Abstract
Mixed-metal oxides play a very important role in many areas of chemistry, physics, materials science, and geochemistry. Recently, there has been a strong interest in understanding phenomena associated with the deposition of oxide nanoparticles on the surface of a second (host) oxide. Here, scanning tunneling microscopy, photoemission, and density-functional calculations are used to study the behavior of ceria nanoparticles deposited on a TiO2(110) surface. The titania substrate imposes nontypical coordination modes on the ceria nanoparticles. In the CeOx/TiO2(110) systems, the Ce cations adopt an structural geometry and an oxidation state (+3) that are quite different from those seen in bulk ceria or for ceria nanoparticles deposited on metal substrates. The increase in the stability of the Ce3+ oxidation state leads to an enhancement in the chemical and catalytic activity of the ceria nanoparticles. The codeposition of ceria and gold nanoparticles on a TiO2(110) substrate generates catalysts with an extremely high activity for the production of hydrogen through the water–gas shift reaction (H2O + CO → H2 + CO2) or for the oxidation of carbon monoxide (2CO + O2 → 2CO2). The enhanced stability of the Ce3+ state is an example of structural promotion in catalysis described here on the atomic level. The exploration of mixed-metal oxides at the nanometer level may open avenues for optimizing catalysts through stabilization of unconventional surface structures with special chemical activity.
Keywords: heterogeneous catalysis, imaging, structural properties, surface reactivity
Mixed-metal oxides play a very important role in many areas of chemistry, physics, materials science, and geochemistry (1–6). In technological applications, they are used in the fabrication of microelectronic circuits, piezoelectric devices, and sensors and as catalysts. Over the years, there has been a strong interest in understanding the behavior of mixed-metal oxides at a fundamental level (1–3). What happens when nanoparticles (NPs) of a given metal oxide are deposited on the surface of a second (host) oxide (3, 7)? In principle, the combination of 2 metals in an oxide matrix can produce materials with novel structural and/or electronic properties. At a structural level, a dopant can introduce stress into the lattice of an oxide host, inducing in this way the formation of defects. On the other hand, the lattice of the oxide host can impose on the dopant element nontypical coordination modes. Finally, metal ↔ metal or metal ↔ oxygen ↔ metal interactions in mixed-metal oxides can give electronic states not seen in single-metal oxides.
In this article, we use photoemission, scanning tunneling microscopy (STM), and calculations based on density-functional theory (DFT) to study the behavior of ceria NPs in contact with TiO2(110). Ceria and titania are among the most widely used oxides in catalysis (1, 4–6, 8–12). These oxides are important components in catalysts used for the production of clean hydrogen through the water–gas shift reaction (H2O + CO → H2 + CO2) and for the oxidation of carbon monoxide (2CO + O2 → 2CO2). Ceria and titania adopt different crystal lattices in their most stable bulk phases, fluorite and rutile, respectively (2, 13). Within the fluorite structure each Ce atom is bonded to 8 O atoms, whereas 6 O atoms surround the Ti atoms in the rutile structure. One of the most interesting properties of ceria is its ability to undergo a conversion between “+4” and “+3” formal oxidation states (13). The surface chemistry and catalytic properties of CeO2 depend on the formation of Ce3+ ions (13), and different approaches are followed to maximize their concentration (4, 5, 8). In the CeOx/TiO2(110) systems, the titania substrate imposes on the ceria NPs nontypical coordination modes with a subsequent change in the relative stability of the Ce3+/Ce4+ oxidation states that leads to a significant enhancement in chemical activity. Furthermore, the deposition of gold NPs on CeOx/TiO2(110) produces surfaces with an extremely high catalytic activity for the water–gas shift reaction and the oxidation of CO. This is quite remarkable because neither Au/TiO2(110) nor Au/CeO2(111) come close to matching the catalytic activity of Au/CeOx/TiO2(110).
Experimental and Theoretical Methods
Microscopy, Photoemission, and Catalytic Tests.
The microscopy studies were carried out in an Omicron variable temperature STM system that is directly attached to a main ultrahigh vacuum (UHV) chamber equipped with optics for low-energy electron diffraction, instrumentation for Auger electron spectroscopy and surface cleaning facilities (9, 14). Chemically etched W tips were used for imaging the surfaces. The TiO2(110) crystal was cleaned by several cycles of Ne+ sputtering (1 keV, 40 min) and annealing (950 K, 5 min), and XPS/AES studies confirmed that there were no surface contaminants after this treatment (15).
Furthermore, the high-resolution STM images of the surface exhibited bright Ti rows separated by 6.5 Å, as typically observed for TiO2(110)-(1x1) (16). Photoemission studies were performed at beamline U7A of the National Synchrotron Light Source (NSLS) at Brookhaven National Laboratory (9) by using a photon energy of 625 eV to collect the O 1s and Ti 2p regions, and 325 eV to collect the Ce 4d, Au 4f, and valence regions. In a separate UHV chamber, we acquired XPS spectra (Ce 3d, Ti 2p, O 1s, and Au 4f regions) and UPS spectra (valence region) using Mg Kα and He-I radiation, respectively. Ce and Au were deposited on TiO2(110) by using metal evaporators (9, 14, 17). The flux of the Au doser was calibrated by depositing Au onto a Mo(100) crystal and measuring the thermal desorption spectra of the admetal (17). The area of the titania surface covered by ceria and gold was estimated by using STM images or a combination of ion-scattering spectroscopy (ISS) and photoemission (9, 17).
