Abstract
A combination of spectroscopies and density functional theory calculations indicate that there are large temperature-dependent absorption spectral changes present in green nitrite reductases (NiRs) due to a thermodynamic equilibrium between a green and a blue type 1 (T1) copper site. The axial methionine (Met) ligand is unconstrained in the oxidized NiRs, which results in an enthalpically favored (ΔH ≈4.6 kcal/mol) Met-bound green copper site at low temperatures, and an entropically favored (TΔS ≈4.5 kcal/mol, at room temperature) Met-elongated blue copper site at elevated temperatures. In contrast to the NiRs, the classic blue copper sites in plastocyanin and azurin show no temperature-dependent behavior, indicating that a single species is present at all temperatures. For these blue copper proteins, the polypeptide matrix opposes the gain in entropy that would be associated with the loss of the weak axial Met ligand at physiological temperatures by constraining its coordination to copper. The potential energy surfaces of Met binding indicate that it stabilizes the oxidized state more than the reduced state. This provides a mechanism to tune down the reduction potential of blue copper sites by >200 mV.
Keywords: electron transfer, reduction potential, thermodynamics, DFT calculation, spectroscopy
Blue copper (also called type 1, T1 copper) (1) active sites are found in a variety of proteins including plastocyanins and azurins that undergo rapid electron transfer (ET) (2–5). The first crystal structures were available for plastocyanin from poplar leaves, which showed an unusual active-site geometry that was a distorted tetrahedron with a short Cu–S(Cys) bond at 2.1 Å, a long Cu–S(Met) bond at 2.8–2.9 Å, and two Cu–N(His) bonds at ≈2.0 Å (6–8). Normally, Cu2+ complexes are square planar because of the Jahn–Teller effect (9), and it was thought that the protein constrained the copper site structure to be in between that of Cu+ (tetrahedral) and Cu2+ (square planar) to facilitate ET. This concept of the protein constraint on the copper site was referred to as an entatic or rack-induced state (10–12). Associated with this unusual active-site structure were novel spectral features relative to normal tetrahedral Cu2+ complexes (1, 13–15). In particular, the blue copper site exhibited an intense lower-energy thiolate π to Cu charge transfer (CT) transition (and a weak higher-energy σ CT transition) reflecting a π bonding interaction of the thiolate with the Cu2+ of the blue copper center (π ground state, Scheme 1A) (16, 17). Normal tetragonal cupric complexes have σ ligand–metal bonds that result in an intense higher-energy σ and a weak lower-energy π ligand to metal CT transition (σ ground state, Scheme 1B) (5).
Scheme 1.
Schematic representation of Cu dx2-y2 and SCys p-orbital ground states of blue copper (plastocyanin, π ground state), resulting in the high-energy weak σ and low-energy strong π CT transitions (A) and normal and green copper (NiR, σ ground state), resulting in the high-energy strong σ and low-energy weak π CT transitions (B).
However, the idea of protein constraint on the oxidized site was questioned both in calculations and spectroscopic studies. Total energy calculations were used to argue that the blue copper ligand set gives a structure similar to the protein site on geometry optimization (18). Photoemission spectroscopic studies on models (19) of the reduced site indicated that the geometric changes predicted based on the change in the electronic structure on oxidation were just those observed experimentally in crystal structure and extended x-ray absorption fine structure (EXAFS) data. In fact it was suggested that the long Cu+–S(Met) bond present in the reduced site was a constraint of the protein. The bond is ≈2.9 Å in the crystal structure of the reduced site (20) rather than the expected distance of ≈2.3 Å (19, 21). The long thioether bond reduces its donor interaction with the copper which is compensated by the thiolate leading to the short Cu–S(Cys) bond of 2.1 Å in the blue copper sites. This long thioether/short thiolate would eliminate all orbital degeneracy of the oxidized site; thus, there would be no Jahn–Teller distortion from the tetrahedral to a tetragonal structure.
The above “coupled distortion” model provided an explanation for a series of blue copper related proteins (i.e., same Cys, Met, 2His ligand set) which changed from blue to green in color in going from plastocyanin to nitrite reductase (NiR) (5, 22, 23). In the latter protein the Cu–S(Met) bond has decreased to 2.45 Å, the Cu–S(Cys) bond has elongated to 2.21 Å, and the S–Cu–S plane has rotated relative to the N–Cu–N plane to a more tetragonal structure (24). Associated with this distortion the thiolate now σ-bonds to the Cu2+ and the CT spectrum exhibits a higher-energy intense σ CT (reflecting a σ ground state) and a lower-energy weaker π CT transition (Scheme 1B), similar to normal tetragonal Cu2+ complexes but inverted relative to the blue copper site in plastocyanin (22).
In our past studies of the green copper site in NiR we observed a large temperature dependence of its absorption spectrum (22). In a study on a loop mutant of Amicyanin, a more limited temperature dependence was observed and assigned to the Boltzmann population of two electronic minima (25). In density functional theory (DFT) studies with a B3LYP hybrid functional, Ryde and coworkers predicated two minima, one similar to the blue copper site and a second similar to the green copper site at comparable energy (26). However, this depends on the functional and amount of Hartree–Fock mixing (5). Based on resonance Raman (rR) data on NiR, two species were suggested to be present (27), but this was refuted by later rR data on the same enzyme (28).
In this study we explore the temperature dependence of the green copper site in NiR by using absorption and rR spectroscopies combined with DFT calculations. These studies show that in contrast to the earlier proposal on the Amicyanin mutant, the temperature dependence in NiR reflects a thermodynamic equilibrium of two species driven by entropy. These studies demonstrate that the thioether–copper bond is unconstrained in NiR and allow us to experimentally determine the strength of this bond. Extension to and the contrast of these results to the behavior of the blue copper site in plastocyanin define the constraints imposed on the blue copper site by the protein and thus provide insight into the entatic/rack nature of the blue copper site and its contribution to ET function.
Results
Low-Temperature Spectroscopy.
