Abstract
We develop and validate a density functional, XYG3, based on the adiabatic connection formalism and the Görling–Levy coupling-constant perturbation expansion to the second order (PT2). XYG3 is a doubly hybrid functional, containing 3 mixing parameters. It has a nonlocal orbital-dependent component in the exchange term (exact exchange) plus information about the unoccupied Kohn–Sham orbitals in the correlation part (PT2 double excitation). XYG3 is remarkably accurate for thermochemistry, reaction barrier heights, and nonbond interactions of main group molecules. In addition, the accuracy remains nearly constant with system size.
Keywords: Becke 3-parameter hybrid functional combined with Lee–Yang–Parr correlation functional, density functional theory, generalized gradient approximation, local density approximation, mean absolute deviation
Density functional theory (DFT) has revolutionized the role of theory by providing accurate first-principles predictions of critical properties for applications in physics, chemistry, biology, and materials science (1). Despite dramatic successes, there remain serious deficiencies, for example, in describing weak interactions (London dispersion), which are so important to the packing of molecules into solids, the binding of drug molecules to proteins, and the magnitude of reaction barriers. We propose here a DFT functional that dramatically improves the accuracy for these properties by including the role of the virtual (unoccupied) states.
Solution of the Schrödinger equation leads to the wavefunction, ψ(r1, r2, …, rN) (2), which depends on the 3N space coordinates and N spin coordinates of N-electrons in the system. Solving for such a wavefunction usually starts with the Hartree–Fock (HF) mean field description involving N self-consistent 1-particle spin-orbitals (in a Slater determinant), which is then used as the basis for expanding the wavefunction in a hierarchy of excited N-electron configurations, by using methods referred to as Møller–Plesset theory (e.g., MP2, MP3, MP4), couple-cluster theory (e.g., CCSD(T)), and quadratic configuration interaction theory (e.g., QCISD(T)), etc. These methods are ab initio but suffer from problems of slow convergence with the size of the basis sets and the configuration expansion lengths, preventing scaling to large systems.
In contrast, DFT is formulated in terms of the 1-particle density, ρ(r), depending on only 3 spatial coordinates rather than 3N, as the fundamental quantity (3, 4). This dramatically simplifies the process of calculating the structures and properties. However, the exact form of the functional, whose solution will lead to the correct density, is not known. Even so, there has been an evolution of successively better approximations to this functional, that has already provided quite good accuracy for many problems (5–15).
Perdew (16) has formulated the hierarchy of DFT approximations as a “Jacob's ladder” rising from the “earth of Hartree” to the “heaven of chemical accuracy.” The first rung of this ladder is the local (spin) density approximation [LDA, e.g., SVWN (4, 5)] and the second rung is the generalized gradient approximation [GGA, e.g., BLYP (6, 7) and PBE (8)]. Although LDA uses densities ρ(r) as local ingredients, GGA employs both the local densities and their gradients ▿ρ(r). The third rung is termed metaGGA [e.g., TPSS (9)], which expands GGA to include further the kinetic energy density τ, and/or the Laplacian of the density ▿2ρ(r). Up to this third rung, they are all local and multiplicative.
The fourth rung of DFT is a hybrid that introduces nonlocality by replacing some portion of the local exchange energy density with the exact (HF-like) exchange energy density. The most popular such hyperGGA flavor is B3LYP (5–7, 10), which has been shown to provide accurate predictions for thermochemistry of small covalent systems (11). However, B3LYP is poor for the predictions of noncovalent bonding interactions (15) and reaction barrier heights (14), with performance degrading dramatically as system sizes increase (12, 13).
The final fifth rung of Jacob's ladder utilizes the unoccupied Kohn–Sham (KS) orbitals (16) in addition to the occupied KS orbitals. This final rung is expected to allow the heaven of chemical accuracy to be achieved for broad applications. However, no such functional based on first principles (17) and practical for general use has been proposed. Empirical versions (18) have led to promising results for thermochemistry and reaction barriers, but they still fail to account for van der Waals interactions.
Here, we develop a fifth-rung functional that incorporates information about the unoccupied KS orbitals [based on the Görling–Levy coupling-constant perturbation expansion to the second order (19)], along with 3 empirical mixing parameters. We demonstrate that this functional is highly accurate for thermochemistry, reaction barriers, and nonbond interactions.
Theory
DFT was placed on a firm theoretical footing by the Hohenberg–Kohn (HK) theorems (3). These HK theorems prove that there exists a total energy functional E[ρ], from which one can obtain the ground state electron density ρ0 by minimizing E[ρ] with respect to the density ρ,
where ρ0 contains all information that can be known about the electronic structure of the system. However, the HK theorems do not specify this true total energy functional.
The most popular implementation of DFT is through the KS method (4), which assumes a noninteracting N-electron system having the same density as the original many-body system. The KS wavefunction can be expressed exactly as a Slater determinant leading to an exact form for the kinetic energy Ts of the noninteracting system and the classic Coulomb energy U. The total energy is then expressed as
where Vext is the external potential energy, and Exc is the exchange-correlation energy, which remains unknown.