The catalytic studies were carried out in a system that combines a batch reactor and a UHV chamber (9, 17). The sample could be transferred between the reactor and UHV chamber without exposure to air. Typically, it was transferred to the batch reactor at ≈298 K, and then the reactant gases were introduced (water–gas shift: 20 Torr of CO and 10 Torr of H2O; CO oxidation: 4 torr of CO and 2 Torr of O2). The catalytic activity for the water–gas shift was measured at 625 K (9, 17), with a temperature of 300 K for the oxidation of CO (10). Product yields were analyzed by gas chromatography or mass spectroscopy (9, 17). The amount of molecules produced was normalized by the active area exposed by the sample. In our reactor, a steady-state regime for the water–gas shift or the oxidation of CO was reached after 2–3 min of reaction time.
DFT Calculations.
Periodic DFT + U calculations were performed by using the VASP code (18) using a (4 × 2) 6-layer thick supercell to model the TiO2(110) surface (19). The adlayer and first 4 layers of the titania slab were allowed to relax during the DFT geometry optimizations. We used the Perdew–Wang 91 GGA functional for exchange-correlation, the projector-augmented wave approach, and plane-waves with a cutoff energy set at 400 eV. We treated the Ti (3s, 3p, 3d, 4s), Ce (4f, 5s, 5p, 5d, 6s), and O (2s,2p) electrons as valence states, whereas the remaining electrons were kept frozen as core states (19). The calculations were performed at the Γ point of the Brillouin zone (18). To reproduce the valence spectra of Ce/TiO2(110), see below, we used Ueff parameters with a value of 4.5 eV for Ce and Ti. This value is close to those used in previous studies for bulk ceria or titania (20). The introduction of the Ueff parameters in the DFT calculations was found to be essential to correctly reproduce the position of the occupied Ce 4f and Ti 3d levels in the valence region, even though the trends found in the energetics for the coadsorption of Ce and O on TiO2(110) were almost the same with or without the Ueff parameters.
Growth of Ceria on TiO2(110)
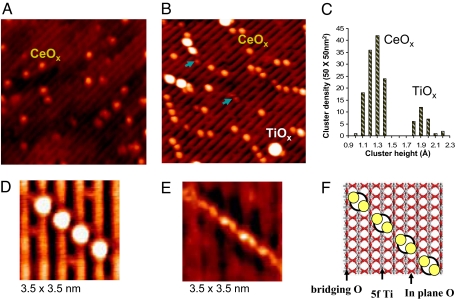
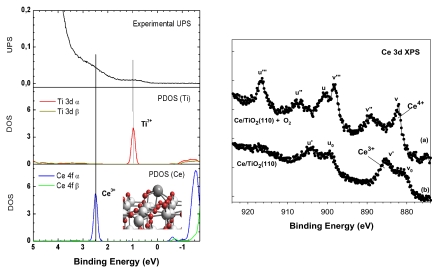
In Fig. 1, we show STM images acquired after depositing cerium on TiO2(110) under different conditions. Fig. 1A corresponds to an image obtained after dosing Ce atoms under ultrahigh vacuum (UHV) at a sample temperature of 298 K. The bright spots have an average height of 1.4 ± 0.2 Å over the flat terrace and correspond to clusters of cerium oxide. The corresponding XPS and UPS spectra indicated that cerium was in an oxidation state of +3, and, consequently, its deposition led to the partial reduction of titanium cations with the appearance of Ti3+. Fig. 2Left shows a typical UPS spectrum for this type of Ce/TiO2(110) surface. The features ≈1 eV can be assigned to Ti3+ centers (1, 16), whereas those at 2–3.5 eV correspond to Ce3+ centers (21). The existence of Ce3+ is corroborated by the Ce 3d XPS data in Fig. 2 Right. The Ce 3d XPS spectrum for the as-prepared Ce/TiO2(110) surface has the distinctive line shape of Ce3+ species (21, 22). Using DFT calculations, we investigated the bonding of Ce atoms to the TiO2(110) surface. The Ce atoms prefer the bonding configuration shown at the bottom of Fig. 2, interacting simultaneously with bridging and in-plane O atoms of the titania substrate. Upon adsorption, Ce formally releases 3 electrons to the oxide host, which move from the 6s and 5d levels in Ce to the lower-energy 3d levels in Ti, reducing 3 Ti4+ cations to Ti3+. The fourth valence electron from Ce is in a 4f level of lower energy than the 3d from Ti and therefore is not transferred, leaving the oxidation state of Ce as 3+. The adsorption of oxygen on the Ce/TiO2(110) surfaces led to the disappearance of Ti3+ sites in the XPS/UPS spectra and in the DFT-calculated density of states. The stability of the Ce3+ cations was verified by their resistance to oxidation under UHV conditions. We had to expose the Ce/TiO2(110) surfaces to an O2 pressure of 1 Torr in the batch reactor to obtain the typical Ce 3d XPS spectrum of Ce4+ cations (21, 22), see Fig. 2 Right.
