Abstract
The role of prostaglandins production in the control of regenerative function of endothelial progenitor cells (EPCs) has not been studied. We hypothesized that activation of cyclooxygenase (COX) enzymatic activity and the subsequent production of prostacyclin (PGI2) is an important mechanism responsible for the regenerative function of EPCs. In the present study, we detected high levels of COX-1 protein expression and PGI2 biosynthesis in human EPCs outgrown from blood mononuclear cells. Expression of COX-2 protein was almost undetectable under basal conditions but significantly elevated after treatment with tumor necrosis factor-α. Condition medium derived from EPCs hyperpolarized human coronary artery smooth muscle cells, similar to the effect of the PGI2 analogue iloprost. The proliferation and in vitro tube formation by EPCs were inhibited by the COX inhibitor indomethacin, or by genetic inactivation of COX-1 or PGI2 synthase (PGIS) with small interfering RNA (siRNA). Impaired tube formation and cell proliferation induced by inactivation of COX-1 were rescued by the treatment with iloprost or selective peroxisome-proliferator activated receptor-δ (PPARδ) agonist, GW501516, but not by the selective PGI2 receptor agonist, cicaprost. Down regulation of PPARδ by siRNA also reduced angiogenic capacity of EPCs. Iloprost failed to reverse PPARδ-siRNA-induced impairment of angiogenesis. Furthermore, transfection of PGIS-siRNA, COX-1-siRNA, or PPARδ-siRNA into EPCs decreased the capillary formation in vivo after transplantation of human EPCs into the nude mice. These results suggest that activation of COX-1-PGI2-PPARδ pathway is an important mechanism underlying pro-angiogenic function of EPCs.
Keywords: adult stem cells, angiogenesis, prostaglandins, peroxisome-proliferator activated receptor, cyclooxygenase
Introduction
Evidence continues to accumulate on the existence of circulating endothelial progenitor cells (EPCs) capable of stimulating angiogenesis and repair of injured endothelium (1–11). However, the mechanisms underlying the reported therapeutic effects of EPCs are poorly understood, thus limiting successful translation of EPC-based therapies into the clinical arena. Arachidonic acid metabolism via cyclooxygenase-1 (COX-1) and/or cyclooxygenase-2 (COX-2) in mature endothelium is of major importance in cardiovascular homeostasis (12, 13). Prostacyclin (PGI2) is a key vasoactive substance released from the endothelium after activation of COX(s) by chemical or physical stimuli (12, 13). Most importantly, PGI2 is known to have a wide range of vasoprotective and therapeutic effects (14). Recently, it has been recognized that PGI2 also has stimulatory effects on angiogenesis (15–19). Despite the fact that a substantial amount of literature is available on functional and therapeutic significance of COX(s) and PGI2 in the vasculature, the role of arachidonic acid metabolism in the regenerative function of EPCs has not been examined. In the present study we hypothesized that activation of COX isoforms and high production of PGI2 are important mechanisms responsible for the regenerative function of EPCs. We provide compelling evidence that the pro-angiogenic effects of human EPCs are in part dependent on the biosynthesis and release of PGI2, and subsequent activation of peroxisome-proliferator activated receptor-δ (PPARδ).
Materials and Methods
An expanded Materials and Methods section is available in Online Data Supplement at http://circres.ahajournals.org.
Isolation, Culturing, and Phenotyping of EPCs
The protocol for collection and use of human blood samples was approved by the Institutional Review Board at the Mayo Clinic. EPCs (late outgrowth) were outgrown 2–3 weeks after culturing of mononuclear cells isolated from the peripheral blood of 15 healthy male subjects (45±4 years old) as previously described (9). Both EPCs and human coronary artery endothelial cells [CAECs; Clonetics, from 4 male donors (28±2 years old)] were cultured in endothelial growth medium-2 (EGM-2, Clonetics). Human coronary artery smooth muscle cells (CSMCs, Clonetics) were cultured in SmGM-2 SingleQuots (Clonetics). All experiments were performed using cells cultured from passages 4 to 8.
Morphological appearance and fluorescent activated cell sorting (FACS) were utilized to define endothelial cell phenotype of EPCs as previously described (1, 9).
NOS Enzyme Activity
The total (including calcium-dependent and –independent) nitric oxide synthase (NOS) enzyme activity of EPCs and CAECs was determined by measuring L-Citrulline synthesis from L- arginine, as previously described (20).
Prostaglandins and Thromboxane Measurement
Subconfluent cells were incubated in EBM-2 (8 ml /100 mm dish) for 24 h. The supernatant (conditioned medium, CM) was collected and immediately mixed with 40 μl of 0.2 mol/L EDTA/PBS, and stored at −80oC. Prostaglandin E2 (PGE2), 6-keto prostaglandin F1α (6-keto PGF1α, the degradation product of PGI2), and thromboxane B2 (TXB2, a breakdown product of TXA2) were assayed using EIA kits (Cayman Chemical Co., 21).
Western Blot Analysis
Western blotting was performed as previously described (22). Goat anti-COX-1, rabbit anti-PGI2 synthase (PGIS), rabbit anti-PPARδ, and goat anti-actin antibodies were obtained from Santa Cruz Biotech, Inc. Rabbit anti-COX-2, PGH-PGE isomerase (PGEI), and TXA2 synthase (TXAS) antibodies were purchased from Cayman Chemical Co. Mouse anti-eNOS and rabbit anti-iNOS antibodies were purchased from BD Transduction Lab. Protein expression was normalized to actin.
Recording of Smooth Muscle Cell Membrane Potentials
Membrane potentials were recorded continuously at room temperature (22°C) on cultured CSMCs using patch clamp techniques as previously described (23).
