Abstract
Fe65 is a binding partner of the Alzheimer's β-amyloid precursor protein APP. The possible involvement of this protein in the cellular response to DNA damage was suggested by the observation that Fe65 null mice are more sensitive to genotoxic stress than WT counterpart. Fe65 associated with chromatin under basal conditions and its involvement in DNA damage repair requires this association. A known partner of Fe65 is the histone acetyltransferase Tip60. Considering the crucial role of Tip60 in DNA repair, we explored the hypothesis that the phenotype of Fe65 null cells depended on its interaction with Tip60. We demonstrated that Fe65 knockdown impaired recruitment of Tip60-TRRAP complex to DNA double strand breaks and decreased histone H4 acetylation. Accordingly, the efficiency of DNA repair was decreased upon Fe65 suppression. To explore whether APP has a role in this mechanism, we analyzed a Fe65 mutant unable to bind to APP. This mutant failed to rescue the phenotypes of Fe65 null cells; furthermore, APP/APLP2 suppression results in the impairment of recruitment of Tip60-TRRAP complex to DNA double strand breaks, decreased histone H4 acetylation and repair efficiency. On these bases, we propose that Fe65 and its interaction with APP play an important role in the response to DNA damage by assisting the recruitment of Tip60-TRRAP to DNA damage sites.
Keywords: Alzheimer, APP, DNA repair
The β-amyloid peptides, main constituents of senile plaques of Alzheimer disease (AD), derive from the proteolytic processing of a type I membrane protein, known as β-amyloid precursor protein (APP) (1). APP functions are not completely understood, and this knowledge could contribute, at least in principle, to the understanding of AD. Possible cues to study the functions of APP could emerge from the analysis of proteins interacting with the short APP cytosolic domain. Several reports indicated that this cytodomain interacts, among the others, with the Fe65 protein (2–4). The latter has the characteristics of an adaptor protein, whose distinctive traits are 3 protein–protein interaction domains, 1 WW and 2 PTB (PhosphoTyrosine Binding) domains (4). The PTB domain located in the C-terminal part of the protein (PTB2) interacts with the cytodomain of APP and of the 2 related proteins APLP1 and APLP2. Similarly, Fe65 has also been found associated with APP intracellular domain (AICD) (5), which is generated, together with the β-amyloid peptides, upon the cleavage of APP by secretases (1).
Experimental evidence from cultured cells suggested 2 possible functions of Fe65, one depending on its presence in the cytosol and another one on its nuclear localization. APP-Fe65 complexes are present in neuronal growth cones (6) and regulate cell motility (7). Considering that Fe65 WW domain interacts with Mena (8) and APP also with mDab1 (9), these findings support the hypothesis that the APP-Fe65 complex is involved in actin-based membrane remodeling, neurite growth and/or synaptic plasticity. The analysis of the phenotypes of APP/APLP1/APLP2 triple KO mice and that of Fe65/Fe65L1 double KO animals support this hypothesis, because they show brain cortical dysplasia associated with altered neuronal migration (10–11).
Another possible but not mutually exclusive model is that based on the presence of Fe65 and Fe65-AICD complex in the nucleus (5, 12–13). In fact, Fe65 or Fe65-AICD complex interact with nuclear proteins such as Tip60, SET, CP2/LSF (13–15) and experimental evidence indicated that they could be involved in transcription activation (16–20). However, several studies raised the question of the specificity of this regulation; in particular, De Strooper and colleagues (21) demonstrated that γ-secretase inhibitors, suppression of presenilins or APP/APLP2 do not induce any significant change in the expression levels of several candidate genes, whereas Fe65 appears to have a rather nonspecific effect on transcription.
We previously observed an increased sensitivity of Fe65 KO MEFs to DNA damaging agents, which is completely rescued by Fe65 reexpression, but not by a Fe65 mutant unable to accumulate in the nucleus (22). Fe65 KO mice also showed an increased sensitivity to ionizing radiations (22). These observations indicate that Fe65 has a role in the cellular response to DNA damage, and in turn suggest that an altered function of the Fe65-APP complex could negatively affect the response of neurons to DNA damage in AD patients.
DNA double-strand-breaks (DSBs) are repaired in eukaryotic cells by 2 different mechanisms, nonhomologous-end-joining and homologous recombination (23). These mechanisms act downstream of the recognition of DNA lesions that are marked by several modifications of surrounding chromatin (24). One of the events common to both pathways is the recruitment of the NuA4 complex, which acetylates histones, in particular histone H4, thus favoring chromatin relaxation at DNA lesions (25). The histone acetyl transferase Tip60, one of the partners of Fe65, has several well-demonstrated roles in DNA repair (26). Among the others, Tip60, in complex with TRRAP, is a key component of the NuA4 complex in which it is responsible for histone H4 acetylation at DNA strand breaks (27).
In this study we explored whether increased sensitivity to DNA damage observed in Fe65 null cells depends on Fe65- Tip60 interaction. By using an experimental system where the I-SceI restriction enzyme induces a DNA DSB at a definite genomic site, we demonstrated that Fe65 suppression decreases Tip60/TRRAP recruitment and Tip60-dependent acetylation of histone H4 at the DNA strand break. These phenomena are accompanied by a significant decrease of DNA repair efficiency. We also demonstrated that the Fe65-APP interaction is required for the function of Fe65 in DNA repair, considering that a Fe65 mutant, unable to interact with APP, fails to rescue the phenotypes observed in Fe65 null cells and, opposite to what observed with WT Fe65, is not associated with intact or damaged chromatin. The requirement of Fe65-APP interaction is further supported by the observation that APP/APLP2 suppression results in a significant decreased of Tip60/TRRAP complex recruitment, of histone H4 acetylation at the DNA damage sites and of the DNA repair efficiency.
