Abstract
Despite the attention focused on mass extinction events in the fossil record, patterns of extinction in the dominant group of marine vertebrates—fishes—remain largely unexplored. Here, I demonstrate ecomorphological selectivity among marine teleost fishes during the end-Cretaceous extinction, based on a genus-level dataset that accounts for lineages predicted on the basis of phylogeny but not yet sampled in the fossil record. Two ecologically relevant anatomical features are considered: body size and jaw-closing lever ratio. Extinction intensity is higher for taxa with large body sizes and jaws consistent with speed (rather than force) transmission; resampling tests indicate that victims represent a nonrandom subset of taxa present in the final stage of the Cretaceous. Logistic regressions of the raw data reveal that this nonrandom distribution stems primarily from the larger body sizes of victims relative to survivors. Jaw mechanics are also a significant factor for most dataset partitions but are always less important than body size. When data are corrected for phylogenetic nonindependence, jaw mechanics show a significant correlation with extinction risk, but body size does not. Many modern large-bodied, predatory taxa currently suffering from overexploitation, such billfishes and tunas, first occur in the Paleocene, when they appear to have filled the functional space vacated by some extinction victims.
Keywords: body size, comparative methods, jaw mechanics, paleoecology, survivorship
Marine ecosystems at the close of the Cretaceous were marked by radical changes, including the devastation of many groups of organisms [planktonic foraminifera and calcareous nannoplankton (1–2)] and complete extirpation of others [†ammonites (2) and many marine reptiles (3); throughout, the dagger symbol indicates extinct groups]. For these reasons, the end-Cretaceous extinction has become a macroevolutionary laboratory for exploring the correlates of extinction risk across a diverse range of clades (2, 4–9), but the effects of this event remain obscure for many groups. The lack of a clear picture is particularly conspicuous for fishes, the dominant vertebrates in marine environments.
Previous work on fishes has centered on intensity—rather than patterns—of extinction during the end-Cretaceous event (e.g., ref. 2), with only a few studies qualitatively addressing selectivity (10–12). Among bony fishes, it has been suggested that epipelagic, predatory families were disproportionately affected (10–11). Both epipelagic and demersal taxa appear to have been hard hit according to a more complicated pattern of selectivity reported for sharks and rays (12). However, the real dynamics of extinction remain unclear for both bony and cartilaginous fishes, because previous analyses rely on qualitative inferences of ecology abstracted from fossils and do not assess the statistical significance of perceived patterns.
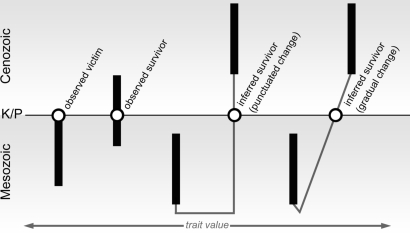
This study marks the first quantitative analysis of extinction selectivity among marine teleost fishes at the close of the Cretaceous by using a newly assembled genus-level database that considers 2 ecologically relevant features of anatomy preserved in fossils: body size and jaw closing mechanical advantage (MA). Body size is a correlate of many aspects of life history and ecology (13–14), and extensive biomechanical research on extant teleosts has established the utility of simple models of jaw mechanics as predictors of diet and trophic level (14–15). This analysis combines a phylogenetic framework with models of trait evolution to account for lineages predicted on the basis of phylogeny but which have not yet been sampled (Fig. 1; see Materials and Methods).
Fig. 1.
Extinction victims and survivors considered by this analysis. Bold black lines represent genus-level lineages, whereas finer gray lines indicate phylogenetic relationships. The vertical axis represents time (K/P indicates Cretaceous/Paleogene boundary), whereas the horizontal axis corresponds to variation in a hypothetical trait value. The first 2 lineages represent the only groups typically incorporated by studies of fossil data: taxa that make their last appearance in the interval preceding the horizon of interest (observed victim) and those that appear on both sides of the horizon (observed survivor). Phylogenies can imply further, unsampled, boundary-crossing lineages, but these are rarely considered. Trait values for inferred survivors are estimated here by using both punctuated (on the left) and gradual (on the right) models of trait evolution.
Here, this dataset is analyzed by using both taxic (5–9) and comparative approaches (16) to address a series of questions concerning the effects of the end-Cretaceous extinction on marine teleosts: (i) Was this event nonrandom (selective) with respect to ecomorphology?; (ii) which anatomical traits, if any, are the correlates of extinction risk?; (iii) how does extinction in this group fit into the larger picture of biotic turnover at the close of the Cretaceous?
Results and Discussion
Extinction Selectivity Among Marine Teleosts.