The adiabatic connection formalism (10, 20–25) provides a rigorous way to define Exc. It assumes an adiabatic path between the fictitious noninteracting KS system (λ = 0) and the physical system (λ = 1) while holding the electron density ρ fixed at its physical λ = 1 value for all λ of a family of partially interacting N-electron systems:
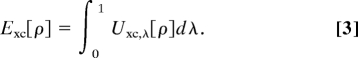 |
Uxc,λ is the potential energy of exchange correlation at intermediate coupling strength λ. The only problem is that the exact integrand Uxc,λ is unknown.
Becke first used this formalism as a practical tool for functional construction (10, 23) by assuming a linear model (23)
and taking Uxc,λ=0 = Exexact, the exact exchange of the KS orbitals, and approximating Uxc,λ=0 ≈ Uxc,λ=0LDA. Becke's half-and-half functional (23) may be approximated by
where we have partitioned ExcLDA = ExLDA + EcLDA and set
The popular Becke's 3-parameter functional modifies Eq. 5 empirically to obtain Eq. 7 (10):
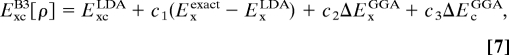 |
where ΔExGGA is the gradient-containing correction terms to the LDA exchange and ΔEcGGA is the gradient-containing correction to the LDA correlation, whereas {c1,c2,c3} are constants fitted against selected experimental thermochemical data. The success of Eq. 7 in achieving high accuracy demonstrates that errors of ExcDFT for covalent bonding arise principally from the λ → 0 or exchange limit, making it important to introduce some portion of exact exchange (10, 23–25).
An alternative to fixing the {a,b} parameters in Eq. 4 is to use the Görling–Levy theory of coupling-constant perturbation expansion (19), in which the initial slope (U′xc,λ=0) is defined by the second-order correlation energy:
We may define EcGL2 as (19):
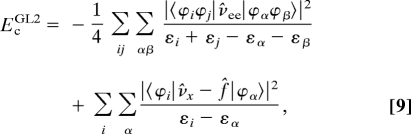 |
where ν̂ee is the electron–electron repulsion operator, ν̂x is the local exchange operator, and f̂ is the Fock-like, nonlocal exchange operator. We may calculate EcGL2 from the KS orbitals with eigenvalues ε, where the subscripts (i, j) and (α, β) denote the occupied and unoccupied KS orbitals, respectively.
Combining Eq. 8 with Eq. 4 leads to:
Eqs. 6 and 10 lead to 2 choices of b, which we combine using empirical parameters, {b1,b2}, to optimize the functional performance:
In principle, EcDFT ≈ (ExcDFT − Exexact) contains a complete description of correlation effects, so that the second term of Eq. 11 may be interpreted as a way to extrapolate the second-order perturbation to infinite order. Hence, we propose to use an empirical formula of the form:
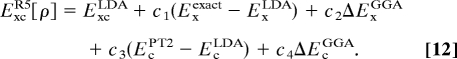 |
In comparison with the Becke 3-parameter scheme (10) of Eq. 7, Eq. 12 is a doubly hybrid DFT that mixes some exact exchange into ExDFT while also introducing a certain portion of EcPT2 into EcDFT. Here, EcPT2contains the double-excitation contributions of EcGL2 (i.e., the first term in Eq. 9). The single-excitation contributions in EcGL2may not be zero, but we absorb them into EcDFT and in the fitting parameters. Eq. 12 presents a fifth-rung functional (R5) that embodies information from both the occupied and the unoccupied KS orbitals as shown in Eq. 9.
In our current applications to test this functional, we calculate the B3LYP wavefunction and use the B3LYP orbitals as the KS orbitals to generate the density and to evaluate the PT2 term. Instead, the original GL2 perturbation theory (19) uses KS orbitals generated from a local exchange-correlation potential (see Eq. 9). Ref. 26 has shown that B3LYP densities are similar to those from CCSD(T) ab initio wavefunctions (for the molecules discussed in ref. 26). Nevertheless, the eigenvalues from B3LYP, whose potential is nonlocal, might differ considerably from those of the KS orbitals obtained from a local potential. Thus, it could be better to use some different set of KS orbitals.
Here, we adopt the LYP correlation functional but constrain c4 = (1 − c3) in Eq. 12. This constraint is not necessary, but it eliminates 1 fitting parameter while excluding compensation from the LDA correlation term. The final 3 parameters {c1,c2,c3} are determined empirically by fitting only to thermochemical data of the G3/99 set, leading to:
We denote this generalized 3-parameter functional as XYG3.
Results and Discussion
Heats of Formation (Thermochemistry).