Results
Fe65 Suppression Prevents the Recruitment of Tip60-TRRAP at DNA Double Strand Breaks.
We have demonstrated that mouse embryo fibroblasts (MEFs) derived from Fe65 null embryos are more sensitive to DNA damage than WT cells (22). The results obtained in KO MEFs, were confirmed in several cell lines where Fe65 knockdown (KD) was induced by RNA interference (Fig. S1).
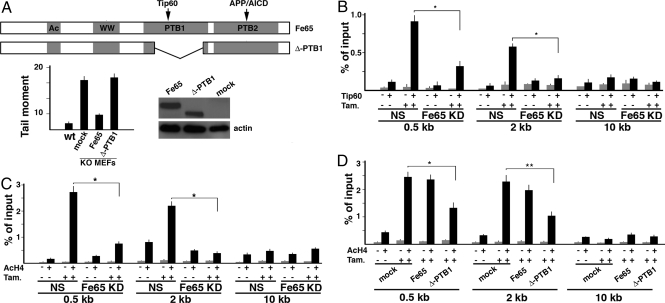
To explore the possible involvement of the Fe65-Tip60 interaction in the increased sensitivity to DNA damage observed in Fe65 KO and KD cells, we analyzed whether a Fe65 mutant lacking the PTB1 domain (Δ-PTB1), and in turn unable to interact with Tip60 (28), can rescue Fe65 KO-associated phenotypes. As shown in Fig. 1A, whereas ectopic expression of Fe65 was able to rescue the phenotype of Fe65 KO MEFs, the Δ-PTB1 mutant failed to rescue the increased sensitivity to DNA damage of these cells.
Fig. 1.
Fe65 suppression affects Tip60 recruitment and histone H4 acetylation at DNA double strand breaks. (A) Fe65 mutant (Δ-PTB1) unable to interact with Tip60 fails to rescue the hypersensitivity to DNA damage of Fe65 KO MEFs. The latter were infected with retroviruses encoding WT Fe65 or Δ-PTB1 mutant or with empty virus (mock). DNA damage after the exposure to 20 μM etoposide for 1 h was measured by Comet assay as described in Materials and Methods. Western blot with myc-Tag antibody demonstrated that WT and mutant Fe65 were expressed at comparable levels. (B) Fe65 knockdown is accompanied by a decreased recruitment of Tip60 at DNA double strand breaks. NIH-GS cells, stably transfected with the inducible form of I-SceI-ER restriction enzyme and with DR-GFP construct (see Materials and Methods) bearing a single I-SceI cleavage site, were treated with 1 μM tamoxifen for 6 h. ChIP was performed with Tip60 antibody (+) or control IgG (−). Immunoprecipitated chromatin was analyzed by real time PCR, using 3 oligonucleotide pairs located at ≈0.5, 2, and 10 kb downstream of the I-SceI cleavage site. *, P < 0.01. (C) Fe65 KD reduced histone H4 acetylation at DNA breaks. Chromatin, immunoprecipitated with an antibody recognizing acetylated histone H4, was analyzed as in B. (D) Overexpression of the Fe65 mutant Δ-PTB1 decreases histone H4 acetylation at DNA breaks. NIH-GS cells were transfected with the vector represented in A. ChIP experiments were performed as described in C. **, P < 0.05
These results suggest that the phenotype of Fe65 KO/KD cells could depended on the interaction of Fe65 with Tip60. To analyze this possibility we exploited the DR-GFP/I-SceI experimental system (29) (see Fig. S2). To this aim, we generated clones of NIH 3T3 cells stably transfected with a DNA construct (DR-GFP) containing 2 nonfunctional GFPs. The upstream (5′) GFP is under the control of the β-actin gene promoter and contains a single recognition site for the I-SceI endonuclease. Considering that no I-SceI sites are present in mammalian genomes, the expression of this enzyme results in generation of a single DNA DSB only at DR-GFP sites (ref. 29 and Fig. S2A). We also generated an I-SceI-ER expression vector, in which the I-SceI cDNA is fused in frame with the cDNA fragment encoding the hormone-binding site of the estrogen receptor. Clones of double stably transfected NIH 3T3 cells (NIH-GS) bearing DR-GFP and I-SceI-ER were pooled and treated with tamoxifen. This treatment induced the activation of I-SceI-ER and, in turn, the cleavage of 5′ GFP (Fig. S2A). In agreement with that observed by others (27), the cleavage induced by I-SceI-ER in NIH-GS cells resulted in the recruitment of Tip60 to the site of DNA damage. In fact, ChIP experiments with Tip60 antibody showed a significant enrichment of Tip60 at the 5′ GFP site upon the induction of I-SceI-ER by tamoxifen. This enrichment was clearly detectable with oligonucleotide pairs amplifying DNA at 0.5 and 2 kb downstream of the cleavage site, but not with the control oligonucleotide pair targeting the region 10 kb downstream of the DSB (Fig. 1B). Interestingly, in cells where Fe65 was knocked down Tip60 recruitment to DSB was strongly decreased (Fig. 1B). Similar to Tip60, its partner TRRAP was recruited to DNA breaks in WT cells. Fe65 KD resulted again in a significant decrease of TRRAP recruitment (Fig. S3A). As a consequence of this impairment of Tip60-TRRAP recruitment to DNA strand breaks, ChIP experiments with acetyl-H4 antibody demonstrated that although in WT cells I-SceI-driven cleavage induced the histone H4 acetylation at the I-SceI site, the concomitant suppression of Fe65 significantly decreased H4 acetylation (Fig. 1C). Furthermore, the expression of the Δ-PTB1 mutant showed a dominant negative effect, strongly reducing Tip60 interaction with damaged DNA (Fig. S3B) and, in turn, H4 acetylation (Fig. 1D).
