Abstract
Purpose
To investigate the intraocular properties and toxicity of IMS2186, a small molecule developed as an anti-choroidal neovascularization (anti-CNV) drug.
Materials and Methods
Cellular toxicity and mechanism of action was tested on cell lines in vitro. Intraocular studies used rabbits for drug dissolution as well as toxicity and rats for the treatment study as well as the toxicity confirmation study. Rabbits' eyes were injected with 2.5 mg of IMS2186 and observed for 36 weeks. Laser-induced CNV in rats was treated with IMS2186, Kenalog, or phosphate-buffered saline (pBS). Fluorescein angiography (FA) and immunohistochemical processing of the globes was performed.
Results
The anti-proliferative IC50 of IMS2186 for human fibroblast cells was 1.0–3.0 μM and 0.3–3.0 μM for human cancer cells; the IC50 of IMS2186 to inhibit endothelial tube formation was 0.1–0.3 μM. The IC50 of IMS2186 for inhibiting the production of pro-inflammatory cytokines was 0.3–1 μM. The IC50 of IMS2186 for inhibiting macrophage migration was 1 μM. These biological properties were not species specific. IMS2186 can be formulated as a suspension for long-lasting release and when delivered intraocularly, no intraocular toxicity was observed by slit lamp exam, fundus exam, intraocular pressure measurements, or by electroretinography. FA showed a reduction in the leakage in eyes treated with IMS2186 and triamcinolone acetonide; DAPI staining also showed significantly less cellularity in IMS2186-treated lesions as compared to PBS (p = 0.0025).
Conclusion
IMS2186 may be a safe intraocular therapeutic agent for intraocular proliferation and angiogenesis.
Keywords: age-related macular degeneration, anti-angiogenic treatment, anti-proliferative treatment, choroidal neovascularization, retina
Introduction
Age-related macular degeneration (AMD) with choroidal neovascularization (CNV) is the leading cause of irreversible vision loss in the elderly population.1,2 A few intravitreal anti-angiogenic medications have been introduced in the last few years, such as pegaptanib sodium (Macugen)3 ranibizumab (Lucentis),4 and bevacizumab (Avastin),5,6 and have shown lesion stabilization or moderate improvement in vision. Although showing benefits, the most effective of these medications are disadvantageous in that they require monthly injections. Also, the clinical use of these treatments, which solely inhibit the vascular endothelial growth factor (VEGF), do not prevent CNV recurrence. This may explain the fact that many eyes do not recover good vision even after long periods of treatment.7
Our goal in this study was to develop a compound with a low solubility to sustain tissue drug levels for a prolonged period of time–and clinically significant anti- proliferative and anti-angiogenic properties. Such a compound could be used for long-term treatment of CNV in AMD, as well as for other proliferative retinal disorders such as proliferative vitreoretinopathy (PVR) or ocular trauma.
IMS2186 is a novel synthetic compound developed as an anti-CNV drug. The mechanism of action, which is still under investigation, is proposed to be the blocking of the cell cycle at G2, and the inhibition of the production PGE2/TNF-a, the latter contributing to anti-inflammatory and anti-angiogenic effects. The purpose of our study was to evaluate the toxicity and intraocular properties of IMS2186 for possible long-acting intraocular use in CNV treatment.
Methods
Physical and Chemical Characterization of IMS2186
IMS2186, or 3-[1-(3-hydroxy-4-methoxyphenyl)-meth-(E)-ylidene]-6-methyl-chroman-4-one, is a nonsteroidal small molecule of 296.33 daltons (Fig. 1) in the form of a fine yellow powder. It can be readily prepared as uniform micronized (diameter <10 μg) crystals by MC fluid jet mill (JetPharma, South Plainfield, NJ, USA) without change in the crystalline structure during the process. The crystal structure of IMS2186, with or without micronization, was also found to be stable in bulk and as a suspension in phosphate-buffered saline (pBS) containing 0.5% carboxylmethyl cellulose for a long duration at room temperature (>6 months). IMS2186 has the melting point of 161°C. It has limited solubility of ∼8 μM in PBS and ∼50 μM in PBS containing 0.5% carboxylmethyl cellulose solution. It has an estimated Log P of 2.9 and has a maximal ultraviolet (UV) absorption at 222 nm. Furthermore, although metabolically stable in plasma (in vitro), it seems to be metabolized quickly when exposed to liver enzymes, as shown in an in vitro rat liver microsome assay, in which a 1-hr incubation of IMS2186 with rat liver microsomal fraction in vitro resulted in an 82% disappearance of this compound.8
FIGURE 1.
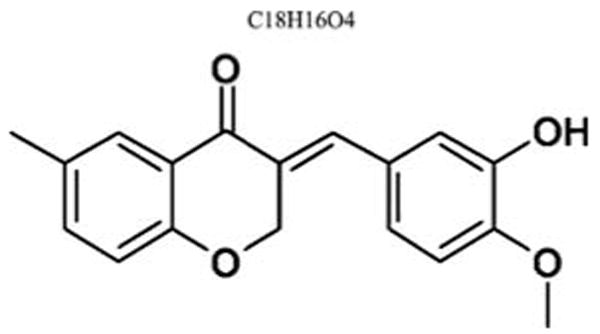
Chemical structure of IMS2186 (3-[1-(3-hydroxy-4-methoxyphenyl)-meth-(E)-ylidene]-6-methyl-chroman-4-one).
Cells
Human fibroblast IMR90, mouse fibroblast NIH3T3, human retinal pigmented epithelium ARPE-19, and the human lung cancer cell line A549 were cultured in modified Eagle's medium (MEM, Invitrogen, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS, Invitrogen) and 0.2% glucose. Human cancer cell lines A2780 and NCI-H460 were cultured in RPMI 1640 (Invitrogen), supplemented with 10% FBS and 2 mM L-Glu. Cells were maintained in a humidified incubator with 5% CO2 at 37°C. HeLa cells were cultured in DMEM (Invitrogen) supplemented with 10% FBS and 2 mM L-Glu. All cell lines were obtained from American Type Culture Collection.
Anchorage-dependent growth (normal liquid cell culture) in 96-well plates was described previously.9 Briefly, 100 μl of cell suspension containing 1–4.0 × 103 cells were plated into each well of a 96-well flat-bottom microtiter plate. The cells were allowed to grow for three days before the cell growth was measured using AlamarBlue staining (recommended 1:10 reagent: cell suspension volume; Trek Diagnostic Systems, Cleveland, OH, USA).
Cell Cycle Analysis
Cell cycle analysis was previously described.9 Briefly, adherent cells, with or without treatment, were detached from the culture dishes using a solution of proteolytic and collagenasic enzymes (Accutase, Innovation Cell Technology, Iowa City, IA, USA) and washed with PBS. Cells were then fixed in 75% (v/v) ethanol at 4°C for at least 1 hr. The cells were washed once with PBS, followed by incubation in PBS containing 40 μg/ml propidium iodine (pI) (BD Bioscience, San Jose, CA, USA) for 15 min at room temperature in the dark. Stained nuclei were analyzed by flow cytometry with 20,000 events per determination.