The Gn paradigm developed by Pople and coworkers provides a hierarchy for extrapolating levels of correlation and basis sets to obtain increasingly accurate thermochemistry (11, 12, 27). To adjust the empirical constants in Gn, they developed a database (DB) of accurate experimental heats of formation that are valuable for developing functionals to describe covalent bonding in the main group molecules. In particular, we use the G3 DB of 223 molecules collected in 1999 (the G3/99 set) (12).
Using XYG3 with the 6-311+G(3df,2p) basis set to calculate the heats of formation of the G3/99 set leads to a mean absolute deviation (MAD) of 1.81 kcal/mol, substantially better than any other DFT methods (Table 1). For comparison, B3LYP leads to MAD = 4.74 kcal/mol. Indeed the G3 method gives MAD = 1.05, whereas G2 gives MAD = 1.88 kcal/mol but at far higher computational cost.
Table 1.
Accuracy of various QM methods for predicting standard enthalpies of formation (Δ fH2980, kcal/mol) for the experimental data of 223 molecules in the G3/99 set
| Functional | MAD | Max (+) | Max (−) |
|---|---|---|---|
| DFT | |||
| XYG3* | 1.81 | 16.67 (SF6) | −6.28 (BCl3) |
| M06-2X* | 2.93 | 20.77 (O3) | −17.39 (P4) |
| M06* | 4.17 | 11.25 (O3) | −25.89 (C2F6) |
| B2PLYP* | 4.63 | 20.37 (n-octane) | −8.01 (C2F4) |
| B3LYP* | 4.74 | 19.22 (SF6) | −8.03 (BeH) |
| M06-L* | 5.82 | 14.75 (PF5) | −27.13 (C2Cl4) |
| BLYP† | 9.49 | 41.0 (C8H18) | −28.1 (NO2) |
| PBE† | 22.22 | 10.8 (Si2H6) | −79.7 (azulene) |
| LDA† | 121.85 | 0.4 (Li2) | −347.5 (azulene) |
| Ab initio | |||
| HF* | 211.48 | 582.72 (n-octane) | −0.46 (BeH) |
| MP2* | 10.93 | 29.21 (Si(CH3)4) | −48.34 (C2F6) |
| QCISD(T)‡ | 15.22 | 42.78 (n-octane) | −1.44 (Na2) |
| G2‡ | 1.88 | 7.2 (SiF4) | −9.4 (C2F6) |
| G3‡ | 1.05 | 7.1 (PF5) | −4.9 (C2F4) |
MADs, in kcal/mol, with the largest positive error (Max(+) energy too high) and the largest negative error (Max(−) energy too low).
*The geometries were optimized by using B3LYP with the 6-311+G(d,p) basis set. Analytical vibrational frequencies were calculated at the same level and scaled by 0.9877 to estimate zero-point energies. Spin-orbit corrections are included. Single point calculations are performed with the 6-311+G(3df,2p) basis set.
†Data are from ref. 28 and computed by using B3LYP/6-311+G(3df,3pd). The geometries were optimized by using B3LYP/6-31G(2df,p). Analytical vibrational frequencies were calculated at the same level and scaled by 0.9854 to estimate zero-point energies.
‡Data are from ref. 29. The QCISD(T) results were obtained by removing the empirical ″high-level corrections″ from the G3 theory to approximate the results of QCISD(full,T)/6-311+G (3d2f,2df,2p) by a series of extrapolations in both the 1-particle and many-particle spaces.
A recent important development in DFT is the M06 family of functionals (M06, M06-2X, M06-HF, and M06-L) (14, 30), which currently provides the highest accuracy with a broad applicability for chemistry. M06, M06-2X, M06-HF are hybrid methods, whereas M06-L is a pure DFT. For the G3/99 set, these methods lead to MAD = 4.17 kcal/mol for M06, 2.93 for M06-2x, and 5.82 for M06-L.
B2PLYP is also a doubly hybrid functional that incorporates a perturbation correction as in Eq. 12, but with different parameters of {c1 = 0.53, c2 = 0.47, c3 = 0.27} (18, 31). The first 2 parameters for the exchange part are normalized to 1.0, which reduces the number of independent fitting parameters to 2. The salient difference between B2PLYP and XYG3 is that B2PLYP employs the DFT portion of Eq. 12 to generate the density used to calculate the DFT energy and orbitals from which the PT2 correction is computed. Such a truncated DFT may give density and orbitals that are dramatically different from the real ones. Thus, using just the DFT portion of B2PLYP leads to MAD = 174.2 kcal/mol for the G3/99, whereas the complete B2PLYP method leads to MAD = 4.63 kcal/mol (with our present basis set). Because the doubly hybrid functionals are rooted within the adiabatic connection theorem (20, 21) and the Görling–Levy theory of coupling-constant perturbation expansion (19), we consider it very important to have accurate KS orbitals to provide an accurate density and the zero-order approximation for perturbation theory.