Fe65 Is Necessary for Efficient DNA Repair.
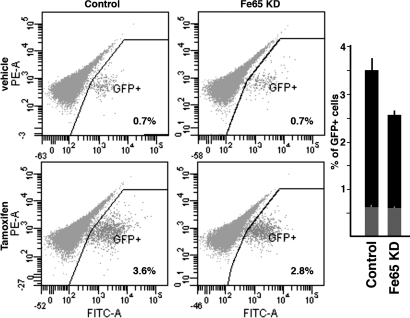
To address the relevance of the Fe65 in DSB repair we measured the efficiency of repair in the same NIH-GS experimental system described before. These cells are stably transfected with DR-GFP and I-SceI-ER. The DR-GFP DNA fragment contains 2 nonfunctional GFPs. The 5′ GFP gene is under the control of the β-actin gene promoter, but it is mutated to generate 2 in-frame stop codons that terminate translation, thereby inactivating the GFP gene. The downstream (3′) GFP is inactivated by upstream and downstream truncations, leaving only ≈500 bp of GFP (see Fig. S2A). When NIH-GS cells were treated with tamoxifen, the resulting DSB induced intrachromosomal gene conversion leading to the reactivation of the 5′ GFP gene. In this system, the extent of repair by homologous recombination was measured by counting GFP positive cells by FACS (see Fig. S2B). Under these conditions, Fe65 KD is accompanied by a small but significant decrease in GFP positive cells, thus clearly indicating Fe65 KD reduced the efficiency of DNA repair (Fig. 2). A significant reduction of repair efficiency was also observed by transfecting the cells with the Δ-PTB1 mutant, thus confirming the dominant negative effect of this protein (Fig. S3C).
Fig. 2.
Fe65 suppression decreases DNA repair efficiency. DNA repair efficiency in NIH-GS cells was measured by counting the percentage of GFP-positive cells 48 h after the exposure of the cells to tamoxifen, which activates I-SceI-ER. Representative FACS output of 1 experiment is shown. (Upper) Cells transfected with nonsilencing control siRNA or Fe65 targeting siRNA and exposed to vehicle. (Lower) Results obtained in cells treated with tamoxifen. The histogram reports the mean values of 3 independent experiments. Gray bars indicate the mean values obtained in the presence of vehicle. The difference between the 2 black bars is significant (P < 0.01).
Interaction with APP Is Required for Fe65 Function in DNA Repair.
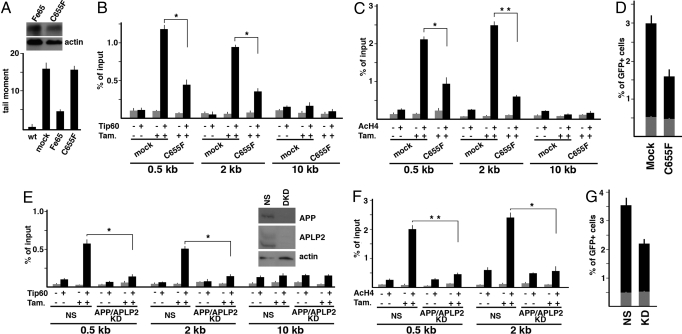
An important issue to be addressed is the possible involvement of APP/AICD in the nuclear functions of Fe65. To explore this point we studied the effects of a Fe65 mutant unable to interact with APP. This mutant carries a point mutation (C655F), which prevents the binding to APP (12). Its retroviral-directed expression in the Fe65 KO MEFs was unable to rescue their increased sensitivity to DNA damage (Fig. 3A). This C655F mutant also showed a strong dominant effect: As shown in Fig. 3 B and C, its transient transfection in the DR-GFP/I-SceI system significantly affected Tip60 recruitment and H4 acetylation at DSB generated by I-SceI. Accordingly, repair efficiency of NIH-GS cells significantly decreased upon the expression of the C655F mutant (Fig. 3D). These results suggest that the interaction with APP/AICD is necessary to allow Fe65 to adequately function in the response to DNA damage. The analysis of the phenotypes induced by the suppression of APP and of its paralogue APLP2 further supported to this hypothesis. In fact, the suppression of APP and APLP2 in NIH-GS cells allowed us to observe a strong decrease of Tip60/TRRAP recruitment at the damaged site and, in turn, a decreased of histone H4 acetylation (Figs. 3 E and F and Fig. S4). Accordingly, DNA damage repair was less efficient in APP/APLP2 KD cells than in cells transfected with nonsilencing siRNA (Fig. 3G).
Fig. 3.
