phosphate-glyoxylic acid (SPG) reaction (31) Nadolol, phentolamine, metoprolol, and ICI118,551 (Sigma) were administered continuously by using Alzet osmotic pumps (Alzet) implanted s.c. Nadolol, phentolamine, and metoprolol were administered in saline, whereas ICI118,551 was administered in 50% saline/50% DMSO. Control mice received pumps administering vehicle alone. Nadolol was given at a dose of 1 mg/kg per day, and phentolamine, metoprolol, and ICI118,551 were given at a dose of 10 mg/kg per day.
Flow Cytometry 25D-1.16 Staining of in Vivo-Infected Cells.
Mice were infected i.v. with 1.2 × 107 pfu of rVV or i.p. with 6 × 107 TCID50 IAV. Twelve hours after infection, spleens were digested with type I collagenase (Worthington Biochemicals) for 1 h at 37 °C. Splenocytes were incubated with FC-receptor-blocking antibody (clone 2.4G2) and stained with the monoclonal antibody 25D-1.16 (which recognizes Kb-SIINFEKL complexes). Flow cytometry was performed with an LSRII (BD) and analyzed with FlowJo software (TreeStar). ICS was performed as described previously (21).
Preparation of pAPC Populations.
Spleens were digested with type I collagenase for 1 h at 37 °C. For pAPC preparations, the first step of the CD8α+ DC isolation kit (Miltenyi Biotec) with AutoMacs purification system (Miltenyi Biotec) was used. This preparation consisted of about 10% CD11c+ cells. For sorting of DC populations, splenocytes were treated with FC-receptor-blocking antibody (clone 2.4G2) and stained with anti-CD11b-FITC (BD), anti-CD11c-PE (BD), and anti-CD8α-Alexa 647 (BD). Cells were sorted using FACSAria (BD), resulting in <95% purity.
Anti-IAV Antibody Titers.
Total IgG titers were determined by ELISA against lysates of PR8-infected MDCK cells as described previously (32).
Lung Viral Titers.
Lungs were homogenized in PBS and normalized to weight. Virus in lung homogenate was measured by TCID50 in MDCK cells. For RT-PCR, RNA was isolated by using an RNeasy mini kit (Qiagen) RT-PCR for matrix was performed by using the Platinum one-step quantitative RT-PCR ThermoScript (Invitrogen). The primers forward, 5′-GGACTGCAGCGTAGACGCTT-3′; reverse, 5′-CATCCTGTTGTATATGAGGCCCAT-3′; and probe, 5′–CTCAGTTATTCTGCTGGTGCACTTGCCA-3′ have been described previously (33).
DC Stimulation and Cytokine Measurement.
Sorted splenic DCs were cultured at 1 × 106/mL with and without 100 ng/mL LPS. No norepinephrine or 10−5 M norepinephrine was added to the cultures, and they were incubated for 24 h. Cells were spun down, and the supernatant was collected and analyzed for IL-12p40 by ELISA (eBioscience) according to the manufacturer's specifications.
RT-PCR for β2-AR Expression.
RNA from sorted splenic DC populations was prepared by using an RNesay mini kit (Qiagen). β2-AR and GAPDH expressions were measured by using Taqman Gene Expression Assays (Applied Biosystems). The β2-AR probe was labeled with FAM, and GAPDH was labeled with VIC to allow for multiplexing of the 2 assays.
Cytokine Measurements in Spleen.
Spleens were harvested on days 1, 2, 3, 5, and 7 after infection and placed in 1.5 mL of PBS containing complete, EDTA-free protease inhibitor mixture (Roche). Spleens were homogenized, and lysate was spun down to collect supernatant. Cytokines were measured by using a Bio-Plex cytokine bead array assay (Bio-Rad) according to the manufacturer's specifications. Assay was read out on a Bio-Plex 200 Suspension Array System (Bio-Rad) and analyzed with Bio-Plex manager software (Bio-Rad).
Statistical Analysis.