PGIS and COX-1 Knockdown by Small Interfering RNA (siRNA)
siRNA against human PGIS, COX-1, or PPARδ (PGIS-siRNA, COX-1-siRNA, or PPARδ-siRNA, respectively), and control siRNA (Ct-siRNA) were obtained from Santa Cruz Biotechnology, Inc. The target sequences are listed in the online data supplement. EPCs at 50% confluence were transfected with 30 nmol/L (optimized concentration) of PGIS- siRNA or COX-1-siRNA (using 30 nmol/ Ct-siRNA as a control), or 100 nmol/L of PPARδ-siRNA (using 100 nmol/L of Ct-siRNA as a control), by use of Lipofectamine 2000 (Invitrogen) in serum-free medium (EBM-2), according to manufacture’s protocol. Fresh EGM-2 was added 6.5 h after transfection, and the cells were analyzed 48 h after transfection.
In Vitro Tube Formation Assay
Endothelial tube formation was assessed using MatrigelTM assay (BD Biosciences) as described (9).
Bromo-2′-deoxyuridine (BrdU) Incorporation Assay
After EPCs were transfected with PGIS-siRNA, COX-1-siRNA, PPARδ-siRNA, or Ct-siRNA, they were subjected to BrdU incorporation assay.
Transplantation of EPCs and In Vivo Capillary Assay
All of the experimental protocols were approved by the Institutional Animal Care and Use Committee of the Mayo Clinic. EPCs were transfected with PGIS-siRNA, COX-1-siRNA, PPARδ-siRNA, or Ct-siRNA for 48h. Cells (5×105) were mixed with 200 nl Matrigel, 30 nl EGM-2, and 20 nl FCS, and were then subcutaneously injected into the flanks of anesthetized 8–12 weeks old athymic nude mice [B6 Cg Foxn1, male; Jackson Laboratory (Bar Harbor, Maine)] (one gel injection per side of flank, two gel injections per mouse). Two weeks later, mice were sacrificed and the grafts were excised for histologic evaluation.
Immunohistochemistry
Formalin-fixed, paraffin-embedded tissue sections were immunostained with mouse anti–human vascular endothelial growth factor receptor-2 (VEGFR-2), mouse anti-eNOS, or mouse IgG (as a control).
Statistical Analysis
Data are presented as mean ± SEM. Differences between mean values of multiple groups were analyzed using ANOVA followed by Tukey test (SigmaStat 2.03 for Windows). Comparison between two groups was made using Student’s t-test. P<0.05 was considered statistically significant.
Results
Characterization of EPCs
Numerous studies on the morphological, functional, and biochemical characteristics of EPCs isolated from circulating blood established two distinct populations of cells: early EPCs and late EPCs (blood outgrowth EPCs) (1, 3–10). In the present study, we focused on blood outgrowth EPCs (7, 9), which have been shown to accelerate angiogenesis and reendothelialization (3, 5). Outgrown colonies appeared 2 weeks after culturing of mononuclear cells in EGM2 (Fig 1A). Confluent cells grew into a monolayer with cobblestone appearance (Fig 1B). FACS analysis revealed that EPCs were positive for endothelial cell surface antigens [VEGFR-2, CD31, and VE-cadherin (VE-cad)]. However, they did not express the myelomonocytic cell marker CD14. Only a small portion of the population expressed the hematopoietic progenitor marker CD34 (Fig 1C).
Figure 1.
Phenotyping of human EPCs. A: A single outgrowth colony formed 2–3 weeks after seeding of mononuclear cells on fibronectin-coated plates (4X magnification). B: Around week 4, confluent cells grew into a monolayer with cobblestone appearance (10X magnification). C: FCAS analysis of cell surface markers on EPCs. Shown are representative data from at least 3 independent experiments for each marker. The open black-lined histograms represent test antibodies, and filled histograms represent the control IgG antibodies. Percentage positive cells are shown in each marker panel. D: Cells were cultured in EBM-2 for 24 h, and assayed for total NOS activity. n=4–7, *P<0.05, compared with CAECs. E: Western blotting for eNOS in cells in the presence or absence of TNF-α for 24 h. n=4, * P<0.05.
Cultured EPCs had significantly lower total NOS enzymatic activity compared to CAECs (Fig 1D). The protein levels of eNOS were also significantly lower in EPCs (Fig 1E). Since tumor necrosis factor-α (TNF-α) is one of the major pro-inflammatory cytokines released during cellular infiltration after ischemia (24), we examined the eNOS expression in response to TNF-α. Treatment with TNF-α reduced eNOS expression in both EPCs and CAECs. The effect of TNF-α was more pronounced in EPCs (Fig 1E), consistent with our previous report (9). iNOS protein was undetectable in both cell types (data not shown).
Profile of Prostaglandin Producing Enzymes and Production of Prostaglandins
In contrast to eNOS, Western blotting demonstrated that protein level of COX-1 was significantly higher in EPCs than in CAECs (Fig 2A). Moreover, the expression of TXAS protein was significantly lower in EPCs (Fig 2C), whereas CAECs and EPCs expressed similar levels of PGIS and PGEI proteins (Fig 2B, D). Under basal conditions, the expression of COX-2 was almost undetectable in both cell types (Fig 2E). TNF-α treatment induced COX-2 expression in both EPCs and CAECs (Fig 2E), but did not change protein levels of COX-1, PGIS, TXAS, and PGEI (data not shown).
Figure 2.

Profiles of prostanoid producing enzymes in EPCs and CAECs. Cells were cultured in EBM-2 for 24 h, protein samples were collected for Western blotting. Data are presented as ratio to CAECs. A-C, n=4–5; D, n=3; * P< 0.05, compared with CAECs. E. Cells were treated with the indicated concentrations of TNF-α for 24 h. n=6, data are presented as ratio to EPCs baseline (EBM-2 alone). * P< 0.05.