The low-temperature absorption spectrum of NiR (Fig. 1, green) is dominated by an intense band at ≈21,700 cm−1 (460 nm) from the T1 site that has been assigned as the S(Cys) → Cu σ charge transfer (CT) transition (22). A weaker S(Cys) → Cu π CT transition is also observed at ≈17,500 cm−1 (570 nm). This is followed by lower-energy ligand field transitions. There is also a S(Met) → Cu CT transition at 25,600 cm−1 (390 nm) (22).
Fig. 1.
Absorption spectra of resting WT NiR at 7 K (green) room temperature, 20 °C (blue).
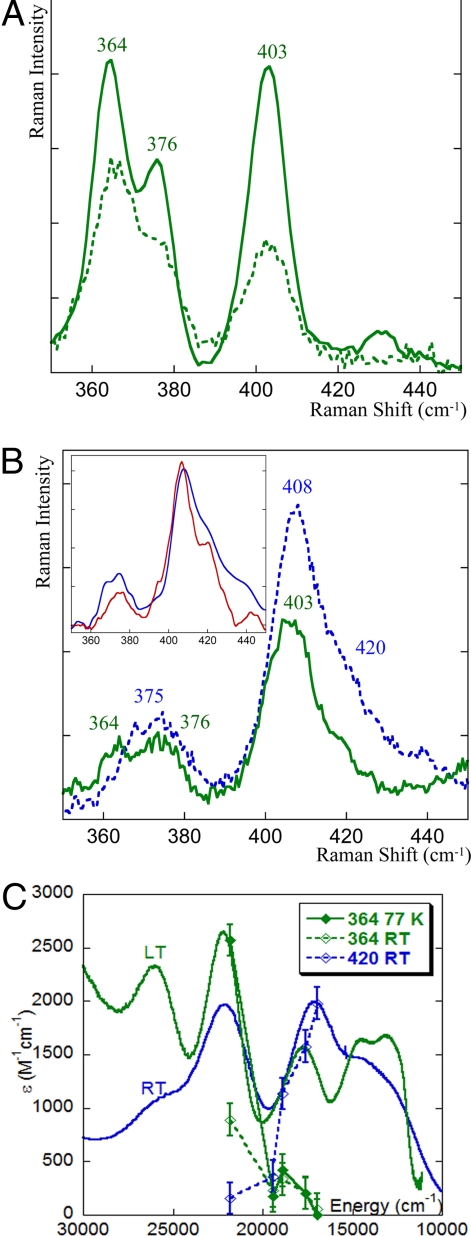
The rR spectrum of NiR excited at 458 nm (into the σ CT band) at 77 K is presented in Fig. 2A (bold green). It has 3 intense peaks at 364, 376, and 403 cm−1 as reported (29). When NiR is excited at 568 nm (into the π CT transition) at 77 K (Fig. 2B, bold green), the same vibrations (364, 376, and 403 cm−1) are enhanced but with a different intensity ratio. This shows that the weak band at 570 nm in the absorption spectrum at low temperature originates from the same species as the 460-nm band, indicating that there is a single species present at 77 K in NiR. This is the green copper center previously defined by spectroscopy (22) and crystallography (24) (reported at 5 K and 100 K, respectively) for this enzyme. Note that the different relative intensities of the vibrations obtained with 568-nm excitation compared with those obtained by excitation into the 458-nm band reflect differences in the π and σ CT excited state distortions (30). The vibrations of the green copper species that are resonance enhanced reflect kinematic mixing of the Cu–S(Cys) vibration over several protein modes, with an effective frequency (intensity-weighted average at 568-nm excitation) (31) of the Cu–S(Cys) bond, i.e., 〈νCu-S〉 of ≈388 cm−1. This is lower than the value for the blue copper sites in plastocyanin where 〈νCu-S〉 = 403 cm−1, reflecting a weaker Cu–S(Cys) bond for the green copper site. The weaker Cu–S(Cys) reflects the stronger donor interaction of the axial Met ligand with the copper in the green site of NiR relative to plastocyanin (Cu–S(Met) bond: 2.45 Å in green copper; 2.8 Å in plastocyanin).
Fig. 2.
Resonance Raman spectra of resting WT NiR excited at 458 nm (A)and 568 nm (B). Data for 77 K are in bold lines and for 25 °C are in dotted lines. (Inset) Resonance Raman spectrum of the pure blue component of NiR (blue spectrum, obtained by subtracting the spectrum of the green species at 77 K, 568 nm from the 25 °C, 568-nm spectrum and renormalization) and of the M182T variant of NiR (red spectrum). (C) Resonance Raman profiles overlaid with the absorption spectra. The 364 cm−1 peak is associated with the green copper species, whereas the 420 cm−1 peak reflects the blue copper component of NiR.
Room Temperature Spectroscopy.
The absorption spectrum at room temperature (Fig. 1, blue) is vastly different from the low-temperature spectrum. There is a significant redistribution of intensity with the band at ≈570 nm having gained intensity and red shifted to ≈600 nm, whereas the absorption band at ≈460 nm has lost ≈50% of its intensity.
As a large temperature dependence was observed in the absorption spectrum of NiR, rR data were also collected at 25 °C. The rR spectrum of NiR, excited at 458 nm at room temperature, has the same features as those obtained at 77 K, but with much lower intensity (Fig. 3A, dotted green), consistent with the loss of absorption intensity of the 460-nm band. However, when NiR is excited at 568 nm at 25 °C, the spectrum changes significantly relative to the low-temperature rR spectrum (Fig. 2B, blue). It now shows an intense peak at 408 cm−1 with a shoulder at 420 cm−1. There is also a broad weak feature at ≈375 cm−1 which lacks the 364 cm−1 feature of the low-temperature green copper site. The vibrational features enhanced by the 568-nm excitation at room temperature are different from those observed at low temperature, for green copper species with the same excitation line and indicate that a second species, in addition to the green copper species, has grown in at elevated temperature.
Fig. 3.