Interaction with APP is necessary for Fe65 function in DNA repair. (A) C655F mutant of Fe65 looses the ability to rescue the increased sensitivity to DNA damage of Fe65 KO MEFs. The latter were transfected with retroviral vectors driving the expression of WT Fe65 or C655F mutant or with empty virus (mock). DNA damage after the exposure to 20 μM etoposide was measured by Comet assay as described in Materials and Methods. Western blot with myc antibody demonstrated that WT and mutant Fe65 were expressed at comparable levels. (B) Overexpression of Fe65 mutant C655F decreases Tip60 recruitment at DNA breaks induced by I-SceI-ER. NIH-GS cells were transfected with the vector encoding C655F or with empty vector (mock). ChIP experiments were performed as described in Fig. 1B. *, P < 0.01. (C) Overexpression of Fe65 mutant C655F decreases histone H4 acetylation at DNA breaks. NIH-GS cells were transfected with the vector encoding C655F or with empty vector (mock). ChIP experiments were performed as described in Fig. 1D. **, P < 0.001. (D) C655F mutant decreases DNA repair efficiency. The experiments were performed as described in Fig. 2. NIH-GS cells were transfected with empty vector (mock) or with the C655F mutant. The histogram reports the mean values of 3 independent experiments. The difference between mock and C655F transfected cells is significant with P < 0.001. (E) APP and APLP2 suppression induces a decrease of Tip60 recruitment at DNA double strand breaks. NIH-GS cells were transfected with nonsilencing (NS) siRNA or with siRNAs targeting APP and APLP2. ChIP were performed and analyzed as described in Fig. 1B. *, P < 0.01 Western blot with APP or APLP2 antibodies is shown. NS, nonsilencing; DKD, double knock down. (F) APP/APLP2 suppression decreases histone H4 acetylation at DNA breaks. ChIP were performed as in Fig. 1C. **, P < 0.001; *, P < 0.01. (G) APP/APLP2 suppression decreases DNA repair efficiency. The experiments were performed as described in Fig. 2. Cells were transfected with nonsilencing (NS) or APP + APLP2 targeting siRNAs (KD). The difference between NS and APP/APLP2 KD cells is significant (P < 0.01).
Fe65 Involvement in DNA Repair Depends on Its Ability to Interact with Intact Chromatin.
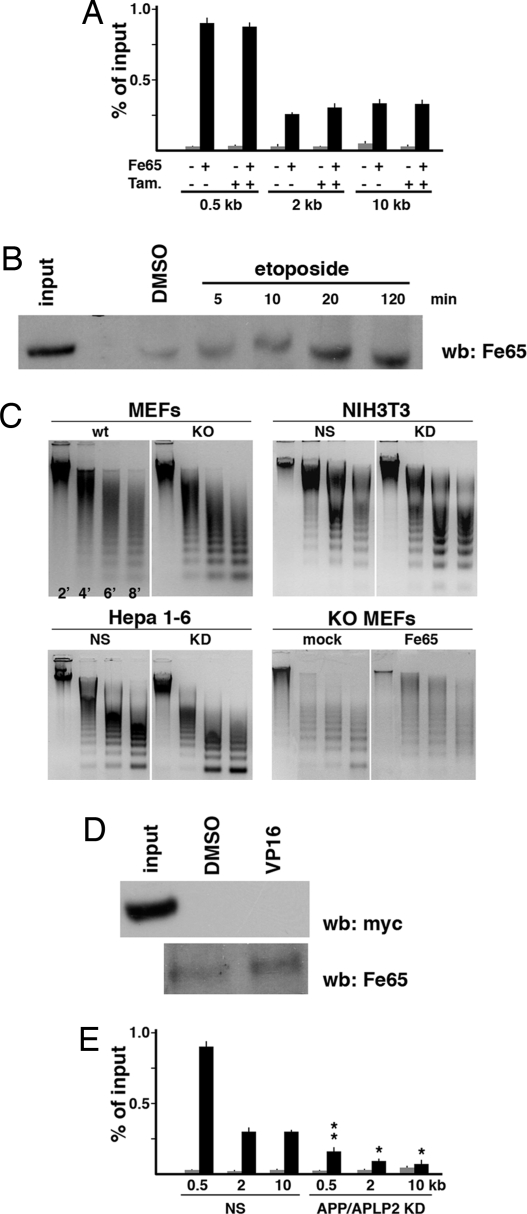
These results suggest that Fe65 plays a significant role in the recruitment of Tip60-TRRAP to the DNA breaks and that this function depends on its interaction with APP. On this basis, we first addressed whether Fe65 was associated to chromatin at DNA strand breaks. ChIP experiments reported in Fig. 4A show that Fe65 was bound to intact chromatin in the region of DR-GFP. Furthermore, in contrast to what observed with Tip60 and TRRAP, its association with chromatin did not significantly change upon DNA cleavage by I-SceI. Then we analyzed Fe65 association with chromatin at a global genomic level. To this aim, we immunoprecipitated cross-linked chromatin from N2A cells with histone H3 antibody. This H3 immunoprecipitated chromatin, approximately representing the whole chromatin, was de-cross-linked and analyzed by Western blot for the presence of Fe65. Fig. 4B shows that Fe65 is indeed associated with intact chromatin and that this association was only slightly increased upon genotoxic stress induced by etoposide (Fig. 4B). We then explored the association of Fe65 with 3 random genomic loci, i.e., those of RNA polymerase II, albumin and GST genes. ChIP experiments demonstrated that endogenous Fe65 was associated to all these sites (Fig. S5).
Fig. 4.