Strikingly, under basal conditions EPCs released a 4-fold higher amount of PGI2 (determined by measuring 6-keto PGF1α) compared to that released from CAECs (Fig 3A). SC560 (0.1μmol/L, a selective COX-1 inhibitor) or COX-1-siRNA significantly reduced PGI2 production in EPCs (Fig 3E, F). The production of PGI2 in EPCs was significantly increased by treatment with TNF-α. However, TNF-α had only a mild stimulatory effect on PGI2 production in CAECs (Fig 3A). The productions of TXA2 (measured as TXB2) and PGE2 were not significantly different between these two cell types under basal conditions (Fig 3B, C). Treatment by TNF-α increased TXA2 and PGE2 production in EPCs (Fig 3B, C). The ratio of PGI2/TXA2 was significantly higher in EPCs under both basal conditions and in the presence of TNF-α (Fig 3D).
Figure 3.
Production of prostanoids in EPCs and CAECs. Cells were cultured in EBM-2 (A-D) in the presence or absence of 0.5 ng/ml TNF-α for 24 h. CMs were collected and assayed for 6-keto PGF1α (A), TXB2 (B), and PGE2 (C). n=3, * P< 0.05. D: Ratio of 6-keto PGF1α/TXB2 under basal EBM-2 and TNF-α conditions. * P< 0.05. n=3. E: EPCs were incubated with EBM-2 (control), or EBM-2 + 0.1 μmol/L SC-560 for 24 h. CMs were collected for measuring of 6-keto PGF1α. Data are present as percentage of control. * P< 0.05. n=5. F: EPCs were transfected with COX-1-siRNA or Ct-siRNA for 48 h. Cells were then incubated with EGM-2 (2 ml/60 mm dish) for 2 h. The supernatants were collected for measurement of 6-keto PGF1α, n=5 *P<0.05. In all panels, open columns present EPCs and hatched columns, CAECs.
Hyperpolarization of Human CSMCs by CM of EPCs
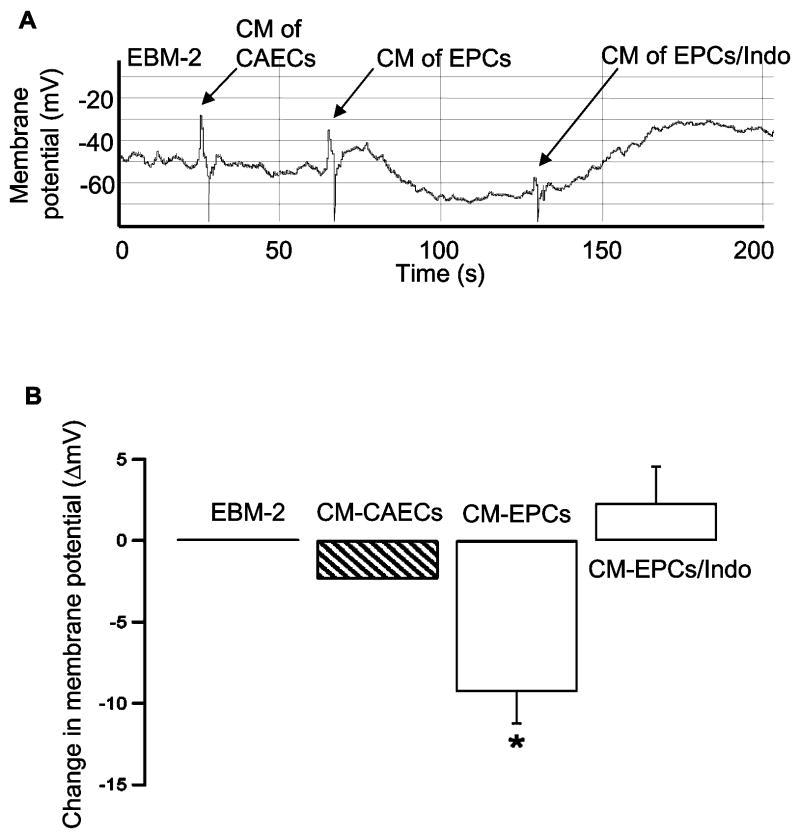
To determine biological activity of prostglandins released by EPCs, we measured the effects of EPCs CM on the membrane potentials of human CSMCs. The membrane potential at baseline (EBM-2) was −40.8 ± 2.9 mV (room temperature). CM obtained from CAECs produced a mild hyperpolarization of CSMCs (−43.1 ± 3.5 mV). However, CM of EPCs produced a significantly stronger hyperpolarizing effect on CSMCs (−50.1 ± 4.5 mV) (Fig 4B). Incubation of EPCs with COX inhibitor indomethacin abolished the effect of CM derived from EPCs on membrane potential (Fig 4), suggesting that these effects were mediated by the products of arachidonic acid metabolism via COX pathway. The hyperpolarization of CSMCs by CM of EPCs was blocked by 0.1 μmol/L iberiotoxin (IBTX, a selective BK channel blocker; data not shown), suggesting that BK channels are involved in the hyperpolarization of the membrane potential. The effect of CM of EPCs was mimicked by 1 μmol/L iloprost (a stable PGI2 analogue), which hyperpolarized membrane potential from −39.3 ± 6.2 mV at baseline to −50.4 ± 5.7 mV (n=3, P<0.05), demonstrating that activation of PGI2 receptors was indeed coupled with hyperpolarization. These results were consistent with previous observation that iloprost activated BK channel in rat CSMCs (25). Since PGI2 was the most abundant prostaglandin present in the CM of EPCs (Fig 3), our findings suggested that the hyperpolarizing effect of CM is most likely mediated by PGI2.
Figure 4.