The G3/99 set consists of 3 subsets of molecules: G2–1 with 56 molecules having up to 3 heavy atoms, G2–2 with 92 additional molecules up to 6 heavy atoms, and G3–3 with 75 additional molecules up to 10 heavy atoms. B3LYP leads to errors that increase dramatically with size (12, 13), with MAD = 2.12 kcal/mol (G2-1), 3.69 (G2-2), and 8.97 (G3-3). B2PLYP [at the 6-311+G(3df,2p) level] does not improve over B3LYP, leading to MADs of 1.85 (G2-1), 3.70 (G2-2) and 7.83 kcal/mol (G3-3). M06-L gives MADs of 3.76 (G2-1), 5.71 (G2-2) and 7.50 kcal/mol (G3-3). This is significantly improved by M06-2X, which includes a doubled portion of exact exchange, leading to MADs of 1.89 (G2-1), 3.22 (G2-2), and 3.36 (G3-3) kcal/mol. For XYG3, we obtain MADs of 1.52 (G2-1), 1.79 (G2-2), and 2.06 kcal/mol (G3-3), which exhibits the best description for larger molecules.
Reaction Barrier Heights.
Zhao and Truhlar compiled several benchmark DBs of barrier heights in 2004 (14, 15, 33), including forward and reverse barrier heights for 19 hydrogen transfer (HT) reactions, 6 heavy-atom transfer (HAT) reactions, 8 nucleophilic substitution (NS) reactions, and 5 unimolecular and association (UM) reactions. We used the 6-311+G(3df,2p) basis set to compute the barriers (see Table 2). Geometries and reference energies were taken from the Truhlar DB web site (14, 15, 33).
Table 2.
Accuracy of various QM methods for energy barriers
| Functional | All (76) | HT38 | HAT12 | NS16 | UM10 |
|---|---|---|---|---|---|
| DFT | |||||
| XYG3* | 1.02 | 0.75 | 1.38 | 1.42 | 0.98 |
| M06-2X† | 1.20 | 1.13 | 1.61 | 1.22 | 0.92 |
| B2PLYP* | 1.94 | 1.81 | 3.06 | 2.16 | 0.73 |
| M06† | 2.13 | 2.00 | 3.38 | 1.78 | 1.69 |
| M06-L† | 3.88 | 4.16 | 5.93 | 3.58 | 1.86 |
| B3LYP* | 4.28 | 4.23 | 8.49 | 3.25 | 2.02 |
| BLYP† | 8.23 | 7.52 | 14.66 | 8.40 | 3.51 |
| PBE† | 8.71 | 9.32 | 14.93 | 6.97 | 3.35 |
| LDA‡ | 14.88 | 17.72 | 23.38 | 8.50 | 5.90 |
| Ab initio | |||||
| HF‡ | 11.28 | 13.66 | 16.87 | 6.67 | 3.82 |
| MP2‡ | 4.57 | 4.14 | 11.76 | 0.74 | 5.44 |
| QCISD(T)‡ | 1.10 | 1.24 | 1.21 | 1.08 | 0.53 |
MADs in kcal/mol for the 76 reactions in the Truhlar database web site (14, 15, 33), which contains the best available ab initio reference data for reaction barrier heights. Here, HT38 refers to the forward and reverse barrier heights for 19 hydrogen transfer reactions; HAT12 refers to the forward and reverse barrier heights for 6 heavy-atom transfer reactions, NS16 refers to the forward and reverse barrier heights for 8 nucleophilic substitution reactions, and UM10 refers to the forward and reverse barrier heights for 5 association and unimolecular reactions.
*Our calculations used the 6-311+G(3df,2p) basis sets with geometries from the Truhlar database web site.
‡Data are from ref. 33.
DFT methods usually underestimate reaction barrier heights. Table 2 shows MAD errors (kcal/mol) of 14.88 (LDA), 8.71 (PBE), and 4.28 (B3LYP). B2PLYP (MAD = 1.94) leads to a substantial improvement, but M06-2X (MAD = 1.20) and XYG3 (MAD = 1.02) are remarkably accurate for all types of reactions for a total of 76 barrier heights. This accuracy is comparable with that of the QCISD(T) ab initio method with the same basis set (1.10 kcal/mol). We emphasize that barrier heights are not included in the XYG3 training set but are included in the M06 training set. Probably it is the presence of ≈80% exact exchange in XYG3 that decreases the self-interaction errors (SIE) of local DFT functionals (25), whereas SIE make local DFT functionals problematic for the stretched partially broken bonds, characteristic of the transition states for chemical reactions.
