Abstract
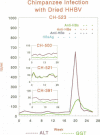
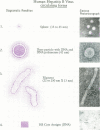
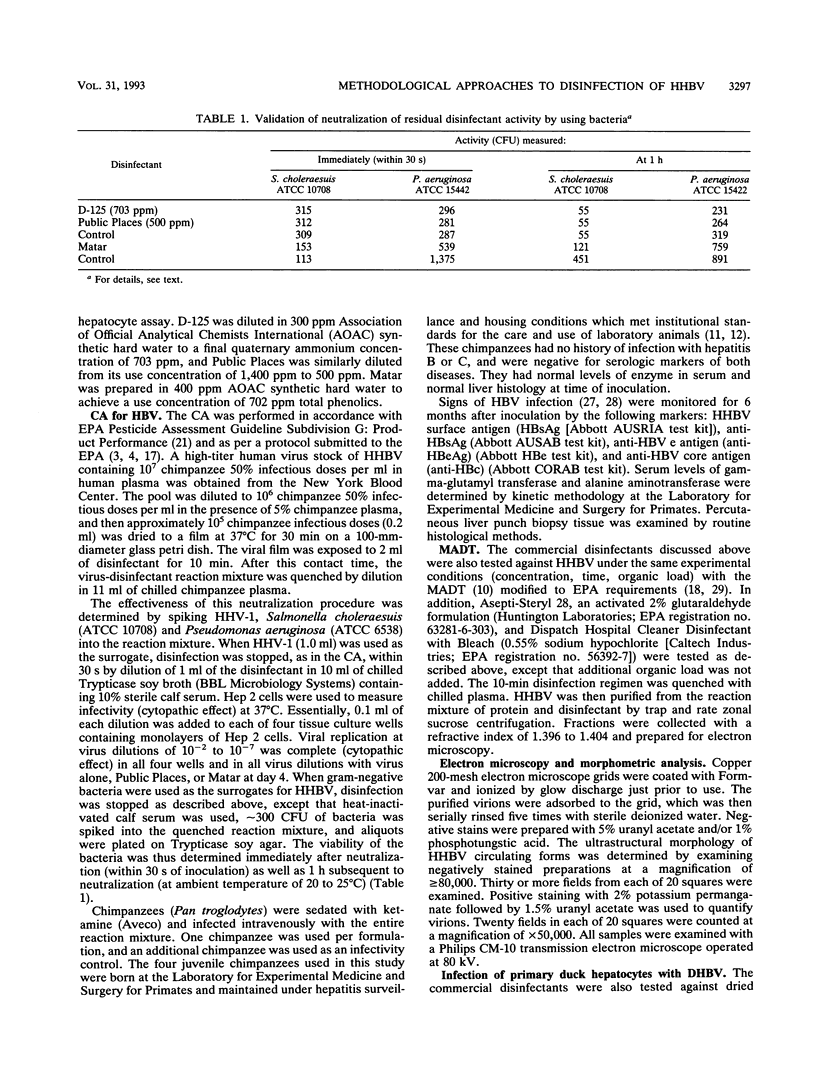
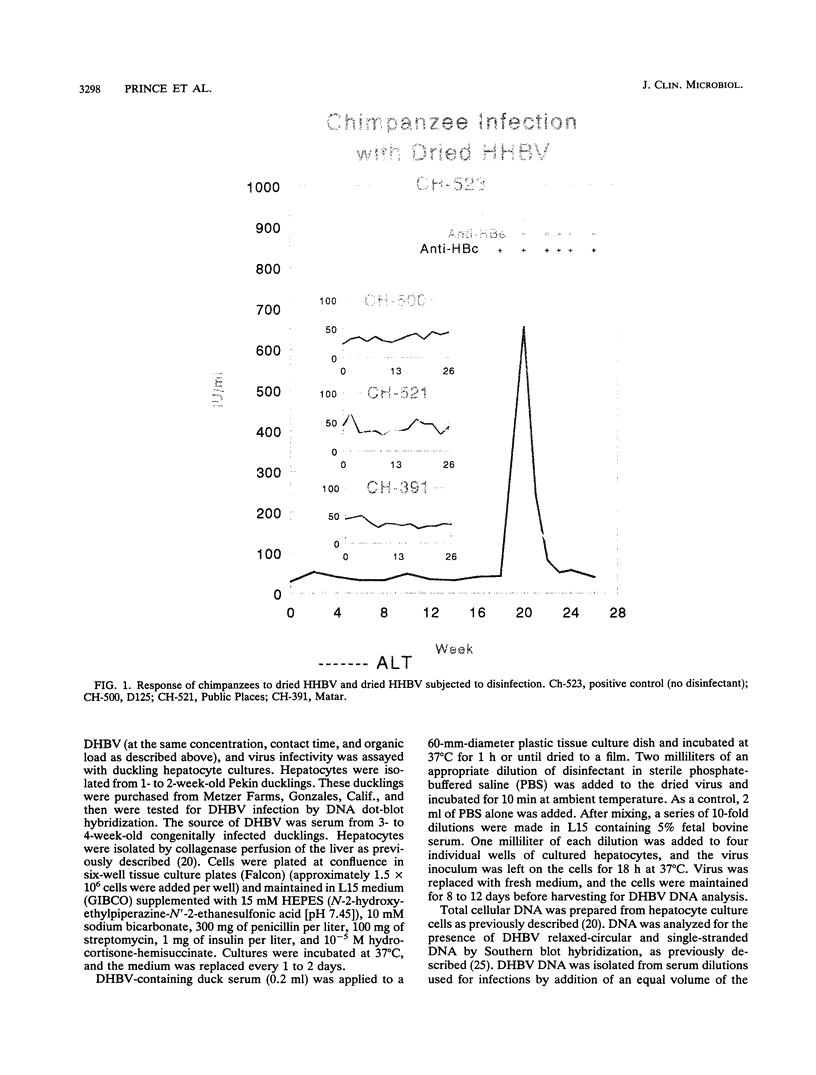
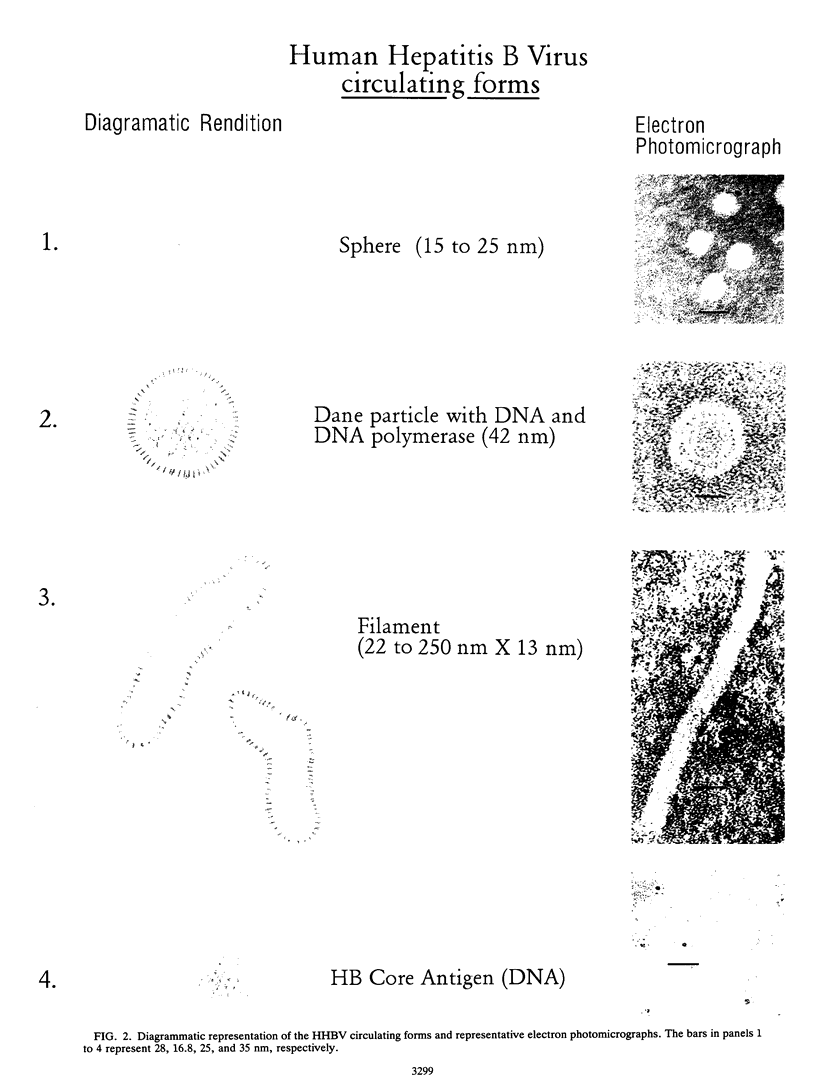
Three commercial disinfectants (two quaternary formulations and one phenolic) were tested against human hepatitis B virus (HHBV). The treated virus was assayed for infectivity by the chimpanzee assay and for morphological alteration by the Morphological Alteration and Disintegration Test. The same agents were tested against duck hepatitis B virus in a duck hepatocyte infectivity assay. It is apparent that human and duck hepatitis viruses were relatively susceptible to disinfection, becoming noninfectious after < or = 10 min of contact with the disinfectant. The Morphological Alteration and Disintegration Test accurately predicted activity in the two infectivity tests. The anti-human hepatitis B virus effect of the low-level quaternary ammonium germicides is a novel finding and suggest that members of the family Hepadnaviridae are relatively susceptible to chemical agents.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bchini R., Capel F., Dauguet C., Dubanchet S., Petit M. A. In vitro infection of human hepatoma (HepG2) cells with hepatitis B virus. J Virol. 1990 Jun;64(6):3025–3032. doi: 10.1128/jvi.64.6.3025-3032.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond W. W., Favero M. S., Petersen N. J., Ebert J. W. Inactivation of hepatitis B virus by intermediate-to-high-level disinfectant chemicals. J Clin Microbiol. 1983 Sep;18(3):535–538. doi: 10.1128/jcm.18.3.535-538.