Fig. 1.
STM results illustrating morphological changes of CeOx on the TiO2(110) surface. (A) STM image (15 × 15 nm) taken after depositing Ce atoms at 298 K in UHV (Vt = 1.3 V and It = 0.05 nA). (B) STM image (15 × 15 nm) acquired after depositing Ce atoms at 600 K and subsequent annealing at 900 K in O2 (PO2 ≈1 × 10−7 Torr) (Vt = 1.2 V and It = 0.07 nA). (C) Height distribution for the spots seen in B. (D and E) Zoomed-in STM images (3.5 × 3.5 nm) of a diagonal array of CeOx taken at different imaging condition of 1.2 V, 0.06 nA and 0.4 V, 0.06 nA, respectively. (F) Model showing possible orientations for the bright protrusions of CeOx in D and E. The dimers of ceria are shown as a combination of white and yellow spheres.
Fig. 2.
Electronic properties of CeOx/TiO2 (110). (Left) (Top) Shown is a UPS spectrum acquired after depositing Ce atoms on TiO2(110) at 298 K. The features marked by vertical lines are not seen on clean stoichiometric TiO2(110). (Middle and Bottom) Displayed are DFT calculated density-of-states (DOS) for a Ce/TiO2(110) surface, including occupied (positive binding energy) and unoccupied states (negative binding energy, states not observable in UPS). The drawing in the Inset shows the bonding configuration of the Ce atoms. Color code for spheres is gray, cerium; red, oxygen; white, titanium. (Right) Ce 3d XPS spectra taken after depositing Ce on TiO2(110) at 298 K (lower), with subsequent exposure to 1 Torr of O2 (upper). The change in the line shape denotes a Ce3+ → Ce4+ transformation (21, 22). The “u” and “v” peaks refer to various final states that are caused by transitions from valence band electrons into Ce 4f states (22).
To avoid the reduction of the titania substrate, Ce atoms were deposited at 600 K and annealed to 900 K under O2 (≈1 × 10−7 Torr) for 5 min. This led to the image shown in Fig. 1B. In this image, the features nonrelated to the ideal TiO2(110) surface can be separated according to their height, as seen in Fig. 1C. Most of the spots (≈80%) have a height of 1.3 ± 0.2 Å. These spots can be attributed to CeOx, after comparing with the image in Fig. 1A for Ce/TiO2(110). A minority of the spots (≈20%) in Fig. 1B have a height of 1.9 ± 0.3 Å. These features were not seen for Ce/TiO2(110) and probably correspond to (1 × 2) reconstructions of TiO2(110) induced by O2 chemisorption (11, 16). They are a consequence of the migration of interstitial Ti atoms from the bulk to the surface of the titania crystal (11, 16). We found them also in blank experiments for O2/TiO2(110). In Fig. 1B, the spots due to CeOx are arranged forming units that are oriented ≈48°, 66°, or 90° with respect to the Ti rows of the oxide substrate. These units were not seen in blank experiments for Ce/TiO2(110) or O2/TiO2(110) and are characteristic of the O2/CeOx/TiO2(110) systems. Close-up images of a ≈44° aligned unit are shown in Fig. 1 D and E. In Fig. 1D, the bright ceria spots are centered on 5f Ti rows and all have a height close to 1.35 Å and a diameter of 6.8 Å. Their size and position suggests that each spot may contain 2 Ce atoms located in between the O bridging and 5f Ti rows. This is confirmed by scanning the same feature with lower imaging bias (0.4 V instead of 1.2 V), where the electron tunneling occurs at different density of states in CeOx/TiO2(110) systems (Fig. 1E). From the STM data, one can construct a structural model consisting of an array of dimers of ceria as displayed in Fig. 1F. According to our measurements of core and valence photoemission, the oxidation state of the Ce atoms inside the dimers is essentially +3. Thus, in the CeOx/TiO2(110) systems, the Ce cations adopt a structural geometry and an oxidation state that are quite different from those seen in bulk ceria (2, 13) or for NPs of ceria deposited on metal substrates (7, 9).
