Abstract
The fossil assemblages of the Late Cretaceous of North America are dominated by large-bodied dinosaur species. Associated skeletons of small dinosaurs are exceedingly rare, and small (<10 kg) carnivorous theropods have not previously been reported from these beds. Here, we describe a small dromaeosaurid from the 75-million-year-old Dinosaur Park Formation of Alberta, Canada. Hesperonychus elizabethae gen. et sp. nov. is represented by a pelvic girdle from an animal weighing ≈1,900 g. Despite its size, the pubes and ilia are coossified, indicating that the animal was somatically mature. This is the smallest carnivorous, nonavian dinosaur known from North America. Phylogenetic analysis of Hesperonychus reveals that it is not closely related to previously described North American dromaeosaurids. Instead, Hesperonychus is a member of the dromaeosaurid clade Microraptorinae, a group containing the 4-winged Microraptor and the feathered Sinornithosaurus, both from the Lower Cretaceous Jehol Group of China. Hesperonychus is the youngest known member of this lineage, extending the temporal range of the clade by 45 million years, and it is the first microraptorine known from North America, providing further evidence for an affinity between the dinosaur faunas of North America and Asia. Study of fossil collections from the Dinosaur Park and Oldman formations of Alberta has revealed numerous isolated bones of small, basal dromaeosaurids, which are tentatively referred to Hesperonychus. These fossils suggest that small dromaeosaurids were a significant component of the carnivore community in this Late Cretaceous biota.
Keywords: Campanian, Dinosaur Park Formation, microraptorinae, theropoda
The vast majority of known nonavian dinosaurs are medium- and large-bodied forms, ranging in size from tens to thousands of kilograms (1). This pattern is especially evident in the Late Cretaceous of North America (2–8). Here, large-bodied (>1,000 kg) dinosaurs dominate fossil assemblages in terms of number of skeletons (2, 3, 7) and number of species (4–6, 8). Small-bodied (<10 kg) carnivorous dinosaurs have not previously been described from these assemblages.
Until now, the smallest carnivorous dinosaur known from the Late Cretaceous of North America was the dromaeosaurid Saurornitholestes langstoni, which weighed ≈10 kg [see Dinosaur Mass Data and Equations in supporting information (SI) Appendix]. Other animals are unlikely to have filled the small-carnivore niche. The alvarezsaurid theropod Albertonykus (9, 10) appears to have been a specialized insectivore (10) rather than a carnivore in the strict sense, and the stagodont marsupials were highly specialized durophages (11, 12) that may have fed on aquatic invertebrates rather than terrestrial prey.
The apparent absence of small, endothermic (or, in the case of dinosaurs, presumably endothermic) carnivores in these Late Cretaceous ecosystems is remarkable: In modern, mammal-dominated terrestrial communities, small-bodied animals outnumber large-bodied animals, both in terms of the number of individuals in a given area (13, 14) and number of species (15, 16). That dinosaurs might have left the small-carnivore niche vacant in North America is still more perplexing when one considers that many small carnivorous dinosaurs are known from Europe, Asia, and Gondwana (17–28). This raises the question of whether small-bodied, endothermic carnivores were truly rare in these assemblages, or whether our picture of these ecosystems is incomplete.
Recently, study of museum collections resulted in the identification of a specimen of a previously unknown dinosaur from the Upper Cretaceous (upper Campanian) Dinosaur Park Formation (29) of Alberta, Canada. The specimen was collected in 1982 but lay unstudied for 25 years. Preparation of the fossil revealed that it represents a new genus of the clade Dromaeosauridae, a group of birdlike, carnivorous theropods (30).
This animal, Hesperonychus elizabethae, gen. et sp. nov., is almost an order of magnitude smaller than any other carnivorous dinosaur known from the Dinosaur Park Formation (see Dinosaur Mass Data and Equations in SI Appendix) or any North American Cretaceous assemblage. This find demonstrates that in the Late Cretaceous of North America, dromaeosaurids exploited the small-carnivore niche.
Surprisingly, Hesperonychus does not appear to be related to previously known North American dromaeosaurids, such as Dromaeosaurus albertensis (31) and Saurornitholestes langstoni (32). Instead, it shares features with the Microraptorinae, a clade of small, basal dromaeosaurids that includes the feathered Sinornithosaurus and the bizarre “4-winged” Microraptor, both from the Lower Cretaceous Jehol Group of China (20–22, 24).
Systematic Paleontology
Systematics are as follows: Theropoda Marsh, 1881; Coelurosauria von Huene, 1914; Maniraptora Gauthier, 1986; Dromaeosauridae Matthew and Brown, 1922; Microraptorinae Senter et al., 2004; Hesperonychus elizabethae gen. et sp. nov.
Holotype
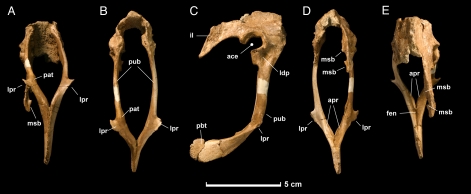
UALVP (University of Alberta Laboratory for Vertebrate Palaeontology, Edmonton) 48778, a partial pelvic girdle comprising the pubes and ilia (Fig. 1).
Fig. 1.