In Vitro Tube Formation Assay
The anti-angiogenesis activity of IMS2186 was tested in vitro by the Matrigel tube formation assay10 using HUVEC (human umbilical vein endothelial cells). Briefly, 50 ml of Matrigel (BD Biosciences, San Jose, CA, USA) was used to coat 96-well microtiter tissue culture plates. The plates were incubated at 37°C for 30 min to allow the Matrigel to settle down and solidify. 3 × 104 HUVEC cells, mixed with the indicated concentrations of IMS2186, were seeded on top of the Matrigel in each well. Vascular endothelial growth factor (VEGF) was added to the cells at 10 ng/ml to stimulate the angiogenesis tube formation. After incubation, at 37°C for 22 hr, tube formation was observed and manually quantified by counting branch points under the microscope.
In Vivo Matrigel Plug Assay
The Matrigel plug angiogenesis assay is a routinely utilized method to evaluate the anti-angiogenic activities of compound in vivo. The anti-angiogenesis activity of IMS2186 was tested using the Matrigel plug assay in mice. 20 μg IMS2186 and 1 μg VEGF were pre-mixed with 0.5 ml Matrigel (BD Biosciences), and the gel mixture was subcutaneously injected into the right flank of nude mice. The gel immediately solidified into a Matrigel plug in vivo due to body temperature. One week post-dosing, the Matrigel plugs in the mice were harvested, and the hemoglobin contents were measured using the Drabkin reagent. Matrigel alone or Matrigel mixed with VEGF were used as negative and positive controls, respectively.
Chamber Cell Migration Assay
The ability of IMS2186 to inhibit immune cell (macrophage) migration was examined using a Boyden chamber-based assay system. A metastatic and migratory melanoma cell line, A2058, was tested as a model for IMS2186 effect in the wound-healing experiment. The QCM™ Chemotaxis 5-μm 96-well cell migration kit (Cat. No. ECM512, Chemicon International, Temecula, CA, USA) was used to assay for chemotaxis. 150 μl media containing MCP-1 (∼10 ng/ml) and different concentrations of IMS2186 were transferred to the 96-well feeder tray (bottom). A cell migration chamber plate, with 5.0 μM pore, was placed over the 96-well feeder tray. A volume of 100 μl of 150,000 THP-1 cells/well, with different concentrations of IMS2186 (same concentration as the corresponding wells in the feeder tray), was transferred to each well on the cell migration chamber plate. The 96-well migration plate was incubated for 1 hr and 30 min in a 37°C and 5% CO2 incubator. After incubation, the migration chamber plate was removed from the plate assembly, and 15 μl of AlamarBlue (Dal1100, Biosource, Camarillo, CA, USA) was added into each well of the feeder tray containing migrated cells. The plate was incubated for 2 hr in a 37°C and 5% CO2 incubator and read using a cytofluor plate reader, Excitation 530 nm, Emission 590 (series 4000).
PGE2 Production Assay
The IMS2186 effect on the LPS-induced PGE2 production from macrophage in vitro was analyzed using a PGE2 ELISA kit (Assay Designs, Ann Arbor, MI, USA). Briefly, THP-1 cells were seeded in a 48-well flat-bottom cell culture plate at the density of 50,000 cells/well. 10 ng/ml PMA was added to the culture medium to induce the differentiation of THP-1 cells. Forty-eight hours later, the cells were fully differentiated and formed monolayers. Different concentrations of IMS2186 in fresh cell culture medium were added to the cells and incubated for 1 hr in a 37°C and 5% CO2 incubator, followed by the addition of 10 ng/ml LPS to the cells. Twenty hours later, cell medium was collected and PGE2 levels in the medium were measured by ELISA.
Intraocular Properties of IMS2186
All animals used in this study were handled in accordance with the guidelines of the University of California San Diego, Office of Veterinary Affairs, and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Intraocular Dissolution and Toxicity of IMS2186
A suspension of raw, non-micronized IMS2186 powder was prepared in 0.5% carboxylmethyl cellulose phosphate-buffered saline (pBS), which smoothly passed through a 27-gauge needle and thus could be used for intravitreal injection.
Six rabbits were used to evaluate the time for complete dissolution of the compound in the eye; only one eye of each rabbit was injected with 2.5 mg of IMS2186 in a volume of 0.05 ml, while the fellow eye was injected with PBS containing 0.5% carboxymethylcellulose and used as control. After injection, the eyes were monitored with indirect ophthalmoscopy, tonometry, slit-lamp biomicroscopy, and fundus photography. The rabbits were observed for 36 weeks before sacrifice, and the size of the vitreous drug depot was documented in relation to the size of the optic nerve head using indirect ophthalmoscopy. Periodic slit lamp, fundus examination, and intraocular pressures were obtained to rule out toxicity as previously described.11,12 Standard full-field electroretinograms (ERGs) were obtained from each rabbit (both eyes) at 4 weeks post injection and at 36 weeks, just before sacrifice,12 in order to evaluate short- and long-term retinal function. Eyes were dilated with 2.5% phenylephrine hydrochloride and 0.5% tropicamide and then dark-adapted for 30 min. The animals were anesthetized with a mixture of ketamine (16 mg/kg) and xylazine (5 mg/kg). The eyes were positioned 6 inches from the Ganzfeld stimulator (LKC Technologies, Gaithersburg, MD, USA). Burian-Allen rabbit ERG bipolar electrodes (Hansen Ophthalmic Development Laboratory, Iowa, IA, USA) were placed on the corneas. Five sweeps of flash were averaged after amplification and digitization with a digital analog converter. For the second stimulus, another five sweeps of flash controlled with neutral density filter 2.0 (Kodak, Rochester, NY, USA) were used. The ERG analysis was based on both the amplitudes and the implicit time measurements of a- and b-waves. A comparison was made between the treated eye and its fellow eye on each of the animals in order to minimize the variation.
Confirmation Toxicity Study and Treatment Study of IMS2186 for Laser-Induced CNV Using Rats
A suspension formulation of the micronized IMS2186, which could pass through a 30-gauge needle, was used for the intraocular efficacy study in the rat CNV model. IMS2186 was micronized, reducing the particle size from 850 microns to less than 10 microns, in order to produce a consistent release profile. We felt that a micronized formulation would have a faster release profile due to a larger surface area and provide a higher free drug level. At the same time, we repeated the toxicity study with the micronized formulation because of the possibility of a higher intraocular free drug level.
Twenty-six Brown Norway rats were randomly divided into three groups to compare the anti-CNV activity of IMS2186 to controls and triamcinolone acetate (Kenalog).