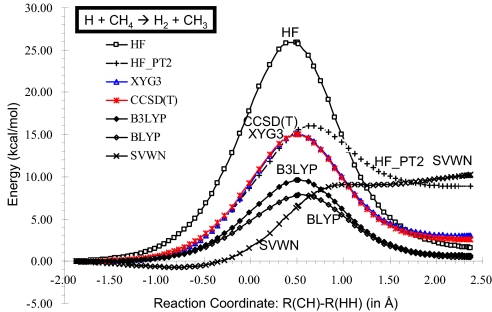
Accurate potential energy surfaces (PES) are essential for using theory to predict chemical processes, but the accuracy depends critically on the level of the theory. Here, we test various methods for describing the H + CH4 → H2 + CH3 reaction. Because of its important roles in CH4/O2 combustion chemistry, this reaction has long been the subject of both experimental and theoretical interest (34). Fig. 1 presents a point-to-point comparison among the results of various methods along the reaction coordinate. We expect that the CCSD(T) curve should be the most accurate, leading to a barrier of 15.03 kcal/mol. Remarkably, XYG3 predicts the barrier of 15.08 kcal/mol, and is within 0.6 kcal/mol of the CCSD(T) results for the entire reaction path.
Fig. 1.
Accuracy of various QM methods for calculating the potential energy surface for the H + CH4 → H2 + CH3 reaction coordinate [defined as R(CH) − R(HH)]. The CCSD(T) calculations using the 6-311++G(3df,2pd) basis set, are expected to be the most accurate. B3LYP calculations were performed self-consistently by using the 6-311+G(3df,2p) basis set. All other results (HF, HF_PT2, XYG3, BLYP, and SVWN) used the density and orbitals from the B3LYP calculation. The XYG3 results superimpose nearly exactly on the CCSD(T) curve.
The LDA (SVWN) reaction path is qualitatively wrong, predicting a barrierless reverse reaction. HF overestimates the barrier height by 10.89 kcal/mol, whereas HF_PT2, which uses exact exchange plus PT2 correlation, overestimates the endothermicity of the reaction by 6.30 kcal/mol. Here, the tendency that BLYP underestimates the barrier heights is seen clearly in Fig. 1, whereas B3LYP, with inclusion of some exact exchange, leads to improved results, but remains inadequate for PES calculations.
Noncovalent Interactions.
The noncovalent interaction DB from Zhao and Truhlar (14, 15) (NCIE31/05) consists of 6 HB complexes, 7 charge-transfer (CT) complexes, 6 dipole interaction (DI) complexes, 7 weak interaction (WI) complexes, and 5 π–π stacking (PPS) complexes. We tested the performance of the XYG3 functional for these noncovalent interactions using the 6-311+G(3df,2p) basis set, with geometries and reference energies taken from the Truhlar DB web site (14, 15).
The errors are summarized in Table 3. We did not include basis set superposition error corrections, which may increase the calculated interaction energies slightly.
Table 3.
Accuracy of various QM methods for predicting noncovalent interactions
| Functional | Total | HB6/04 | CT7/04 | DI6/04 | WI7/05 | PPS5/05 |
|---|---|---|---|---|---|---|
| DFT | ||||||
| M06-2X* | 0.30 | 0.45 | 0.36 | 0.25 | 0.17 | 0.26 |
| XYG3† | 0.32 | 0.38 | 0.64 | 0.19 | 0.12 | 0.25 |
| M06* | 0.43 | 0.26 | 1.11 | 0.26 | 0.20 | 0.21 |
| M06-L* | 0.58 | 0.21 | 1.80 | 0.32 | 0.19 | 0.17 |
| B2PLYP† | 0.75 | 0.35 | 0.75 | 0.30 | 0.12 | 2.68 |
| B3LYP† | 0.97 | 0.60 | 0.71 | 0.78 | 0.31 | 2.95 |
| PBE‡ | 1.17 | 0.45 | 2.95 | 0.46 | 0.13 | 1.86 |
| BLYP‡ | 1.48 | 1.18 | 1.67 | 1.00 | 0.45 | 3.58 |
| LDA‡ | 3.12 | 4.64 | 6.78 | 2.93 | 0.30 | 0.35 |
| Ab initio | ||||||
| HF† | 2.08 | 2.25 | 3.61 | 2.17 | 0.29 | 2.11 |
| MP2‡ | 0.64 | 0.99 | 0.47 | 0.29 | 0.08 | 1.69 |
| QCISD(T)‡ | 0.57 | 0.90 | 0.62 | 0.47 | 0.07 | 0.95 |
MADs in kcal/mol for the 31 cases in the Truhlar web site DB (14, 15), which contains the best available information from ab initio calculations of noncovalent interactions (NCIE31/05). This consists of 6 HB complexes, 7 CT complexes, 6 DI complexes, 7 WI complexes, and 5 PPS complexes. The WI and PPS are dominated by London dispersion.
*Data are from ref. 14.
†Our calculations used the 6-311+G(3df,2p) basis sets with geometries from the Truhlar DB web site. Counterpoise corrections for possible basis set superposition errors were not included.
‡Data are from ref. 15.
We expect that the QCISD(T) ab initio method provides the highest accuracy, leading to a MAD = 0.57 kcal/mol. Remarkably M06-2X (MAD = 0.30) and XYG3 (MAD = 0.32) have half this error including WI and PPS for which London dispersion dominates. Also M06 (MAD = 0.43) and M06-L (MAD = 0.58) perform well for all 5 sets. Note that these nonbond interactions were not included in the XYG3 training set but were included in the M06 training set.