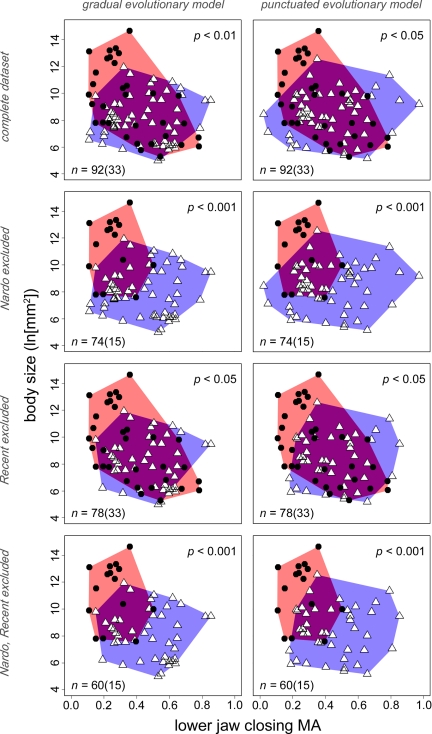
Randomization tests reject the null hypothesis that extinction victims represent an ecomorphologically random subset of taxa present in the final stage of the Cretaceous (Maastrichtian; 65.5–70.6 million years ago). This conclusion is robust to variation in the composition of the dataset (variants described in Materials and Methods) and model of morphological change (punctuated or gradual) used to infer traits of boundary-crossing lineages implied by phylogeny, with significance values ranging from P < 0.05 to P < 0.001 (Fig. 2 and Table 1). Extinction victims span the range of anatomical values on both axes but are concentrated in the upper left-hand corner of all plots. Fishes in this region share large body sizes and low MA jaws that, in nearly all cases, bear large, fang-like teeth (Fig. 3). Significantly, no lineages in this region of morphospace survive from the Maastrichtian into the Paleocene (Fig. 2).
Fig. 2.
Distribution of marine teleost survivors (open triangles, blue envelope) and victims (filled circles, red envelope) of the end-Cretaceous extinction, showing the effect of excluding some dataset partitions (vertical axis) and different models of character evolution used to estimate trait values for inferred boundary-crossing lineages (horizontal axis). The distribution of survivors and victims is significantly different regardless of these permutations (significance indicated in upper right-hand corner of the plots). The number of genera is indicated in the lower left-hand corner of the plots; the figure in parentheses indicates the number of victims. Dataset partitions are as follows: Nardò: taxa making their last appearance in the imprecisely dated Nardò fossil assemblage; Recent, boundary crossing lineages inferred on the basis of extant taxa alone (i.e., no Cenozoic body fossil record).
Table 1.
Results of logistic regressions examining selectivity among marine teleost genera during the end-Cretaceous extinction, conducted on raw (phylogenetically uncorrected) data
| Excluded sets | n | Mode | Prandom | AWS | AWMA | AWS+MA | PS,MA (best fit) | Odds ratioS,MA (best fit) |
|---|---|---|---|---|---|---|---|---|
| None | 92 (33) | G | <0.01 | 0.60 | 0.050 | 0.35 | 0.0071, – | −0.2904, – |
| P | <0.05 | 0.53 | 0.14 | 0.33 | 0.031, – | −0.2223, – | ||
| Nardò | 74 (15) | G | <0.001 | 0.020 | 2.2·10−5 | 0.98* | 0.00027, 0.015 | −0.8382, 8.757 |
| P | <0.001 | 0.035 | 7.1·10−5 | 0.96* | 0.00036, 0.019 | −0.7859, 7.176 | ||
| Recent | 78 (33) | G | <0.05 | 0.28 | 0.33 | 0.38 | 0.14, 0.12 | −0.1740, 2.230 |
| P | <0.05 | 0.40 | 0.25 | 0.35 | 0.034, – | −0.2174, – | ||
| Nardò, Recent | 60 (15) | G | <0.001 | 0.012 | 0.0022 | 0.99* | 0.0025, 0.011 | −0.6956, 9.550 |
| P | <0.001 | 0.015 | 0.0018 | 0.98* | 0.0019, 0.020 | −0.6588, 9.514 |
For sample sizes (n), the figure in parentheses indicates the number of the total representing victims. Mode refers to model of evolutionary change; gradual (G) or punctuated (P). Significance values given in Prandom indicate the probability that the distribution of victims and survivors is random. Columns marked AW give Akaike weights for the regression models indicated in subscript (abbreviations for models: S, body size; MA, jaw closing MA). These values indicate relative support for their corresponding models. AW of the best fitting model for a given dataset partition appears in boldface, and is marked with an asterisk when its model is a substantially better fit than the alternatives. Only values for the factors of the best-fitting model are given for PS,MA and the odds ratio. The first value in these 2 columns applies to S and the second (where present) to MA.