Holotype pelvic girdle (UALVP 48778) of Hesperonychus elizabethae from the late Campanian Dinosaur Park Formation of Alberta, Canada. (A) Ventral view. (B) Anterior view. (C) Right lateral view. (D) Posterior view. (E) Dorsal view. ace, acetabulum; apr, pubic apron; fen, fenestra; il, ilium; ldp, lateral depression; lpr, lateral process; msb, medial shelf of brevis fossa; pat, pathology; pbt, pubic boot; pub, pubis.
Referred Material
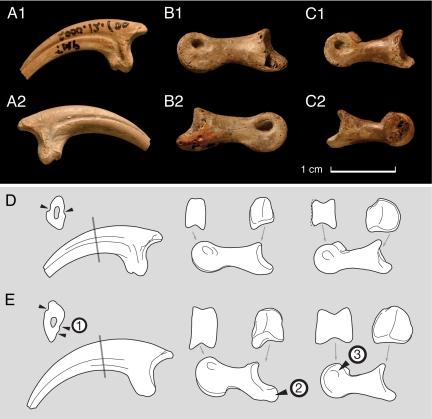
A number of isolated pedal phalanges are tentatively referred to Hesperonychus (see Specimen Data in SI Appendix and Fig. 2). Phalanx II-1 is represented by TMP (Royal Tyrrell Museum of Palaeontology, Drumheller, AB, Canada) 1989.116.65, TMP 1966.19.22, and UALVP 50687. Phalanx II-2 is represented by TMP 1992.36.61 and TMP 1983.67.7. Phalanx II-3 is represented by TMP 1979.10.6, TMP 1980.16.1880, TMP 1990.107.15, TMP 1995.092.0009, TMP 2000.12.100, and UALVP 50686.
Fig. 2.
Pedal phalanges, cf. Hesperonychus. (A) Pedal phalanx II-3 (TMP 2000.12.100), from Dinosaur Provincial Park, Canada (reversed from left) in medial (A1) and lateral (A2) views. (B) Right pedal phalanx II-2 (TMP 1983.67.7) in medial (B1) and lateral (B2) views. (C) Right pedal phalanx II-1 (1989.116.65) in medial (C1) and lateral (C2) views. (D and E) cf. Hesperonychus specimens (D) compared with Saurornitholestes langstoni (E), a typical member of the Eudromaeosauria. Derived features characterizing the Eudromaeosauria include (1) paired grooves on the medial surface of the ungual that are shifted ventrally relative to the lateral groove; (2) phalanx II-2 with an elongate heel of the proximal articular surface, and (3) phalanx II-1 with strong dorsal projecton of the distal articular surface. Absence of these eudromaeosaur features indicates that these fossils are from a basal dromaeosaur, rather than juveniles of Saurornitholestes or Dromaeosaurus.
Etymology
The name Hesperonychus derives from hesperus (Latin, west) and onychos (Greek, claw). The specific epithet elizabethae honors the late Dr. Elizabeth Nicholls, who discovered the holotype.
Horizon and Locality
The holotype was collected from exposures of the Dinosaur Park Formation located on the south side of the Red Deer River, ≈20 km east of Dinosaur Provincial Park, Alberta. The Dinosaur Park Formation was deposited during the middle of the late Campanian, between 76.5 and 74.8 Ma (29). Referred material was collected from Dinosaur Provincial Park and surrounding badlands and other locations in southern Alberta, including Devil's Coulee, Manyberries, Onefour, Irvine, and Sandy Point (Specimen Data in SI Appendix); all are within 200 km of the type locality. All specimens for which precise stratigraphic data are available come from a narrow chronostratigraphic interval that encompasses the Dinosaur Park Formation and coeval beds of the uppermost Oldman Formation (Specimen Data in SI Appendix). This interval represents no more than 1.7 million years of time (29).
Diagnosis
Small dromaeosaurid characterized by the following autapomorphies: pubic peduncle of ilium with medial surface deeply excavated; posterior wing of ilium with medial shelf split to form anterior and posterior processes; lateral tubercles of pubis wing-like and curving anteriorly; pubis with fossa on lateral surface ventral to acetabulum; pubic apron shifted onto posterior surface of pubis; pubic symphysis teardrop-shaped in lateral view; ischiadic process of pubis reduced to a narrow lamina.
Description and Comparisons
Hesperonychus is a remarkably small theropod. By using a regression of pubis length against body mass, the holotype is estimated to have weighed ≈1,900 g, approximately half the weight of a domestic cat. Despite the small size of the animal, the pubes and ilia are completely fused to each other, indicating that the animal was somatically mature.