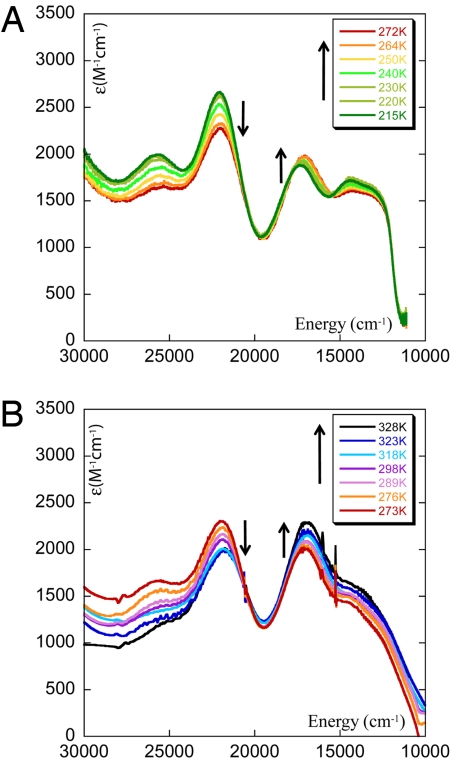
Temperature dependence of the absorption spectra of resting WT NiR in buffer with ethylene glycol (40:60) (A) and in buffer solution (B).
Subtraction of the low-temperature green copper spectrum (scaled down by 50%; see below) from the room temperature data and renormalization yields the spectrum of the pure high-temperature component (Fig. 2B Inset, blue) which has 〈νCu-S〉 of ≈407 cm−1. This is significantly increased from the 〈νCu-S〉 ≈388 cm−1 for the green copper species, indicating that the species that grows in at elevated temperatures has a stronger Cu–S(Cys) bond. A mutant of NiR has been reported where the axial Met ligand has been replaced by a threonine residue (M182T) (29, 32). This mutation results in a blue T1 copper site with 〈νCu-S〉 of ≈408 cm−1. The rR spectrum of the high-temperature species (Fig. 2B Inset, bold blue) is essentially the same as that of the mutant. Note that the rR profile (Fig. 2C) of the 364-cm−1 mode (associated with only the green copper component) indicates that it is strongly enhanced by the 460 nm CT band and only weakly enhanced by the 600-nm CT band. This profiles well with the low-temperature absorption spectrum of the green species, which has an intense σ CT band at 460 nm and a weak π CT band at 570 nm. It decreases in intensity with increasing temperature reflecting the decrease in the amount of green site present. The profile for the 420-cm−1 mode, characteristic of the high-temperature species, shows that it is strongly enhanced by the 600-nm π CT band and not by the 460-nm σ CT band. The very different Raman profile of the 420-cm−1 vibration indicates that it must originate from a second blue copper component of the T1 site in NiR.
The high 〈Cu–S〉 vibration frequency and the intense π CT transition for the room temperature species show that it is a blue copper site as in plastocyanin that has a relatively strong Cu–S(Cys) bond reflecting a relatively weak Cu–S(Met) bond. The 〈νCu-S〉 of ≈407 cm−1 for the blue species in NiR is higher than that of plastocyanin, which has axial Cu–S(Met) ≈2.8 Å (〈νCu-S〉 of ≈403 cm−1) but lower than that of fungal laccases (〈νCu-S〉 of ≈413 cm−1) that has no axial ligand (5). From the coupled distortion model (5, 22, 23) there is a direct correlation between the observed 〈νCu-S〉 associated with the Cu–S(Cys) bond and the Cu-S(Met) bond length. The longer Met has a decreased donor interaction with the copper that is compensated by an increase in the Cu–S(Cys) bond. The 〈νCu-S〉 of the blue component of NiR indicates that its Cu–S(Met) bond length/strength is in-between that of plastocyanin and fungal laccases. Thus, the rR data clearly show the presence of two distinct chromophores (green and blue copper sites) at room temperature and only one chromophore (green copper) at low temperature.
Thermodynamics.
A series of solution absorption spectra were collected over a wide range of temperatures (215–323 K; Fig. 4 A and B). The data show that the intensity of the 460-nm band decreases whereas the intensity of the 600-nm band increases with increasing temperature. This could indicate a Boltzmann population of a second local minimum having a different geometry of the T1 site (as has been proposed in ref. 25) or the presence of two T1 sites in NiR in a thermodynamic equilibrium where one site is enthalpically favored and the other is entropically favored.
Fig. 4.
Plot of ln Keq vs. 1/T, where Keq ≈ 1 at 298 K.
Using the absorption spectrum of a pure green site (absorption spectrum at 7 K in Fig. 1, green) and the data for the M182T mutant of NiR as representative of a blue site (29) the relative amounts of the blue and the green species were obtained from the temperature-dependent absorption spectra of NiR (Fig. 3). Note that at 55 °C (328 K) there are 65% blue copper sites (which continues to increase with temperature) and 35% green sites demonstrating that there is a thermodynamic equilibrium and not a Boltzmann population of two levels, where for the latter a maximum of 50% population of the higher-energy level is possible. The relative populations of the blue and the green copper sites have been used to obtain the equilibrium constant, Keq, for the green to blue conversion at various temperatures (Eq. 1). A plot of the ln Keq vs. 1/T (Fig. 4) resulted in a ΔH ≈ 4.6 kcal/mol from the slope and ΔS ≈ 15 cal/molK (TΔS = 4.5 kcal/mol at 298 K) from the intercept, for the green to blue component conversion of NiR. Thus, the green species is enthalpically favored and the blue species is entropically favored. As a consequence, <215 K (i.e., TΔS = 3.2 kcal/mol), only the green species is present and contributes to the absorption and resonance Raman spectra as Keq is dominated by the ΔH term. The fraction of the blue form increases with temperature and at room temperature (298 K) the Keq between these two forms is close to unity, i.e., ΔG = 0. These observed ΔH and ΔS for the blue/green T1 copper site equilibrium are analyzed by using DFT calculations in the analysis section.
Analysis
Oxidized Site.