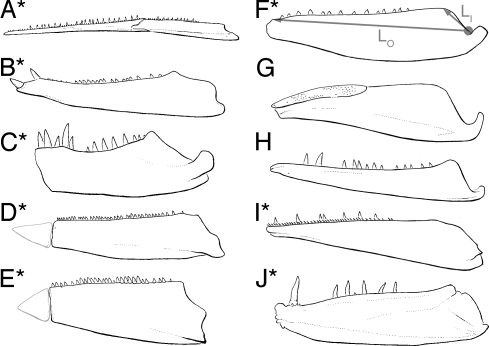
Fig. 3.
Jaws belonging to victims that fall outside the envelope of survivors in all data partitions (see Fig. 2). (A) †Belonostomus. (B) †Protosphyraena. (C) †Xiphactinus. (D) †Saurodon. (E) †Saurocephalus. (F) †Pachyrhizodus. (G) †Pentanogmius. (H) †Apateodus. (I) †Cimolichthys. (J) †Enchodus. Jaws marked with an asterisk are from taxa where gut contents from that genus or a closely related form indicate predation on large, nektonic prey (SI Appendix; see also Dataset S1 for unprocessed measurements). Measurements used to calculate jaw closing mechanical advantage (input lever: LI; output lever: LO; MA = LI/LO) are shown in F. Predentary bones of †Saurodon and †Saurocephalus were not used in calculations of MA, and are shown in light gray here. Images are not to the same scale.
Studies of living fishes illuminate the functional significance of the trait values common to these victims. Body size covaries positively with prey size in fishes (14), whereas low MA values are characteristic of fishes that employ rapid strikes to capture evasive prey (14–15). Large bodies and mechanically “fast” jaws suggest that these fishes were predators on large, active prey. Direct dietary evidence corroborates this inference. Fishes or pelagic cephalopods are known as gut contents from 4 (17–18) of the 10 genera that fall outside the envelope of survivors in all data partitions (Fig. 2), whereas similar prey is known for close relatives of another 4 victims [supporting information (SI) Appendix].
Correlates of Extinction Risk.
Two approaches were taken to investigate the nonrandom distribution of victims in ecomorphospace. The first of these treated all taxa as independent data points [the taxic approach typically applied to extinction in fossil datasets (5–9)], whereas the second used comparative methods [the approach typically applied to extinction in modern datasets (16, 19–20)]. Taxic approaches highlight differences in raw trait values between victim and survivor pools, summarizing the features that distinguish those groups. However, such analyses can deliver misleading interpretations of the correlates of extinction risk, because trait values of related taxa are not statistically independent due to common ancestry (16). This problem becomes clear in a hypothetical “worst-case” scenario, where (i) only those taxa with a particular trait go extinct, and (ii) all of these victims form a clade to the exclusion of all other taxa studied. These closely related victims will share many aspects of biology that might influence survivorship apart from the focal trait, but a taxic analysis would nevertheless isolate that one feature as a clear correlate of extinction risk. In contrast, a comparative analysis that considered the phylogenetic distribution of the trait would not find a significant relationship, because it would only recognize a single link between the character and elevated vulnerability.
It should be apparent from the foregoing example why studies that treat taxa as independent data points are expected to show elevated rates of type I error when relevant characters show a phylogenetic pattern (16). This prediction is borne out by analyses of extinction risk in modern taxa, where fewer significant correlates of vulnerability are inferred when shared evolutionary history is considered (19–20). Despite its associated problems, I have included a taxic analysis here to: (i) demonstrate how interpretations of extinction correlates are sensitive to the methods applied, (ii) deliver a set of results comparable to those given by other paleobiological studies, and (iii) provide a clear picture of how victims differ from survivors, even though distinguishing attributes might not represent significant predictors of vulnerability. This final result is particularly relevant in a paleobiological context, because it highlights devastated regions of ecomorphospace that might be populated in successive geological intervals as newly evolving groups fill the functional roles once held by victims.
For the taxic analysis, the raw dataset was examined by using logistic regression models that evaluated the relationship between the 2 anatomical traits and the binary response variable (extinction/survival). A series of models were fitted to each of the raw dataset variants by using maximum likelihood, with the fit of competing models assessed by using Akaike weights (AW). Most dataset iterations were best fit by a model involving both body size and jaw MA, rather than either variable in isolation (Table 1). In cases where they do provide the best fit, single-variable models are not supported substantially better than more complicated ones. Body size is always the most important factor and is significant in all dataset configurations except those excluding lineages with no Cenozoic fossil record and fitted with models including both traits, where P is above the 0.05 threshold (gradual, P = 0.14; punctuated, P = 0.11; SI Appendix). MA is a significant extinction correlate in only half of the dataset variants when analyzed with regression models also incorporating body size and is always less important than that factor. When analyzed in isolation, MA is a significant factor in 5 of 8 dataset partitions (SI Appendix), but the fit of this model is almost always substantially worse than those incorporating body size.