Effects of CM of EPCs and CAECs on the resting membrane potentials of CSMCs A: A representative tracing of membrane potentials was recorded continuously from CSMCs showing the effects of CMs derived from CAECs, EPCs, or EPCs + indomethacin (Indo, 10 μmol/L). B: Change in membrane potential (ΔmV) after treatment with CMs derived from CAECs, EPCs or EPCs+Indo is shown in the bar graph. Treatment with CM of EPCs significantly hyperpolarized the membrane potential by 9.29±1.95 mV, which was reversed in the presence of 10 μmol/L Indo. n=5–6. * P<0.05, compared with EBM-2, CM-CAECs, or CM-EPCs+Indo.
Cell Proliferation
In the next series of experiments, we examined the role of PGI2 in the regenerative function of EPCs. EPCs proliferation was inhibited by 10 nmol/L indomethacin (Fig 5A), and this effect was reversed by iloprost (3 nmol/L). COX-1 inhibitor SC560 (1 or 5 nmol/L) inhibited EPCs growth in a concentration-dependent manner, while the same concentrations of COX-2 inhibitor NS398 had no significant effect on EPCs proliferation (Fig 5B). Most importantly, PGIS-siRNA or COX-1-siRNA also significantly decreased EPC growth and BrdU incorporation (Fig 6A-E). Iloprost rescued the decreased cell proliferation induced by PGIS-siRNA or COX-1-siRNA (Fig 6C-F).
Figure 5.
Role of COX-1 in EPC proliferation. A. EPCs (20000/well of 24-well plate, seeded in triplicate) were cultured in EGM-2 in the absence (control) or presence of indomethacin (Indo), or Indo + iloprost (ilopr) for 3 days. The number of attached cells in each well was counted in a hemocytometer. A: n=7. *P<0.05, compared to the other 2 groups. **P<0.05, compared to control. B: EPCs were treated with the indicated concentrations of SC560 or NS398 for 3 days. n=3, *P<0.05, compared with EGM-2 alone (control). **P<0.05, compared to other 4 groups.
Figure 6.
Role of PPARδ in PGI2-mediated EPC proliferation. EPCs were transfected with 30 nmol/L Ct-siRNA or PGIS-siRNA (A) or COX-1-siRNA (B) for 48 h. Protein samples were collected for Western blotting. A, n=5, *P<0.05, compared to Ct-siRNA. B, n=3, *P<0.05, compared to Ct-siRNA. C: After EPCs were treated with PGIS- siRNA or Ct-siRNA for 24 h, cells were seeded on 24-well plates (20000/well, in triplicate) and cultured in EGM-2 in the absence or presence of 3 μmol/L iloprost for 48 h. Number of attached cells was counted (n=4–6, **P<0.05, compared with 2 other groups, * P<0.05, compared with Ct-siRNA). D: After EPCs were transfected with COX-1-siRNA or Ct-siRNA for 24 h, cells were seeded in 24-well plates and treated with 3 μmol/L of iloprost or GW501516, or 1 μmol/L cicaprost for 48 h (n=5–12, *P<0.05, compared with COX-1-siRNA, cox-1-siRNA+cicapr, or Ct-siRNA, **P<0.05, compared with Ct-siRNA). E: EPCs were transfected with PGIS-siRNA, COX-1-siRNA, or Ct-siRNA for 48h, then seeded on 96-well plates and assayed for BrdU incorporation [n=3, *P<0.05, compared with control (EGM-2 alone) or Ct-siRNA]. F: After EPCs were transfected with COX-1-siRNA for 30 h, cells were seeded on 96-well plates and incubated in the absence (EGM-2 alone) or presence of indicated treatments for 14 h. Cells were then labeled with BrdU in the same treatment as before, for 24 h (n=5, *P<0.05, compared with COX-1-siRNA in EGM-2 alone or COX-1-siRNA+cicaprost). G: EPCs were transfected with 100 nmol/L of PPARδ-siRNA or Ct-siRNA for 48, protein samples were assayed for Western blotting. Quantification of 4 independent experiments is presented under the representative blot (*P<0.05, compared to Ct-siRNA). H: EPCs were transfected with PPARδ-siRNA or Ct-siRNA for 30 h, cells were then seeded in 96-well plates and cultured in the absence (EGM-2 alone) or presence of 3 μmol/L iloprost for 14 h. Cells were then labeled with BrdU in the same incubations as before, for 24 h (n=5, *P<0.05, compared with Ct-siRNA).
Since iloprost and PGI2 activate PGI2 receptor and PPARs (18, 26, 27), we further investigated the mechanisms of PGI2-dependent mitogenesis. It has been shown that PPARδ mediates PGI2-induced angiogenesis (28) and endothelial survival (18). Therefore, we examined the role of PPARδ in the PGI2-mediated angiogenesis in EPCs. COX-1-siRNA transfected EPCs were treated with 3 μM GW501516 (a selective PPARδ agonist), or 1 μM cicaprost [a selective PGI2 receptor agonist with almost no binding activity for PPARs (26, 27)]. GW501516 and iloprost rescued cell proliferation in EPCs transfected with COX-1-siRNA, whereas cicaprost did not have any effect (Fig 6D, F). In contrast, both cicaprost (1μM) and iloprost (3 μM) significantly increased cAMP in the EPCs by 2.3±0.8 and 2.7±0.5 fold, respectively (data not shown), demonstrating a similar stimulating effect of these compounds on PGI2 receptor. Thus, these results point to an essential role of PPARδ in the PGI2-dependent, and iloprost-induced mitogenesis. Furthermore, down regulation of PPARδ in EPCs by PPARδ-siRNA decreased cell proliferation (Fig 6G, H). The rescue effect of iloprost was abolished in the cells treated with PPARδ-siRNA (Fig 6H), thereby confirming that PPARδ is the major PPAR isoform responsible for the mitogenic effect of PGI2 and iloprost.
