The inner ear's sensory cells, the hair cells, mediate mechanotransduction, which enables us to experience sound and movements of the head. Protruding from the apical portion of a hair cell, a hair bundle is formed from actin-rich stereocilia, which are arrayed with increasing length in a staircase formation; mechanical stimuli that deflect the bundle toward the tallest stereocilia open cation-selective transduction channels and initiate mechanotransduction. Integral for the sensing of mechanical tension, a filamentous structure called the tip link spans from the tip of one stereocilium to the side of the next taller one. The molecular identity of the tip link has been hotly debated by the hair-cell field, and the article by Schwander et al. (1) in this issue of PNAS provides a significant step toward a final settlement of this issue. To appreciate the full ramifications of this study and understand the different layers of possible conclusions, it makes sense to step back and recall what we know about tip links.
A mechanical stimulus such as sound is transmitted within the inner ear to hair cells and causes the bundle to tilt back and forth. The stereocilia are arranged so that a tilt in the excitatory direction causes the tip links to be pulled taut, at which point transduction channels at the base of the tip link (2) open and allow cations to flow into the hair cell (Fig. 1). Genetic evidence in zebrafish and mouse (3–6), in combination with localization and in vitro interaction studies (7), suggested that 2 extracellular adhesion proteins, cadherin 23 (CDH23) and protocadherin 15 (PCDH15), constitute the tip link. According to this model, the tip link is made up of a parallel dimer of PCDH15 on the lower end of a tip link and an equivalent dimer of CDH23 at the upper end, with the 2 homodimers binding to each other through their most distal cadherin domains. However, final conclusive proof that CDH23 and PCDH15 comprise the tip link is still missing. In the case of CDH23, one of the main reasons for skepticism derives from the fact that the protein is also involved in the development of the hair bundle, as evidenced by disorganized stereocilia in various mouse CDH23 mutants. Hence, hearing loss and lack of mechanotransduction in a CDH23 mutant animal does not necessarily prove a direct involvement of CDH23 in mechanotransduction, the legitimate argument being that the entire hair bundle never developed sufficiently to allow mechanotransduction in the first place.
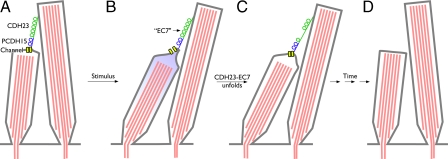
Fig. 1.
Disruption of tip links in salsa mutant. (A) Hair-cell transduction channels are gated by the tip links, formed of PCDH15 and CDH23. At rest, channels are mostly closed. (B) Mechanical stimulus increases tip-link tension and opens channels. (C) In salsa mutant, EC7 unfolds and channels close prematurely. (D) Over time, this mutant mysteriously leads to loss of tip links and, later, degeneration of hair cells.
One way to tackle this problem would be experimentally separate the role of CDH23 in development from its putative role in mechanotransduction. The study by Schwander et al. (1) delivers exactly that.
The salsa mouse strongly supports the role of CDH23 in mature hearing.
In a large-scale recessive chemical mutagenesis screen, Schwander et al. (1) fished out the salsa mouse, which harbors a mutation in the CDH23 gene that causes progressive hearing loss. In contrast to all other mouse CDH23 mutants, the salsa mouse has normally developed hair cells and hair bundles until postnatal day (P) 28; any defects in hair cell mechanotransduction therefore cannot be blamed on a defective development.
Sequence analysis revealed that salsa mice bear a novel point mutation in the seventh extracellular cadherin domain of the CDH23 gene, impairing the ability of this domain, and this domain only, to bind calcium. CDH23 has 27 of these cadherin domains, of which only the first interacts with PCDH15 (7). It is therefore not surprising that the effect of a mutation in the seventh cadherin domain is subtle and has no effect on hair bundle morphogenesis. Although the hair bundle appears normal until P28, tip links and CDH23 immunoreactivity nevertheless start to disappear after P10, accompanied by increased threshold for hearing. These mice eventually become completely deaf.
To understand the phenotype of the salsa mouse, we have to understand the effects of a cadherin-domain mutation. We know from studies with classical cadherins that such mutations can lead to a loss in adhesion (8), perhaps caused by destabilization of the cadherin domain. Moreover, the effects of such mutations are likely exacerbated by mechanical stress (9). Based on this observation, we suggest the following plausible scenario for the progressive loss of tip links in salsa mice. Initially, salsa mice form essentially normal tip links. However, when the hair bundle is exposed to sound-induced mechanical stress, after development of the entire peripheral auditory system, salsa tip links cannot withstand the mechanical strain and rupture more frequently than those in their WT counterparts. There is some evidence for that suggestion in the electrophysiological recordings; immediately after mechanical stimulation, hair cells in salsa mice at P7 have normal transduction-current amplitudes (1). However, channels close more rapidly in salsa mice, particularly after large stimuli, which might have occurred if the mutated cadherin repeat had unfolded. Perhaps unfolded cadherin repeats allow tip links to come apart. Moreover, biochemical evidence suggests that CDH23 with the salsa mutation has a lower affinity for PCDH15 (1), suggesting that the tip links might more readily rupture upon repeated mechanical stimulation.