The variable temperature spectroscopic data indicate that there are two forms of oxidized T1 sites of NiR that are in a thermodynamic equilibrium. They differ in Cu–S(Cys) bond strength based on the rR data. One has a dominant S(Cys) → Cu σ CT and a relatively weak Cu-S(Cys) bond (〈νCu-S(Cys)〉 = ≈388 cm−1) reflecting a short Cu–S(Met) bond consistent with the structure of the green copper center reported in the low-temperature crystal structure (33). The Cu–S(Cys) bond of the second species, which appears at higher temperature and has a dominant S(Cys) → Cu π CT transition, is stronger (〈νCu-S(Cys)〉 = ≈413 cm−1) than that of the green copper site. In fact, it is even slightly stronger than that of the blue copper site in plastocyanin [which has a Cu–S(Met) distance of 2.8 Å] (8), but weaker than that of the blue copper sites in Fet3p and fungal laccases that have no axial ligand (34–36). This indicates that the blue component in NiR has a very weak Cu–S(Met) bond. Note that the crystal structure of a NiR with atomic resolution (PDB ID code 1OE1) (37) (blue absorption at room temperature, crystal structure at 100 K) reveals that the thioether has two conformations: one with a Cu–S(Met) distance of 2.45 Å (major) and a second with 4.26 Å (minor). The thermodynamic parameters obtained in Results indicate that the green species of NiR is enthalpically favored, whereas the blue species of NiR is entropically favored. DFT calculations are used here to evaluate the bond strength of this axial thioether ligand, and its contribution to the enthalpy and entropy of the oxidized T1 copper site.
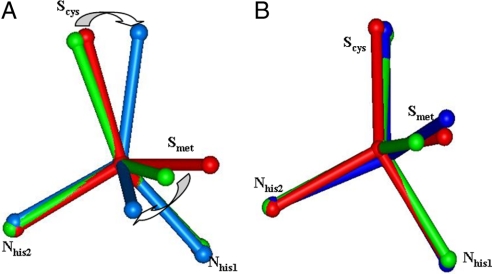
The geometry optimized structure with no constraints (Fig. 5A green) reproduced the crystal structure [PDB ID code 1ZV2 (33) at 100 K; Fig. 5 red] of the green copper site of Rs NiR (the deviations of the calculated bond distances and angles are within 0.04Å and 5°, repectively). It has a flattened tetrahedral (toward square planar) structure with a Cu–S(Cys) distance of 2.18 Å and a Cu–S(thioether) distance of 2.41 Å (Fig. 5A green). On elimination of the thioether ligand, the reoptimized Cu–S(Cys) bond length decreases by 0.05 Å. The copper is now in a trigonal ligand field, and has a structure in reasonable agreement (within 0.04 Å and 3°) with the copper sites found in the fungal laccases and Fet3p. These two structures can be considered to be the limits of the coupled distortion coordinate [i.e., unconstrained relatively strong Cu–S(Met) bond and no Cu–S(Met) bond, respectively].
Fig. 5.
The geometry of oxidized (A) and reduced type 1 Cu sites (B) (only Cu and coordinated N, S atoms are shown for clarity, and the arrows indicate the major angle changes). Red, crystal structure of NiR at 100 K, short Cu–S(Met) bond length; green, fully geometry optimized structure, short Cu–S(Met) bond length; blue, partial geometry-optimized structure with Cu–S(thioether) distance constrained at 4 Å of the long bond length.
The calculated total electronic energy for Cu–S(thioether) bond dissociation for the green copper site is 6.8 kcal/mol. On loss of the thioether (Eq. 2), the enthalpy change is 5.4 kcal/mol (Table 1, top row, in parentheses, where solvent effects were included) and the entropy gain is 39 cal/(mol·K) (TΔS = 11.7 kcal/mol at 298 K; Table 1, top row). The calculated enthalpy change is consistent with the experimental results (ΔHexp = 4.6 kcal/mol). However, the calculated entropy gain is larger than experimentally observed (TΔS = 4.5 kcal/mol at 298 K). This reflects the fact that in the NiR protein the thioether still remains near the active site as it is covalently linked into the polypeptide chain. This is consistent with the lower 〈νCu-S(thiolate)〉 for the blue component of NiR relative to the fungal laccases (no axial ligand) reflecting the presence of a very weak Cu–S(Met) bond.
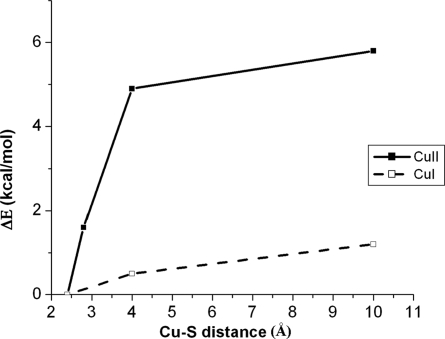
Fig. 6 solid line shows the effect of elongation of the Cu–S(thioether) bond from 2.4 Å of the green site to longer distances (i.e., constrained Cu–S(Met) with optimization of the rest of the structure). By 4 Å (modeling the blue component of NiR) the Met dissociation energy is <2 kcal/mol. Geometry optimization of the structure with the Cu–S(thioether) bond at 4.0 Å leads to a trigonal ligand field with a shorter copper thiolate bond (dCu–S(Cys) = 2.13 Å; Fig. 5A blue) relative to the calculated unconstrained green site (dCu–S(Cys) = 2.18 Å). In going to the calculated 4.0 Å Cu–S(Met) blue structure, the S(Cys)–Cu–S(Met) plane rotates further out of the N(His)–Cu–N(His) plane, which reflects a larger distortion toward a tetrahedral structure. This 4.0-Å blue species has essentially the same structure as the copper site without an axial ligand. On loss of the axial ligand from the 4-Å structure, the entropy change is 20 cal/(mol·K) [TΔS = 6.0 kcal/mol at 298 K (Table 1, bottom row); note that this calculation does not include solvation] which reflects the gain of rotational and translational freedom. This is approximately half the calculated entropy change of 39 cal/(mol·K) (TΔS = 11.7 kcal/mol at 298 K; Table 1, first row) for Met loss from the green species. In going from the green species to the 4.0-Å component, the calculated entropy change is 5.7 kcal/mol (i.e., the difference between the above two entropy terms). This is now consistent with the experimentally determined entropic contribution for the green to blue conversion in NiR (TΔSexp = 4.5 kcal/mol), which favors the blue species at elevated temperature. As shown in supporting information (SI) Fig. S1, this entropy gain reflects an increase in the number of low-energy normal modes in the 4.0-Å blue species (dCu–S(thiether) = 4.0 Å) relative to the green species (dCu–S(thiether) = 2.4 Å). The population of these low-energy modes results in the larger entropic stabilization of the 4.0-Å blue species.