In Vitro Angiogenesis
The in vitro tube formation by EPCs was reduced by indomethacin. This inhibitory effect was reversed by 1 nmol/L iloprost (Fig 7A, B). Genetic inactivation of PGIS and COX-1 by siRNA also significantly impaired the tube formation by EPCs (Fig 7C, D). Iloprost and GW501516, but not cicaprost, reduced the impairment of in vitro angiogenesis induced by COX-1-siRNA (Fig 7D). Furthermore, genetic inactivation of PPARδ caused a decrease in the tube formation by EPCs, which was not reversed by iloprost or GW501516 (Fig 7E). These results indicated that angiogenic effect of PGI2 and iloprost is primarily mediated by activation of PPARδ.
Figure 7.

In vitro tube formation. A-B: EPCs were incubated in EBM-2 in the absence (control) or presence of indomethacin (Indo), or Indo+iloprost (Ilopr) for 18 h. Cells were then seeded on MatrigelTM coated well and continued the treatment for another 4 h. A, representative images of tube formation. B: quantification of tube formation (n=4–6, *P<0.05, compared with all other groups, **P<0.05, compared to control). C-E: After transfected with Ct-siRNA, PGIS-siRNA, or COX-1-siRNA (C, D), or PPARδ (E) for 48 h, EPCs were treated with 3 μmol/L of iloprost or GW501516, or 1 μmol/L cicaprost for 18 h. Tube formation assay was then performed in the same incubations as before. D, Data are presented as % of control (n=3–6, *P<0.05, compared to COX-1-siRNA or COX-1-siRNA+cicapr, **P<0.05, compared with PGIS-siRNA, COX-1-siRNA, or COX-1-siRNA+cicapr). E, n=3, *P<0.05, compare to Ct-siRNA.
In Vivo Angiogenesis
To further examine the role of PGI2 in the angiogenic capacity of EPCs, we transplanted EPCs treated with COX-1-siRNA, PGIS-siRNA, or Ct-siRNA, into the nude mice. Two weeks later, capillaries positive for human VEGFR-2 were formed in the gel plugs, many of them containing red blood cells (Fig 8A-D). Transfection of PGIS-siRNA or COX-1-siRNA significantly reduced the in vivo capillary formation in these preparations (Fig 8C, D, E). Down regulation of PPARδ expression also impaired the in vivo angiogenesis (Fig 8F), supporting the concept that COX-1- PGI2 -PPARδ pathway in human EPCs plays an important role in angiogenesis.
Figure 8.
In vivo capillary formation. EPCs were treated with 30 nmol/L Ct-siRNA (A, B), PGIS-siRNA (C), or COX-1-siRNA (D) for 48 h, and then transplanted with Matrigel into nude mice. The gel plugs were excised 2 weeks after transplantation. A, control staining for mouse IgG. B-D, immunostaining for human VEGFR-2 (40X magnification). The arrow heads indicate capillaries, whereas the capillaries containing red blood cells are indicated by arrows. E, quantification of capillary formation (data presented as % of Ct-siRNA), n=4–7, *P<0.05, compared with Ct-siRNA. F: EPCs were transfected with Ct-siRNA or PPARδ-siRNA (n=4, 2 pairs at a concentration of 100 nmol/L, 2 pairs at 30 nmol/L) for 48 h, and transplanted into the nude mice (n=4, *P<0.05, compared to Ct-siRNA).
Discussion
The results of the present study demonstrate that the pro-angiogenic function of human EPCs is critically dependent on arachidonic acid metabolism and biosynthesis of PGI2. We report several novel findings: 1) EPCs release high levels of PGI2, and this is associated with intrinsically high levels of COX-1 expression; 2) production of TXA2 and PGE2 is low and similar between EPCs and CAECs; 3) the in vitro and in vivo angiogenic capacity of EPCs is dependent on the endogenous production of PGI2 in EPCs; 4) the PGI2-dependent angiogenic function of EPCs is mediated by activation of PPARδ rather than PGI2 receptor. These results suggest that COX-1-PGI2-PPARδ is an important signaling pathway in the regenerative function of EPCs.
In this study, we found a striking difference between PGI2 production in human EPCs and in human CAECs. Since biochemical and functional heterogeneity among human primary endothelial cells is well established, our results may not be generalized to include comparison between EPCs and endothelial cells from other vascular beds. Nonetheless, the study provides strong evidence that PGI2 is critical for EPCs’ angiogenic function and vascular protection. Previous studies have established that vasodilator effect of PGI2 is mediated in part by hyperpolarization of membrane potential in vascular smooth muscle cells (29, 30). Since PGI2 was the most abundant product of arachidonic acid metabolism detected in our experiments, we examined the effect of CMs (obtained from both EPCs and CAECs) on membrane potential of smooth muscle cells. As anticipated, EPCs CM has strong hyperpolarizing properties, which are significantly greater than those of CAECs CM. Most notably, treatment of EPCs with indomethacin abolished the effect of CM on membrane potential suggesting that activity of COX(s) is critical for the paracrine effect of EPCs on smooth muscle cells. Hyperpolarization of membrane potential is one of the key mechanisms that produce smooth muscle relaxation. Our results thus suggest that EPCs may cause vasodilatation and increase local blood flow by paracrine-induced hyperpolarization.