Fe65 is associated with intact and damaged chromatin and this association depends on its ability to interact with APP. (A) Fe65 is associated with intact and cleaved DR-GFP locus in NIH-GS cells. The latter were treated with tamoxifen or left untreated to activate I-SceI-ER. Cross-linked chromatin was immunoprecipitated with Fe65 antibody or control IgG (−). Association of Fe65 with DR-GFP locus was measured by Real time PCR as reported in Fig. 1. (B) Fe65 is associated with intact and damaged chromatin. N2A cells were treated with 100 μM etoposide or left untreated for the indicated times. Cross-linked chromatin was immunoprecipitated with histone H3 antibody. De-cross-linked chromatin was analyzed by Western blot with Fe65 antibody. Input indicates the nonimmunoprecipitated cross-linked extract. (C) Fe65 knock out or knockdown induce chromatin decondensation. Chromatin from the indicated cell lines, in which Fe65 was suppressed by gene KO or by RNA interference, was digested with 2 mM micrococcal nuclease for the indicated times. Digested DNA was examined by agarose gel electrophoresis. Fe65 KO or KD result in the accumulation of the smallest bands of nucleosomal ladder. Retroviral-directed reexpression of WT Fe65 restored the normal cleavage pattern (Lower Right). (D) C655F mutant of Fe65 is not associated with chromatin. NIH 3T3 cells were transfected with myc-tagged C655F mutant. Association of C655F mutant with chromatin was analyzed as in B. (Upper) The C655F mutant was not associated to chromatin either in cells treated with 100 μM etoposide (VP16) or not treated (DMSO). (Lower) Reblot of the filter shown in Upper. Endogenous Fe65 is associated with chromatin. (E) APP/APLP2 suppression hampers the association of Fe65 with chromatin. NIH-GS cells were transfected with nonsilencing (NS) or APP + APLP2 targeting siRNAs. ChIP were performed with control IgG (gray bars) or with Fe65 antibody (black bars) and with the oligonucleotide pairs used in Fig. 1. **, P < 0.001; **, P < 0.01.
The association of Fe65 with chromatin is functionally relevant, as demonstrated by the analysis of chromatin state in Fe65 KO and KD cells. In fact, chromatin from Fe65 KO MEFs or Fe65 KD cells was more accessible to micrococcal nuclease digestion, than chromatin from WT cells (Fig. 4C), thus demonstrating that Fe65 suppression leads to a significant degree of chromatin de-condensation. This phenotype was rescued by Fe65 reexpression in KO MEFs (Fig. 4C).
We then addressed whether Fe65 association with chromatin requires the interaction with APP. Opposite to what observed with WT Fe65, C655F mutant is not associated to immunoprecipitated, intact or damaged chromatin (Fig. 4D and Fig. S6), thus suggesting a convincing explanation for why this mutant failed to rescue the phenotype observed in Fe65 KO MEFs. Furthermore, in APP/APLP2 KD cells we observed a significant decrease of Fe65 associated with chromatin (Fig. 4F), thus clearly indicating that the interaction of Fe65 with APP is necessary to allow its association to chromatin.
Discussion
In a previous study, we demonstrated that ablation of Fe65 gene is associated with an increased sensitivity to DNA damaging agents. This observation suggested a possible function of Fe65 in the molecular machinery of the cellular response to genotoxic stress. In the present study, we addressed the question of whether the phenotype provoked by Fe65 suppression is a consequence of an altered function of one of its partners, Tip60. This hypothesis was suggested by the notion that Tip60 is a major player in the molecular machinery of DNA repair, thus Fe65 could have some role in the regulation of Tip60 functions.
We made several main observations: (i) knockdown of Fe65 provokes a significant decrease in the recruitment of Tip60 and TRRAP to DNA double strand breaks; (ii) accordingly, Fe65 suppression also provokes a dramatic decrease of histone H4 acetylation of lesioned chromatin and, in turn, (iii) the impairment of DNA repair efficiency. Furthermore, we demonstrated that the interaction of Fe65 with APP is necessary to allow Fe65 to associate with chromatin and therefore to play its role in the response to DNA damage. Based on these results, we propose the hypothesis that Fe65 and its interaction with APP play a crucial role in the association of the NuA4 complex containing Tip60 and TRRAP to DNA lesion.
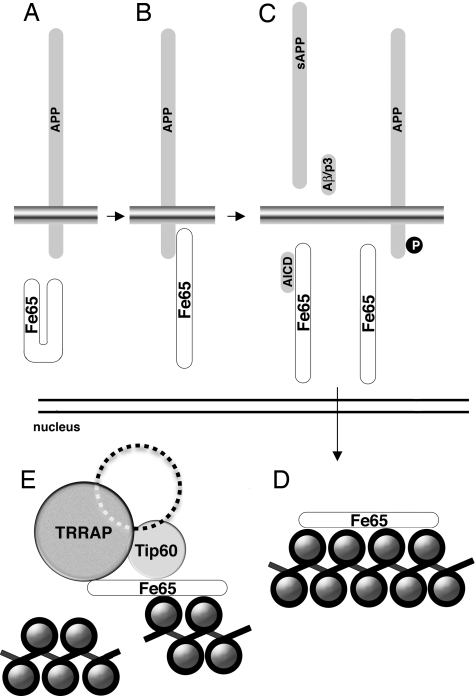
Although further studies are necessary to definitively address the mechanisms of the Fe65 involvement in DNA repair and chromatin remodeling, available knowledge allow us to propose the model reported in Fig. 5. The first step of this model is the interaction of Fe65 with APP. Our results demonstrate that a Fe65 mutant unable to interact with APP fails to rescue the phenotype induced by Fe65 KO. Moreover, the same mutant has a clear dominant negative effect on the recruitment of Tip60-TRRAP at the DNA lesion and, in turn, on the DNA repair efficiency of the cells (see Fig. 3). One possible explanation of the loss of function of the C655F mutant could be based on the model proposed by Cao and Südhof (28). This model suggests that the interaction of Fe65 with APP induces a conformational change leading Fe65 from a “closed” to an “open” conformation. The “closed” conformation could be due to an intramolecular interaction between the WW domain-containing region and the PTB domain region (28). The APP cytodomain opens this structure by competing for the binding of the WW domain with the PTB2. A Fe65 deletion mutant, lacking the entire PTB2 domain, is, similarly to the C655F mutant, completely unable to interact with APP. However, this mutant protein, rescues the phenotype of Fe65 KO cells (data not shown). On this basis it could be speculated that this Δ-PTB2 mutant is in a constitutive “open” conformation, thus rescuing the absence of Fe65. On the contrary, the C655F mutant could be in a constitutive “closed” conformation, therefore being unable to rescue the effects of Fe65 suppression. The loss of the function of this C655F mutant is likely because, opposite to what observed with WT Fe65, it is unable to interact with the chromatin (see Fig. 4D and Fig. S6), whereas it is still able to interact with Tip60 (Fig. S7), thus explaining why it exerts a strong dominant negative effect.