In all cases, the odds ratio (the analogue of the slope in a standard linear regression) for body size is negative, ranging from −0.8382 to −0.1738, indicating an inverse relationship with survivorship. The broad range of values spanned by the odds ratios arises from inclusion/exclusion of the Campanian–Maastrichtian Nardò fossil assemblage. When incorporated in the dataset, this fauna increases the number of small-bodied taxa making their last appearance in the Maastrichtian, resulting in a shallower slope. These small-bodied genera are no longer considered when the Nardò fauna is excluded, and the distribution of extinction victims becomes more skewed toward large-bodied taxa. The same phenomenon also underlies the elevated significance levels for nonrandom patterns of survivorship for those subsets excluding Nardò. The odds ratio for MA in all regression models incorporating that trait are greater than zero, indicating a positive relationship between this variable and survivorship; genera with low lever ratios (fast jaws) are more likely to be extinction victims than those fishes with higher MA values.
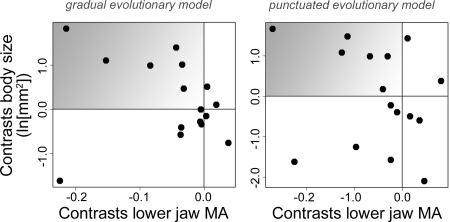
To correct for phylogenetic autocorrelation, phylogenetically independent contrasts (PIC) were estimated for 2 variants of the dataset (complete gradual, complete punctuated) by using 2 approaches implemented in the software package CAIC (21). The first of these treated extinction as a continuous character with only end-member states observed (CRUNCH algorithm), whereas the other considered it as a binary, categorical trait (BRUNCH algorithm). These 2 sets of PIC deliver a consistent picture of extinction risk that differs from what would be inferred from raw genus data. The significant relationship between body size and extinction risk inferred from phylogenetically uncorrected data are not apparent in analyses of PIC (CRUNCH analysis: gradual, P = 0.32; punctuated, P = 0.88; univariate regression of body size on extinction, forced through origin; BRUNCH analysis: gradual, P = 0.19; punctuated, P = 0.51; one-tailed t test of body-size shifts associated with extinction; Fig. 4), but the relationship between MA and extinction is significant in all cases, with lower jaw MA associated with elevated extinction risk (CRUNCH analysis: gradual, P = 0.0054; punctuated, P = 0.010; univariate regression of MA on extinction, forced through origin; BRUNCH analysis: gradual, P = 0.011; punctuated, P = 0.022; one-tailed t test of MA shifts associated with extinction; Fig. 4). This relationship is particularly striking, because MA is not a significant predictor of extinction risk in the same 2 variants of the dataset when raw genus values are considered. The failure of the comparative analysis to recover body size as a significant correlate of vulnerability appears attributable to the clustering of many of the largest extinction victims within a few clades (†Ichthyodectiformes, Aulopiformes).
Fig. 4.
Independent contrasts for body size and MA, showing shifts in these traits for extinction victims (either individual genera or higher clades) relative to their nearest surviving relatives. Patterns recovered from analyses of phylogenetically uncorrected data predict that points should be clustered in the shaded quadrant (i.e., victims should have larger body sizes and lower MA values). Shifts are significantly biased toward decreased MA for both datasets (gradual, P = 0.011; punctuated, P = 0.022; one-tailed t test), but neither demonstrates a significant bias in body-size shifts (gradual, P = 0.19; punctuated, P = 0.51; one-tailed t test).
Shifts in the correlates of extinction risk inferred under different analytical frameworks seen here raise questions about the interpretation of results derived from phylogenetically uncorrected trait values in other studies. Such datasets are common in paleobiological analyses of extinction because phylogenies are generally unavailable for fossil invertebrates. However, there is strong evidence that many of the factors isolated by these studies as predictors of vulnerability show a nonrandom phylogenetic distribution (22). It is uncertain how interpretations of extinction risk for these groups might change when shared evolutionary heritage is considered (but see refs. 19–20); further work is clearly needed (16).
Reliability of Inferred Extinctions at the End Cretaceous.
Review of the fossil records of putative victims indicates that the most reliable inferences of extinction apply to: (i) large-bodied genera and (ii) genera with low MA values. This is significant, because it is these same taxa that appear to be selected against during the end-Cretaceous extinction regardless of the approach used to infer the correlates of vulnerability.
Of the 33 genera making their last appearance in the Maastrichtian, at least 17 are singletons (i.e., they occur only in this stage; this number ranges from 17 to 20 owing to taxonomic uncertainties surrounding specimens from the type Maastrichtian). Remaining extinction victims are known from at least one additional stage before the Maastrichtian. The disparity in body-size and MA distributions of singleton and multistage extinction victims is clear; on average, singletons are smaller than multistage victims, with higher jaw MA (SI Appendix). This difference is significant and robust to the exclusion or variable taxonomic treatment of the problematic forms from the type Maastrichtian (Kolmogorov–Smirnov and Mann–Whitney U tests, respectively, for: MA, P = 0.0027–0.015, 0.0048–0.030; body size, P = 4.0·10−5−0.0065, 9.2·10−6−0.00021). Most importantly, victims with the largest body sizes and lowest jaw MA are known from several stages, and many of these occur in every stage between their first and last appearance in the fossil record (SI Appendix). The apparently high preservation potentials of these genera suggest that their absence from Cenozoic deposits is genuine, instead of an artifact of nonpreservation. This implies that the extinctions of these taxa are the most reliable in this analysis. The fact that few of these genera have close relatives in Cenozoic deposits or the Recent fauna bolsters this conclusion. Such relatives would decrease apparent selectivity by indicating additional boundary-crossing lineages with similar morphologies, but their absence points to the total extinction of the extended clades to which the victims belong.