Several lines of evidence suggest that under our experimental conditions, the majority of PGI2 was generated by activation of COX-1: a) under basal conditions, EPCs expressed high levels of COX-1 whereas COX-2 protein was almost undetectable; b) inhibition of COX-1 reduced EPCs proliferation, whereas COX-2 inhibitor did not affect cell proliferation; c) the inhibitory effect of PGIS-siRNA on angiogenic response was not statistically different from the inhibition of angiogenesis induced by siRNA designed to inactivate COX-1; and d) production of PGI2 was significantly reduced by a COX-1 selective inhibitor, SC560, or COX-1-siRNA. However, we wish to point out that at the present time, the relative degree of COX-1-PGI2 pathway contribution [as compared to COX-2 or other paracrine mechanisms (31)] to angiogenic function of EPCs is difficult to determine. Based on our findings with selective pharmacological or genetic inhibition of COX-1 or PGIS, it appears likely that mechanisms other than COX-1-PGI2 pathway are also contributors to the ability of EPCs to stimulate angiogenesis.
There are two major signalling pathways responsible for the vascular effects of PGI2. The classic PGI2 signalling is mediated via a G-protein-coupled cell membrane receptor, leading to an activation of adenylyl cyclase and an increase in cAMP (32, 33). Stimulation of this pathway by PGI2 enhances mitogenic effects of growth factors (other than PGI2) or cross activation of RAS/RAF/MEK/ERK mitogenic pathway (33). PGI2 may also stimulate angiogenesis by activation of PPARs (18, 28, 32, 34). PGI2 analogs have been shown to induce DNA binding and transcriptional activation by PPARα and PPARδ (27). Recent studies suggest that activation of PPARδ by PGI2 is responsible for regulation of angiogenesis and apoptosis in endothelial cells (18, 27, 28, 34, 35). The colocolization of COX/ PGIS at the nuclear membrane is consistent with the ability of endogenous PGI2 to activate nuclear receptors (32). We also detected the perinuclear distribution of PGIS in EPCs (He and Katusic unpublished observation). In the present study, we found that the impairment of angiogenesis by inactivation of COX-1 was reversed by a selective agonist of PPARδ, GW501516. This effect was similar to the effect of iloprost, suggesting that activation of PPARδ is a major mechanism underlying the effect of iloprost. In contrast, cicaprost (a PGI2 analogue that dose not activate PPARδ) did not rescue the COX-1-siRNA phenotype, supporting the concept that PGI2 receptor plays a minor role in PGI2-induced angiogenesis. Furthermore, down regulation of PPARδ also inhibited angiogenic function of EPCs. Iloprost failed to rescue the impairment of angiogenesis induced by PPARδ-siRNA, strongly suggesting that PPARδ is the major mediator responsible for PGI2-dependent angiogenesis. GW501516 also failed to correct the inhibitory effect of PPARδ-siRNA, confirming the effectiveness of this siRNA. Most importantly, the results of in vivo experiments reinforced our conclusion that COX-1-PGI2-PPARδ pathway is an important signaling mechanism in the angiogenic function of EPCs.
Interaction between transplanted human EPCs and endogenous mouse endothelium has not been fully characterized in our in vivo experiments. Two mechanisms may account for the reduced angiogenesis by genetically manipulated human EPCs, impaired angiogenic capacity of transplanted EPCs per se, and/or reduced ability of EPCs to stimulate angiogenic function of existing mouse endothelium. The current literature suggests that EPCs produce and release well established angiogenic molecules including VEGF (8) thereby supporting the concept that paracrine stimulation of existing endothelium is an important mechanism of EPCs-induced angiogenesis. However, with regard to in vivo angiogenic effect of human EPCs, our results do not allow any conclusion regarding the relative contribution of transplanted EPCs per se, versus angiogenic stimulation of existing mouse endothelium by paracrine effects of EPCs.
TNF-α is one of the most important pro-inflammatory cytokines present in the ischemic tissues (24). TNF-α treatment increases PGIS expression in bovine endothelial cells (36). In the present study, however, we did not detect the induction of PGIS by TNF-α in human EPCs or human CAECs. The reason for this discrepancy between bovine and human endothelial cells is not immediately apparent, but could be due to the species differences. Interestingly, TNF-α stimulated COX-2 expression in both EPCs and CAECs by a similar magnitude, however, TNF-α had significantly more pronounced stimulatory effects on PGI2 production in EPCs. It is therefore likely that COX-2 activity is an important component of angiogenic activity of EPCs exposed to pro-inflammatory environment. The exact mechanisms underlying the high production of PGI2 in EPCs activated by TNF-α are unclear but could be explained by an elevated expression of TNF-α receptors, increased mobilization of arachidonic acid (37), or high antioxidant capacity of EPCs protecting PGIS from inactivation by peroxynitrite (9, 38). Indeed, previous studies demonstrated that human EPCs had a high level of manganese superoxide dismutase expression (9), and low intracellular concentration of reactive oxygen species (39). These findings coupled with detected low enzymatic activity of NOS suggest that EPCs may be able to minimize production of peroxynitrite, which is generated by a chemical reaction between superoxide anion and NO in ischemic tissue. This could enhance the ability of EPCs to robustly increase PGI2 production in response to TNF-α thus securing preservation of strong vascular protective and pro-angiogenic effects of EPCs under the conditions of oxidative stress.
This study is the first to demonstrate the importance of arachidonic acid metabolism and biosynthesis of PGI2 in the mediation of pro-angiogenic and vasodilator effect of human EPCs. The results provide a novel insight into the mechanism of PGI2 dependent angiogenesis in EPCs. Our observations suggest that adverse cardiovascular effects of COX(s) inhibitors may involve interference of these compounds with regenerative program of EPCs.
Supplementary Material
Acknowledgments
Sources of Funding:
This work was supported by the National Institute of Health grants HL-53524, HL-66958, and by the Mayo Foundation.
Footnotes
Disclosures: None.