WI and PPS lead to notorious failures for common DFT methods because dispersion interactions are lacking in the correlation functionals. The PT2 term in XYG3 reduces this error, but B2PLYP was not able to describe the PPS complexes. It was suggested that this might be because the PT portion (≈27%) is too small to overcome the repulsion from the DFT part (35). However, we suspect that it is because the orbitals from the truncated DFT in B2PLYP stray too far from the real KS orbitals.
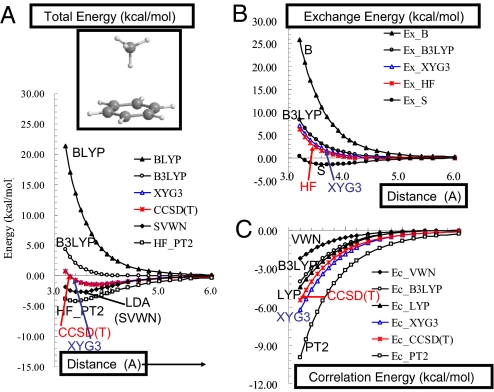
Fig. 2 compares the intermolecular potentials of the CH4–C6H6 complexes calculated by XYG3 and CCSD(T) (36), along with some other DFT results. Proper description of such potential energy curves is very important for describing the binding of ligands to biological systems, because steric constraints might prevent the ligand from adopting its optimum geometry. We find that XYG3 results compare extremely well with those of CCSD(T), with deviations generally <0.1 kcal/mol.
Fig. 2.
Enlarged figure for CH4-C6H6 system (see Fig. S1). (A) The intermolecular potentials for the CH4–C6H6 complexes from various methods. The most accurate is expected to be CCSD(T), which is at the complete basis set limit from ref. 36. XYG3 nearly superimposes on CCSD(T). B3LYP calculations were performed self-consistently by using the 6-311+G(3df,2p) basis set. All other results used density and orbitals from B3LYP. (B) The exchange part of the interaction energy. The HF result is expected to be the most accurate. The exchange part of XYG3 nearly superimposes on the HF. (C) The correlation part of the interaction energy. The CCSD(T) results are expected to be the most accurate. XYG3 is closest among the DFT.
Fig. 2B shows the exchange contributions to the noncovalent interaction energy. Here, we see that XYG3 agrees closely with HF, which we expect to be the most accurate. We note here that Slater exchange (S) leads to a spurious well, whereas the GGA correction (Becke88) causes the potential curve to be far too repulsive.
Fig. 2C shows that correlation (attractive) contributions to the noncovalent interaction energy. Here, we see that XYG3 agrees closely with CCSD(T), which we expect to be the most accurate. Note that PT2 by itself is too attractive. Thus, it is the combination of PT2 with LYP that provides the excellent correlation description in XYG3.
That the exchange and correlation parts of XYG3 independently fit what are expected to be the most accurate descriptions indicates that XYG3 gets the right answer for the right reason with a correct description of the fundamental physics.
Summary
We develop here the extension of DFT to the fifth rung of the Perdew Jacob ladder hierarchy. This is done through a construction based on the adiabatic connection formalism using the Görling–Levy coupling-constant perturbation expansion to the second order. This leads to a doubly hybrid functional, XYG3, that uses exact exchange to improve the quality of DFT exchange at the exchange limit (λ = 0), while using both occupied and unoccupied KS orbitals through double-excitation contributions from the PT2 term. In this work, we use the KS orbitals and eigenvalues from a self-consistent B3LYP calculation to compute the PT2 term, which is supplemented with a fraction of LYP to provide the complete correlation energy. Other choices of the virtual orbitals would be possible.
XYG3 contains 3 empirical parameters: (i) the proportion of exact exchange (normalized with the portion of LDA exchange), (ii) the proportion of GGA exchange correction, and (iii) the proportion of PT2 (normalized with the portion of LYP correlation), which determined by using only thermochemical data. In addition to the accuracy of XYG3 for thermochemistry, we find that it is remarkably accurate for the energy along the reaction pathway including reaction barrier heights and for nonbond interactions, neither of which were included in the training set. This suggests that XYG3 captures a consistent description of the physics.
XYG3 does have limitations. Approximate functionals may break the variational principle, leading to energies lower than exact. This can be a serious problem for the PT2 term when there is near-degeneracy of the orbitals as, for example, in the system containing transition metals.
It is also important to consider the scaling of such DFT methods to judge the practicality for application to large systems. Thus, pure DFT methods scale most favorably with size. Including the exact exchange as in B3LYP leads a formal scaling as N4, whereas including the PT2 term leads to a formal scaling as N5, just as for MP2. Linear scaling methods have been developed for MP2 (37–39) that dramatically accelerate calculations for large molecules, and we expect that these can be used with XYG3. See SI for additional information
Supplementary Material
Acknowledgments.