Table 1.
Calculated free energies for loss of an axial Met ligand [constrained at different Cu–S(Met) distances] from an oxidized copper site at room temperature
| Model, Å* | ΔE† | ΔH‡ | ΔS | TΔS | ΔG |
|---|---|---|---|---|---|
| 2.4§ | 10.4 (6.8) | 9.1 (5.4) | 39 (42) | 11.7 (12.5) | −2.6 (7.1) |
| 2.8¶ | 8.9 (5.2) | 7.7 | 35 | 10.4 | −2.7 |
| 4.0¶ | 6.2 (1.9) | 5.5 | 20 | 6.0 | −0.2 |
*Oxidized Cu site model: [Cu(Im)2(SCH3)(S(CH3)2)]+.
†Total electronic energy change for loss of Met ligand.
‡ΔE with zero-point and thermal corrections included: H = Eelectronic + EZPE + Evib + Erot + Etrans + RT. ZPE = zero point energy. The results of PCM calculations are shown in the parentheses. ΔE, ΔH, TΔS, and ΔG are in kcal/mol, and ΔS is in cal/mol·K.
§The dCu–S(thioether) distance is obtained from full geometry optimization.
¶The dCu–S(thioether) distance is constrained as indicated.
Fig. 6.
Potential energy surface as a function of Cu–S(thioether) distance (solvation included using a PCM with ε = 4.0). Solid line, oxidized Cu site; dashed line, reduced Cu site.
We now consider the blue copper site in plastocyanin with the Cu–S(Met) constrained at 2.8 Å. The optimized structure reproduces the classic blue copper site in plastocyanin, with the copper in a trigonal ligand field and a short copper thiolate bond (dCu–S(Cys) = 2.15 Å). The calculated enthalpy and entropy changes for the Met loss for the plastocyanin blue copper site are essentially the same as for the green species (Table 1, first and second row), where plastocyanin is enthalpically destabilized by ≈1 kcal/mol. Importantly, if plastocyanin were unconstrained one would expect a similar behavior to that of NiR with loss of the axial ligand due to increased entropy at elevated temperature. However, experimentally, the oxidized blue copper sites in plastocyanins and related proteins show no temperature-dependent absorption behavior. This reflects constraints by the protein environment on the plastocyanin active site, referred to as the entatic/rack state (see below).
Reduced Site.
Full optimization of the reduced model of the green copper site gave a tetrahedral ligand field with a Cu–S(thioether) distance of 2.5 Å (Fig. 5B green), similar to the crystal structure of the reduced NiR at 100 K (33) (Fig. 5B red). Removal of the thioether ligand and reoptimization gives a geometry very similar to that of the reduced T1 site in Fet3p, with the copper ion in the N(His)–N(His)–S(Cys) plane. Elongation of the thioether bond results in a very small energy change (ΔE ≈ 1 kcal/mol; Fig. 6, dashed line). This leads to a very small enthalpy change ((ΔH ≈ 1 kcal/mol) associated with the loss of an axial Met bond. However, the entropy change for this process is similar to that of the oxidized copper site (TΔS = 10.9 kcal/mol at 298 K; Table 2, first row). Further, in comparing the results for the fully optimized structure to those for the constrained dCu–S(thiether) = 4.0 Å structure, the entropy change is ≈4.5 kcal/mol at 298 K [TΔS = 10.9 kcal/mol (Table 2, top row) and 6.4 kcal/mol (Table 2, bottom row); note these calculations do not include solvation], indicating a similar entropy change in going from a green to a blue site in the reduced NiR as determined above for the oxidized site. Thus, for the reduced site the entropy term should be dominant and the Met ligand should not be bound to the copper at room temperature. A low ΔH and large TΔS is also calculated for loss of a Met ligand for the reduced blue copper site in plastocyanin [Table 2, second row: the values for the plastocyanin site (2.9 Å) to convert to a 4-Å Cu–S(Met) distance (as in NiR) are ΔH = 0.6 kcal/mol, TΔS = 3.7 kcal/mol]. The fact that this does not occur based on the room temperature crystal structure (20, 38) again reflects a protein constraint on the reduced copper site in plastocyanin.
Table 2.
Calculated free energies for loss of an axial Met ligand [constrained at different Cu–S(Met) distances] from a reduced copper site at room temperature
| Model, Å* | ΔE† | ΔH‡ | ΔS | TΔS | ΔG |
|---|---|---|---|---|---|
| 2.5§ | 5.8 (0.5) | 4.7 (0) | 37 (38) | 10.9 (11.3) | −6.2 (11.9) |
| 2.9¶ | 5.0 (0.4) | 3.8 | 34 | 10.1 | −6.3 |
| 4.0¶ | 4.6 (0) | 3.2 | 21 | 6.4 | −3.2 |
*Reduced Cu site model: [Cu(Im)2(SCH3)(S(CH3)2)].
†Total electronic energy change for loss of Met ligand.
‡ΔE with zero-point and thermal corrections included: H = Eelectronic + EZPE + Evib + Erot + Etrans + RT. ZPE = zero point energy. The results of PCM calculations are shown in the parentheses. ΔE, ΔH, TΔS, and ΔG are in kcal/mol, and ΔS is in cal/mol·K.
§The dCu–S(thioether) distance is obtained from full geometry optimization.
¶The dCu–S(thioether) distance is constrained as indicated.