At the same time, these patterns suggest a taphonomic bias against the preservation of small-bodied and, to a lesser degree, high-MA taxa. Spurious patterns of selectivity might arise if victims with such traits are underrepresented in the dataset. However, this filter applies to victims and survivors alike, and although the number of these unsampled Maastrichtian genera is unknown, it is certain that small-bodied survivors implied by phylogeny are underrepresented in this study. When coupled with the apparent inverse relationship between the ecomorphological traits considered here (Fig. 2), this biases the analysis against recovering a relationship between increased body size/lower MA and extinction risk.
The relative paucity of small survivors derives from 2 conservative rules used in the assembly of the dataset. First, in cases where clade intrarelationships are unclear, only a single boundary-crossing lineage was reconstructed (SI Appendix). As phylogenetic hypotheses within these radiations mature, the number of boundary-crossers will either remain the same or increase; they can never decrease. Second, and more importantly, this analysis has only considered boundary-crossing lineages within clades that are represented by at least one Mesozoic taxon. Many teleost groups are therefore excluded here because they are unknown from pre-Cenozoic deposits, even though the presence of these groups before the end Cretaceous is implied by large-scale (i.e., interordinal level) phylogenetic analyses. This is particularly true for the supraordinal group Acanthomorpha; recent molecular phylogenies, combined with the presence of tetraodontiforms (a derived group nested within acanthomorphs) in Upper Cretaceous rocks, draws no fewer than 7 additional lineages into the Mesozoic (23). These guidelines were applied to clades whose members, nearly without exception, have vastly smaller body sizes than the largest extinction victims. Better-resolved phylogenies and the discovery of Mesozoic exemplars for groups previously unknown from sediments of that age will likely increase the ecomorphological disparity between victims and survivors.
Evolutionary Significance: Synthesizing Patterns of Extinction Selectivity at the End Cretaceous.
Biological context for exploring patterns of vulnerability inferred for fossil fishes comes from the large body of research targeting patterns of selectivity during and ecosystem change after the end-Cretaceous extinction. However, any interpretations made here must be coupled with an important caveat: The current analysis lacks the stratigraphic resolution to reject the possibility that extinctions might be spread throughout the Maastrichtian, rather than clustered at the end of the stage (see ref. 6).
Among macroscopic marine invertebrates, it has been argued that preferential survival of detritivores [bivalves (5), but see ref. 6 and echinoids (7)] and strong selection against photosynthetic groups [corals (8)] stem from a temporary halt in photosynthesis precipitated by bolide impact-induced darkness (1, 4). However, patterns of selectivity with respect to these ecological traits might be complicated by “hitchhiking” on population-level features including geographic range, which appears to be the dominant determinant of survivorship for some invertebrate groups (refs. 8 and 9, but see ref. 7). Despite these ambiguities, multiple lines of evidence—including a sharp decline in the accumulation rate of fish teeth in deep-sea sediment cores—point to drastic postimpact changes in open-ocean ecosystems that persisted millions of years into the Paleocene before higher trophic levels fully recovered (1). When combined with empirical (24) and theoretical (25) ecological studies, this emerging picture of ecosystem perturbation implies that fish extinctions might have been driven by bottom-up trophic dynamics triggered by a decline in primary productivity (11).
The clearest pattern delivered by this study is the complete extirpation of large-bodied fishes with biomechanically fast jaws (Figs. 2 and 3). Alternative analytical approaches isolate different combinations of these traits as underlying correlates of extinction (independent contrasts: jaw mechanics alone; raw data: body size and often, but not always, jaw mechanics), but it is nevertheless clear that the ecomorphologies of victims differed substantially from those of survivors. Elevated extinction intensity among teleosts appearing to occupy higher trophic levels is consistent with the collapse of oceanic food webs, corroborating earlier hypotheses implicating diet as an important determinant of survivorship among fishes (10–11).