This experiment evaluated laser-induced CNV lesions: IMS2186 group (9 rats), triamcinolone acetonide (Kenalog) group (9 rats), and PBS group (8 rats). Only one eye of each rat was used for the study. The rats were anesthetized for all procedures with an intraperitoneal injection of ketamine (23 mg/kg) and xylazine (5 mg/kg). The pupils werefully dilated as described above. The fundus was visualized using a 5.4-mm rat fund us laser lens (Ocular Instruments, Bellevue, WA, USA) with 2.5% hydroxypropylmethylcellulose solution.
A diode laser (Oculight SLx; IridexCo., Mountain View, CA, USA) with an 810-nm wavelength was used to place 5 to 6 burns concentrically around the optic nerve with a distance from the disc of 2 to 3 disc diameters. Laser parameters were: 330 mW power, 75 μM spot size and 100 ms exposure. Only the burns with observed cavitations and air bubble formation, indicating the rupture of the Bruch's membrane, were counted as valid burns, i.e., burns that would effectively induce CNV.13,14 Immediately after the laser, the eyes received an intravitreal injection of the pre-determined compound in a volume of 2.0 μl. The dose used was 100 μg/eye for IMS2186 (2.0 μl of the solution of 50 μg/μl), 80 μg/eye for triamcinolone acetonide (Kenalog) and 50 μl of PBS containing 0.5% carboxymethylcellulose. All compounds were delivered through a 30-gauge needle connected to a calibrated Hamilton syringe dispenser (Hamilton Company, Reno, NV, USA).
Two weeks after laser and intravitreal drug injection, all rats had fluorescein angiography (FA) examinations, using a confocal scanning laser ophthalmoscope(Heidelberg Retinal Angiograph; Heidelberg Engineering, Carlsbad, CA, USA) to determine CNV formation.12 Two milligrams of sodium fluorescein in a 0.05-ml volume (12.5% sodium fluorescein solution) was injected into the sublingual vein. The rats were sacrificed three days after angiography, and the globes were enucleated and fixed in 4% paraformaldehyde for 2 hr prior to immunohistochemical processing.
For the evaluation of fluorescein angiographic leakage, the laser burns seen on the fluorescein angiograms were graded into four levels by the degree of leakage using the nearby normal fundus background fluorescein as a standard comparison. In brief, no recognition of the laser burn was graded as zero (0), mild intensity increase as one (1), moderated intensity increase as two (2), and marked increase as three (3); the evaluation was done by two ophthalmologists with the identity of samples masked. There was a consensus reached on most lesions, and, in case of discrepancy, average lesion scores were used for statistical calculations.
To confirm the lack of toxicity of the same total dose concentration in the eyes of rats as rabbits (1.6 mg/ml), 4 eyes of 2 rats were analyzed, using light microscopy. Rats were sacrificed as described above, and eyes were placed in a 2% paraformaldehyde/2.5% glutaraldehyde fixative at 4°C overnight. Eyes were grossed the next day by bisecting the globes perpendicular to the long ciliary nerve through the optic nerve. The cornea, lens, ciliary body, retina, choroid, and sclera were evaluated for toxicity. Structures appeared normal outside the lasered posterior pole. One-half of each eye was placed into a pre-labeled cassette in 10% formalin and processed for histological analysis, while the other half was returned to the fixative.
We evaluated CNV by immunohistochemical processing using staining by FITC-isolectin for endothelial cells15 and DAPI (4′,6-diamidino-2-phenylindole dihydrochloride) staining16,17 to determine overall lesion cellularity. This work was done by flat mounting the posterior poles and acquiring and quantifying the stained images using a confocal microscope for two-dimensional area measurements. For our quantitative analysis of the rat CNV lesions, 2-D tiled images were acquired in Image Pro-Plus 5.1 (Media Cybernetics Inc., Silver Spring, Maryland) using an Olympus Mal Confocal microscope to evaluate the flat area of the laser scars. All images were collected at a 672 × 512 pixel resolution and a depth of 16 bits per channel. Voxel dimensions were 0.772 pixels/μm for the x- and y-axes. Using a 10 × objective, images were acquired under two different fluorescent filters and captured using a Hamatsu digital camera. A continuous pattern was set up by defining the sides of the desired scanned area, setting the left, top, right, and bottom boundaries in order to produce the desired 5 × 5 tiled image of the 5 to 7 laser-induced CNV lesions. The endothelial cell marker, isolectin B4, was excited at 505 nm for 20 ms while emission at 530 nm was recorded for 20 ms. The DAPI nuclear dye was excited for 2 ms at 358 nm and recorded for 2 ms at 461 nm. The DAPI image was placed on top of the FITC image in a direct overlay to produce a double-stained composite image for visualization purposes. All images were done using 2 × 2 binning. The two-dimensional images were then analyzed (each filter separately) in ImageJ 1.37v (Wayne Rasband, NIH), using the freehand tool to outline our area of interest. The area was measured using the NIH ImageJ software.
For statistical analysis, differences of the lesion grades and differences of the lesion areas from the FITC-isolectin and DAPI measurements among groups were analyzed by repeated measurement techniques to correct for multiple comparisons and more than one lesion in each eye using JMP fit model platform (SAS Institute, Carey, NC, USA) to correct for the lack of lesion independence within any given eye. All analyses were conducted at a 0.05 significance level.
Results
Biological Characterization of IMS2186
IMS2186 has anti-proliferative properties. We assessed the anti-proliferative activity of IMS2186 in in vitro settings, and the results showed that IMS2186 inhibits cell growth in an in vitro setting, including tumor cells (NIH H460, A2780, HeLa cells, etc.) (Fig. 2A and Table 1), as well as non-transformed fibroblasts (IMR90, NIH3T3, etc.) and retinal pigment epithelial cells (RPE-19) (Fig. 2B and Table 2) from different species (mouse and human). The preliminary mechanism study shows that the treatment of the cancer H460 cells resulted in G2/M phase cell cycle arrest (Fig. 2C), and suggests that the anti-proliferative activity of IMS2186 is mediated by cell cycle blocking.
FIGURE 2.