The pubic peduncle of the ilium is long anteroposteriorly and has a deep fossa medially, which extends onto the medial surface of the pubis. The lateral surface of the pubic peduncle is convex, with no trace of a cuppedicus fossa. The ilia are strongly inclined medially and may have contacted each other dorsally along the midline. Unlike the situation in Velociraptor, in which the ilia diverge posteriorly and the dorsal margin is laterally everted (30, 33, 34), the posterior alae of the ilia would have been approximately parallel, and the postacetabular blade projects vertically. As in basal paravians (23, 35–37), the posterior wing of the ilium is tapered and curved ventrally in lateral view. Along the dorsal edge of the posterior ala, there is a distinct tubercle, a common maniraptoran feature (36). The brevis shelf projects ventrolaterally away from the posterior blade of the ilium. Medially, the medial shelf of the brevis fossa is split into separate anterior and posterior processes, a condition unique to Hesperonychus. The acetabulum is similar to those of other dromaeosaurids in that it lacks a prominent supracetabular crest (30, 36). However, anteriorly, the contribution of the ilium to the acetabulum is broad, and the anterior rim projects strongly laterally, as it does in Unenlagia (36). The medial opening of the acetabulum is partially closed, as it is in other Dromaeosauridae (36). The acetabulum opens dorsolaterally rather than laterally, as is the case in Velociraptor (38), suggesting the ability to partially abduct the hindlimbs. This morphology is of interest in light of proposals that Microraptor gui abducted its feathered hindlimbs to function as airfoils (24).
As in many other paravians (20, 23, 24, 30, 33–35, 37), the pubic shaft projects posteroventrally. The proximal end of the pubis has a distinct scar on its anterior surface, in the same position as the large tubercle found in Velociraptor (33). The proximal end also bears a deep depression on its lateral surface, just ventral and anterior to the acetabulum. The ischiadic peduncle of the pubis is mediolaterally compressed and its contact with the ischium is reduced to a thin blade of bone. The distal shaft of the pubis sharply curves posteriorly, as it does in the Jehol dromaeosaurids Microraptor (24) and Sinornithosaurus (20); Unenlagia shows a similar curvature but it is more weakly developed (35). Proximally, the shaft of the pubis is mediolaterally compressed. Unlike the condition in Velociraptor, where the distal shaft of the pubis is anteroposteriorly flattened (33, 34), the distal shaft is subcircular in section. The right pubic shaft has an unusual swelling not seen on the left, apparently representing a well-healed fracture. On the lateral surface of each pubic shaft, there is a distinct process, as in other microraptorines (20–22, 24). However, in the new taxon, these processes are larger, winglike, and curve anteriorly. The laminae that form the pubic apron are greatly reduced, and their midline contact is all but lost, resulting in a deep, broad pelvic canal. Unusually, these laminae are located on the posterior surface of the shaft rather than on the medial surface, as is the case in other deinonychosaurs (21–23, 30, 33–35). At their ends, the pubes fuse to form a symphysis. No distinct anterior or posterior processes are present. Instead, the symphysis is spatulate in lateral view, as in other microraptorines (20, 24).
In addition to the holotype, numerous small pedal phalanges have been recovered from the Dinosaur Park and Oldman formations (Fig. 2 A–C), which are tentatively referred to Hesperonychus.
Phalanx II-1, the proximal phalanx, is long and slender, as in basal deinonychosaurs (20–24, 39) (Fig. 2C). Its distal articular surface is broad and spool-shaped in distal view (Fig. 2D), as in other dromaeosaurids (30). However, in medial view, the distal articular surface is weakly expanded and subcircular (Fig. 2D). In contrast, the distal articular surface is dorsally extended in Eudromaeosauria (Fig. 2E). The proximal articular surface has a small medial cotyle.
Phalanx II-2, the penultimate phalanx, resembles those of basal deinonychosaurs (20–22, 39) in being relatively long and gracile, with a short proximal articular heel and weak expansion of the proximal and distal articular surfaces (Fig. 2B). In ventral view, the heel is triangular, as in Sinovenator and saurornitholestines. Ventrally, the flexor tendon groove is only weakly developed, in contrast to the condition in saurornitholestines, in which this groove is prominent.
Phalanx II-3, the ungual phalanx (Fig. 2A), resembles the sickle claw of basal dromaeosaurids such as Rahonavis (39) and microraptorines (20, 22). In cross-section, the claw is broad for its depth; the medial surface is almost flat, and the lateral surface is highly convex (Fig. 2D), resulting in a semilunate cross-section. A similar cross-section occurs in Rahonavis UA (University of Antananarivo, Antananarivo, Madagascar) 8656 and the troodontid Sinovenator IVPP (Institute of Vertebrate Paleontology and Paleoanthropology, Beijing, Peoples Republic of China) V12615. In contrast, derived dromaeosaurids (Eudromaeosauria), including Saurornitholestes (Fig. 2E), Velociraptor (33, 34), Deinonychus (40), and Utahraptor (41), have a more blade-like claw. Phalanx II-3 is also distinguished by the more symmetrical arrangement of the vascular grooves. In Eudromeosauria, the lateral groove is shifted toward the dorsal edge of the claw, and the medial groove is shifted ventrally (40, 41) This asymmetry is only weakly developed in the ungual phalanges of the new taxon (Fig. 2), although it is developed to a greater degree than in troodontids. Eudromaeosauridae also exhibit an accessory vascular groove on the medial surface of pedal ungual II, which is absent from the fossils described here (Fig. 2).