We thank Prof. D. H. Zhang (Dalian Institute of Chemical Physics, Dalian, China) for providing the CCSD(T) results of the potential energy curves for the H + CH4 → H2 + CH3 reaction. This work was supported by National Natural Science Foundation of China Grants 20525311, 20533030, 20423002, and 10774126; Ministry of Science and Technology of China Grants 2007CB815206 and 2004CB719902, with partial support by National Science Foundation Grants ECS-0609128 and CTS-0608889) and Office of Naval Research (ONR)–Defense Advanced Research Projects Agency Grants PROM N00014-06-1-0938 and N00014-05-1-0778). The computation facilities of the Materials and Process Simulation Center (MSC) used in these studies have been supported by grants from the Army Research Office–Defense University Research Instrumentation Program (DURIP) and ONR–DURIP.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0901093106/DCSupplemental.
References
- 1.Chong DP. Recent Advances in Density Functional Methods. Singapore: World Scientific; 1997. Pts I and II. [Google Scholar]
- 2.Szabo A, Ostlund NS. Modern Quantum Chemistry: Introduction to Advanced Electronic Structure Theory. New York: MacMillan; 1982. [Google Scholar]
- 3.Hohenberg P, Kohn W. Inhomogeneous electron gas. Phys Rev B. 1964;3:864–871. [Google Scholar]
- 4.Kohn W, Sham JL. Self-consistent equations including exchange and correlation effects. Phys Rev A. 1965;4:1133–1138. [Google Scholar]
- 5.Vosko SH, Wilk L, Nusair M. Accurate spin-dependent electron liquid correlation energies for local spin density calculations: A critical analysis. Can J Phys. 1980;58:1200–1211. [Google Scholar]
- 6.Becke AD. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev A. 1988;38:3098–3100. doi: 10.1103/physreva.38.3098. [DOI] [PubMed] [Google Scholar]
- 7.Lee C, Yang W, Parr RG. Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B. 1988;37:785–789. doi: 10.1103/physrevb.37.785. [DOI] [PubMed] [Google Scholar]
- 8.Perdew JP, Burke K, Ernzerhof M. Generalized gradient approximation made simple. Phys Rev Lett. 1996;77:3865–3868. doi: 10.1103/PhysRevLett.77.3865. [DOI] [PubMed] [Google Scholar]
- 9.Tao J, et al. Meta-generalized gradient approximation: Non-empirical construction and performance of a density functional. Philos Mag. 2007;87:1071–1084. [Google Scholar]
- 10.Becke AD. Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys. 1993;98:5648–5652. [Google Scholar]
- 11.Curtiss LA, Raghavachari K, Redfern PC, Pople JA. Assessment of Gaussian-2 and density functional theories for the computation of enthalpies of formation. J Chem Phys. 1997;106:1063–1079. [Google Scholar]
- 12.Curtiss LA, Raghavachari K, Redfern PC, Pople JA. Assessment of Gaussian-3 and density functional theories for a larger experimental test set. J Chem Phys. 2000;112:7374–7383. doi: 10.1063/1.2039080. [DOI] [PubMed] [Google Scholar]
- 13.Wodrich MD, Corminboeuf C, Schleyer PvR. Systematic errors in computed alkane energies using B3LYP and other popular DFT functionals. Org Lett. 2006;8:3631–3634. doi: 10.1021/ol061016i. [DOI] [PubMed] [Google Scholar]
- 14.Zhao Y, Truhlar DG. Density functionals with broad applicability in chemistry. Theor Chem Acc. 2008;120:215–241. doi: 10.1021/ar700111a. [DOI] [PubMed] [Google Scholar]
- 15.Zhao Y, Truhlar DG. Design of density functionals that are broadly accurate for thermochemistry, thermochemical kinetics, and nonbonded interactions. J Phys Chem A. 2005;109:5656–5667. doi: 10.1021/jp050536c. [DOI] [PubMed] [Google Scholar]
- 16.Perdew JP, et al. Prescription for the design and selection of density functional approximations: More constraint satisfaction with fewer fits. J Chem Phys. 2006;123 doi: 10.1063/1.1904565. 062201–1-9. [DOI] [PubMed] [Google Scholar]
- 17.Constantin LA, Pitarke JM, Dobson JF, Garcia-Lekue A, Perdew JP. High-level correlated approach to the jellium surface energy, without uniform-gas input. Phys Rev Lett. 2008;100 doi: 10.1103/PhysRevLett.100.036401. 036401–036404. [DOI] [PubMed] [Google Scholar]
- 18.Grimme S. Semiempirical hybrid density functional with perturbative second-order correlation. J Chem Phys. 2006;124 doi: 10.1063/1.2148954. 034108–1-16. [DOI] [PubMed] [Google Scholar]