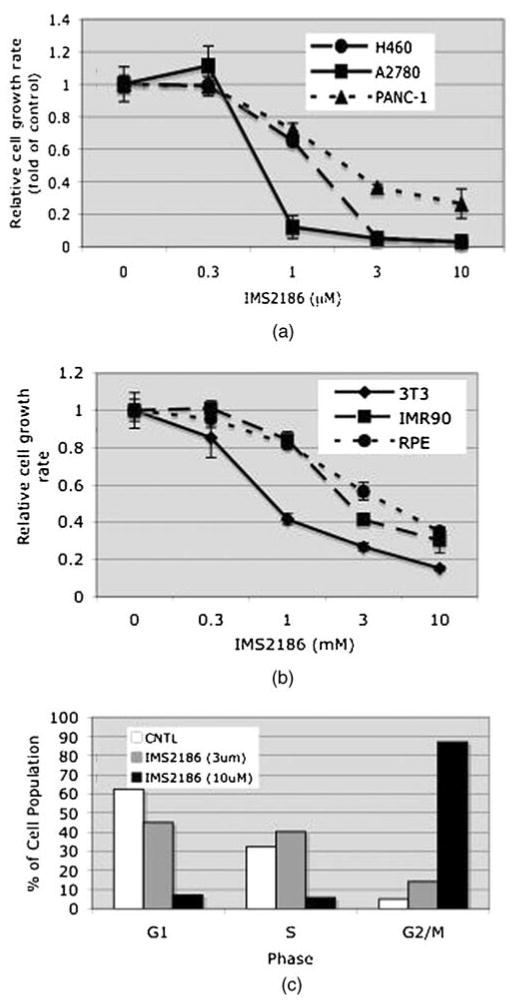
Anti-proliferative effect of IMS2186. (A) Correlation between the IMS2186 dose and the cell growth rate for three different cancer cell lines. Error bars indicate standard deviations (SD). (B) Correlation between the IMS2186 dose and the cell growth rate for non-transformed fibroblast and RPE-19 cell lines. Error bars indicate SD. (C) IMS2186 inhibition of different phases of normal retinal cell cycle. CNTL is a negative (not treated) control; G1 is period during interphase before synthesis and mitosis; S: DNA synthesis and chromosomal replication phase; G2/M: post-synthesis period of interphase/mitosis (cell division).
TABLE 1. Anti-proliferative activity of IMS2186 in various human cancer cell lines.
| Cancer cell line | IC50 (μM) |
|---|---|
| Ovarian (A2780) | 0.3–1 |
| Ovarian (SKOV3) | 0.3∼ |
| Ovarian (OVCAR3) | ∼ 3 |
| Lung (NCI-1299) | 0.3–1 |
| NSCL (A549) | 1–3 |
| NSCL (H460) | ∼ 0.5 |
| Colon (HCT116) | 1–3 |
| Pancreas (PANC-1) | 1–3 |
TABLE 2. Anti-proliferative activity of IMS2186 in various non-cancer cells.
| Cell | IC50 (μM) |
|---|---|
| Mouse fibroblasts (NIH 3T3) | 0.5–1 |
| Human fibroblasts (IMR90) | 1–3 |
| Human retina pigment epithelium (RPE) | ∼ 3 |
IMS2186 has anti-angiogenic properties. Angiogenesis is the process by which new blood capillaries are formed. Abnormal angiogenesis is found to play an essential role in wet AMD development, in which an abnormal growth of new blood vessels under the retina causes fluid leakiness that damages the macula, eventually leading to the impairment or loss of vision. IMS2186 was found to inhibit angiogenesis with an IC50 of 0.1 μM (Fig. 3A) in vitro. Also, as shown in Figure 3B, IMS2186 significantly inhibited the VEGF-induced blood vessel formation in the Matrigel plug in an in vivo setting.
FIGURE 3.
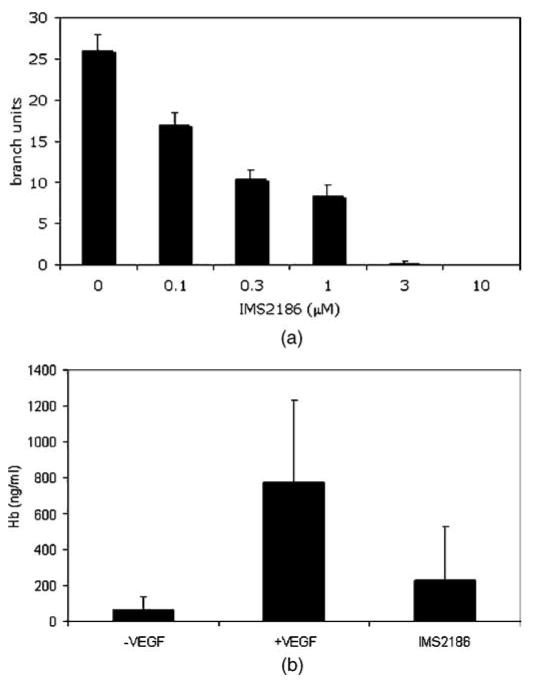
Anti-angiogenic effect of IMS2186 in vitro (tube formation) and in vivo (Matrigel plug assay in mice). (A) Negative correlation between the amount of HUVEC tube formation and the dose of IMS218. Note that dose over 1 micromolar completely inhibited branch formation. The results represent the mean ± SD (n = 3). (B). Anti-angiogenic effect of IMS2186 in vivo. Compared to the control group, VEGF enhanced angiogenesis (p = 0.03), while IMS2186 treatment reduced angiogenesis to the level which was not different from that of the control group (p = 0.4).
IMS2186 inhibits cell migration. Cell migration has been demonstrated to play a role in many biological processes such as inflammation (macrophages, etc.), angiogenesis (endothelial cells), and scar formation (fibroblasts, etc.), and thus in exudative AMD pathogenesis. We assessed IMS2186 for the inhibition of cell migration in two cell migration assays: wound healing and chamber assay. As shown in Figure 4A, IMS2186 inhibited the migration of the cells in the wound-healing assay, demonstrating its anti-migration properties. Control cells, which were not exposed to IMS2186, migrated and covered the wound within 20 hr. IMS2186 was also able to inhibit THP-1 migration into the lower chamber, as shown in Figure 4B, suggesting that IMS2186 can potentially inhibit macrophage mobilization.
FIGURE 4.
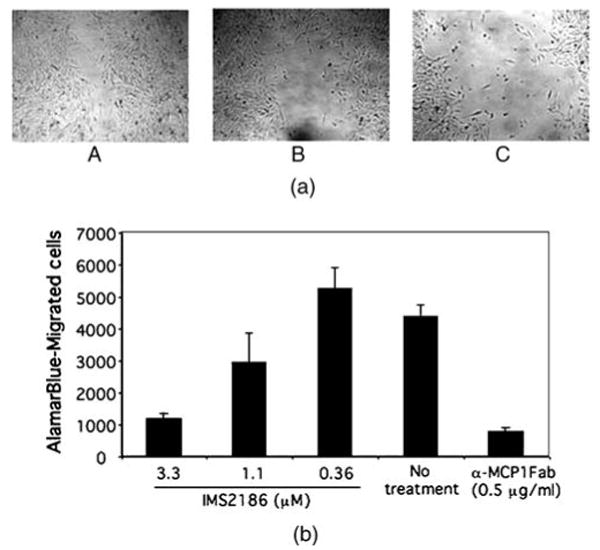
Wound-healing assay of A2058 migration and cell migration assay in vitro. (A) Photographs of the wound were taken 22 hr after treatment with IMS2186 and represent the cell migration rate for the following: (a) no treatment with IMS2186, (b) treatment with 3 μM of IMS2186, (c) treatment with 10 μM of IMS2186. (B) Average of three biological samples is shown, and the error bar represents the standard deviation (SD). Monocyte chemotactic protein-1 (MCP-1) Fab specifically blocks macrophages and monocyte migration. The cell migration blockage of 3.3 μM IMS2186 is comparable to MCP-1 Fab blockage.