Statistical analysis was performed where appropriate with Prism 4 software (Graphpad Software) using an unpaired t test (for 2 groups) or 1-way ANOVA (for 3 groups). P values less than 0.05 were considered significant.
T Cell Proliferation Assays.
OT-1 CD8+ T cells were purified (>99% purity) from spleen and lymph nodes of either OT-1 Rag−/− or OT-1 CD45.1 Rag−/− mice by using CD8a+ T cell isolation kit (Miltenyi Biotec) with the AutoMacs purification system (Miltenyi Biotec). Cells were labeled with 2mM CFSE (Molecular Probes), and 5 × 106 cells were transferred into C57BL/6 mice by i.v. injection. Two days following virus challenge, spleens were harvested, stained for anti-Vb5 PE (BD) and anti-CD45.1 PE-Cy7 (eBioscience), and analyzed on a LSRII (BD). Division was calculated using FlowJo software (Treestar). To measure T cell division ex vivo, OT-1 T cells were plated at 1 × 105 cells per well in a 96-well plate. Irradiated pAPCs were added at varying concentrations. After 48 h of stimulation, 0.25mCi of [3H]-thymidine was added to each well. Twenty-four h later, incorporation of 3H into DNA was measured using a FilterMate Harvester (Perkin-Elmer) and β-scintillation counting by using a 1450 TriLux Microbeta Counter (Perkin-Elmer).
Supplementary Material
Acknowledgments.
Deborah Tokarchick and Glennys Reynoso provided excellent technical assistance. We are grateful to David Topham (University of Rochester Medical Center, Rochester, NY) for his generous gift of WSN-OVA and Richard Webby (St Jude's Children Research Hospital, Memphis, TN) for the PR8-OVA. We would also like to thank Virginia Sanders for help in establishing the 6-OHDA model and Steve Cole for the SPG protocol. This work was generously supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0808851106/DCSupplemental.
References
- 1.Ader R, Felten D, Cohen N. Interactions between the brain and the immune system. Annu Rev Pharmacol Toxicol. 1990;30:561–602. doi: 10.1146/annurev.pa.30.040190.003021. [DOI] [PubMed] [Google Scholar]
- 2.Sternberg EM. Neural regulation of innate immunity: A coordinated nonspecific host response to pathogens. Nat Rev Immunol. 2006;6:318–328. doi: 10.1038/nri1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calvo W. The innervation of the bone marrow in laboratory animals. Am J Anat. 1968;123:315–328. doi: 10.1002/aja.1001230206. [DOI] [PubMed] [Google Scholar]
- 4.Reilly FD, McCuskey PA, Miller ML, McCuskey RS, Meineke HA. Innervation of the periarteriolar lymphatic sheath of the spleen. Tissue Cell. 1979;11:121–126. doi: 10.1016/0040-8166(79)90012-0. [DOI] [PubMed] [Google Scholar]
- 5.Williams JM, Felten DL. Sympathetic innervation of murine thymus and spleen: A comparative histofluorescence study. Anat Rec. 1981;199:531–542. doi: 10.1002/ar.1091990409. [DOI] [PubMed] [Google Scholar]
- 6.Livnat S, Felten SY, Carlson SL, Bellinger DL, Felten DL. Involvement of peripheral and central catecholamine systems in neural-immune interactions. J Neuroimmunol. 1985;10:5–30. doi: 10.1016/0165-5728(85)90031-1. [DOI] [PubMed] [Google Scholar]
- 7.Brown GL, Gillespie JS. The output of sympathetic transmitter from the spleen of the cat. J Physiol. 1957;138:81–102. doi: 10.1113/jphysiol.1957.sp005839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sautel M, Sacquet J, Vincent M, Sassard J. NE turnover in genetically hypertensive rats of Lyon strain. II. Peripheral organs. Am J Physiol. 1988;255:H736–H741. doi: 10.1152/ajpheart.1988.255.4.H736. [DOI] [PubMed] [Google Scholar]