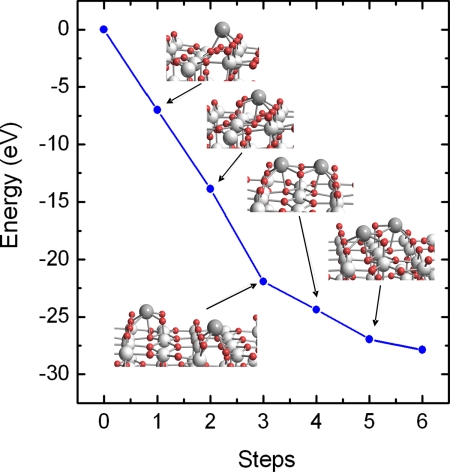
Using DFT, we investigated the process of adsorption–oxidation for Ce deposited on TiO2(110). Fig. 3 shows the calculated energy pathway for such a process. The adsorption energy of atomic Ce is very high (ΔE = −7.23 eV). On its most stable adsorption site, Ce interacts with 2 bridging and 1 in-plane O atoms (Fig. 3, step1). The fact that 3 electrons move from high to lower energy levels, Ce(5d16s2) → Ti(3d1), explains in part the high adsorption energy of Ce. The adsorption process of atomic Ce could be described as
The dissociation of O2 near the adsorbed Ce is a highly exothermic process (ΔE = −6.66 eV). The final structure is a unit of CeO2 over TiO2(110), where the O atoms are adsorbed on top of in-plane Ti atoms and strongly interact with the Ce atom (Fig. 3, step 2). The oxidation state of the Ce in this configuration is 4+
Such CeO2 monomers could be assigned to the smallest spots observed in STM at very low coverages of ceria. For the surface in Fig. 1B, possible CeO2 monomers are denoted by arrows and, as predicted by the DFT calculations, they are not located at the center of the Ti rows. The CeO2 monomers are excellent sites for the adsorption of a second Ce atom to form dimers (Fig. 3, step 4). The increment in the adsorption energy with respect to the adsorption on a clean surface is due to the fact that 1 of the 3 electrons released by the incoming Ce does not go to the high-energy 3d levels of Ti but rather to the 4f of Ce4+ from the CeO2 monomer, which is reduced to Ce3+
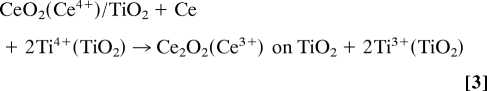 |
The addition of oxygen to the Ce2O2 unit generates Ce2O3.
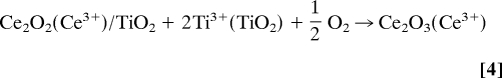 |
on TiO2, and the ceria dimer adopts a configuration (Fig. 3, step 5) where shared oxygen leads to a diagonal arrangement in agreement with the results of STM (Fig. 1 E and F). In the presence of oxygen, the complete oxidation of Ce has to be considered, going from a Ce2O3 dimer to 2 CeO2 monomers (Fig. 3, step 6). Differently from the previous step, here, there are not high-energy Ti 3d electrons but 2 electrons in low-energy Ce 4f states. The process is exothermic but only by −0.92 eV, 3 times less than the energy released in the previous step. This means that as long as Ti3+ species exist, O2 will prefer to adsorb and dissociate on them because the stabilization energy for the system is much higher. Therefore, even though the oxidation process of dimers is favorable, the other site is preferred for the adsorption and dissociation of O2. This illustrates the complex interplay that one can have when dealing with the electronic and chemical properties of a mixed-metal oxide.
Fig. 3.
DFT calculated energy pathway for the adsorption and oxidation of Ce on TiO2(110). The following steps were examined: (1) adsorption of Ce; (2) O2 adsorption-dissociation and formation of the first monomer (CeO2); (3) adsorption of a second cerium atom; (4) formation of the first Ce2O2 dimer; (5) adsorption of ½O2 and formation of the Ce2O3 dimer; and (6) adsorption of ½O2 and formation of 2 CeO2 monomers.
For applications in catalysis, the relative stability of the Ce3+/Ce4+ oxidation states of ceria is a very important issue (1, 4–6, 13, 23, 24). We calculated the energy released by the reactions
for both bulk ceria and Ce2O3 dimers deposited on TiO2(110). The ΔE for the oxidation process, (the reaction shown in 5), was −2.56 eV in the case of bulk Ce2O3 and −0.92 eV for the Ce2O3 dimers bonded to titania. The reduction of CeO2/TiO2(110) by CO, (the reaction shown in 6), was a very exothermic process with a ΔE of −2.35 eV. In contrast, the ΔE for the corresponding reaction of bulk CeO2 was −0.71 eV. These trends were confirmed by comparing reduction/oxidation experiments on CeO2 (111) and CeOx/TiO2(110). For example, a CeO2(111) surface did not undergo reduction under an atmosphere of 20 Torr of CO at 400 K (17), but a CeO2/TiO2(110) surface (formed by exposing CeOx/TiO2(110) to 1 Torr of O2 at 298 K) was completely transformed into Ce2O3/TiO2(110). The high stability of the Ce3+ cations in CeOx/TiO2(110) is a consequence of their nontypical coordination mode and the effect of Ce (4f)–O (2p)–Ti (3d) bonding interactions.