Fig. 5.
Fe65 involvement in chromatin remodeling and DNA damage repair. Available results support the hypothesis that Fe65 exists in 2 conformations. (A–D) The interaction with the cytodomain of APP induces Fe65 from a “close” (A) to an “open” (B) conformation. The cleavage of APP or its phosphorylation may cause the release of Fe65 from the membrane anchor (C) thus allowing its association with chromatin (D). (E) In basal conditions, chromatinized Fe65 appears to have a general role in favoring chromatin condensation. Chromatin associated Fe65 is necessary for the recruitment of Tip60-TRRAP-containing complex at DNA double strand breaks. Loss of function of the Fe65-APP machinery induces a significant decrease of DNA repair efficiency.
Further evidence supporting the involvement of APP in the nuclear function of Fe65 comes from the results showing that the suppression of APP/APLP2 leads to the impairment of Fe65 functions. In fact, in APP/APLP2 KD cells the association of Fe65 to chromatin is strongly reduced and accordingly the recruitment of Tip60-TRRAP to damaged DNA, histone H4 acetylation and DNA damage repair are decreased.
The second step of our model is the translocation of “active” Fe65 from its APP anchor site to the chromatin. One possible mechanism for this event implies the proteolytic processing of APP followed by the release of AICD-Fe65 from the membrane. Another possibility is that posttranslational modifications of APP and/or Fe65 cause the release of Fe65 and its nuclear translocation. For example, the phosphorylation of Thr-668 of APP is an interesting candidate as a trigger mechanism inducing the release of Fe65 (30).
Our results indicate that, in basal conditions, nuclear Fe65 is associated to chromatin. As mentioned before, this association requires the interaction with APP, because a mutant Fe65 unable to interact with APP is not associated to chromatin and APP/APLP2 suppression decreases the association to chromatin of endogenous Fe65. The Fe65 KO or KD leads to a decondensation of the chromatin structure, therefore, at least in basal conditions, Fe65 seems to favor a condensed state of the chromatin. This Fe65 function remains to be studied in details. However, it seems to be distinct from that involved in the response of the cells to DNA damage and in the repair of the lesions. Chromatin fiber decompaction could depended at least in part on histone H4 acetylation, likely catalyzed by Tip60 (31), thus Fe65 could also negatively regulate, via its interaction with Tip60, global histone acetylation and in turn the chromatin state. However, it is not expected that the phenotype observed in Fe65 KD cells, i.e., reduced efficiency of DNA repair (see Fig. 2), is a consequence of chromatin decompaction, considering that, at least in principle, decompacted chromatin should favor and not hamper DNA repair. This paradox could be explained by hypothesizing that Fe65 has 2 related but distinct effects on chromatin: On intact chromatin, Fe65 favors the compaction of the chromatin, whereas, upon DNA double strand break, it stimulates the recruitment of Tip60, the acetylation of histone H4 and in turn the local relaxation of the chromatin. This possibility is reasonable if we consider that Fe65 is an adaptor molecule, which can interact with several different ligands. The Δ-PTB1 mutant has also a strong dominant negative effect. This dominant effect is in part due to the fact the this mutant was found associated with chromatin (Fig. S6C) and could be the consequence of the titration of some other partners of Fe65, which may be necessary for the proper function of Fe65. Among these Fe65 partners, possibly involved in the phenomena we have described, there are the Set protein and the Abl tyrosine kinase. Both of them interact with the WW domain of Fe65 (14, 32) and could be involved in the nuclear functions of Fe65. Set is, in fact, a component of the INHAT complex (inhibitor of histone acetyl transferase) (33) and nuclear Abl has a well-known role in the cellular response to DNA damage (34).
The third step concerns with the mechanisms through which Fe65 favors the recruitment of Tip60-TRRAP to the damaged DNA. Because Fe65 is associated to chromatin in basal conditions, one hypothesis is that Tip60-TRRAP containing complex is recruited to DNA lesion by binding Fe65 that is already on the chromatin. This hypothesis implies a signal inducing the interaction between Fe65 and Tip60-TRRAP. This signal could be the phosphorylation of Fe65, which was demonstrated to take place few minutes after the genotoxic stress (22).
The study of APP has tried to address the possible role of dysfunction of this molecule in the pathogenesis of AD. Our results suggest that the involvement of the Fe65-APP complex in the response of the cells to DNA damage and in the DNA repair machinery should be taken into account as a possible mechanism contributing to neuronal dysfunction observed in AD pathology. This possibility deserves further attention, also considering that numerous studies have pointed to the accumulation of DNA lesions, including double strand breaks mostly because of oxidative damage, in mild cognitive impairment and in AD. These observations indicate that DNA damage occurs in all of the steps of the disease progression and DNA repair defects could significantly contribute to neurodysfunction and neurodegeneration observed in dementia (35).