IMS2186 is also found to inhibit the production of pro-inflammatory cytokines such as PGE2 (Fig. 5). Inflammatory response was believed to be a major causal factor in a number of ocular pathogenesis, contributing to aberrant angiogenesis and fibrosis.
FIGURE 5.
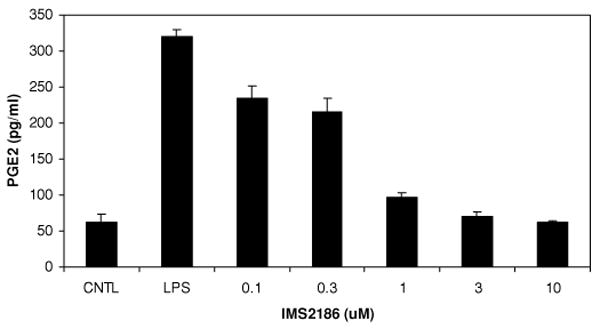
IMS2186 reduces PGE2 production in vitro. The effect of IMS2186 on LPS-induced PGE2 production by THP-1 cells was evaluated in vitro by ELISA. Differentiated THP-1 cells were treated with indicated concentrations of IMS2186 in the presence of 1 μg/ml LPS. Cells treated with LPS alone were used as an induction control (LPS). PGE2 level in cells without LPS treatment was designated as the basal control (CNTL). IMS2186 inhibited PGE2 production in a dose-dependent manner.
The above biological properties of IMS2186 are summarized in Table 3. Some of these phenotypes of IMS2186 have been confirmed in cell systems from more than one species. So far, no species specificity for the activity of IMS2186 has been observed; however, this needs to be validated by testing the compound in different animal models for in vivo pharmacological evaluation.
TABLE 3. Summary of the in vitro biological properties of IMS2186.
| Cell systems tested | IC50 (μM) | Assay type | Species | |
|---|---|---|---|---|
| Anti-proliferation | Cancer cell lines, primary fibroblasts, hepatocytes (rat), ARPE, etc. | 1–3 | Proliferation (AlamarBlue— Biosource International), cell cycle analysis | Human, mouse |
| Anti-migration | THP-1, HUVEC, A2058 | 1–3 | Wound healing (Valster et al., 2005) Chamber migration assay (Meter et al., 2005) | Human, mouse |
| Anti-angiogenesis | HUVEC | 0.1–0.5 | Matrigel tube formation (Min et al., 2004) | Human |
| Anti-inflammation | THP-1 | 0.1–0.5 | Production of PGE-2 | Human, mouse |
THP-1: Human macrophage. HUVEC: Primary human endothelial cells.
Intraocular Properties of IMS2186
Dissolution and Toxicity
In rabbits, the yellow repository of the drug sat in the vitreous near the injection site with minimal dispersion and did not affect the visual axis (Fig. 6A and 6B). We estimated that half of the IMS2186 had dissolved at 14 weeks, but the drug depot was still visible until the end of this experiment (36 weeks). Histology revealed normal intraocular structures, with the retinal and ciliary body at 4 and 36 weeks (Fig. 7A and 7B). Evaluation of the rat eyes treated with IMS2186 for CNV reveals no evidence of toxicity, using the micronized formulation at days 3 and 14 post injection in areas outside the laser lesions. Histology at 2 weeks post-treatment revealed no differences between the treated and untreated eyes.
FIGURE 6.
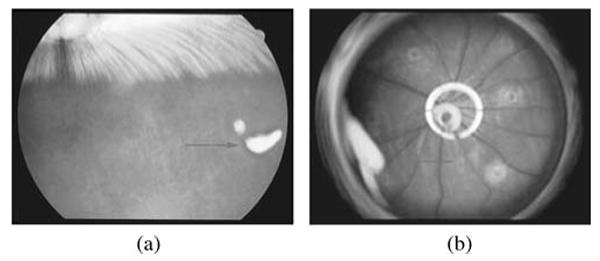
Appearance of the IMS2186 depot in the vitreous of rabbit and rat eyes. This figure presents a fundus photo of a rabbit's eye (A) and rat's eye (B) with IMS2186 depot in the vitreous. Red arrows points toward the IMS2186 yellow dense depot.
FIGURE 7.
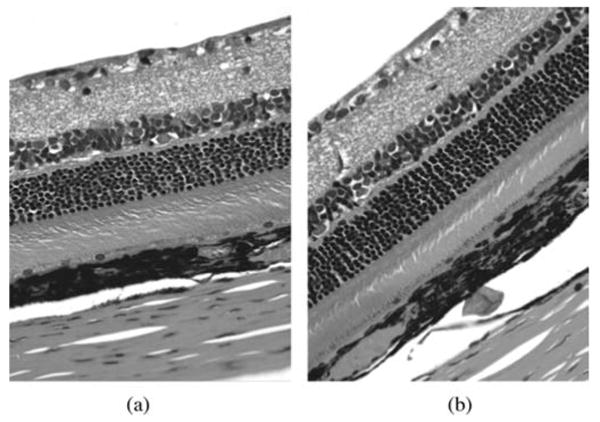
Results of histopathology in treated and untreated rats' eyes. This figure shows retinal structure under light microscopy in rats at 2 weeks. The control eye is injected with vehicle (A), and IMS2186 treated eye (B) did not show any structural abnormalities of retina layers (G: ganglion cells, IN: inner nuclear cells, ON: outer nuclear cells, RPE: retinal pigment epithelium cells, Ch: choroids).
No toxicity by slit lamp, fundus exam, or intraocular pressure determinations was observed in the rabbit eyes with IMS2186 during the entire 36-week course of study. Mean intraocular pressure at week 1 was 14.2 ± 3 mmHg in treated eyes versus 17.5 ± 3.6 mmHg in control eyes (p > 0.05); and at week 7 was 18.3 ± 6.7 mmHg in treated eyes versus 17 ± 3.3 mmHg in control eyes (p > 0.05). Full-field electroretinography (ERG) was obtained at week 4 and week 36. There were no statistically significant differences between recordings obtained from control and IMS2186 treated eyes (Table 4). The waveforms were normal in both groups (Fig. 8).
TABLE 4. Full-field ERG results in rabbits.
| Timeframe | a-Wave implicit time (ms) | a-Wave amplitude (μV) | b-Wave implicit time (ms) | b-Wave amplitude (μV) | |
|---|---|---|---|---|---|
| 1 month | Treated eye | 15.3 ± 1.6 | 3.4 ± 1.9 | 62.7 ± 7.9 | 102.6 ± 32.1 |
| Control eye | 15.3 ± 1.6 | 4.0 ± 1.5 | 62.7 ± 7.4 | 103.2 ± 23.6 | |
| p-value | 1.0 | 0.5 | 1.0 | 0.95 | |
| 9 months | Treated eye | 16.0 ± 0 | 5.2 ± 1.8 | 54.7 ± 10.3 | 129.2 ± 43.4 |
| Control eye | 16.0 ± 0 | 2.8 ± 2.0 | 59.3 ± 8.9 | 105.7 ± 38.6 | |
| p-value | 1.0 | 0.08 | 0.3 | 0.1 |
FIGURE 8.