Catalytic Activity of Au/CeOx/TiO2(110)
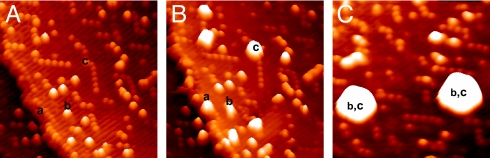
The WGS reaction is a critical catalytic process for the production of clean hydrogen in the chemical industry (4, 5). There is a continuous search for catalysts with a better WGS activity (4–6, 9). As shown below, the deposition of gold NPs on CeOx/TiO2(110) yielded surfaces with an extremely high catalytic activity for the WGS. Fig. 4 displays STM images acquired from the same surface area before (Fig. 4A) and after (Fig. 4B) depositing gold on CeOx/TiO2(110) at 298 K with subsequent annealing to 600 K (Fig. 4C). The CeOx/TiO2(110) was preannealed under O2 (≈1 × 10−7 Torr) at 900 K and had a morphology similar to that seen in Fig. 1B. The deposition of Au at room temperature, ≈0.2 monolayer, produced 3-dimensional metal particles anchored to steps of the titania surface, “a” sites, to the (1 × 2) reconstructions of TiO2(110), “b” sites, and to the CeOx dimers, “c” sites. Annealing to 600 K produced large particles of Au that were simultaneously located on b and c sites. Au NPs with a diameter as large as 5.9 nm and a height of 1.3 nm were seen, but smaller metal particles were also present on the CeOx/TiO2(110) surface. On this surface, the dispersion of the Au NPs was substantially larger than seen on a pure TiO2(110) surface where Au mainly binds to the steps (10, 15).
Fig. 4.
Morphology of Au/CeOx/TiO2 (110). (A) STM image of a CeOx/TiO2(110) surface. Ce was deposited at 600 K under an atmosphere of O2 (≈1 × 10−7 Torr) and then the sample was annealed at 900 K in O2. (B) STM image for a Au/CeOx/TiO2(110) surface. The gold was deposited on the same area shown in A at 298 K. Approximately 6% of the surface was covered with Au. (C) STM image obtained after annealing the system in B to 600 K. All of the STM images correspond to an area of 20 × 20 nm and imaging conditions of Vt = 1.5 V and It = 0.03 nA.
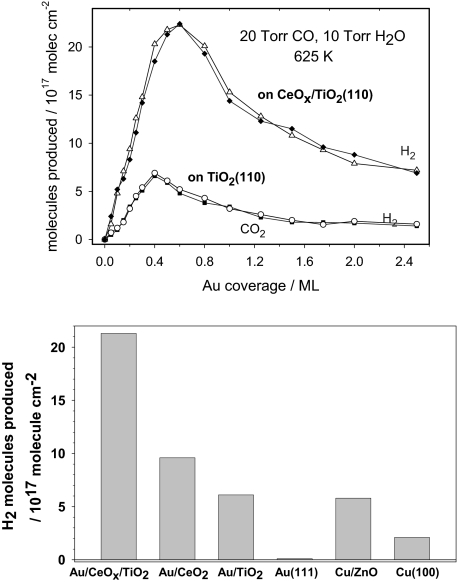
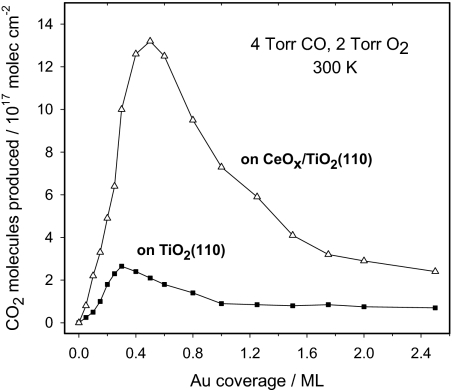
Neither CeOx/TiO2(110) nor Au(111) were able to catalyze the WGS. However, Au/CeOx/TiO2(110) surfaces are outstanding catalysts for the WGS as shown in Fig. 5. We performed test experiments in which Au/TiO2(110) surfaces were prepared following the same steps used for the synthesis of Au/CeOx/TiO2(110) but without the deposition of cerium. The Au/TiO2(110) systems were good WGS catalysts, see Fig. 5 Upper, but they did not come close to match the activity of Au/CeOx/TiO2(110). The same is valid when comparing with the WGS activities of Au/CeO2(111) (17), CeOx/Au(111) (9), Cu/ZnO(0001) (17), and copper single crystals (17, 25). Cu/ZnO is the most common WGS catalyst used in the industry (5, 17, 25), and copper is the best pure-metal catalyst (25, 26). For the Au/CeOx/TiO2(110) catalyst in Fig. 5, one could assume that the concentration of active sites is proportional to the number of ceria regions in contact with gold NPs. Because only 12% of the titania support was covered by ceria, as measured by ion scattering spectroscopy (ISS), the Au/CeOx/TiO2(110) catalyst must be at least 300 times more active than a Cu (100) surface on a per-active-site basis.*
Fig. 5.