The linear dimensions of the referred specimens range from 71% to 105% of the dimensions of the corresponding elements in the microraptorine Sinornithosaurus millennii (IVPP V12811), whereas the pubis of the holotype is 80% of the length of the pubis in IVPP V12811 (see Specimen Data in SI Appendix). Thus, the referred elements and the holotype of Hesperonychus belong to individuals of comparable size. The pedal phalanges are virtually identical to the pedal phalanges of digit II in Sinornithosaurus and Microraptor. However, they lack features found in the Eudromaeosauria, indicating that they do not represent juveniles of Saurornitholestes langstoni or Dromaeosaurus albertensis, the eudromaeosaurs known from the Dinosaur Park assemblage (31, 32). As described above, the second pedal digit of Eudromaeosauria is characterized by numerous derived characters (blade-like ungual with asymmetrically arranged vascular grooves; elongate proximal articular heel of phalanx II-2; dorsally extended medial condyle of phalanx II-1) that are conspicuously absent in these specimens (Fig. 2 D and E). The presence of these synapomorphies in a juvenile eudromaeosaur, the holotype of Bambiraptor feinbergorum (42), demonstrates that the absence of such features cannot be explained as the result of ontogenetic changes in morphology. For these reasons, we interpret the referred specimens as coming from a small-bodied basal dromaeosaur. In the absence of an associated skeleton, referral to Hesperonychus must be considered tentative, but this identification is consistent with the available evidence.
Systematics
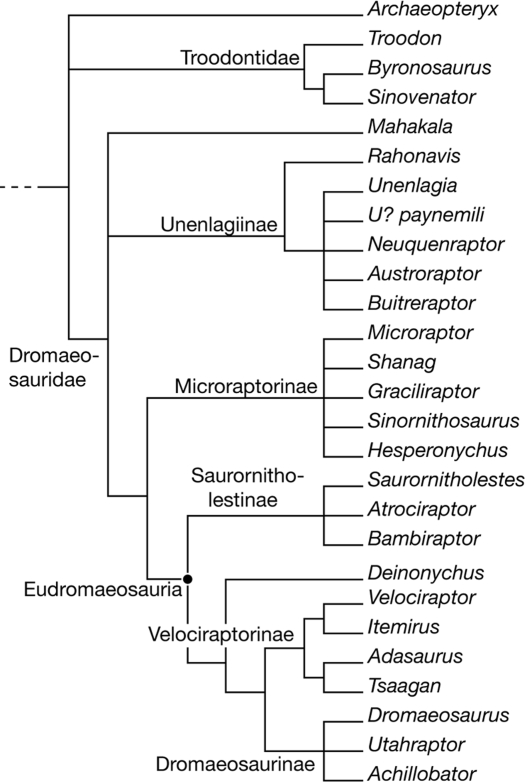
Phylogenetic analysis places Hesperonychus in the dromaeosaurid clade Microraptorinae (Fig. 3). Referral of Hesperonychus to the Microraptorinae is supported by the presence of a lateral process of the pubis, strong posterior curvature of the pubic shaft, and a spatulate pubic foot.
Fig. 3.
Strict consensus of 5,292 most parsimonious trees resulting from phylogenetic analysis of 23 in-group taxa, 4 out-group taxa, and 114 characters. H. elizabethae was found to be part of the clade Microraptorinae. Tree length = 223, consistency index = 0.5430, retention index = 0.6930, rescaled consistency index = 0.3791.
This study agrees with previous studies in recovering the clades Microraptorinae, Velociraptorinae, Dromaeosaurinae, and Unenlagiinae (26–28, 43, 44), although the membership of some of these clades differs in our analysis. Shanag ashile, for instance, was found to belong to the Microraptorinae, and Adasaurus mongoliensis was found to be a member of the Velociraptorinae (Fig. 3). Another result of this study is the recovery of a clade containing 3 taxa from the Campanian of North America: Saurornitholestes langstoni, Atrociraptor marshalli, and Bambiraptor feinbergorum. This clade is here named Saurornitholestinae. Bambiraptor has been referred to the Microraptorinae in some studies (43), but our analysis shows that it is not a member of this clade. Although Bambiraptor resembles microraptorines in having a curved pubis, this resemblance is superficial: It is the pubic symphysis that is curved, not the pubic shaft (as is the case in Microraptorinae). Rather, the animal shares derived features with Saurornitholestes (prominent depression caudal to the accessory antorbital fenestra, ridge on the medial surface of the ischium). Saurornitholestinae was found to be the sister taxon of a clade consisting of Velociraptorinae, Dromaeosaurinae, and Deinonychus (Fig. 3). These taxa and the Saurornitholestinae form a monophyletic group (26–28, 30, 43, 44) of derived dromaeosaurids, here termed Eudromaeosauria.
Discussion and Conclusions
The recognition of Hesperonychus results in a remarkable extension of the temporal range of the Microraptorinae. Previously, the geologically youngest known microraptorines came from the Jiufotang Formation of northeastern China's Jehol Group (24) (≈120 Ma) (45). The discovery of Hesperonychus in the Dinosaur Park Formation (≈75 Ma) (29) therefore extends the range of the clade by ≈45 million years, more than half the length of the Cretaceous. It remains unknown whether Hesperonychus represents the persistence of a 4-winged morphology, as seen in Microraptor gui (24), or a flightless form, such as Sinornithosaurus (20). The latter seems more likely in light of the fact that Hesperonychus approached Sinornithosaurus in size. Surprisingly, there appears to have been little evolution in body size in the Microraptorinae during their long history. The discovery of Hesperonychus indicates that microraptorines continued to exploit the small predator niche for at least 50 million years, whereas the Eudromaeosauria continued to exploit the medium- and large-bodied predator niche over the same span of time (27). In this respect, the evolution of the Dromaeosauridae appears to have been surprisingly conservative.