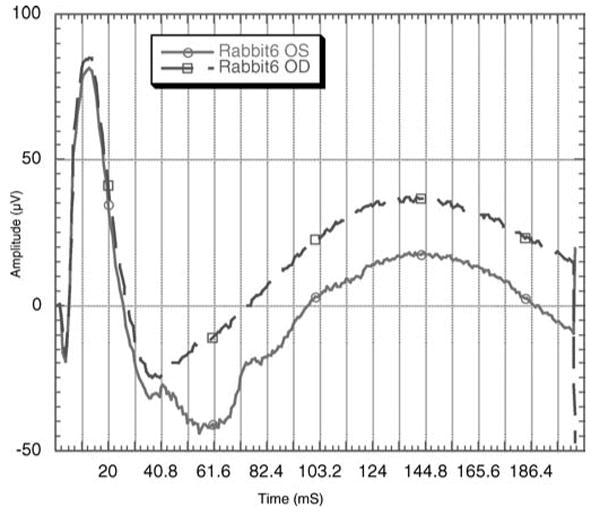
ERG tracings from a rabbit whose right eye (OD) received 2.5 mg of IMS2186 intravitreal injection while the left eye (OS) received PBS intravitreal injection. The ERG tracings were recorded at the 9th month after the injection, demonstrating normal waveforms and timing.
Evaluation of the Efficacy of Treatment of CNV in Laser-Induced CNV Rats
Fluorescein angiographic analysis of the leakage of rats with laser-induced CNV showed a reduction in leakage in eyes treated with IMS2186 as well as in eyes treated with Kenalog as compared to the leakage in eyes with PBS (Kenalog 1.35 versus PBS 2.09, p = 0.03) (Fig. 9); (IMS2186 1.86 versus PBS 2.09, (p = 0.05); the mean leaking grade). The beneficial effect of Kenalog was not found to be better than IMS2186 (p = 0.09).
FIGURE 9.
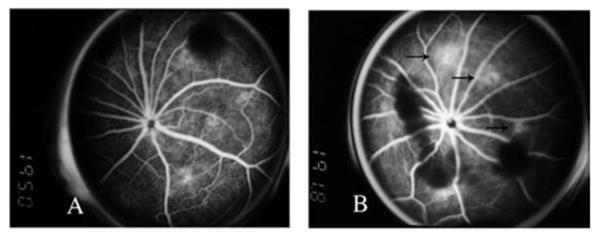
The fluorescein angiography. The left panel (A) shows the fluorescein angiograph at week 2 from an eye treated with IMS2186, demonstrating a lack of active leaking, while the right panel (B), at the similar timeframe, from an eye treated with PBS, demonstrated active leaking (arrows).
Vascularity was determined by confocal microscopic analysis with FITC-isolectin staining and was expressed by staining the area. IMS2186 showed a trend of less endothelial cell staining compared to PBS (p = 0.13; one-tail comparison of means) as well as Kenalog (p = 0.025; one-tail comparison of means). There was no difference in efficacy between Kenalog and IMS2186 (p = 0.44, two-tail comparison of means).
DAPI staining was used to determine the cellular proliferation/infiltration into the CNV lesions. This measurement shows the antiproliferative activity; the less proliferation, the better the organization and residual function of the retina over the CNV lesion. The area of the lesions stained by DAPI showed significantly less cellularity in the IMS2186 and Kenalog groups than those in the PBS group (p = 0.0025 and p = 0.0025, one-tail comparison of means; Figs. 10 and 11). We found that IMS2186 had a 30% reduction of lesion area compared to PBS in the lesion area measurement.
FIGURE 10.
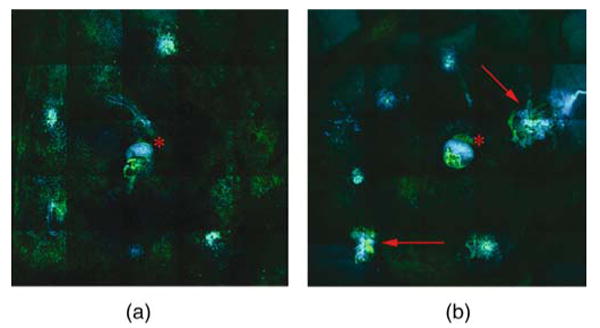
Flat-mount rat's retina composite labeled with DAPI and FITC-I. Flat-mount preparations to better visualize the size, vascularity, and cellularity of laser-induced CNV in rat eyes. Composite image shows vasculature labeled in green by endothelial marker, FITC-conjugated isolectin IB4, and cell nuclei labeled in blue by DAPI (general nuclear stain) demonstrating cell growth and migration. The lesions in the IMS2186-treated eye are smaller and less intensely labeled (A), though the lesions in the untreated eye are larger and more intensely stained (red arrows) (B). The red asterisk shows staining of the optic nerve head in both images.
FIGURE 11.
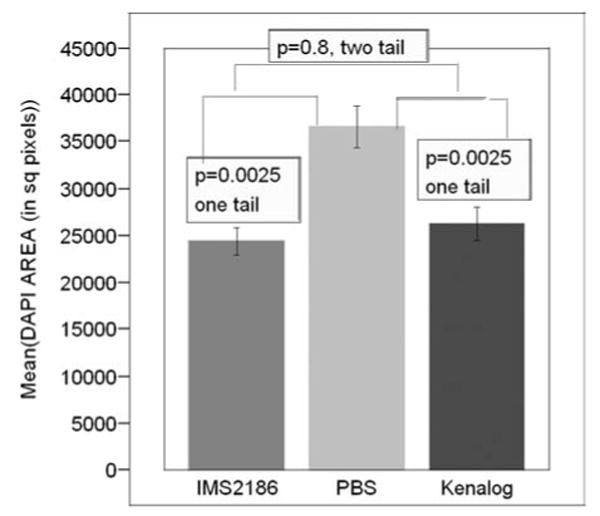
Comparison of the reduction in proliferation between the two groups by DAPI staining. This figure shows a graphical comparison of area of proliferation among groups measured in square pixels (y-axis) stained by DAPI. The largest area was observed in the PBS group, and both IMS2186 and Kenalog showed significantly less area of staining (less proliferation). Also, IMS2186 showed slightly less area of proliferation than Kenalog, although this difference was not statistically significant.
Discussion
We have evaluated IMS2186 for its biological properties that were designed to treat several important patho-physiological processes occurring in exudative age-related macular degeneration (AMD) in order to explore the potential utility as treatments for eye diseases such as wet AMD.