References
- 1.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 2.Assmus B, Schachinger V, Teupe C, Britten M, Lehmann R, Dobert N, Grunwald F, Aicher A, Urbich C, Martin H, Hoelzer D, Dimmeler S, Zeiher AM. Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI) Circulation. 2002;106:3009–3017. doi: 10.1161/01.cir.0000043246.74879.cd. [DOI] [PubMed] [Google Scholar]
- 3.Yoon CH, Hur J, Park KW, Kim JH, Lee CS, Oh IY, Kim TY, Cho HJ, Kang HJ, Chae IH, Yang HK, Oh BH, Park YB, Kim HS. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: the role of angiogenic cytokines and matrix metalloproteinases. Circulation. 2005;112:1618–1627. doi: 10.1161/CIRCULATIONAHA.104.503433. [DOI] [PubMed] [Google Scholar]
- 4.He T, Smith LA, Harrington S, Nath KA, Caplice NM, Katusic ZS. Transplantation of circulating endothelial progenitor cells restores endothelial function of denuded rabbit carotid arteries. Stroke. 2004;35:2378–2384. doi: 10.1161/01.STR.0000141893.33677.5d. [DOI] [PubMed] [Google Scholar]
- 5.Kong D, Melo LG, Mangi AA, Zhang L, Lopez-Ilasaca M, Perrella MA, Liew CC, Pratt RE, Dzau VJ. Enhanced inhibition of neointimal hyperplasia by genetically engineered endothelial progenitor cells. Circulation. 2004;109:1769–1775. doi: 10.1161/01.CIR.0000121732.85572.6F. [DOI] [PubMed] [Google Scholar]
- 6.Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003;9:702–712. doi: 10.1038/nm0603-702. [DOI] [PubMed] [Google Scholar]
- 7.Lin Y, Weisdorf DJ, Solovey A, Hebbel RP. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest. 2000;105:71–77. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ, Hwang KK, Oh BH, Lee MM, Park YB. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol. 2004;24:288–293. doi: 10.1161/01.ATV.0000114236.77009.06. [DOI] [PubMed] [Google Scholar]
- 9.He T, Peterson TE, Holmuhamedov EL, Terzic A, Caplice NM, Oberley LW, Katusic ZS. Human endothelial progenitor cells tolerate oxidative stress due to intrinsically high expression of manganese superoxide dismutase. Arterioscler Thromb Vasc Biol. 2004;24:2021–2027. doi: 10.1161/01.ATV.0000142810.27849.8f. [DOI] [PubMed] [Google Scholar]
- 10.Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, Krasich R, Temm CJ, Prchal JT, Ingram DA. Re-defining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–1809. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gulati R, Jevremovic D, Witt TA, Kleppe LS, Vile RG, Lerman A, Simari RD. Modulation of the vascular response to injury by autologous blood-derived outgrowth endothelial cells. Am J Physiol Heart Circ Physiol. 2004;287:H512–H517. doi: 10.1152/ajpheart.00063.2004. [DOI] [PubMed] [Google Scholar]
- 12.Alfranca A, Iniguez MA, Fresno M, Redondo JM. Prostanoid signal transduction and gene expression in the endothelium: Role in cardiovascular diseases. Cardiovasc Res. 2006;70:446–456. doi: 10.1016/j.cardiores.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 13.Grosser T, Fries S, FitzGerald GA. Biological basis for the cardiovascular consequences of COX-2 inhibition: therapeutic challenges and opportunities. J Clin Invest. 2006;116:4–15. doi: 10.1172/JCI27291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu KK, Liou JY. Cellular and molecular biology of prostacyclin synthase. Biochem Biophys Res Commun. 2005;338:45–52. doi: 10.1016/j.bbrc.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 15.Buchanan FG, Chang W, Sheng H, Shao J, Morrow JD, DuBois RN. Up-regulation of the enzymes involved in prostacyclin synthesis via Ras induces vascular endothelial growth factor. Gastroenterology. 2004;1275:1391–1400. doi: 10.1053/j.gastro.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 16.Pichiule P, Chavez JC, LaManna JC. Hypoxic regulation of angiopoietin-2 expression in endothelial cells. J Biol Chem. 2004;279:12171–1280. doi: 10.1074/jbc.M305146200. [DOI] [PubMed] [Google Scholar]
- 17.Murohara T, Horowitz JR, Silver M, Tsurumi Y, Chen D, Sullivan A, Isner JM. Vascular endothelial growth factor/vascular permeability factor enhances vascular permeability via nitric oxide and prostacyclin. Circulation. 1998;97:99–107. doi: 10.1161/01.cir.97.1.99. [DOI] [PubMed] [Google Scholar]
- 18.Liou JY, Lee S, Ghelani D, Matijevic-Aleksic N, Wu KK. Protection of endothelial survival by peroxisome proliferator-activated receptor-{delta} mediated 14-3-3 upregulation. Arterioscler Thromb Vasc Biol. 2006;26:1481–1487. doi: 10.1161/01.ATV.0000223875.14120.93. [DOI] [PubMed] [Google Scholar]
- 19.Gately S, Li WW. Multiple roles of COX-2 in tumor angiogenesis: a target for antiangiogenic therapy. Semin Oncol. 2004;31:2–11. doi: 10.1053/j.seminoncol.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 20.d’Uscio LV, Milstien S, Richardson D, Smith L, Katusic ZS. Long-term vitamin C treatment increases vascular tetrahydrobiopterin levels and nitric oxide synthase activity. Circ Res. 2003;92:88–95. doi: 10.1161/01.res.0000049166.33035.62. [DOI] [PubMed] [Google Scholar]