Exposure to loud sound causes temporary hearing loss in humans, which may be in part caused by breakage of tip links (10, 11). A healthy hair cell recovers from such a trauma by simply rerigging the tip link, but what happens in salsa mice? Perhaps tip links break substantially more often. The extensive loss of hair cells caused by apoptosis at later stages raises the possibility of a connection between tip-link regeneration and cell death. Frequent rupture of tip links is likely answered by the hair cell with an ongoing effort to regenerate the tip links; in the salsa mice, however, it is as if one tries to repair a machine with a faulty component that caused the machine's malfunction in the first place. It will break again. The hair cell might continue its efforts until it ultimately collapses under the stress caused by these futile efforts. It seems plausible that the hearing loss described in the salsa mutant arises from an overlap of initial loss of mechanotransduction caused by tip link rupture and the permanent loss of hearing caused by the irreparable loss of hair cells (Fig. 1).
The above-mentioned model is also supported by the finding that vestibular function in the salsa mice is not affected. Both hearing and balance rely on hair cell function, but auditory hair cells are exposed to far higher frequencies, i.e., up to 50 kHz, making those tip links more likely to break. Vestibular hair cells work at very low frequencies, likely minimizing the destabilizing and ultimately devastating effect of this mutation. In agreement with this model, Schwander et al. (1) also find that tip link loss starts in the basal, high-frequency portion of the cochlea. Future electrophysiological experiments should examine whether high-frequency stimuli can cause transducer currents to decline more rapidly in salsa mice.
By cleanly distinguishing the developmental and mechanotransduction roles of CDH23, the salsa mouse strongly supports the role of CDH23 in mature hearing. Furthermore, it suggests a plausible interpretation of progressive hearing loss, where an overactive regeneration effort of the hair cell leads to a disastrous meltdown of the entire system. This concept is especially relevant considering the fact that the human deafness syndrome DFNB12 is caused by a similar mutation in the CDH23 gene, also displaying progressive hearing loss. The salsa mouse is therefore an excellent model in which to tackle progressive hearing loss in humans.
Acknowledgments.
Work in P.G.G.'s laboratory is supported by National Institutes of Health Grants R01 DC002368, R01 DC007602, R21 DC008801, and P30 DC005983. J.-B.S. is supported by National Institutes of Health Grant K99/R00 DC009412.
Footnotes
The authors declare no conflict of interest.
See companion article on page 5252.
References
- 1.Schwander M, et al. A mouse model for nonsyndromic deafness (DFNB12) links hearing loss to defects in tip links of mechanosensory hair cells. Proc Natl Acad Sci USA. 2009;106:5252–5257. doi: 10.1073/pnas.0900691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beurg M, Fettiplace R, Nam J-H, Ricci AJ. Localization of inner hair cell mechanotransducer channels using high-speed calcium imaging. Nat Neurosci. 2009 doi: 10.1038/nn.2295. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bolz H, et al. Mutation of CDH23, encoding a new member of the cadherin gene family, causes Usher syndrome type 1D. Nat Genet. 2001;27:108–112. doi: 10.1038/83667. [DOI] [PubMed] [Google Scholar]
- 4.Di Palma F, et al. Mutations in Cdh23, encoding a new type of cadherin, cause stereocilia disorganization in waltzer, the mouse model for Usher syndrome type 1D. Nat Genet. 2001;27:103–117. doi: 10.1038/83660. [DOI] [PubMed] [Google Scholar]
- 5.Siemens J, et al. Cadherin 23 is a component of the tip link in hair-cell stereocilia. Nature. 2004;428:950–955. doi: 10.1038/nature02483. [DOI] [PubMed] [Google Scholar]
- 6.Sollner C, et al. Mutations in cadherin 23 affect tip links in zebrafish sensory hair cells. Nature. 2004;428:955–959. doi: 10.1038/nature02484. [DOI] [PubMed] [Google Scholar]
- 7.Kazmierczak P, et al. Cadherin 23 and protocadherin 15 interact to form tip-link filaments in sensory hair cells. Nature. 2007;449:87–91. doi: 10.1038/nature06091. [DOI] [PubMed] [Google Scholar]
- 8.Prakasam A, Chien YH, Maruthamuthu V, Leckband DE. Calcium site mutations in cadherin: Impact on adhesion and evidence of cooperativity. Biochemistry. 2006;45:6930–6939. doi: 10.1021/bi060213m. [DOI] [PubMed] [Google Scholar]
- 9.Sotomayor M, Schulten K. The allosteric role of the Ca2+ switch in adhesion and elasticity of C-cadherin. Biophys J. 2008;94:4621–4633. doi: 10.1529/biophysj.107.125591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Husbands JM, Steinberg SA, Kurian R, Saunders JC. Tip-link integrity on chick tall hair cell stereocilia following intense sound exposure. Hear Res. 1999;135:135–145. doi: 10.1016/s0378-5955(99)00101-x. [DOI] [PubMed] [Google Scholar]
- 11.Zhao Y, Yamoah EN, Gillespie PG. Regeneration of broken tip links and restoration of mechanical transduction in hair cells. Proc Natl Acad Sci USA. 1996;93:15469–15474. doi: 10.1073/pnas.93.26.15469. [DOI] [PMC free article] [PubMed] [Google Scholar]