The pathogenesis of choroidal neovascularization (CNV) in AMD is very complicated and involves a number of biological changes. Excessive cell proliferation, fibrosis (scarring), and inflammatory processes are critical to wet AMD pathogenesis. Proliferation processes result in an excess formation of fibrous tissue in the subretinal area of the eyes, which contributes to an impaired central vision. Also, the release of migration inducers, such as MCP-1 by RPE cells, is proposed to cause the accumulation of macrophages in the eyes that release various cytokines to further aggravate the disease. In addition, leukocyte-released growth factors are found to be involved in the formation of new abnormal blood vessels in wet AMD. PGE2 is one of the well-known pro-inflammatory mediators that play an important role in various inflammatory diseases. Recently, it was found that PGE2 is also involved in the development of AMD. For example, VEGF stimulates HUVEC cells to secret PGE2, which in turn further enhances the VEGF-mediated angiogenesis signaling.18 Thus, inhibition of PGE2 production by IMS2186 may contribute to the amelioration of inflammation as well as angiogenesis in AMD. In this study, we elected to use triamcinolone acetonide as a positive control because the animal model is different from human macular degeneration, and inflammation is a major component of this CNV model. Although triamcinolone has its limitations and disadvantages on human macular degeneration patients,19 it has been shown effective on laser-induced rodent CNV models.20,21,22 In humans, however, we note that there is an effect of triamcinolone on the CNV lesion size, where clinical studies have shown a decrease in lesion size with the use of intravitreal triamcinolone.23 It would not be possible to use bevacizumab or ranibizumab as positive controls because of the species specificity of these anti-VEGF compounds. We also stress that we used another control (vehicle) as a negative control, which is what also enables us to evaluate the potential efficacy of IMST2186 in laser-induced choroidal neovascularization.
Current options for exudative AMD treatment, such as Lucentis (ranibizumab), Macugen (pegaptanib sodium), or Avastin (bevacizumab) are pure VEGF inhibitors. They reduce leakage from newly formed abnormal vessels by binding to the VEGF and therefore block the induction and maintenance of neovascularization. We hypothesized that, with currently available anti-VEGF drugs, even after anatomically successful treatment, when subretinal fluid is resolved and the retina is grossly restored to its normal anatomical configuration, the visual outcomes are often mediocre (in the range of 20/70–20/80) because of proliferation and gliosis, which result in retinal disorganization combined with RPE-atrophy.
For these reasons, we chose to develop and test IMS2186, which has inhibitory effects in vitro, on not only angiogenesis, but also on the mobilization of macrophages and the proliferation of fibroblasts and other cell types. IMS2186 can arrest cell growth at the G2 phase, and hence acts more upstream of the angiogenesis, rather than simply blocking the VEGF. We are hopeful that this drug will allow inhibition, not only of neovascularization, but also of cell proliferation, inflammation, and gliosis. IMS2186 may potentially reduce scarring and could possibly provide better visual outcomes after treatment.
We also wanted to develop a drug to overcome the other shortcomings of current anti-VEGF drugs for CNV treatment, specifically their short half-life, which requires monthly intravitreal injections to sustain therapeutic levels of the drug. Our study showed that a micronized (crystal size <10 microns) suspension formulation of IMS2186 could potentially serve as a sustained-release intraocular treatment. The physicochemical structure of IMS2186 showed also that free (soluble) drug concentration of IMS2186 in a physiological media (e.g., vitreous or saline) is 8–10 μM above the IC50s for all the aforementioned biological activities (ranging 0.1– 3.0 μM, depending the type of assays). All these properties suggest that an intraocular injection of IMS2186 could be a long-lasting effective treatment for CNV in AMD, with a reduction in scarring and related visual loss.
We recognize that the formulation of drug we tested in the rabbit toxicity and dissolution study was the non-micronized form, which lasted in animal eyes up to 36 weeks (9 months) and was non-toxic intravitreally for this period of time. We elected to refine our formulation by micronizing the compound to provide a more consistent release of drug. We confirmed the lack of toxicity for the micronized formulation with the same calculated total drug concentration (16 mg/ml) in the rat eye.
In a clinical situation, a smaller amount of micronized formulation could be injected into the eye and would be expected to last about 3–4 months, allowing ophthalmologists to have control over dosing and drug accumulation.
We have further plans to evaluate the compound on more relevant eye origin cell lines such as choroidal or retinal endothelial cells or pericytes in vitro and detailed pharmacokinetics and release properties of micronized IMS2186 in vivo, as this long-acting intravitreally injectable drug has multiple biological properties that are advantageous in the treatment of CNV in AMD and possibly in the treatment of other disorders of intraocular proliferation and angiogenesis.
Acknowledgments
We acknowledge the financial support of the Unrestricted Retina Research Fund of UCSD Jacobs Retina Center to WRF and LC. This work was also supported in part by Research to Prevent Blindness (Freeman). Doctors Dehua Yu, Michelle Hysell, Guohong Liu, Ning Ke, James E. Macdonald, Qi-Xiang Li, and Flossie Wong-Staal are employees of Immusol Inc.
Footnotes
Publisher's Disclaimer: Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf
This article maybe used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden.
The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
Contributor Information
Iryna A. Falkenstein, Joan and Irwin Jacobs Retina Center, Department of Ophthalmology, Shiley Eye Center, University of California, San Diego, California, USA
Lingyun Cheng, Joan and Irwin Jacobs Retina Center, Department of Ophthalmology, Shiley Eye Center, University of California, San Diego, California, USA.
Flossie Wong-Staal, Immusol Inc., San Diego, California, USA.
Ajay M. Tammewar, Joan and Irwin Jacobs Retina Center, Department of Ophthalmology, Shiley Eye Center, University of California, San Diego, California, USA
Erin C. Barron, Joan and Irwin Jacobs Retina Center, Department of Ophthalmology, Shiley Eye Center, University of California, San Diego, California, USA
Gabriel A. Silva, Joan and Irwin Jacobs Retina Center, Department of Ophthalmology, Shiley Eye Center, University of California, San Diego, California, USA
Qi-Xiang Li, Immusol Inc., San Diego, California, USA.
Dehua Yu, Immusol Inc., San Diego, California, USA.
Michelle Hysell, Immusol Inc., San Diego, California, USA.
Guohong Liu, Immusol Inc., San Diego, California, USA.
Ning Ke, Immusol Inc., San Diego, California, USA.