Materials and Methods
Fe65 KO MEFs, NIH3T3, Hepa 1–6 and N2A cells were cultured as reported in SI Text. Fe65, APP and APLP2 silencing was obtained as described in SI Text. NIH-GS clones were obtained as described in Fig. S2.
WT Fe65 and Δ-PTB1 and C655F mutants were cloned in the pBABE-puro vector and the retroviruses were produced in 293 LinX cells (see also SI Text).
Etoposide (VP-16, Calbiochem, 100 mM stock in Me2SO) was used at 20 or 100 μM for the indicate times. 4-Hydroxytamoxifen (Sigma, 100 μM stock in 95% ethanol) was used at 1 μM.
Antibodies are reported in SI Text. Neutral comet assay was carried out as described in ref. 22. Chromatin immunoprecipirtatin was performed as described in ref. 14 (see SI Text).
Fe65 interaction with chromatin was also measured by immunoprecipitating cross-linked chromatin with histone H3 as described in SI Text. MNase experiments were carried out as described in SI Text. For FACS analysis NIH-GS were resuspended in PBS at 200,000 cells per mL and GFP positive cells were counted with a FACScanto (BD Biosciences) instrument. Each experiment was performed at least in triplicate by counting 30,000 events per sample. The Student's t test was used to measure statistical significance.
Supplementary Material
Acknowledgments.
We thank Caterina Missero, Nicola Zambrano and Domenico Grieco for reading the manuscript and helpful suggestions. The work was supported by grants from the U.S. Alzheimer Association, Associazione Italiana Per La Ricerca Sul Cancro, and the Italian Ministry of Health.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0810869106/DCSupplemental.
References
- 1.Reinhard C, Hébert SS, De Strooper B. The amyloid-beta precursor protein: Integrating structure with biological function. EMBO J. 2005;24:3996–4006. doi: 10.1038/sj.emboj.7600860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fiore F, et al. The regions of the Fe65 protein homologous to the phosphotyrosine interaction/phosphotyrosine binding domain of Shc bind the intracellular domain of the Alzheimer's amyloid precursor protein. J Biol Chem. 1995;270:30853–6. doi: 10.1074/jbc.270.52.30853. [DOI] [PubMed] [Google Scholar]
- 3.Borg JP, Ooi J, Levy E, Margolis B. The phosphotyrosine interaction domains of X11 and FE65 bind to distinct sites on the YENPTY motif of amyloid precursor protein. Mol Cell Biol. 1996;16:6229–6241. doi: 10.1128/mcb.16.11.6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zambrano N, et al. Interaction of the phosphotyrosine interaction/phosphotyrosine binding-related domains of Fe65 with wild-type and mutant Alzheimer's beta-amyloid precursor proteins. J Biol Chem. 1997;272:6399–6405. doi: 10.1074/jbc.272.10.6399. [DOI] [PubMed] [Google Scholar]
- 5.Cupers P, Orlans I, Craessaerts K, Annaert W, De Strooper B. The amyloid precursor protein (APP)-cytoplasmic fragment generated by gamma-secretase is rapidly degraded but distributes partially in a nuclear fraction of neurones in culture. J Neurochem. 2001;78:1168–1178. doi: 10.1046/j.1471-4159.2001.00516.x. [DOI] [PubMed] [Google Scholar]
- 6.Sabo SL, Ikin AF, Buxbaum JD, Greengard P. The amyloid precursor protein and its regulatory protein, FE65, in growth cones and synapses in vitro and in vivo. J Neurosci. 2003;23:5407–5415. doi: 10.1523/JNEUROSCI.23-13-05407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sabo SL, Ikin AF, Buxbaum JD, Greengard P. The Alzheimer amyloid precursor protein (APP) and FE65, an APP-binding protein, regulate cell movement. J Cell Biol. 2001;153:1403–1414. doi: 10.1083/jcb.153.7.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ermekova KS, et al. The WW domain of neural protein FE65 interacts with proline-rich motifs in Mena, the mammalian homolog of Drosophila enabled. J Biol Chem. 1997;272:32869–77. doi: 10.1074/jbc.272.52.32869. [DOI] [PubMed] [Google Scholar]
- 9.Trommsdorff M, Borg JP, Margolis B, Herz J. Interaction of cytosolic adaptor proteins with neuronal apolipoprotein E receptors and the amyloid precursor protein. J Biol Chem. 1998;273:33556–60. doi: 10.1074/jbc.273.50.33556. [DOI] [PubMed] [Google Scholar]
- 10.Herms J, et al. Cortical dysplasia resembling human type 2 lissencephaly in mice lacking all three APP family members. EMBO J. 2004;23:4106–4115. doi: 10.1038/sj.emboj.7600390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guénette S, et al. Essential roles for the FE65 amyloid precursor protein-interacting proteins in brain development. EMBO J. 2006;25:420–431. doi: 10.1038/sj.emboj.7600926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Minopoli G, et al. The beta-amyloid precursor protein functions as a cytosolic anchoring site that prevents Fe65 nuclear translocation. J Biol Chem. 2001;276:6545–6550. doi: 10.1074/jbc.M007340200. [DOI] [PubMed] [Google Scholar]
- 13.Cao X, Südhof TC. A transcriptionally active complex of APP with Fe65 and histone acetyltransferase Tip60. Science. 2001;293:115–120. doi: 10.1126/science.1058783. [DOI] [PubMed] [Google Scholar]