Discussion
The oxidized green copper site of NiR at low temperature is tetragonally distorted with a strong predominantly σ bonding interaction of the thiolate ligand with the copper. Temperature-dependent rR and absorption data indicate that the thioether ligand in NiR partially dissociates at higher temperatures resulting in a trigonal blue copper site, which has a strong π bonding interaction of the thiolate ligand with the copper. Experimentally the Cu2+-thioether bond strength in NiR has been determined here to be 4.6 kcal/mol, and at room temperature the gain in entropy (TΔS = 4.6 kcal/mol) on thioether dissociation approximately equals the enthalpy change due to loss of this ligand which results in the formation of a ≈1:1 mixture of green and blue copper species. This indicates that the T1 site in NiRs is unconstrained, allowing the association and dissociation of the Met ligand at low and high temperatures, respectively. The relatively unconstrained nature of the T1 site of NiR may reflect the long Cys–Xn–His loop, which would allow flexibility and limit intraloop H-bonding. DFT calculations reproduce the experimentally observed ΔH and ΔS of this process. Further, in the reduced state, calculations indicate that the axial Met–Cu bond is very weak with a ΔH of ≈1 kcal/mol for dissociation. However, the TΔS is comparable to that of the oxidized site (≈4.6 kcal/mol observed experimentally, at room temperature) and hence is the dominant contributor to ΔG at room temperature for the reduced site. Thus, whereas the oxidized form exists as a 1:1 mixture of blue and green species, the reduced T1 site in NiR likely exists as one species with no thioether-Cu bonding interaction at room temperature.
It is important to emphasize that our model for the large temperature dependence in NiR is conceptually different from that invoked previously to explain the more limited temperature dependence of absorption data in a loop mutation of Amicyanin (25). It had been proposed that there is a low-lying second electronic minimum of the T1 site that becomes Boltzmann populated with increasing temperature. However, this only allows for the maximum of a 1:1 population of the two species at high temperatures. Alternatively, our experimental results show that at elevated temperatures (55 °C, 328 K) the population of the blue site is 65 ± 5%, indicating that there is in fact a thermodynamic equilibrium between the two species.
Classic blue copper sites like plastocyanin have elongated Met–Cu bonds at ≈2.8 Å and do not show the large temperature-dependent behavior observed for NiR. These results show that, in contrast to the green/blue equilibrium of sites in NiR, the thioether is constrained in plastocyanin. Importantly, in plastocyanins the Met ligand stays bound at room temperature in both oxidation states. Having a constrained thioether ligand has been referred to as an entatic (11) or rack state (10) for these classic blue copper sites. From the crystal structures (7, 20) and spectroscopies (19) the thioether is bound to the copper at similar distances in both oxidation states. Thus, the protein opposes the entropy effect of loss of a relatively weak Met ligand. From Fig. 6 and Tables 1 and 2, the Met has a stronger bonding interaction with the oxidized than the reduced site. This can lead to a >200 mV lowering of the reduction potential in these classic blue copper sites relative to sites without the axial methionine ligand. Indeed the fungal laccases and Fet3p have reduction potentials that range from 470 to 770 mV, higher than those of plastocyanin (370 mV) and azurin (310 mV).
The green T1 copper sites in NiRs have a dominant σ ground state at low temperature and at elevated temperatures a blue T1 copper site is also present with a dominant π ground state (Scheme 1). This is in contrast with other “perturbed” T1 copper sites like Cucumber Basic Blue (CBB) and Pseudoazurin (PA) that have both σ and π character (23) but no temperature-dependent behavior (30). This implies that there is a single species at all temperatures and the thioether remains bound to the copper in these active sites, although at a shorter distance relative to plastocyanin, which leads to the mixed π/σ character. Thus, these sites are also constrained and represent different points along the coupled distortion coordinate. This can play a role in fine tuning the reduction potential of these sites because these have different ΔEs of the Cu–S(Met) bond (Fig. 6).
In summary, the large temperature dependence observed in the absorption and rR spectra in green NiRs reflect an unconstrained T1 site where entropy favors the dissociation of the relatively weak axial Met ligand at higher temperature. The ΔH and TΔS of Met binding are comparable in the oxidized state (≈4.5 kcal/mol), whereas, the ΔH in the reduced state is much lower. For the blue copper sites in the plastocyanins and azurins, the protein overcomes the entropy increase associated with ligand loss. This stabilizes the oxidized more than the reduced site and can tune the reduction potential down by >200 mV.
Materials and Methods
Materials.
All reagents were of the highest grade commercially available and used without further purification. Rhodobacter sphaeroides (Rs) NiR was isolated and purified (pH ≈ 7.2) as reported (32, 39, 40). Glassed samples for low-temperature absorption experiments were prepared by adding 50% (vol/vol) buffer/glycerol-(O-d)3 or buffer/ethylene glycol. Addition of glycerol/ethylene glycol had no effect on the EPR spectrum. The approximate concentration of samples used for spectroscopy was 0.5 mM in phosphate buffer, pH ≈ 7.1. A temperature-independent buffer (41) was also used for the spectroscopic studies and exhibited identical results as those in phosphate buffer. Additionally we have studied the pH effect on Rs NiR and have observed that the T1 site is unperturbed with change in pH. For spectroscopic details see SI Text.
Computational Details.
DFT calculations (spin unrestricted for Cu2+) were performed by using Gaussian 03 (42). Geometries were optimized by using a hybrid functional involving BP86 (43, 44) with 38% Hartree–Fock exchange, tight SCF convergence criteria (10−8 au) and a mixed triple-zeta/double zeta (TZVP on Cu and S and 6–31G* on the other atoms) basis set (45). Wave function stability checks were performed to confirm that the calculations corresponded to the ground state. For the potential energy surfaces, single-point calculations using a polarized continuum method (PCM) (46) were applied to the geometry optimized vacuum structures. X-ray structure of plastocyanin or NiR [PDB ID codes 1PLC (7) and 1OE1 (47), respectively] were used to obtain starting coordinates. Computational models are discussed in SI Text.
Supplementary Material
Acknowledgments.
This work was supported by National Science Foundation Grant CHE 0446304 (to E.I.S.) and National Institutes of Health Grant EB00326929 (to C.P.S). S.G. and X.X. received William S. Johnson fellowships.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0900995106/DCSupplemental.