Water-gas shift catalysis. (Upper) Water–gas shift activity of Au/TiO2(110) and Au/CeOx/TiO2(110) as a function of Au coverage. The area of TiO2(110) covered by CeOx was measured with ISS, before depositing gold, and found to be ≈12% of the clean substrate. The reported values for the production of H2 (blue curve) and CO2 (black curve) were obtained after exposing the catalysts to 20 Torr of CO and 10 Torr of H2O at 625 K for 5 min. The number of H2 and CO2 molecules produced is normalized by the sample surface area. (Lower) Comparison of the water–gas shift activity of Cu(100), Au(111), and 0.5 mL of Au supported on TiO2(110), CeO2(111) or CeOx/TiO2(110). The data for Cu(100), Cu/ZnO(0001) and Au/CeO2(111) were taken from ref. 17.
Postreaction characterization of the Au/CeOx/TiO2(110) surfaces with XPS showed the presence of metallic Au and Ce3+ cations. An identical result was found in in situ measurements of X-ray absorption spectroscopy for Au/CeOx/TiO2 powders under WGS reaction conditions. The high catalytic activity of Au/CeOx/TiO2(110) can be attributed to the special chemical properties of the supported Ce2O3 dimers and cooperative effects at ceria–gold interfaces. Usually, the rate-determining step in the WGS reaction is the dissociation of water (9, 17, 26). Isolated NPs of gold cannot dissociate this molecule (27, 28). We found that the Ce3+ sites present in CeOx/TiO2(110) easily dissociate water, but, upon exposure to CO, highly stable HCOx species were formed on the oxide surface and there was no production of H2 or CO2 gas. In Au/CeOx/TiO2(110), one has a bifunctional catalyst: The adsorption and dissociation of water takes place on the oxide, CO adsorbs on gold NP sites, and all subsequent reaction steps occur at oxide–metal interfaces. Au NPs do catalyze the reaction of OH with CO to yield a HOCO intermediate and then H2 and CO2 (27). Previous studies for the WGS on Au–CeO2 catalysts point to a direct participation of the oxide in the reaction process (4, 5, 9). Our results illustrate the tremendous impact that an optimization of the chemical properties of nanoceria can have on the activity of a WGS catalyst.
Nowadays, the oxidation of CO on Au/TiO2 catalysts is receiving a lot of attention (1, 10, 29, 30). Fig. 6 displays the CO oxidation activity of Au/TiO2(110) and Au/CeOx/TiO2(110) as a function of Au coverage. In the case of Au/TiO2(110) a maximum in the production of CO2 is seen at a Au coverage of ≈0.3 mL. Previous studies have shown that there is a marked size effect on the catalytic activity, with Au clusters in the range of 3–3.5 nm exhibiting the maximum reactivity (10, 29). Our STM studies for Au/TiO2(110) and Au/CeOx/TiO2(110) also show a strong variation in catalytic activity with Au particle size. In all cases, Au/CeOx/TiO2(110) is a much better catalyst for the oxidation of CO than Au/TiO2(110). If the maximum catalytic activities seen in Fig. 6 are normalized by the number of Au atoms present on the oxide supports (10, 29), we estimate turnover frequencies (TOFs) for CO oxidation of 2.1 molecules per site−1 s−1 for Au/TiO2(110) and 6.2 molecules per site−1 s−1 for Au/CeOx/TiO2(110). These should be taken as lower limits for the TOFs because we probably overestimated the number of exposed Au active sites. Here, we are following previous studies (10, 29) that estimate the TOFs assuming total dispersion of Au on the oxide substrate. In any case, the TOF of Au/CeOx/TiO2(110) is already larger than the TOF found, under similar conditions of pressure and temperature, for the oxidation of CO on a highly active Au ultrathin film supported on a reduced TiOx substrate: ≈4 molecules per site−1 s−1 (30).
Fig. 6.
CO oxidation activity of Au/TiO2(110) and Au/CeOx/TiO2(110) as a function of Au coverage. The area of TiO2(110) covered by CeOx was measured with ISS, before depositing gold, and found to be ≈16% of the clean substrate. The reported values for the production of CO2 were obtained after exposing the catalysts to 4 Torr of CO and 2 Torr of O2 at 300 K for 5 min. The number of CO2 molecules produced is normalized by the sample surface area.
The rate-limiting step for the oxidation of CO on Au/oxide surfaces is the activation and dissociation of the O2 molecule (31–33). The oxide probably helps in the stabilization of an OC·O2 intermediate and the breaking of the O–O bond. The structure of the titania supported ceria nanoparticles should facilitate their direct interaction with an OC·O2 intermediate. Postreaction surface analysis with XPS showed the presence of a significant amount of Ce3+ in the Au/CeOx/TiO2(110) catalysts. This and a relatively high dispersion of Au (Fig. 4B) could be responsible for the superior activity of Au/CeOx/TiO2(110) during the oxidation of CO at room temperature.