The most prominent teleostean casualties of the end-Cretaceous extinction include the predatory †pachycormids, †pachyrhizodontids, †ichthyodectiforms, †enchodontids, and †cimolichthyids, all of which are equipped with high-aspect-ratio caudal fins and fusiform bodies that imply fast swimming and sustained cruising. Taken together, these fishes appear to be the ecological analogues of modern, large-bodied predatory teleosts such as scombroids (tunas, mackerels, cutlassfishes, and the wahoo), xiphioids (billfishes), sphyraenids (barracudas), and carangoids (jacks and dolphinfishes). Significantly, all of these extant groups make their first appearance in the early Paleogene (26), suggesting that they might have radiated to fill the functional roles vacated by extinction victims (11). Coincident with the origin of these modern predators are polyphyletic proliferations of large-bodied, predatory osteoglossomorphs in marine environments (27). These short-lived (Paleocene–Eocene) diversifications are particularly striking because extant osteoglossomorphs are freshwater fishes, and all Mesozoic body fossils assigned to this clade derive exclusively from freshwater deposits (27). The pattern of decimation and subsequent replacement in teleosts is mirrored in chondrichthyans, where there is conspicuous extinction and replacement among large-bodied, predatory sharks centered on the Cretaceous–Paleogene boundary (12).
Ironically, the very same groups that seem to have diversified into emptied ecospace at the dawn of the Cenozoic now face the greatest risks of extinction from overexploitation; commercial fisheries disproportionately target large, predatory taxa (28). Paralleling patterns of extinction selectivity at the end of the Cretaceous, studies that correct for differential harvesting intensity find increased vulnerability of large-bodied fishes occupying high trophic levels (29). Part of this decline in modern groups appears attributable to the strong inverse correlation between body size and both (i) rates of recruitment and (ii) adult production per spawning adult, relationships that directly contradict the common notion that the high fecundity of larger fishes buffers them against extinction threats (30). The mechanisms driving these 2 biodiversity collapses separated by 65 million years clearly differ, but congruent patterns of risk imply that some aspects of fish ecomorphology might consistently correlate with elevated extinction vulnerability regardless of the ultimate factors causing population decline.
Materials and Methods
Database Compilation and Phylogenetic Framework.
The genus-level database assembled for this study contains body size and lower jaw-closing MA for 249 teleost genera (227 known as fossils plus 22 based on Recent material alone). Jaw-closing MA is the ratio of the closing inlever to the outlever (14) (Fig. 4F). Maximum body size is represented by lateral area. Measurements for fossil genera are from specimens or the literature, whereas those for extant taxa derive from preserved material. The Dataset SI contains all raw data, including citations and specimen numbers.
Thirty-eight genera are represented by Maastrichtian fossils, and of these, 5 are also found in Cenozoic deposits. The incorporation of phylogenetic information implies 54 additional lineages spanning the Cretaceous–Paleogene boundary, for a total of 92 lineages present in the Maastrichtian. The sensitivity of results was tested by analyzing 3 “pruned” datasets in addition to the complete dataset: (i) a subset (n = 74) excluding taxa from the imprecisely dated Campanian–Maastrichtian assemblage from Nardò, Italy (n = 18) from the suite of Maastrichtian taxa, but retaining the lineages crossing the Cretaceous-Paleogene boundary implied by these taxa; (ii) a subset (n = 78) excluding lineages crossing the Cretaceous–Paleogene boundary represented only by Recent taxa (i.e., those groups unknown as body fossils from Cenozoic deposits; n = 14); (iii) the intersection of subsets (i) and (ii) (n = 60). The exclusion of groups with no Cenozoic fossils is a conservative measure adopted for the following reasons: (i) this approach seeks equivalency in the face of potential taphonomic biases that are not reflected in collections of Recent material; (ii) the prevalence of random walks in evolutionary trajectories (31) indicates that the monotonic change assumed by the models of trait-value evolution used here (see below) are unlikely for intervals exceeding 65 million years.
Estimates of Trait Values in Inferred Survivors.
Anatomical characters for lineages inferred to have survived the end-Cretaceous extinction based on phylogeny were derived from conditions at the nodes immediately bounding that lineage above (“descendant”) and below (“ancestor”) (Fig. 1), requiring reconstruction of ancestral states. Weighted squared-change parsimony (WSP), as implemented in Mesquite (32), was used to estimate conditions at internal nodes. WSP estimates are equivalent to maximum-likelihood reconstructions using a Brownian motion model (33). Two modes of morphological change were considered: punctuated and gradual. For punctuated evolution, all branch lengths were set to equal length. For gradual evolution, character values were estimated by using WSP on a tree incorporating branch lengths derived from stratigraphy. In the case of punctuated change, the anatomical attributes of the lineage are those of the node or terminal bounding it above. For gradual change, trait values of the lineage at the point where it crosses the Cretaceous–Paleogene boundary are estimated as the weighted average of the values of the bounding nodes by the following formula:
where Th is the trait value of the lineage at the extinction horizon, Tb is the trait value at the node bounding the lineage immediately below the horizon, Ta is the trait value at the node bounding the lineage immediately above the horizon, Lb is the length of the branch between the horizon and the node immediately below it, and Lt is the total length of the branch.