References
- 1.Malkin R, Malmstrom BG. The state and function of copper in biological systems. Adv Enzymol Relat Areas Mol Biol. 1970;33:177–244. doi: 10.1002/9780470122785.ch4. [DOI] [PubMed] [Google Scholar]
- 2.Adman ET. Copper protein structures. Adv Protein Chem. 1991;42:145–197. doi: 10.1016/s0065-3233(08)60536-7. [DOI] [PubMed] [Google Scholar]
- 3.Gray HB. Long-range electron-transfer in blue copper proteins. Chem Soc Rev. 1986;15(1):17–30. [Google Scholar]
- 4.Holm RH, Kennepohl P, Solomon EI. Structural and functional aspects of metal sites in biology. Chem Rev (Washington, DC) 1996;96:2239–2314. doi: 10.1021/cr9500390. [DOI] [PubMed] [Google Scholar]
- 5.Solomon EI, Szilagyi RK, George SD, Basumallick L. Electronic structures of metal sites in proteins and models: Contributions to function in blue copper proteins. Chem Rev (Washington, DC) 2004;104(2):419–458. doi: 10.1021/cr0206317. [DOI] [PubMed] [Google Scholar]
- 6.Colman PM, et al. X-ray crystal structure analysis of plastocyanin at 2.7 Å resolution. Nature. 1978;272:319–324. [Google Scholar]
- 7.Guss JM, Bartunik HD, Freeman HC. Accuracy and precision in protein structure analysis: restrained least-squares refinement of the structure of poplar plastocyanin at 1.33 ang resolution. Acta Crystallogr B Struct Sci B. 1992;48(6):790–811. doi: 10.1107/s0108768192004270. [DOI] [PubMed] [Google Scholar]
- 8.Guss JM, Freeman HC. Structure of oxidized poplar plastocyanin at 1.6 A resolution. J Mol Biol. 1983;169(2):521–563. doi: 10.1016/s0022-2836(83)80064-3. [DOI] [PubMed] [Google Scholar]
- 9.Jahn H, Teller E. Stability of degenerate electronic states in polyatomic molecules. Phys Rev A. 1936;49:874. [Google Scholar]
- 10.Malmstrom BG. Rack-induced bonding in blue-copper proteins. Eur J Biochem. 1994;223(3):711–718. doi: 10.1111/j.1432-1033.1994.tb19044.x. [DOI] [PubMed] [Google Scholar]
- 11.Williams RJP. Energised (entatic) states of groups and of secondary structures in proteins and metalloproteins. Eur J Biochem. 1995;234(2):363–381. doi: 10.1111/j.1432-1033.1995.363_b.x. [DOI] [PubMed] [Google Scholar]
- 12.Gray HB, Malmstrom BG, Williams RJP. Copper coordination in blue proteins. J Biol Inorg Chem. 2000;5(5):551–559. doi: 10.1007/s007750000146. [DOI] [PubMed] [Google Scholar]
- 13.Malmstrom BG, Reinhammar B, Vanngard T. The state of copper in stellacyanin and laccase from the lacquer tree Rhus vernicifera. Biochim Biophys Acta. 1970;205(1):48–57. doi: 10.1016/0005-2728(70)90060-5. [DOI] [PubMed] [Google Scholar]
- 14.Gray HB, Solomon EI. Electronic structures of blue copper centers in proteins. Metal Ions Biol. 1981;3:1–39. [Google Scholar]
- 15.Solomon EI, Lowery MD. Electronic structure contributions to function in bioinorganic chemistry. Science. 1993;259(5101):1575–1581. doi: 10.1126/science.8384374. [DOI] [PubMed] [Google Scholar]
- 16.Gewirth AA, Solomon EI. Electronic structure of plastocyanin: Excited state spectral features. J Am Chem Soc. 1988;110(12):3811–3819. [Google Scholar]
- 17.Solomon EI, et al. Electronic structure of the oxidized and reduced blue copper sites: Contributions to the electron transfer pathway, reduction potential, and geometry. Inorg Chim Acta. 1996;243(1–2):67–78. [Google Scholar]
- 18.Ryde U, Olsson MHM, Pierloot K, Roos BO. The cupric geometry of blue copper proteins is not strained. J Mol Biol. 1996;261(4):586–596. doi: 10.1006/jmbi.1996.0484. [DOI] [PubMed] [Google Scholar]
- 19.Guckert JA, Lowery MD, Solomon EI. Electronic structure of the reduced blue copper active site: Contributions to reduction potentials and geometry. J Am Chem Soc. 1995;117(10):2817–2844. [Google Scholar]
- 20.Guss JM, Harrowell PR, Murata M, Norris VA, Freeman HC. Crystal structure analyses of reduced (copper I) poplar plastocyanin at six pH values. J Mol Biol. 1986;192(2):361–387. doi: 10.1016/0022-2836(86)90371-2. [DOI] [PubMed] [Google Scholar]
- 21.Solomon EI, Randall DW, Glaser T. Electronic structures of active sites in electron transfer metalloproteins: Contributions to reactivity. Coord Chem Rev. 2000;200:595–632. [Google Scholar]
- 22.LaCroix LB, et al. Electronic structure of the perturbed blue copper site in nitrite reductase: Spectroscopic properties, bonding, and implications for the entatic/rack state. J Am Chem Soc. 1996;118(33):7755–7768. [Google Scholar]
- 23.LaCroix LB, et al. Spectroscopic and geometric variations in perturbed blue copper centers: Electronic structures of stellacyanin and cucumber basic protein. J Am Chem Soc. 1998;120(37):9621–9631. [Google Scholar]
- 24.Adman ET, Godden JW, Turley S. The structure of copper-nitrite reductase from Achromobacter cycloclastes at five pH values, with NO2- bound and with type II copper depleted. J Biol Chem. 1995;270(46):27458–27474. doi: 10.1074/jbc.270.46.27458. [DOI] [PubMed] [Google Scholar]
- 25.Comba P, Mueller V, Remenyi R. Interpretation of the temperature-dependent color of blue copper protein mutants. J Inorg Biochem. 2004;98(5):896–902. doi: 10.1016/j.jinorgbio.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 26.Pierloot K, DeKerpel JOA, Ryde U, Olsson MHM, Roos BO. Relation between the structure and spectroscopic properties of blue copper proteins. J Am Chem Soc. 1998;120(50):13156–13166. [Google Scholar]