Additionally, Hesperonychus is the first definitive member of the Microraptorinae known from North America. This results in a geographic range extension for the clade, and provides further evidence of an affinity between the dinosaur faunas of North America and Asia (46, 47).
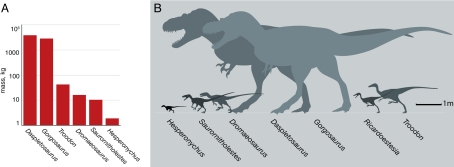
The discovery of Hesperonychus also alters our understanding of the predator community in the Late Cretaceous of North America. It now appears that North American carnivorous dinosaurs ranged widely in body size, from <2 kg to many tonnes, >4 orders of magnitude (Fig. 4). The large difference in size between Hesperonychus and other, contemporary dromaeosaurids is consistent with the suggestion that dinosaurian predators were highly segregated in terms of body size so as to reduce competition for prey (48).
Fig. 4.
Size of H. elizabethae and other carnivorous dinosaurs from the Dinosaur Park assemblage of Alberta. (A) Comparison of body mass of H. elizabethae and other carnivorous dinosaurs (a mass estimate is not given for Ricardoestesia, which is known only from teeth and dentaries). (B) Relative size of Hesperonychus and other predatory dinosaurs from the assemblage.
Despite the absence of associated skeletons, Hesperonychus may have been a relatively common animal in the Dinosaur Park fauna. The most common element, phalanx II-3, is represented by at least 10 specimens in the TMP collections. For the same element, Saurornitholestes is known from ≈30 isolated specimens; only 2 Dromaeosaurus specimens have been recognized. Therefore, considering that the small bones of Hesperonychus have a low preservation potential and that their size makes them easy to overlook in the field, fossils of this microraptorine are relatively common. It remains to be seen whether microraptorine elements will be recognized in other North American fossil assemblages.
Whereas Hesperonychus is diminutive when compared with contemporary theropods, by the standards of Mesozoic mammals it was a relatively large animal. The metatherian Eodelphis cutleri is perhaps the largest mammal in the Dinosaur Park assemblage, but it weighed just 600 g (49). Ironically, therefore, our discovery of a small theropod in the assemblage actually emphasizes the lack of substantial overlap in body size between dinosaurs and mammals in this fauna. The patterns seen in the Dinosaur Park assemblage are therefore consistent with the conventional wisdom that dinosaurian predation and competition limited the evolution of large body size in Mesozoic mammals, the existence of uncommon “giant” taxa such as Gobiconodontidae (50) notwithstanding. Relatively few Mesozoic mammals approached or exceeded 500–5,000 g (51), the size of the smallest nonavian dinosaurs, and few if any nonavian dinosaurs were <100 grams (24), the size of the average Mesozoic mammal (51). However, one could also argue that nonavian dinosaurs were precluded from evolving small body size by mammalian competition. Such interpretations are not mutually exclusive.
That the existence of Hesperonychus remained undetected until now, >100 years after the discovery of the Dinosaur Park Formation assemblage, underscores the degree to which preservational and collecting biases alter our picture of dinosaur-dominated faunas. The Late Cretaceous of the Western Interior boasts one of the world's richest and most intensively studied Mesozoic terrestrial fossil assemblages, but skeletons of small vertebrates are vanishingly rare (2, 3, 7). In contrast, isolated microfossils document the presence of a rich fauna of small, terrestrial vertebrates. This fauna included amphibians, squamates, birds, mammals (52–58), and, as shown here, small dromaeosaurids. Only a small percentage of these taxa are known from associated remains, demonstrating the existence of a strong taphonomic bias against the preservation of small vertebrate skeletons (2, 3, 59). Historically, this problem has been compounded by a collecting bias that has favored the recovery of large, relatively complete dinosaur skeletons. The existence of similar biases in other fossil assemblages has doubtless served to obscure the diversity, abundance, and ecological importance of the small-bodied members of the Dinosauria.
Materials and Methods
Mass was estimated for Hesperonychus by regressing mass estimates for Archaeopteryx and dromaeosaurids against pubis length by using ordinary least-squares (OLS) regression (see Dinosaur Mass Data and Equations in SI Appendix) to generate an allometric equation. Systematics of Hesperonychus were established by coding the holotype into a morphological data matrix (see Data Matrix in SI Appendix) of 114 characters (Taxa and Characters in SI Appendix) with 23 in-group taxa. The matrix includes previously published (26–28, 36, 43, 44, 60, 61) and heretofore unpublished characters. Four out-group taxa were used to polarize the characters. Out-group taxa used were the troodontids Troodon, Byronosaurus, and Sinovenator and the basal avian Archaeopteryx. Phylogenetic analysis was performed in PAUP* version 4b10 in branch and bound search mode. Five characters were ordered, and all characters were equally weighted. Tree statistics were calculated with uninformative characters excluded.
Supplementary Material
Acknowledgments.