Summary and Conclusions
Scanning tunneling microscopy, photoemission, and density-functional calculations were used to study the behavior of ceria nanoparticles deposited on a TiO2(110) surface. The titania substrate imposes nontypical coordination modes on the ceria nanoparticles. In the CeOx/TiO2(110) systems, the Ce cations adopt an structural geometry and an oxidation state (+3) that are quite different from those seen in bulk ceria or for ceria nanoparticles deposited on metal substrates. The increase in the stability of the Ce3+ oxidation state leads to an enhancement in the chemical and catalytic activity of the ceria nanoparticles. The codeposition of ceria and gold nanoparticles on a TiO2(110) substrate generates catalysts with an extremely high activity for the production of hydrogen through the water–gas shift reaction (H2O + CO → H2 + CO2) or for the oxidation of carbon monoxide (2CO + O2 → 2CO2). Our results illustrate the high impact that an optimization of the chemical properties of nanoceria can have on the activity of a WGS or CO oxidation catalyst. This approach should be valid in general for catalysts that contain ceria as part of a mixed-metal oxide (4–7), opening new directions for tuning catalytic activity by coupling appropriate pairs of oxides. The key issue is to take advantage of the complex interactions that occur in a mixed-metal oxide at the nanometer level.
Acknowledgments.
We thank M. Pérez for thought-provoking discussions about the mechanism for the WGS reaction on Au/CeOx/TiO2(110). The work performed at Brookhaven National Laboratory was supported by the U.S. Department of Energy, Office of Basic Energy Sciences under contract DE-AC02-98CH10886. J.E. is grateful to the Instituto de Tecnología Venezolana para el Petróleo for partial support of the work carried out at the Universidad Central de Venezuela. The work done at Seville was funded by Ministry of Science and Innovation Grant MAT2008-04918 and Barcelona Supercomputing Center—Centro Nacional de Supercomputación (Spain).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
It is usually assumed that all atoms of a flat Cu(100) surface, 1.53 × 1015 atoms cm−2, are active in the WGS reaction (25). The structural model in Fig. 1F was used to calculate the area occupied by a Ce2O3 dimer on the TiO2(110) surface. Using this and the ceria coverage determined from ISS measurements (≈12% of the titania surface was covered), we find that the concentration of Ce in the Au/CeOx/TiO2(110) catalyst of Fig. 5 was in the order of 0.03 × 1015 atoms cm−2. This is an upper limit to the actual concentration of the active sites, because not all of the Ce atoms had a Au particle nearby (see Fig. 4 B and C). After subtracting the WGS activity of Au/TiO2(110) from that of Au/CeOx/TiO2(110), one finds that the Au/ceria sites are at least 300 times more active than the atoms in a Cu(100) surface.
References
- 1.Zhou B, Hermans S, Somorjai GA, editors. Nanotechnology in Catalysis. New York: Kluwer–Plenum; 2004. [Google Scholar]
- 2.Rao CNR, Raveau B. Transition Metal Oxides: Structure, Properties and Synthesis of Ceramic Oxides. 2nd Ed. New York: Wiley; 1998. [Google Scholar]
- 3.Knauth P, Schoonman J, editors. Nanocrystalline Metals and Oxides: Selected Properties and Applications. Berlin: Springer; 2002. [Google Scholar]
- 4.Fu Q, Saltsburg H, Flytzani-Stephanopoulos M. Active nonmetallic Au and Pt species on ceria-based water–gas shift catalysts. Science. 2003;301:935–938. doi: 10.1126/science.1085721. [DOI] [PubMed] [Google Scholar]
- 5.Burch R. Gold catalysts for pure hydrogen production in the water–gas shift reaction: Activity, structure and reaction mechanism. Phys Chem Chem Phys. 2006;8:5483–5500. doi: 10.1039/b607837k. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez ID, Navarro RM, Alvarez-Galvan MC, Rosa F, Fierro JLG. Performance enhancement in the water–gas shift reaction of platinum deposited over a cerium-modified TiO2 support. Catal Commun. 2008;9:1759–1765. [Google Scholar]
- 7.Fernández-García M, Martinez-Arias A, Hanson JC, Rodriguez JA. Nanostructured oxides in chemistry: Characterization and properties. Chem Rev. 2004;104:4063–4104. doi: 10.1021/cr030032f. [DOI] [PubMed] [Google Scholar]
- 8.Esch F, et al. Electron localization determines defect formation on ceria substrates. Science. 2005;309:752–755. doi: 10.1126/science.1111568. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez JA, et al. Activity of CeOx and TiOx nanoparticles grown on Au(111) in the water–gas shift reaction. Science. 2007;318:1757–1760. doi: 10.1126/science.1150038. [DOI] [PubMed] [Google Scholar]