- 27.Dooley DM, Moog RS, Liu MP, Payne WJ, LeGall J. Resonance Raman spectra of the copper-sulfur chromophores in Achromobacter cycloclastes nitrite reductase. J Biol Chem. 1988;263(29):14625–14628. [PubMed] [Google Scholar]
- 28.Han J, et al. Resonance Raman excitation profiles indicate multiple Cys Cu charge transfer transitions in type 1 copper proteins. J Am Chem Soc. 1993;115(10):4256–4263. [Google Scholar]
- 29.Basumallick L, et al. Spectroscopic Studies of the Met182Thr mutant of nitrite reductase: Role of the axial ligand in the geometric and electronic structure of blue and green copper sites. J Am Chem Soc. 2003;125(48):14784–14792. doi: 10.1021/ja037232t. [DOI] [PubMed] [Google Scholar]
- 30.Xie X, et al. A variable temperature spectroscopic study on P. pantotrophus Pseudoazurin: Effects of protein constraints on the blue Cu site. J Inorg Biochem. doi: 10.1016/j.jinorgbio.2009.04.012. in press. [DOI] [PubMed] [Google Scholar]
- 31.Blair DF, et al. Resonance Raman studies of blue copper proteins: Effects of temperature and isotopic substitutions. Structural and thermodynamic implications. J Am Chem Soc. 1985;107(20):5755–5766. [Google Scholar]
- 32.Olesen K, et al. Spectroscopic, kinetic, and electrochemical characterization of heterologously expressed wild-type and mutant forms of copper-containing nitrite reductase from Rhodobacter sphaeroides 2.4.3. Biochemistry. 1998;37(17):6086–6094. doi: 10.1021/bi971603z. [DOI] [PubMed] [Google Scholar]
- 33.Jacobson F, et al. Structures of the oxidized and reduced forms of nitrite reductase from Rhodobacter sphaeroides 2.4.3 at high pH: Changes in the interactions of the type 2 copper. Acta Crystallogr D. 2005;61(9):1190–1198. doi: 10.1107/S0907444905017488. [DOI] [PubMed] [Google Scholar]
- 34.Ducros V, et al. Crystal structure of the type-2 Cu depleted laccase from Coprinus cinereus at 2.2 Å resolution. Nat Struct Biol. 1998;5:310–316. doi: 10.1038/nsb0498-310. [DOI] [PubMed] [Google Scholar]
- 35.Solomon EI, Sundaram UM, Machonkin TE. Multicopper oxidases and oxygenases. Chem Rev. 1996;96(7):2563–2606. doi: 10.1021/cr950046o. [DOI] [PubMed] [Google Scholar]
- 36.Taylor AB, Stoj CS, Ziegler L, Kosman DJ, Hart PJ. The copper-iron connection in biology: Structure of the metallo-oxidase Fet3p. Proc Natl Acad Sci USA. 2005;102(43):15459–15464. doi: 10.1073/pnas.0506227102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ellis MJ, Dodd FE, Sawers G, Eady RR, Hasnain SS. Atomic resolution structures of native copper nitrite reductase from Alcaligenes xylosoxidans and the active site mutant Asp92Glu. J Mol Biol. 2003;328(2):429. doi: 10.1016/s0022-2836(03)00308-5. [DOI] [PubMed] [Google Scholar]
- 38.Inoue T, et al. Structure comparison between oxidized and reduced plastocyanin from a fern, Dryopteris crassirhizoma. Biochemistry. 1999;38(42):13853–13861. doi: 10.1021/bi990502t. [DOI] [PubMed] [Google Scholar]
- 39.Veselov A, Olesen K, Sienkiewicz A, Shapleigh JP, Scholes CP. Electronic structural information from Q-band ENDOR on the type 1 and type 2 copper liganding environment in wild-type and mutant forms of copper-containing nitrite reductase. Biochemistry. 1998;37(17):6095–6105. doi: 10.1021/bi971604r. [DOI] [PubMed] [Google Scholar]
- 40.Zhao Y, et al. Catalytic function and local proton structure at the type 2 copper of nitrite reductase: The correlation of enzymatic pH dependence, conserved residues, and proton hyperfine structure. Biochemistry. 2002;41(23):7464–7474. doi: 10.1021/bi0256274. [DOI] [PubMed] [Google Scholar]
- 41.Sieracki NY, Hwang HJ, Lee MK, Garner DK, Lu Y. A temperature independent pH (TIP) buffer for biomedical biophysical applications at low temperatures. Chem Commun (Cambridge, UK) 2008;7:823–825. doi: 10.1039/b714446f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frisch MJ, et al. Gaussian 03. Wallingford, CT: Gaussian; 2004. Revision C. 02. [Google Scholar]
- 43.Becke AD. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev A. 1988;38:3098. doi: 10.1103/physreva.38.3098. [DOI] [PubMed] [Google Scholar]
- 44.Perdew JP. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys Rev B. 1986;33:8822. doi: 10.1103/physrevb.33.8822. [DOI] [PubMed] [Google Scholar]
- 45.Szilagyi RK, Metz M, Solomon EI. Spectroscopic calibration of modern density functional methods using [CuCl4]2- J Phys Chem A. 2002;106(12):2994–3007. [Google Scholar]
- 46.Cossi M, Scalmani G, Rega N, Barone V. New developments in the polarizable continuum model for quantum mechanical and classical calculations on molecules in solution. J Chem Phys. 2002;117:43. [Google Scholar]
- 47.Ellis MJ, Dodd FE, Sawers G, Eady RR, Hasnain SS. Atomic resolution structures of native copper nitrite reductase from Alcaligenes xylosoxidans and the active site mutant Asp92Glu. J Mol Biol. 2003;328(2):429–438. doi: 10.1016/s0022-2836(03)00308-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.