Resampling-Based Tests of Extinction Selectivity.
For each variant of the raw dataset described above, all taxa were combined into a single pool. Two bootstrap samples (equal in size to the number of victims and survivors, respectively) were drawn with replacement, and the F ratio (MANOVA) computed for this pair. A total of 1,000 pseudoreplicates were generated, giving a distribution of F ratios. The F ratio calculated from the empirical distribution of extinction victims and survivors was compared with this distribution to assess significance. These procedures, as well as logistic regressions (below), were executed in R (34).
Taxic Analysis of Raw Genus Data.
Three competing models were examined for each variant of the raw dataset by using logistic regression, with their parameters estimated via maximum likelihood: (i) extinction ≈ size + MA; (ii) extinction ≈ size; (iii) extinction ≈ MA. Model fit was assessed by using Akaike weights.
Phylogenetically Independent Contrasts.
Two sets of phylogenetically independent contrasts (PIC) were calculated by using the program CAIC (21). Only Maastrichtian taxa were considered, and the cladogram used is a composite derived from clade-specific analyses plus a “backbone” drawn from large-scale surveys of fish phylogeny (SI Appendix). There are uncertainties surrounding branch lengths deep within the teleost tree, so all branches were set equal. Results were examined a posteriori by regressing PIC values against nodal values for the corresponding character; none of these had a slope significantly different from zero, indicating that these branch lengths do not violate assumptions of the model used to estimate PIC. The first set of PIC was generated by the BRUNCH algorithm, and consisted of trait value shifts associated with the origin of clades that suffer extinction at the end Cretaceous. Selectivity was assessed by testing whether the mean shift was directionally biased (the expected shift for body size is positive, whereas that for MA is negative). The CRUNCH algorithm, which treated extinction as a continuous variable scored for 2 states, was used to estimate the second set of PIC. Selectivity was assessed by regressing the response variable (body size, MA) contrasts against predictor variable (extinction) contrasts; regressions were forced through the origin (35).
Supplementary Material
Acknowledgments.
M. Coates, M. Foote, D. Jablonski, and 2 anonymous reviewers provided critical comments on this paper. K. Swagel made radiographs of Recent fishes. This work was supported by the Lerner–Gray Fund for Marine Research, a Hinds Fund Grant, an Evolving Earth Grant, a National Science Foundation Graduate Research Fellowship (award DGE-0228235), and an Environmental Protection Agency STAR Fellowship (award FP916730).
Footnotes
The author declares no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0808468106/DCSupplemental.
References
- 1.D'Hondt S. Consequences of the Cretaceous/Paleogene mass extinction for marine ecosystems. Annu Rev Ecol Evol Syst. 2005;36:295–317. [Google Scholar]
- 2.MacLeod N, et al. The Cretaceous–Tertiary biotic transition. J Geol Soc London. 1997;154:265–292. [Google Scholar]
- 3.Bardet N. Extinction events among Mesozoic marine reptiles. Hist Biol. 1994;7:313–324. [Google Scholar]
- 4.Sheehan PM, Hansen TA. Detritus feeding as a buffer to extinction at the end of the Cretaceous. Geology. 1986;14:868–870. [Google Scholar]
- 5.Rhodes MC, Thayer CW. Mass extinctions: Ecological selectivity and primary production. Geology. 1991;19:877–880. [Google Scholar]
- 6.Jablonski D, Raup DM. Selectivity of end-Cretaceous marine bivalve extinctions. Science. 1995;279:1327–1330. doi: 10.1126/science.11536722. [DOI] [PubMed] [Google Scholar]
- 7.Smith AB, Jeffery CH. Selectivity of extinction among sea urchins at the end of the Cretaceous period. Nature. 1998;392:69–71. [Google Scholar]
- 8.Kiessling W, Baron-Szabo RC. Extinction and recovery patterns of scleractinian corals at the Cretaceous–Tertiary boundary. Palaeogeogr Palaeoclimatol Palaeoecol. 2004;214:195–223. [Google Scholar]
- 9.Jablonski D. Extinction and the spatial dynamics of biodiversity. Proc Natl Acad Sci USA. 2008;105:11528–11535. doi: 10.1073/pnas.0801919105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cavin L, Martin M. Les Actinoptérygiens et la limite Crétacé–Tetiarie. Geobios Mém Spec. 1995;19:183–188. [Google Scholar]
- 11.Cavin L. Effects of the Cretaceous–Tertiary boundary event on bony fishes. In: Buffetaut E, Koeberl C, editors. Geological and Biological Effects of Impact Events. Berlin: Springer; 2001. pp. 141–158. [Google Scholar]