- 19.Görling A, Levy M. Correlation-energy functional and its high-density limit obtained from a coupling-constant perturbation expansion. Phys Rev B. 1993;47:13105–13113. doi: 10.1103/physrevb.47.13105. [DOI] [PubMed] [Google Scholar]
- 20.Langreth DC, Perdew JP. Exchange-correlation energy of a metallic surface: Wave-vector analysis. Phys Rev B. 1977;15:2884–2902. [Google Scholar]
- 21.Gunnarsson O, Lundqvist B. Exchange and correlation in atoms, molecules, and solids by the spin-density-functional formalism. Phys Rev B. 1976;13:4274–4298. [Google Scholar]
- 22.Kurth S, Perdew JP. Density-functional correction of random-phase-approximation correlation with results for jellium surface energies. Phys Rev B. 1999;59:10461–10468. [Google Scholar]
- 23.Becke AD. A new mixing of Hartree–Fock and local density-functional theories. J Chem Phys. 1993;98:1372–1377. [Google Scholar]
- 24.Perdew JP, Ernzerhof M, Burke K. Rationale for mixing exact exchange with density functional approximations. J Chem Phys. 1996;105:9982–9985. [Google Scholar]
- 25.Mori-Sanchez P, Cohen AJ, Yang WT. Self-interaction-free exchange-correlation functional for thermochemistry and kinetics. J Chem Phys. 2006;124 doi: 10.1063/1.2179072. 091102–1-4. [DOI] [PubMed] [Google Scholar]
- 26.Cremer D. Density functional theory: Coverage of dynamic and non-dynamic electron correlation effects. Mol Phys. 2001;99:1899–1940. [Google Scholar]
- 27.Curtiss LA, Raghavachari K, Trucks GW, Pople JA. Gaussian-2 theory for molecular energies of first- and second-row compounds. J Chem Phys. 1991;94:7221–7230. [Google Scholar]
- 28.Staroverov VN, Scuseria GE, Tao J, Perdew JP. Comparative assessment of a new nonempirical density functional: Molecules and hydrogen-bonded complexes. J Chem Phys. 2003;119:12129–12137. [Google Scholar]
- 29.Wu JM, Xu X. The X1 method for accurate and efficient prediction of heats of formation. J Chem Phys. 2007;127:214105–214113. doi: 10.1063/1.2800018. [DOI] [PubMed] [Google Scholar]
- 30.Zhao Y, Truhlar DG. A new local density functional for main-group thermochemistry, transition metal bonding, thermochemical kinetics, and noncovalent interactions. J Chem Phys. 2006;125 doi: 10.1063/1.2370993. 194101–1-17. [DOI] [PubMed] [Google Scholar]
- 31.Schwabe T, Grimme S. Towards chemical accuracy for the thermodynamics of large molecules: New hybrid density functionals including non-local correlation effect. Phys Chem Chem Phys. 2006;8:4398–4401. doi: 10.1039/b608478h. [DOI] [PubMed] [Google Scholar]
- 32.Kümmel S, Kronik L. Orbital-dependent density functionals: Theory and applications. Rev Mod Phys. 2008;80:3–59. [Google Scholar]
- 33.Zhao Y, González-García N, Truhlar DG. Benchmark database of barrier heights for heavy atom transfer, nucleophilic substitution, association, and uni-molecular reactions and its use to test theoretical methods. J Phys Chem A. 2005;109:2012–2018. doi: 10.1021/jp045141s. [DOI] [PubMed] [Google Scholar]
- 34.Zhang LL, Lu YP, Lee S-Y, Zhang DH. A transition state wave packet study of the H+CH4 reaction. J Chem Phys. 2007;127 doi: 10.1063/1.2812553. 234313–1-7. [DOI] [PubMed] [Google Scholar]
- 35.Schwabe T, Grimme S. Double-hybrid density functionals with long-range dispersion corrections: Higher accuracy and extended applicability. Phys Chem Chem Phys. 2007;9:3397–3406. doi: 10.1039/b704725h. [DOI] [PubMed] [Google Scholar]
- 36.Takatani T, Sherrill CD. Performance of spin-component-scaled Møller–Plesset theory (SCS-MP2) for potential energy curves of noncovalent interactions. Phys Chem Chem Phys. 2007;9:6106–6114. doi: 10.1039/b709669k. [DOI] [PubMed] [Google Scholar]
- 37.Kobayashi M, Imamura Y, Nakai H. Alternative linear-scaling methodology for the second-order Møller–Plesset perturbation calculation based on the divide-and-conquer method. J Chem Phys. 2007;127 doi: 10.1063/1.2761878. 074103–1-8. [DOI] [PubMed] [Google Scholar]
- 38.Häser M, Almlöf J. Laplace transform techniques in Møller–Plesset perturbation theory. J Chem Phys. 1992;96:489–494. [Google Scholar]
- 39.Rauhut G, Pulay P. Considerations regarding the local treatment of Laplace transform MPPT. Chem Phys Lett. 1996;248:223–227. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.