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman S. P., Hutchinson E. P., Scott E. M., McDermott L. M. Death, injury and revival of chemically treated Bacillus subtilis spores. J Appl Bacteriol. 1983 Feb;54(1):91–99. doi: 10.1111/j.1365-2672.1983.tb01305.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Tsuzuki M., Koshimizu K., Toyama H., Yoshihara N., Shikata T., Abe K., Mizuno K., Otomo N., Oda T. Susceptibility of hepatitis B virus to disinfectants or heat. J Clin Microbiol. 1984 Aug;20(2):214–216. doi: 10.1128/jcm.20.2.214-216.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krugman S., Giles J. P., Hammond J. Hepatitis virus: effect of heat on the infectivity and antigenicity of the MS-1 and MS-2 strains. J Infect Dis. 1970 Nov;122(5):432–436. doi: 10.1093/infdis/122.5.432. [DOI] [PubMed] [Google Scholar]
- Moor-Jankowski J., Mahoney C. J. Chimpanzees in captivity: humane handling and breeding within the confines imposed by medical research and testing. Position paper for the Jane Goodall Institute Workshop on Psychological Well-Being of Captive Chimpanzees 1st to 3rd December, 1987. J Med Primatol. 1989;18(1):1–26. doi: 10.1002/ajp.1350180102. [DOI] [PubMed] [Google Scholar]
- Muchmore E. Hepatitis surveillance standards for hepatitis studies in a chimpanzee colony. Dev Biol Stand. 1980;45:13–21. [PubMed] [Google Scholar]
- Murray S. M., Freiman J. S., Vickery K., Lim D., Cossart Y. E., Whiteley R. K. Duck hepatitis B virus: a model to assess efficacy of disinfectants against hepadnavirus infectivity. Epidemiol Infect. 1991 Jun;106(3):435–443. doi: 10.1017/s0950268800067480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedigo N. G., Vernon M. W., Curry T. E., Jr Characterization of a computerized semen analysis system. Fertil Steril. 1989 Oct;52(4):659–666. doi: 10.1016/s0015-0282(16)60982-2. [DOI] [PubMed] [Google Scholar]
