Abstract
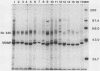
Immunochemical properties of the major outer membrane protein (MOMP) of 16 strains of Chlamydia psittaci isolated from psittacine birds, budgerigars, a pigeon, turkeys, humans, cats, a muskrat, sheep, and cattle and a strain of C. trachomatis, L2/434/Bu, were compared by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and by immunoblotting analysis with hyperimmunized rabbit antisera to strains of parrot, turkey, feline, and bovine origin. The MOMPs of the strains showed variation in molecular weights and immunological specificities. Fifteen of the C. psittaci strains were classified into two avian and two mammalian types based on immunological specificity of the MOMP, whereas the other strain was not classified in this study. Immunological classification based on specificity of the MOMP by immunoblotting proved to be a valuable method to classify various strains of C. psittaci.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker J. A. A VIRUS OBTAINED FROM A PNEUMONIA OF CATS AND ITS POSSIBLE RELATION TO THE CAUSE OF ATYPICAL PNEUMONIA IN MAN. Science. 1942 Nov 20;96(2499):475–476. doi: 10.1126/science.96.2499.475. [DOI] [PubMed] [Google Scholar]
- Banks J., Eddie B., Sung M., Sugg N., Schachter J., Meyer K. F. Plaque reduction technique for demonstrating neutralizing antibodies for Chlamydia. Infect Immun. 1970 Oct;2(4):443–447. doi: 10.1128/iai.2.4.443-447.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batteiger B. E., Newhall W. J., 5th, Terho P., Wilde C. E., 3rd, Jones R. B. Antigenic analysis of the major outer membrane protein of Chlamydia trachomatis with murine monoclonal antibodies. Infect Immun. 1986 Sep;53(3):530–533. doi: 10.1128/iai.53.3.530-533.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell H. D., Kromhout J., Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981 Mar;31(3):1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell H. D., Kuo C. C., Kenny G. E. Antigenic analysis of Chlamydiae by two-dimensional immunoelectrophoresis. I. Antigenic heterogeneity between C. trachomatis and C. psittaci. J Immunol. 1975 Oct;115(4):963–968. [PubMed] [Google Scholar]
- Caldwell H. D., Schachter J. Antigenic analysis of the major outer membrane protein of Chlamydia spp. Infect Immun. 1982 Mar;35(3):1024–1031. doi: 10.1128/iai.35.3.1024-1031.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba N., Arikawa J., Takashima I., Hashimoto N. Isolation and serological survey of chlamydiosis in feral pigeons and crows in Hokkaido. Nihon Juigaku Zasshi. 1984 Apr;46(2):243–245. doi: 10.1292/jvms1939.46.243. [DOI] [PubMed] [Google Scholar]
- Eb F., Orfila J. Serotyping of Chlamydia psittaci by the micro-immunofluorescence test: isolates of ovine origin. Infect Immun. 1982 Sep;37(3):1289–1291. doi: 10.1128/iai.37.3.1289-1291.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eb F., Orfila J. Typage de Chlamydia psittaci par la micromémethode en immunofluorescence. Ann Microbiol (Paris) 1981 Jan-Feb;132A(1):81–96. [PubMed] [Google Scholar]
- Esen A., Conroy J. M., Wang S. Z. A simple and rapid dot-immunobinding assay for zein and other prolamins. Anal Biochem. 1983 Jul 15;132(2):462–467. doi: 10.1016/0003-2697(83)90035-0. [DOI] [PubMed] [Google Scholar]
- FRASER C. E., BERMAN D. T. TYPE-SPECIFIC ANTIGENS IN THE PSITTACOSIS-LYMPHOGRANULOMA VENEREUM GROUP OF ORGANISMS. J Bacteriol. 1965 Apr;89:943–948. doi: 10.1128/jb.89.4.943-948.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushi H., Nojiri K., Hirai K. Monoclonal antibody typing of Chlamydia psittaci strains derived from avian and mammalian species. J Clin Microbiol. 1987 Oct;25(10):1978–1981. doi: 10.1128/jcm.25.10.1978-1981.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayston J. T., Wang S. New knowledge of chlamydiae and the diseases they cause. J Infect Dis. 1975 Jul;132(1):87–105. doi: 10.1093/infdis/132.1.87. [DOI] [PubMed] [Google Scholar]