We thank Clive Coy for his skillful preparation of the holotype. For discussions, we thank David Evans, Mark Norell, Anthony Russell, Jessica Theodor, Alan Turner, Xu Xing, and the University of Calgary Vertebrate Morphology Research Group. We also thank the anonymous reviewers whose suggestions have greatly improved this manuscript. We appreciate the assistance of James Gardner (Royal Tyrrell Museum of Palaeontology, Drumheller, AB, Canada), Farish Jenkins (Museum of Comparative Zoology, Cambridge, MA), Walter Joyce (Yale Peabody Museum, New Haven, CT), Scott Hartman (Wyoming Dinosaur Center, Thermopolis, WY), Mark Norell (American Museum of Natural History, New York), Ken Stadtman (Brigham Young University, Provo, UT), Brandon Strilisky (TMP), and Xing Xu (Institute of Vertebrae Paleontology and Paleoanthropology, Beijing, Peoples Republic of China) for specimen access and T. Miyashita (University of Alberta Laboratory for Vertebrate Paleontology, Edmonton) for assistance with collections. This work was supported by a National Science Foundation Graduate Research Fellowship, the Alberta Ingenuity Graduate Studentship, the University of Calgary Dean's Entry Grant (to N.R.L.), and Natural Sciences and Engineering Research Council Discovery Grant 203091-02 (to P.J.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0811664106/DCSupplemental.
References
- 1.Sereno PC. The origin and evolution of dinosaurs. Annu Rev Earth Planet Sci. 1997;25:435–489. [Google Scholar]
- 2.Brown B. The Hell Creek beds of the Upper Cretaceous of Montana: Their relation to contiguous deposits, with faunal and floral lists and a discussion of their correlation. Bull Am Mus Nat Hist. 1907;23:823–845. [Google Scholar]
- 3.Dodson P. Sedimentology and taphonomy of the Oldman Formation (Campanian), Dinosaur Provincial Park, Alberta (Canada) Palaeogeogr Palaeoclimatol Palaeoecol. 1971;10:21–74. [Google Scholar]
- 4.Ryan MJ, Russell AP. Dinosaurs of Alberta (exclusive of Aves) In: Tanke DH, Carpenter K, editors. Mesozoic Vertebrate Life. Bloomington, IN: Indiana Univ Press; 2001. pp. 279–297. [Google Scholar]
- 5.Russell D, Manabe M. Synopsis of the Hell Creek (uppermost Cretaceous) dinosaur assemblage. Geol Soc Am Spec Paper. 2002;361:169–176. [Google Scholar]
- 6.Weishampel DB, et al. In: Dinosaur Distribution. The Dinosauria. Second Ed. Weishampel DB, Dodson P, Osmólska H, editors. Berkeley, CA: Univ of California Press; 2004. pp. 517–606. [Google Scholar]
- 7.Currie PJ, Russell DA. The geographic and stratigraphic distribution of articulated and associated dinosaur remains. In: Currie PJ, Koppelhus EB, editors. Dinosaur Provincial Park: A Spectacular Ancient Ecosystem Revealed. Bloomington, IN: Indiana Univ Press; 2005. pp. 202–220. [Google Scholar]
- 8.Longrich NR. A new, large ornithomimid from the Dinosaur Park Formation of Alberta, Canada: Implications for the study of dissociated dinosaur remains. Palaeontology. 2008;51(4):983–997. [Google Scholar]
- 9.Hutchinson JR, Chiappe LM. The first known Alvarezsaurid (Theropoda: Aves) from North America. J Vertebr Paleontol. 1998;18(3):447–450. [Google Scholar]
- 10.Longrich NR, Currie PJ. Albertonykus borealis, a new alvarezsaur (Dinosauria: Theropoda) from the Early Maastrichtian of Alberta, Canada: Implications for the systematics and ecology of the Alvarezsauridae. Cretaceous Res. 2009;30:239–252. [Google Scholar]
- 11.Clemens WA., Jr . Marsupialia. In: Lilligraven JA, Kielan-Jaworowska , Clemens WA Jr, editors. Mesozoic mammals: The First Two-Thirds of Mammalian History. Berkeley, CA: Univ of California Press; 1979. pp. 192–220. [Google Scholar]
- 12.Kielan-Jaworowska Z, Cifelli RL, Luo Z-X. Mammals from the Age of Dinosaurs: Origins, Evolution and Structure. New York: Columbia Univ Press; 2004. p. 648. [Google Scholar]
- 13.Damuth J. Population density and body size in mammals. Nature. 1981;290:699–700. [Google Scholar]
- 14.Carbone C, Gittleman JL. A common rule for the scaling of carnivore density. Science. 2002;295:2273–2276. doi: 10.1126/science.1067994. [DOI] [PubMed] [Google Scholar]
- 15.Hutchinson GE, Macarthur RH. A theoretical ecological model of size distributions among species of animals. Am Nat. 1959;93:117–125. [Google Scholar]
- 16.Brown JH, Nicoletto PF. Spatial scaling of species composition: Body masses of North American land mammals. Am Nat. 1991;138(6):1478–1512. [Google Scholar]