- 9.Fuchs BA, Albright JW, Albright JF. Beta-adrenergic receptors on murine lymphocytes: Density varies with cell maturity and lymphocyte subtype and is decreased after antigen administration. Cell Immunol. 1988;114:231–245. doi: 10.1016/0008-8749(88)90318-8. [DOI] [PubMed] [Google Scholar]
- 10.Petitto JM, Huang Z, McCarthy DB. Molecular cloning of NPY-Y1 receptor cDNA from rat splenic lymphocytes: Evidence of low levels of mRNA expression and [125I]NPY binding sites. J Neuroimmunol. 1994;54:81–86. doi: 10.1016/0165-5728(94)90234-8. [DOI] [PubMed] [Google Scholar]
- 11.Ricci A, et al. alpha1-adrenergic receptor subtypes in human peripheral blood lymphocytes. Hypertension. 1999;33:708–712. doi: 10.1161/01.hyp.33.2.708. [DOI] [PubMed] [Google Scholar]
- 12.Sanders VM, et al. Differential expression of the beta2-adrenergic receptor by Th1 and Th2 clones: Implications for cytokine production and B cell help. J Immunol. 1997;158:4200–4210. [PubMed] [Google Scholar]
- 13.Maestroni GJ. Sympathetic nervous system influence on the innate immune response. Ann N Y Acad Sci. 2006;1069:195–207. doi: 10.1196/annals.1351.017. [DOI] [PubMed] [Google Scholar]
- 14.Maestroni GJ. Short exposure of maturing, bone marrow-derived dendritic cells to norepinephrine: Impact on kinetics of cytokine production and Th development. J Neuroimmunol. 2002;129:106–114. doi: 10.1016/s0165-5728(02)00188-1. [DOI] [PubMed] [Google Scholar]
- 15.Kostrzewa RM, Jacobowitz DM. Pharmacological actions of 6-hydroxydopamine. Pharmacol Rev. 1974;26:199–288. [PubMed] [Google Scholar]
- 16.Rice PA, Boehm GW, Moynihan JA, Bellinger DL, Stevens SY. Chemical sympathectomy increases numbers of inflammatory cells in the peritoneum early in murine listeriosis. Brain Behav Immun. 2002;16:654–662. doi: 10.1016/s0889-1591(02)00016-8. [DOI] [PubMed] [Google Scholar]
- 17.Hall NR, et al. Effects of 6-hydroxydopamine upon primary and secondary thymus dependent immune responses. Immunopharmacology. 1982;5:39–48. doi: 10.1016/0162-3109(82)90035-2. [DOI] [PubMed] [Google Scholar]
- 18.Callahan TA, Moynihan JA. Contrasting pattern of cytokines in antigen- versus mitogen-stimulated splenocyte cultures from chemically denervated mice. Brain Behav Immun. 2002;16:764–773. doi: 10.1016/s0889-1591(02)00029-6. [DOI] [PubMed] [Google Scholar]
- 19.Dunn AJ, Powell ML, Meitin C, Small PA., Jr Virus infection as a stressor: Influenza virus elevates plasma concentrations of corticosterone, and brain concentrations of MHPG and tryptophan. Physiol Behav. 1989;45:591–594. doi: 10.1016/0031-9384(89)90078-4. [DOI] [PubMed] [Google Scholar]
- 20.Sheridan JF, et al. Stress-induced neuroendocrine modulation of viral pathogenesis and immunity. Ann N Y Acad Sci. 1998;840:803–808. doi: 10.1111/j.1749-6632.1998.tb09618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen W, Norbury CC, Cho Y, Yewdell JW, Bennink JR. Immunoproteasomes shape immunodominance hierarchies of antiviral CD8(+) T cells at the levels of T cell repertoire and presentation of viral antigens. J Exp Med. 2001;193:1319–1326. doi: 10.1084/jem.193.11.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mylin LM, et al. Quantitation of CD8(+) T-lymphocyte responses to multiple epitopes from simian virus 40 (SV40) large T antigen in C57BL/6 mice immunized with SV40, SV40 T-antigen-transformed cells, or vaccinia virus recombinants expressing full-length T antigen or epitope minigenes. J Virol. 2000;74:6922–6934. doi: 10.1128/jvi.74.15.6922-6934.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fruci D, et al. Quantifying recruitment of cytosolic peptides for HLA class I presentation: Impact of TAP transport. J Immunol. 2003;170:2977–2984. doi: 10.4049/jimmunol.170.6.2977. [DOI] [PubMed] [Google Scholar]