- Pugh J. C., Summers J. W. Infection and uptake of duck hepatitis B virus by duck hepatocytes maintained in the presence of dimethyl sulfoxide. Virology. 1989 Oct;172(2):564–572. doi: 10.1016/0042-6822(89)90199-2. [DOI] [PubMed] [Google Scholar]
- Tabor E., Purcell R. H., Gerety R. J. Primate animal models and titered inocula for the study of human hepatitis A, hepatitis B, and non-A, non-B hepatitis. J Med Primatol. 1983;12(6):305–318. [PubMed] [Google Scholar]
- Thraenhart O., Kuwert E. K., Scheiermann N., Dermietzel R., Paar D., Maruhn D., Alberti A., Richter H. J., Hotz J. Comparison of the morphological alteration and disintegration test (MADT) and the chimpanzee infectivity test for determination of hepatitis B virucidal activity of chemical disinfectants. Zentralbl Bakteriol Mikrobiol Hyg B. 1982;176(5-6):472–484. [PubMed] [Google Scholar]
- Tuttleman J. S., Pugh J. C., Summers J. W. In vitro experimental infection of primary duck hepatocyte cultures with duck hepatitis B virus. J Virol. 1986 Apr;58(1):17–25. doi: 10.1128/jvi.58.1.17-25.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenza F. P., Muchmore E. The clinical chemistry of chimpanzees. I. Determination of aminotransferase baseline values for hepatitis studies. J Med Primatol. 1982;11(6):342–351. [PubMed] [Google Scholar]
- Valenza F. P., Muchmore E. The clinical chemistry of chimpanzees: II. Gamma glutamyl transferase levels in hepatitis studies. J Med Primatol. 1985;14(6):305–315. [PubMed] [Google Scholar]