- 17.Wagner A. New contributions concerning the ancient world's fauna from the lithographic slate; V. Compsognathus longipes Wagn. (Translated from German) Abhandlungen der Bayerischen Akademie der Wissenschaften. 1861;9:30–38. [Google Scholar]
- 18.Russell DA, Dong Z-M. A nearly complete skeleton of a new troodontid dinosaur from the Early Cretaceous of the Ordos Basin, Inner Mongolia, People's Republic of China. Can J Earth Sci. 1993;30:2163–2173. [Google Scholar]
- 19.Chen P, Dong Z, Zhen S. An exceptionally well preserved theropod dinosaur from the Yixian Formation of China. Nature. 1997;391:147–152. [Google Scholar]
- 20.Xu X, Wang X-L, Wu X-C. A dromaeosaurid dinosaur with filamentous integument from the Yixian Formation of China. Nature. 1999;401:262–266. [Google Scholar]
- 21.Xu X, Zhou Z, Wang X. The smallest known non-avian theropod dinosaur. Nature. 2000;408:705–708. doi: 10.1038/35047056. [DOI] [PubMed] [Google Scholar]
- 22.Hwang SH, Norell MA, Ji Q, Gao K. New specimens of Microraptor zhaoianus (Theropoda: Dromaeosauridae) from northeastern China. Am Mus Nov. 2002;3381:1–44. [Google Scholar]
- 23.Xu X, Norell MA, Wang X-L, Makovicky PJ, Wu X-C. A basal troodontid from the Early Cretaceous of China. Nature. 2002;415:780–784. doi: 10.1038/415780a. [DOI] [PubMed] [Google Scholar]
- 24.Xu X, et al. Four winged dinosaurs from China. Nature. 2003;421:335–340. doi: 10.1038/nature01342. [DOI] [PubMed] [Google Scholar]
- 25.Xu X. A new dromaeosaur (Dinosauria: Theropoda) from the Early Cretaceous Yixian Formation of Western Liaoning. Vertebr PalAsiatica. 2004;42(2):111–119. [Google Scholar]
- 26.Makovicky PJ, Apestegula S, Agnolin FL. The earliest dromaeosaurid theropod from South America. Nature. 2005;437:1007–1011. doi: 10.1038/nature03996. [DOI] [PubMed] [Google Scholar]
- 27.Turner AH, Hwang SH, Norell MA. A small derived theropod from Öösh, Early Cretaceous, Baykhangor Mongolia. Am Mus Nov. 2007;3557:1–27. [Google Scholar]
- 28.Turner AH, Pol D, Clarke J, Erickson GM, Norell M. A basal dromaeosaurid and size evolution preceding avian flight. Science. 2007;317:1378–1381. doi: 10.1126/science.1144066. [DOI] [PubMed] [Google Scholar]
- 29.Eberth DA. The geology. In: Currie PJ, Koppelhus EB, editors. Dinosaur Provincial Park: A Spectacular Ancient Ecosystem Revealed. Bloomington, IN: Indiana Univ Press; 2005. pp. 54–82. [Google Scholar]
- 30.Norell MA, Makovicky PJ. Dromaeosauridae. In: Weishampel DB, Dodson P, Osmólska H, editors. The Dinosauria. Berkeley, CA: Univ of California Press; 2004. pp. 196–209. [Google Scholar]
- 31.Matthew WD, Brown B. The family Deinodontidae, with notice of a new genus from the Cretaceous of Alberta. Bull Am Mus Nat Hist. 1922;66:367–385. [Google Scholar]
- 32.Sues H.-D. A new small theropod dinosaur from the Cretaceous of Alberta. Zool J Linnean Soc. 1978;62:381–400. [Google Scholar]
- 33.Norell MA, Makovicky PJ. Important features of the dromaeosaur skeleton: Information from a new specimen. Am Mus Nov. 1997;3215:1–28. [Google Scholar]
- 34.Norell MA, Makovicky PJ. Important features of the dromaeosaurid skeleton II: Information from newly collected specimens of Velociraptor mongoliensis. Am Mus Nov. 1999;3282:1–45. [Google Scholar]
- 35.Novas FE, Puerta PF. Evidence concerning avian origins from the Late Cretaceous of Patagonia. Nature. 1997;387:390–392. [Google Scholar]
- 36.Novas FE. Avian traits in the ilium of Unenlagia comahuensis (Maniraptora, Avialae) In: Currie PJ, Koppelhus EB, Shugar MA, Wright JL, editors. Feathered Dragons: Studies on the Transition From Dinosaurs to Birds. Bloomington, IN: Indiana Univ Press; 2004. pp. 301–342. [Google Scholar]
- 37.Wellnhofer P. Archaeopteryx: The Ancient Bird of Solnhofen (Translated from German) Munich: Verlag Dr. Friedrich Pfeil; 2008. pp. 1–256. [Google Scholar]
- 38.Longrich N. Structure and function of hindlimb feathers in Archaeopteryx lithographica. Paleobiology. 2006;32(3):417–431. [Google Scholar]
- 39.Forster CA, Sampson SD, Chiappe LM, Krause DW. The theropodan ancestry of birds: New evidence from the Late Cretaceous of Madagascar. Science. 1998;279:1915–1919. doi: 10.1126/science.279.5358.1915. [DOI] [PubMed] [Google Scholar]