- Hirai K., Itoh K., Yamashita T., Fukushi H., Hayashi Y., Kuzuya M., Shimakura S., Hashimoto A., Akiyama K. Prevalence of Chlamydia psittaci in pet birds maintained in public places or in close human contact. Nihon Juigaku Zasshi. 1983 Dec;45(6):843–845. doi: 10.1292/jvms1939.45.843. [DOI] [PubMed] [Google Scholar]
- Kuo C. C., Chen H. H., Wang S. P., Grayston J. T. Identification of a new group of Chlamydia psittaci strains called TWAR. J Clin Microbiol. 1986 Dec;24(6):1034–1037. doi: 10.1128/jcm.24.6.1034-1037.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MANIRE G. P., MEYER K. F. The toxins of the psittacosis-lymphogranuloma group of agents; differentiation of strains by the toxin neutralization test. J Infect Dis. 1950 May-Jun;86(3):241–250. doi: 10.1093/infdis/86.3.241. [DOI] [PubMed] [Google Scholar]
- Meyer K. F. The host spectrum of psittacosis-lymphogranuloma venereum (PL) agents. Am J Ophthalmol. 1967 May;63(5 Suppl):1225–1246. doi: 10.1016/0002-9394(67)94105-0. [DOI] [PubMed] [Google Scholar]
- Mitzel J. R., Wright G. G., Swack N. S. Cross immunity among strains of Chlamydia psittaci. Proc Soc Exp Biol Med. 1970 Dec;135(3):944–946. doi: 10.3181/00379727-135-35176. [DOI] [PubMed] [Google Scholar]
- Perez-Martinez J. A., Storz J. Antigenic diversity of Chlamydia psittaci of mammalian origin determined by microimmunofluorescence. Infect Immun. 1985 Dec;50(3):905–910. doi: 10.1128/iai.50.3.905-910.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Public Health Weekly Reports for OCTOBER 6, 1944. Public Health Rep. 1944 Oct 6;59(40):1299–1329. [PMC free article] [PubMed] [Google Scholar]
- Schachter J., Banks J., Sugg N., Sung M., Storz J., Meyer K. F. Serotyping of Chlamydia. I. Isolates of ovine origin. Infect Immun. 1974 Jan;9(1):92–94. doi: 10.1128/iai.9.1.92-94.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter J., Banks J., Sugg N., Sung M., Storz J., Meyer K. F. Serotyping of Chlamydia: isolates of bovine origin. Infect Immun. 1975 May;11(5):904–907. doi: 10.1128/iai.11.5.904-907.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter J. Immunofluorescent cross-absorption tests for detecting antigenic differences between Bedsonia isolates. Rev Int Trach. 1968;45(4):321–330. [PubMed] [Google Scholar]
- Schachter J., Meyer K. F. Lymphogranuloma venereum. II. Characterization of some recently isolated strains. J Bacteriol. 1969 Sep;99(3):636–638. doi: 10.1128/jb.99.3.636-638.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter J., Ostler H. B., Meyer K. F. Human infection with the agent of feline pneumonitis. Lancet. 1969 May 31;1(7605):1063–1065. doi: 10.1016/s0140-6736(69)91703-6. [DOI] [PubMed] [Google Scholar]
- Shewen P. E. Chlamydial infection in animals: a review. Can Vet J. 1980 Jan;21(1):2–11. [PMC free article] [PubMed] [Google Scholar]
- Spalatin J., Fraser C. E., Connell R., Hanson R. P., Berman D. T. Agents of psittacosis-lymphogranuloma venereum group isolated from muskrats and snowshoe hares in Saskatchewan. Can J Comp Med Vet Sci. 1966 Sep;30(9):260–264. [PMC free article] [PubMed] [Google Scholar]
- Spears P., Storz J. Biotyping of Chlamydia psittaci based on inclusion morphology and response to diethylaminoethyl-dextran and cycloheximide. Infect Immun. 1979 Apr;24(1):224–232. doi: 10.1128/iai.24.1.224-232.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAMURA A., HIGASHI N. PURIFICATION AND CHEMICAL COMPOSITION OF MENINGOPNEUMONITIS VIRUS. Virology. 1963 Aug;20:596–604. doi: 10.1016/0042-6822(63)90284-8. [DOI] [PubMed] [Google Scholar]
- Wang S. P., Kuo C. C., Barnes R. C., Stephens R. S., Grayston J. T. Immunotyping of Chlamydia trachomatis with monoclonal antibodies. J Infect Dis. 1985 Oct;152(4):791–800. doi: 10.1093/infdis/152.4.791. [DOI] [PubMed] [Google Scholar]
- Yamashita T., Hirai K. Isolation of Chlamydia psittaci from imported psittacine birds in Japan. Nihon Juigaku Zasshi. 1981 Aug;43(4):561–563. doi: 10.1292/jvms1939.43.561. [DOI] [PubMed] [Google Scholar]