- 21.Gurbel PA, Murugesan SR, Lowry DR, Serebruany VL. Plasma thromboxane and prostacyclin are linear related and increased in patients presenting with acute myocardial infarction. Prostaglandins Leukot Essent Fatty Acids. 1999;61:7–11. doi: 10.1054/plef.1999.0064. [DOI] [PubMed] [Google Scholar]
- 22.He T, Weintraub NL, Goswami PC, Chatterjee P, Flaherty DM, Domann FE, Oberley LW. Redox factor-1 contributes to the regulation of progression from G0/G1 to S by PDGF in vascular smooth muscle cells. Am J Physiol Heart Cir Physiol. 2003;285:H804–H812. doi: 10.1152/ajpheart.01080.2002. [DOI] [PubMed] [Google Scholar]
- 23.Lu T, Katakam PV, VanRollins M, Weintraub NL, Spector AA, Lee HC. Dihydroxyeicosatrienoic acids are potent activators of Ca(2+)-activated K(+) channels in isolated rat coronary arterial myocytes. J Physiol. 2001;534:651–667. doi: 10.1111/j.1469-7793.2001.t01-1-00651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vandervelde S, van Luyn MJ, Tio RA, Harmsen MC. Signaling factors in stem cell-mediated repair of infarcted myocardium. J Mol Cell Cardiol. 2005;39:363–376. doi: 10.1016/j.yjmcc.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 25.Lu T, Wang XL, He T, Zhou W, Kaduce TL, Katusic ZS, Spector AA, Lee HC. Impaired arachidonic acid-mediated activation of large-conductance Ca2+-activated K+ channels in coronary arterial smooth muscle cells in Zucker Diabetic Fatty rats. Diabetes. 2005;54:2155–63. doi: 10.2337/diabetes.54.7.2155. [DOI] [PubMed] [Google Scholar]
- 26.Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- 27.Forman BM, Chen J, Evans RM. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc Natl Acad Sci U S A. 1997;94:4312–7. doi: 10.1073/pnas.94.9.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim H, Gupta RA, Ma WG, Paria BC, Moller DE, Morrow JD, DuBois RN, Trzaskos JM, Dey SK. Cyclo-oxygenase-2-derived prostacyclin mediates embryo implantation in the mouse via PPARdelta. Genes Dev. 1999;13:1561–74. doi: 10.1101/gad.13.12.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanaka Y, Horinouchi T, Koike K. New insights into beta-adrenoceptors in smooth muscle: distribution of receptor subtypes and molecular mechanisms triggering muscle relaxation. Clin Exp Pharmacol Physiol. 2005;32:503–514. doi: 10.1111/j.1440-1681.2005.04222.x. [DOI] [PubMed] [Google Scholar]
- 30.Tanaka Y, Koike K, Toro L. MaxiK channel roles in blood vessel relaxations induced by endothelium-derived relaxing factors and their molecular mechanisms. J Smooth Muscle Res. 2004;40:125–153. doi: 10.1540/jsmr.40.125. [DOI] [PubMed] [Google Scholar]
- 31.He T, Peterson TE, Katusic ZS. Paracrine mitogenic effect of human endothelial progenitor cells: role of interleukin-8. Am J Physiol Heart Circ Physiol. 2005;289:H968–72. doi: 10.1152/ajpheart.01166.2004. [DOI] [PubMed] [Google Scholar]
- 32.Lim H, Dey SK. A novel pathway of prostacyclin signaling-hanging out with nuclear receptors. Endocrinology. 2002;143:3207–3210. doi: 10.1210/en.2002-220159. [DOI] [PubMed] [Google Scholar]
- 33.Dumaz N, Marais R. Integrating signals between cAMP and the RAS/RAF/MEK/ERK signalling pathways. Based on the anniversary prize of the Gesellschaft fur Biochemie und Molekularbiologie Lecture delivered on 5 July 2003 at the Special FEBS Meeting in Brussels. FEBS J. 2005;272:3491–3504. doi: 10.1111/j.1742-4658.2005.04763.x. [DOI] [PubMed] [Google Scholar]
- 34.Piqueras L, Reynolds AR, Hodivala-Dilke KM, Alfranca A, Redondo JM, Hatae T, Tanabe T, Warner TD, Bishop-Bailey D. Activation of PPAR{beta}/{delta} Induces Endothelial Cell Proliferation and Angiogenesis. Arterioscler Thromb Vasc Biol. 2007;27:63–69. doi: 10.1161/01.ATV.0000250972.83623.61. [DOI] [PubMed] [Google Scholar]
- 35.Pola R, Gaetani E, Flex A, Aprahamian TR, Bosch-Marcé M, Losordo DW, Smith RC, Pola P. Comparative analysis of the in vivo angiogenic properties of stable prostacyclin analogs: a possible role for peroxisome proliferator-activated receptors. J Mol Cell Cardiol. 2004;36:363–370. doi: 10.1016/j.yjmcc.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 36.Hara S, Miyata A, Yokoyama C, Inoue H, Brugger R, Lottspeich F, Ullrich V, Tanabe T. Isolation and molecular cloning of prostacyclin synthase from bovine endothelial cells. J Biol Chem. 1994;269:19897–19903. [PubMed] [Google Scholar]
- 37.Kronke M, Adam-Klages S. Role of caspases in TNF-mediated regulation of cPLA(2) FEBS Lett. 2002;531:18–22. doi: 10.1016/s0014-5793(02)03407-5. [DOI] [PubMed] [Google Scholar]
- 38.Nie H, Wu JL, Zhang M, Xu J, Zou MH. Endothelial nitric oxide synthase-dependent tyrosine nitration of prostacyclin synthase in diabetes in vivo. Diabetes. 2006;55:3133–3141. doi: 10.2337/db06-0505. [DOI] [PubMed] [Google Scholar]
- 39.Dernbach E, Urbich C, Brandes RP, Hofmann WK, Zeiher AM, Dimmeler S. Antioxidative stress-associated genes in circulating progenitor cells: evidence for enhanced resistance against oxidative stress. Blood. 2004;104:3591–3597. doi: 10.1182/blood-2003-12-4103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.