James E. Macdonald, Immusol Inc., San Diego, California, USA
William R. Freeman, Joan and Irwin Jacobs Retina Center, Department of Ophthalmology, Shiley Eye Center, University of California, San Diego, California, USA
References
- 1.Ferris FL, Fine SL, Hyman L. Age-related macular degeneration and blindness due to neovascular maculopathy. Arch Ophthalmol. 1984;102:1640–1642. doi: 10.1001/archopht.1984.01040031330019. [DOI] [PubMed] [Google Scholar]
- 2.Klein R, Klein BE, Linton KL. Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology. 1992;99:933–943. doi: 10.1016/s0161-6420(92)31871-8. [DOI] [PubMed] [Google Scholar]
- 3.Gragoudas ES, Adamis AP, Cunningham ET, Feinsod M, Guyer DR. VEGF inhibition study in ocular neovascularization clinical trial group. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004;351:2805–2816. doi: 10.1056/NEJMoa042760. [DOI] [PubMed] [Google Scholar]
- 4.Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY, MARINA study group Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 5.Spaide RF, Laud K, Fine HF, Klancnik JM, Meyerle CB, Yannuzzi LA, Sorenson J, Slakter J, Fisher YL, Cooney MJ. Intravitreal bevacizumab treatment of choroidal neovascularization secondary to age-related macular degeneration. Retina. 2006;26:383–390. doi: 10.1097/01.iae.0000238561.99283.0e. [DOI] [PubMed] [Google Scholar]
- 6.Avery RL, Pieramici DJ, Rabena MD, Castellarin AA, Nasir MA, Giust MJ. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology. 2006;113:363–372. doi: 10.1016/j.ophtha.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 7.Freeman WR, Falkenstein I. Avastin and new treatments for AMD: Where are we? Retina. 2006;26:853–858. doi: 10.1097/01.iae.0000244722.35073.7c. [DOI] [PubMed] [Google Scholar]
- 8.Giuliano C, Jairaj M, Zafiu CM, Laufer R. Direct determination of unbound intrinsic drug clearance in the microsomal stability assay. Drug Metab Dispos. 2005;33:1319–1324. doi: 10.1124/dmd.105.005033. [DOI] [PubMed] [Google Scholar]
- 9.Yu D, MacDonald J, Josephs S, Liu Q, Nguy V, Tor Y, Wong-Staal F, Li QX. MDDD, a 4,9-diazapyrenium derivative is selective toxic to glioma by inducing growth arrest at G0/G1 without p53. Investigational New Drugs. 2006;24:489–498. doi: 10.1007/s10637-006-7590-1. [DOI] [PubMed] [Google Scholar]
- 10.Min JK, Han KY, Kim EC, Kim YM, Lee SW, Kim OH, Kim KW, Gho YS, Kwon YG. Capsaicin inhibits in vitro and in vivo angiogenesis. Cancer Res. 2004;64:644–651. doi: 10.1158/0008-5472.can-03-3250. [DOI] [PubMed] [Google Scholar]
- 11.Cheng L, Hostetler KY, Chaidhawangul S, Gardner MF, Beadle JR, Toyoguchi M, Bergeron-Lynn G, Freeman WR. Treatment of prevention of herpes simplex virus retinitis with intravitreally injectable crystalline 1-o-hexadecylpropanediol-3-phospho-ganciclovir. Invest Ophthalmol Vis Sci. 2002;43:515–521. [PubMed] [Google Scholar]
- 12.Lu S, Cheng L, Hostetler KY, Koh HJ, Beadle JR, Davidson MC, Freeman WR. Intraocular properties of hexadecyloxypropyl-cyclic-cidofovir in Guinea pigs. J Ocul Pharmacol Ther. 2005;21:205–209. doi: 10.1089/jop.2005.21.205. [DOI] [PubMed] [Google Scholar]
- 13.Koh HJ, Bessho K, Cheng L, Bartsch DU, Jones TR, Bergeron-Lynn G, Freeman WR. Inhibition of choroidal neovascularization in rats by the urokinase-derived peptide A6. Invest Ophthalmol Vis Sci. 2004;45:635–640. doi: 10.1167/iovs.03-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El Bradley M, Cheng L, Bartsch DU, Appelt K, Rodanant N, Bergeron-Lynn G, Freeman WR. Preventive versus treatment effect of AG3340, a potent matrix metalloproteinase inhibitor in a rat model of choroidal neovascularization. J Ocul Pharmacol Ther. 2004;20:217–236. doi: 10.1089/1080768041223657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He S, Ding Y, Zhou J, Krasnoperov V, Zozulya S, Kumar SR, Ryan SJ, Gill PS, Hinton DR. Soluble EphB4 regulates choroidal endothelial cell function and inhibits laser-induced choroidal neovascularization. Invest Ophthalmol Vis Sci. 2005;46:4772–4779. doi: 10.1167/iovs.05-0502. [DOI] [PubMed] [Google Scholar]
- 16.Silva GA, Czeisler C, Niece KL, Beniash E, Harrington DA, Kessler JA, Stupp SI. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science. 2004;303:1352–1355. doi: 10.1126/science.1093783. [DOI] [PubMed] [Google Scholar]
- 17.Ho M, Yu D, Davidson MC, Silva GA. Comparison of standard surface chemistries for culturing mesenchymal stem cells prior to neural differentiation. Biomaterials. 2006;27:4333–4339. doi: 10.1016/j.biomaterials.2006.03.037. [DOI] [PubMed] [Google Scholar]
- 18.Tamura K, Sakurai T, Kogo H. Relationship between prostaglandin E2 and vascular endothelial growth factor (VEGF) in angiogenesis in human vascular endothelial cells. Vascul Pharmacol. 2006;44:411–416. doi: 10.1016/j.vph.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 19.Geltzer A, Turalba A, Vedula SS. Surgical implantation of steroids with antiangiogenic characteristics for treating neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2007 Oct;17(4):CD005022. doi: 10.1002/14651858.CD005022.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kato A, Kimura H, Okabe K, Okabe J, Kunou N, Nozaki M, Ogura Y. Suppression of laser-induced choroidal neovascularization by posterior sub-tenon administration of triamcinolone acetonide. Retina. 2005;25(4):503–509. doi: 10.1097/00006982-200506000-00017. [DOI] [PubMed] [Google Scholar]
- 21.Ciulla TA, Criswell MH, Danis RP, Fronheiser M, Yuan P, Cox TA, Csaky KG, Robinson MR. Choroidal neovascular membrane inhibition in a laser-treated rat model with intraocular sustained release triamcinolone acetonide microimplants. Br J Ophthalmol. 2003;87(8):1032–1037. doi: 10.1136/bjo.87.8.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ciulla TA, Criswell MH, Danis RP, Hill TE. Intravitreal triamcinolone acetonide inhibits choroidal neovascularization in a laser-treated rat model. Arch Ophthalmol. 2001;119(3):399–404. doi: 10.1001/archopht.119.3.399. [DOI] [PubMed] [Google Scholar]
- 23.Lee J, Freeman WR, Azen SP, Chung EJ, Koh HJ. Prospective, randomized clinical trial of intravitreal triamcinolone treatment of neovascular age-related macular degeneration: One-year results. Retina. 2007;27(9):1205–1213. doi: 10.1097/IAE.0b013e31815ec367. [DOI] [PubMed] [Google Scholar]