- 10.Valden M, Lai X, Goodman DW. Onset of catalytic activity of gold clusters on titania with the appearance of nonmetallic properties. Science. 1998;281:1647–1650. doi: 10.1126/science.281.5383.1647. [DOI] [PubMed] [Google Scholar]
- 11.Wendt S, et al. The role of interstitial sites in the Ti3d defect state in the band gap of titania. Science. 2008;320:1755–1759. doi: 10.1126/science.1159846. [DOI] [PubMed] [Google Scholar]
- 12.Diwald O, Thompson TL, Goralski EG, Walck SD, Yates JT. The effect of nitrogen ion implantation on the photoactivity of TiO2 rutile single crystals. J Phys Chem B. 2004;108:52–57. [Google Scholar]
- 13.Trovarelli A. Catalytic properties of ceria and CeO2-containing materials. Catal Rev Sci Eng. 1996;38:439–520. [Google Scholar]
- 14.Ma S, Rodriguez JA, Hrbek J. STM study of the growth of cerium oxide nanoparticles on Au(111) Surf Sci. 2008;602:3272–3279. [Google Scholar]
- 15.Park JB, Conner SF, Chen DA. Bimetallic Pt-Au clusters on TiO2(110): Growth, surface composition, and metal-support interactions. J Phys Chem C. 2008;112:5490–5500. [Google Scholar]
- 16.Diebold U. The surface science of titanium dioxide. Surf Sci Rep. 2003;48:53–229. [Google Scholar]
- 17.Rodriguez JA, et al. Water gas shift reaction on Cu and Au nanoparticles supported on CeO2(111) and ZnO(0001̄): Intrinsic activity and importance of support interactions. Angew Chem Int Ed. 2007;46:1329–1332. doi: 10.1002/anie.200603931. [DOI] [PubMed] [Google Scholar]
- 18.Kresse G, Furthmuller J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput Mater Sci. 1996;6:15–50. doi: 10.1103/physrevb.54.11169. [DOI] [PubMed] [Google Scholar]
- 19.Graciani J, Alvarez LJ, Rodriguez JA, Sanz JF. N doping of rutile TiO2(110) surface: A theoretical DFT study. J Phys Chem C. 2008;112:2624–2631. [Google Scholar]
- 20.Fabris S, de Gironcoli S, Baroni S, Vicario G, Balducci G. Taming multiple valency with density functionals: A case study of defective ceria. Phys Rev B. 2005;71 041101. [Google Scholar]
- 21.Liu G, Rodriguez JA, Hrbek J, Dvorak J, Peden CHF. Electronic and chemical properties of Ce0.8Zr0.2O2(111) surfaces: Photoemission, XANES, density-functional, and NO2 adsorption studies. J Phys Chem B. 2001;105(32):7762–7770. [Google Scholar]
- 22.Pfau A, Schierbaum KD. The electronic-structure of stoichiometric and reduced CeO2 surfaces—An XPS, UPS and HREELS study. Surf Sci. 1994;321:71–80. [Google Scholar]
- 23.Wang X, et al. Ceria-based catalysts for the production of H2 through the water–gas-shift reaction: Time-resolved XRD and XAFS studies. Top Catal. 2008;49:81–88. [Google Scholar]
- 24.Jacobs G, et al. Water–gas shift: Comparative screening of metal promoters for metal/ceria systems and role of the metal. Appl Catal A. 2004;258:203–214. [Google Scholar]
- 25.Nakamura J, Campbell JM, Campbell CT. Kinetics and mechanism of the water–gas shift reaction catalyzed by the clean and Cs-promoted Cu(110) surface—A comparison with Cu(111) J Chem Soc Faraday Trans. 1990;86:2725–2734. [Google Scholar]
- 26.Gokhale AA, Dumesic JA, Mavrikakis M. On the mechanism of low-temperature water gas shift reaction on copper. J Am Chem Soc. 2008;130:1402–1414. doi: 10.1021/ja0768237. [DOI] [PubMed] [Google Scholar]
- 27.Liu P, Rodriguez JA. Water–gas-shift reaction on metal nanoparticles and surfaces. J Chem Phys. 2007;126:164705. doi: 10.1063/1.2722747. [DOI] [PubMed] [Google Scholar]
- 28.Ojifinni RA, et al. Water-enhanced low-temperature CO oxidation and isotope effects on atomic oxygen-covered Au(111) J Am Chem Soc. 2008;130:6801–6812. doi: 10.1021/ja800351j. [DOI] [PubMed] [Google Scholar]
- 29.Bamwenda GR, Tsubota S, Nakamura T, Haruta M. The influence of the preparation methods on the catalytic activity of platinum and gold supported on TiO2 for CO oxidation. Catal Lett. 1997;44:83–87. [Google Scholar]
- 30.Chen MS, Goodman DW. The structure of catalytically active gold on titania. Science. 2004;306:252–255. doi: 10.1126/science.1102420. [DOI] [PubMed] [Google Scholar]
- 31.Hernandez NC, Sanz JF, Rodriguez JA. Unravelling the origin of the high-catalytic activity of supported Au: A density-functional theory-based interpretation. J Am Chem Soc. 2006;128:15600–15601. doi: 10.1021/ja0670153. [DOI] [PubMed] [Google Scholar]
- 32.Molina LM, Hammer B. Active role of oxide support during CO oxidation at Au/MgO. Phys Rev Lett. 2003;90:206102. doi: 10.1103/PhysRevLett.90.206102. [DOI] [PubMed] [Google Scholar]
- 33.Remediakis IN, Lopez N, Norskov JK. CO oxidation on rutile-supported Au nanoparticles. Angew Chem Int Ed. 2005;44:1824–1826. doi: 10.1002/anie.200461699. [DOI] [PubMed] [Google Scholar]