- 14.Telese F, et al. Transcription regulation by the adaptor protein Fe65 and the nucleosome assembly factor SET. EMBO Rep. 2005;6:77–82. doi: 10.1038/sj.embor.7400309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zambrano N, Minopoli G, de Candia P, Russo T. The Fe65 adaptor protein interacts through its PID1 domain with the transcription factor CP2/LSF/LBP1. J Biol Chem. 1998;273:20128–33. doi: 10.1074/jbc.273.32.20128. [DOI] [PubMed] [Google Scholar]
- 16.Baek SH, et al. Exchange of N-CoR corepressor and Tip60 coactivator complexes links gene expression by NF-kappaB and beta-amyloid precursor protein. Cell. 2002;110:55–67. doi: 10.1016/s0092-8674(02)00809-7. [DOI] [PubMed] [Google Scholar]
- 17.Bao J, et al. Suppression of beta-amyloid precursor protein signaling into the nucleus by estrogens mediated through complex formation between the estrogen receptor and Fe65. Mol Cell Biol. 2007;27:1321–1333. doi: 10.1128/MCB.01280-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.von Rotz RC, et al. The APP intracellular domain forms nuclear multiprotein complexes and regulates the transcription of its own precursor. J Cell Sci. 2004;117:4435–4448. doi: 10.1242/jcs.01323. [DOI] [PubMed] [Google Scholar]
- 19.Pardossi-Piquard R, et al. Presenilin-dependent transcriptional control of the Aβ-degrading enzyme neprilysin by intracellular domains of betaAPP and APLP. Neuron. 2005;46:541–554. doi: 10.1016/j.neuron.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Liu Q, et al. Amyloid precursor protein regulates brain apolipoprotein E and cholesterol metabolism through lipoprotein receptor LRP1. Neuron. 2007;56:66–78. doi: 10.1016/j.neuron.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hébert SS, et al. Regulated intramembrane proteolysis of amyloid precursor protein and regulation of expression of putative target genes. EMBO Rep. 2006;7:739–745. doi: 10.1038/sj.embor.7400704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Minopoli G, et al. Essential roles for Fe65, Alzheimer amyloid precursor-binding protein, in the cellular response to DNA damage. J Biol Chem. 2007;282:831–835. doi: 10.1074/jbc.C600276200. T. [DOI] [PubMed] [Google Scholar]
- 23.Shrivastav M, De Haro LP, Nickoloff JA. Regulation of DNA double-strand break repair pathway choice. Cell Res. 2008;18:134–147. doi: 10.1038/cr.2007.111. [DOI] [PubMed] [Google Scholar]
- 24.Escargueil AE, Soares DG, Salvador M, Larsen AK, Henriques JA. What histone code for DNA repair? Mutat Res. 2008;658:259–270. doi: 10.1016/j.mrrev.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Downs JA, Allard S, Jobin-Robitaille O, Javaheri A, Auger A, Bouchard N, Kron SJ, Jackson SP, Côté J. Binding of chromatin-modifying activities to phosphorylated histone H2A at DNA damage sites. Mol Cell. 2004;16:979–990. doi: 10.1016/j.molcel.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 26.Squatrito M, Gorrini C, Amati B. Tip60 in DNA damage response and growth control: Many tricks in one HAT. Trends Cell Biol. 2006;16:433–442. doi: 10.1016/j.tcb.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 27.Murr R, et al. Histone acetylation by Trrap-Tip60 modulates loading of repair proteins and repair of DNA double-strand breaks. Nat Cell Biol. 2006;8:91–99. doi: 10.1038/ncb1343. [DOI] [PubMed] [Google Scholar]
- 28.Cao X, Südhof TC. Dissection of amyloid-β precursor protein-dependent transcriptional activation. J Biol Chem. 2004;279:24601–11. doi: 10.1074/jbc.M402248200. [DOI] [PubMed] [Google Scholar]
- 29.Richardson C, Elliott B, Jasin M. Chromosomal double-strand breaks introduced in mammalian cells by expression of I-Sce I endonuclease. Methods Mol Biol. 1999;113:453–463. doi: 10.1385/1-59259-675-4:453. [DOI] [PubMed] [Google Scholar]
- 30.Ando K, Iijima KI, Elliott JI, Kirino Y, Suzuki T. Phosphorylation-dependent regulation of the interaction of amyloid precursor protein with Fe65 affects the production of beta-amyloid. J Biol Chem. 2001;276:40353–61. doi: 10.1074/jbc.M104059200. [DOI] [PubMed] [Google Scholar]
- 31.Shogren-Knaak M, et al. Histone H4–K16 acetylation controls chromatin structure and protein interactions. Science. 2006;31:844–847. doi: 10.1126/science.1124000. [DOI] [PubMed] [Google Scholar]
- 32.Zambrano N, et al. The beta-amyloid precursor protein APP is tyrosine-phosphorylated in cells expressing a constitutively active form of the Abl protoncogene. J Biol Chem. 2001;276:19787–92. doi: 10.1074/jbc.M100792200. [DOI] [PubMed] [Google Scholar]
- 33.Seo SB, et al. Regulation of histone acetylation and transcription by INHAT, a human cellular complex containing the set oncoprotein. Cell. 2001;104:119–130. doi: 10.1016/s0092-8674(01)00196-9. [DOI] [PubMed] [Google Scholar]
- 34.Kharbanda S, et al. Activation of the c-Abl tyrosine kinase in the stress response to DNA-damaging agents. Nature. 1995;376:785–788. doi: 10.1038/376785a0. [DOI] [PubMed] [Google Scholar]
- 35.Lovell MA, Markesbery WR. Oxidative DNA damage in mild cognitive impairment and late-stage Alzheimer'sdisease. Nucleic Acids Res. 2007;35:7497–7504. doi: 10.1093/nar/gkm821. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.