- 24.Callahan TA, Moynihan JA. The effects of chemical sympathectomy on T-cell cytokine responses are not mediated by altered peritoneal exudate cell function or an inflammatory response. Brain Behav Immun. 2002;16:33–45. doi: 10.1006/brbi.2000.0618. [DOI] [PubMed] [Google Scholar]
- 25.Leo NA, Callahan TA, Bonneau RH. Peripheral sympathetic denervation alters both the primary and memory cellular immune responses to herpes simplex virus infection. Neuroimmunomodulation. 1998;5:22–35. doi: 10.1159/000026323. [DOI] [PubMed] [Google Scholar]
- 26.Templeton A, Nguyen G, Ash JD, Straub RH, Carr DJ. Chemical sympathectomy increases susceptibility to ocular herpes simplex virus type 1 infection. J Neuroimmunol. 2008;197:37–46. doi: 10.1016/j.jneuroim.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Duijn G, Langedijk JP, de Boer M, Tager JM. High yields of specific hybridomas obtained by electrofusion of murine lymphocytes immunized in vivo or in vitro. Exp Cell Res. 1989;183:463–472. doi: 10.1016/0014-4827(89)90405-9. [DOI] [PubMed] [Google Scholar]
- 28.Jenkins MR, Webby R, Doherty PC, Turner SJ. Addition of a prominent epitope affects influenza A virus-specific CD8+ T cell immunodominance hierarchies when antigen is limiting. J Immunol. 2006;177:2917–2925. doi: 10.4049/jimmunol.177.5.2917. [DOI] [PubMed] [Google Scholar]
- 29.Topham DJ, Castrucci MR, Wingo FS, Belz GT, Doherty PC. The role of antigen in the localization of naive, acutely activated, and memory CD8(+) T cells to the lung during influenza pneumonia. J Immunol. 2001;167:6983–6990. doi: 10.4049/jimmunol.167.12.6983. [DOI] [PubMed] [Google Scholar]
- 30.Fu TM, et al. An endoplasmic reticulum-targeting signal sequence enhances the immunogenicity of an immunorecessive simian virus 40 large T antigen cytotoxic T-lymphocyte epitope. J Virol. 1998;72:1469–1481. doi: 10.1128/jvi.72.2.1469-1481.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sloan EK, Tarara RP, Capitanio JP, Cole SW. Enhanced replication of simian immunodeficiency virus adjacent to catecholaminergic varicosities in primate lymph nodes. J Virol. 2006;80:4326–4335. doi: 10.1128/JVI.80.9.4326-4335.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benton KA, et al. Heterosubtypic immunity to influenza A virus in mice lacking IgA, all Ig, NKT cells, or gamma delta T cells. J Immunol. 2001;166:7437–7445. doi: 10.4049/jimmunol.166.12.7437. [DOI] [PubMed] [Google Scholar]
- 33.Hunzeker J, Padgett DA, Sheridan PA, Dhabhar FS, Sheridan JF. Modulation of natural killer cell activity by restraint stress during an influenza A/PR8 infection in mice. Brain Behav Immun. 2004;18:526–535. doi: 10.1016/j.bbi.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 34.Maestronl JGM, Mazzola P. Langerhans cells b2-adrenoceptors: Role in migration, cytokine production, Th priming and contact hypersensitivity. J Neuroimmunol. 2003;144:91–99. doi: 10.1016/j.jneuroim.2003.08.039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.