- 12.Kriwet J, Benton MJ. Neoselachian (Chondrichthyes, Elasmobranchii) diversity across the Cretaceous–Tertiary boundary. Palaeogeogr Palaeoclimatol Palaeoecol. 2004;214:181–194. [Google Scholar]
- 13.Peters RH. The Ecological Implications of Body Size. Cambridge, UK: Cambridge Univ Press; 1983. [Google Scholar]
- 14.Wainwright PC, Richard BA. Predicting patterns of prey use from morphology in fishes. Environ Biol Fishes. 1995;44:97–113. [Google Scholar]
- 15.Wainwright PC, Bellwood DR. Ecomorphology of feeding in coral reef fishes. In: Sale PF, editor. Coral Reef Fishes: Dynamics and Diversity in a Complex Ecosystem. San Diego: Academic; 2002. pp. 33–55. [Google Scholar]
- 16.Purvis A. Using phylogenies to study extinction. Annu Rev Ecol Evol Syst. 2008;39:301–319. [Google Scholar]
- 17.Viohl G. Piscivorous fishes of the Solnhofen lithographic limestone. In: Boucot AJ, editor. Evolutionary Paleobiology of Behavior and Coevolution. Amsterdam: Elsevier; 1990. pp. 287–303. [Google Scholar]
- 18.Everhart MJ. Oceans of Kansas: A natural history of the Western Interior Sea. Bloomington, IN: Indiana Univ Press; 2005. [Google Scholar]
- 19.Purvis A, Gittleman JL, Cowlishaw G, Mace GM. Predicting extinction risk in declining species. Proc R Soc Ser B. 2000;267:1947–1952. doi: 10.1098/rspb.2000.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fisher DO, Blomberg SP, Owens IPF. Extrinsic versus intrinsic factors in the decline and extinction of Australian marsupials. Proc R Soc Ser B. 2003;270:1801–1808. doi: 10.1098/rspb.2003.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Purvis A, Rambaut A. Comparative analysis by independent contrasts (CAIC): An Apple Macintosh application for analyzing comparative data. Comput Appl Biosci. 1995;11:247–251. doi: 10.1093/bioinformatics/11.3.247. [DOI] [PubMed] [Google Scholar]
- 22.Hunt G, Roy K, Jablonski D. Species-level heritability reaffirmed: A comment on “On the heritability of geographic range sizes”. Am Nat. 2005;166:129–135. doi: 10.1086/430722. [DOI] [PubMed] [Google Scholar]
- 23.Smith WL, Wheeler WC. Venom evolution widespread in fishes: A phylogenetic roadmap for the bioprospecting of piscine venoms. J Hered. 2006;97:206–217. doi: 10.1093/jhered/esj034. [DOI] [PubMed] [Google Scholar]
- 24.Ware DM, Thomson RE. Bottom-up ecosystem trophic dynamics determine fish production in the northeast Pacific. Science. 2005;308:1280–1284. doi: 10.1126/science.1109049. [DOI] [PubMed] [Google Scholar]
- 25.Solé RV, Montoya JM, Erwin DH. Recovery after mass extinction: Evolutionary assembly in large-scale biosphere dynamics. Philos Trans R Soc Ser B. 2002;357:697–707. doi: 10.1098/rstb.2001.0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patterson C. Osteichthyes: Teleostei. In: Benton MJ, editor. The Fossil Record 2. London: Chapman & Hall; 1993. pp. 621–656. [Google Scholar]
- 27.Bonde N. Osteoglossomorphs of the marine Lower Eocene of Denmark with remarks on other Eocene taxa and their importance for palaeobiogeography. Geol Soc London Spec Pub. 2008;295:253–310. [Google Scholar]
- 28.Myers RA, Worm B. Extinction, survival or recovery of large predatory fishes. Philos Trans R Soc London Ser B. 2005;360:13–20. doi: 10.1098/rstb.2004.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reynolds JD, Dulvy NK, Goodwin NB, Hutchings JA. Biology of extinction risk in marine fishes. Proc R Soc Ser B. 2005;272:2337–2344. doi: 10.1098/rspb.2005.3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Denney NH, Jennings S, Reynolds JD. Life-history correlates of maximum population growth rates in marine fishes. Proc R Soc London Ser B. 2002;269:2229–2237. doi: 10.1098/rspb.2002.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hunt G. The relative importance of directional change, random walks, and stasis in the evolution of fossil lineages. Proc Natl Acad Sci USA. 2007;104:18404–18408. doi: 10.1073/pnas.0704088104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maddison WP, Maddison DR. Mesquite: A modular system for evolutionary analysis, version 1.05. 2004. http://mesquiteproject.org.
- 33.Maddison WP. Squared-change parsimony reconstruction of ancestral states for continuous characters on a phylogeny. Syst Zool. 1991;40:304–314. [Google Scholar]
- 34.R Core Development Team. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2008. www.R-project.org. [Google Scholar]
- 35.Garland T, Harvey PH, Ives AR. Procedures for the analysis of comparative data using phylogenetically independent contrasts. Syst Biol. 1992;41:18–32. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.