- 40.Ostrom JH. Osteology of Deinonychus antirrhopus, an unusual theropod from the Lower Cretaceous of Montana. Peabody Mus Nat Hist Bull. 1969;30:1–165. [Google Scholar]
- 41.Kirkland JI, Gaston R, Burge D. A large dromaeosaur (Theropoda) from the Lower Cretaceous of Eastern Utah. Hunteria. 1993;2:1–16. [Google Scholar]
- 42.Burnham DA. New information on Bambiraptor feinbergi (Theropoda: Dromaeosauridae) from the Late Cretaceous of Montana. In: Currie PJ, Koppelhus EB, Shugar MA, Wright JL, editors. Feathered Dragons: Studies on the Transition from Dinosaurs to Birds. Indianapolis, IN: Indiana Univ Press; 2004. pp. 67–111. [Google Scholar]
- 43.Senter P. A new look at the phylogeny of Coelurosauria (Dinosauria: Theropoda) J Syst Palaeontol. 2007;5(4):1–35. [Google Scholar]
- 44.Novas FE, Pol D, Canale JI, Porfiri JD, Calvo JO. A bizarre Cretaceous theropod dinosaur from Patagonia and the evolution of Gondwanan dromaeosaurids. Proc R Soc B. 2008 doi: 10.1098/rspb. 2008.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He HY, et al. Timing of the Jiufotang Formation (Jehol Group) in Liaoning, northeastern China, and its implications. Geophys Res Lett. 2004;31:1–4. [Google Scholar]
- 46.Russell DA. The role of Central Asia in dinosaurian biogeography. Can J Earth Sci. 1993;30:2002–2012. [Google Scholar]
- 47.Holtz TR, Chapman RE, Lamanna MC. Mesozoic biogeography of Dinosauria. In: Weishampel DB, Dodson P, Osmólska H, editors. The Dinosauria. Second Ed. Berkeley, CA: Univ of California Press; 2004. pp. 627–642. [Google Scholar]
- 48.Van Valkenburgh B, Molnar RE. Dinosaurian and mammalian predators compared. Paleobiology. 2002;28(4):527–543. [Google Scholar]
- 49.Gordon CL. A first look at estimating body size in dentally conservative marsupials. J Mamm Evol. 2003;10(1/2):1–21. [Google Scholar]
- 50.Hu Y, Meng J, Wang Y, Li C. Large Mesozoic mammals fed on young dinosaurs. Nature. 2005;433:149–152. doi: 10.1038/nature03102. [DOI] [PubMed] [Google Scholar]
- 51.Alroy J. Cope's Rule and the dynamics of body mass evolution in North American fossil mammals. Science. 1998;280:731–734. doi: 10.1126/science.280.5364.731. [DOI] [PubMed] [Google Scholar]
- 52.Estes R. Fossil vertebrates from the Late Cretaceous Lance Formation, Eastern Wyoming. Univ California Pub Dept Geol Sci. 1964;49:1–180. [Google Scholar]
- 53.Sahni A. The vertebrate fauna of the Judith River Formation, Montana. Bull Am Mus Nat Hist. 1972;147(6):323–412. [Google Scholar]
- 54.Brinkman DB. Paleoecology of the Judith River Formation (Campanian) of Dinosaur Provincial Park, Alberta, Canada: Evidence from vertebrate microfossil localities. Palaeogeogr Palaeoclimatol Palaeoecol. 1990;78:37–54. [Google Scholar]
- 55.Peng J, Russell AP, Brinkman DB. Vertebrate microsite assemblages (exclusive of mammals) from the Foremost and Oldman Formations of the Judith River Group (Campanian) of Southeastern Alberta: An illustrated guide. Prov Mus Alberta Nat Hist Occasional Paper. 2001;25:1–54. [Google Scholar]
- 56.Caldwell MW. The squamates: Origins, phylogeny, and paleoecology. In: Currie PJ, Koppelhus EB, editors. Dinosaur Provincial Park: A Spectacular Ancient Ecosystem Revealed. Bloomington, IN: Indiana Univ Press; 2005. pp. 235–248. [Google Scholar]
- 57.Longrich NR. An ornithurine-dominated avifauna from the Belly River Group (Campanian, Upper Cretaceous) of Alberta, Canada. Cretaceous Research. 2009;30:161–177. [Google Scholar]
- 58.Sankey JT, Brinkman DB, Fox RC, Eberth DA. Patterns of distribution of mammals in the dinosaur park formation and their paleobiological significance. In: Currie PJ, Koppelhus EB, editors. Dinosaur Provincial Park: A Spectacular Ancient Ecosystem Revealed. Bloomington: Indiana Univ Press; 2005. pp. 437–449. [Google Scholar]
- 59.Dodson P. A faunal review of the Judith River (Oldman) Formation, Dinosaur Provincial Park, Alberta. Mosasaur. 1983;1:89–118. [Google Scholar]
- 60.Currie PJ. New information on the anatomy and relationships of Dromaeosaurus albertensis (Dinosauria: Theropoda) J Vertebr Paleontol. 1995;15(3):576–591. [Google Scholar]
- 61.Currie PJ, Varricchio DJ. A new dromaeosaurid from the Horseshoe Canyon Formation (Upper Cretaceous) of Alberta, Canada. In: Currie PJ, Koppelhus EB, Shugar MA, Wright JL, editors. Feathered Dragons. Indianapolis: Indiana Univ Press; 2004. pp. 112–132. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.