Abstract
Background
The General Population Nutrition Intervention Trial was a randomized primary esophageal and gastric cancer prevention trial conducted from 1985 to 1991, in which 29 584 adult participants in Linxian, China, were given daily vitamin and mineral supplements. Treatment with “factor D,” a combination of 50 μg selenium, 30 mg vitamin E, and 15 mg beta-carotene, led to decreased mortality from all causes, cancer overall, and gastric cancer. Here, we present 10-year follow-up after the end of active intervention.
Methods
Participants were assessed by periodic data collection, monthly visits by village health workers, and quarterly review of the Linxian Cancer Registry. Hazard ratios (HRs) and 95% confidence intervals (CIs) for the cumulative effects of four vitamin and mineral supplementation regimens were calculated using adjusted proportional hazards models.
Results
Through May 31, 2001, 276 participants were lost to follow-up; 9727 died, including 3242 from cancer (1515 from esophageal cancer and 1199 from gastric cancer). Participants who received factor D had lower overall mortality (HR = 0.95, 95% CI = 0.91 to 0.99; P = .009; reduction in cumulative mortality from 33.62% to 32.19%) and gastric cancer mortality (HR = 0.89, 95% CI = 0.79 to 1.00; P = .043; reduction in cumulative gastric cancer mortality from 4.28% to 3.84%) than subjects who did not receive factor D. Reductions were mostly attributable to benefits to subjects younger than 55 years. Esophageal cancer deaths between those who did and did not receive factor D were not different overall; however, decreased 17% among participants younger than 55 (HR = 0.83, 95% CI = 0.71 to 0.98; P = .025) but increased 14% among those aged 55 years or older (HR = 1.14, 95% CI = 1.00 to 1.30; P = .47). Vitamin A and zinc supplementation was associated with increased total and stroke mortality; vitamin C and molybdenum supplementation, with decreased stroke mortality.
Conclusion
The beneficial effects of selenium, vitamin E, and beta-carotene on mortality were still evident up to 10 years after the cessation of supplementation and were consistently greater in younger participants. Late effects of other supplementation regimens were also observed.
CONTEXT AND CAVEATS
Prior knowledge
The undernourished population of Linxian, China, has high rates of cancer of the esophagus and gastric cardia. In the General Population Nutrition Intervention Trial of 1985–1991, 29 584 Linxian villagers aged 40–69 years were given daily supplements of one or more of four vitamin and mineral combinations. “Factor D,” which contained selenium, vitamin E, and beta-carotene, was associated with reduced mortality overall, from cancer, and from gastric cancer.
Study design
For 15-year follow-up, villagers were interviewed monthly concerning their health and registered cancer deaths were reviewed to look for sustained and delayed effects of the vitamin supplements. Hazard ratios for death by cancer, stroke and other causes were calculated for the four supplement combinations.
Contribution
Ten years after the end of the trial, participants who took factor D still had a 5% reduction in total mortality and 11% reduction in gastric cancer; these effects were concentrated among participants younger than 55 years. Esophageal cancer decreased 17% in participants younger than 55 years, but increased 14% in those older than 55 years.
Implications
Sustained benefits were associated with a combination of selenium, vitamin E, and beta-carotene supplementation. More subtle long-term effects were also observed for other vitamin supplements.
Limitations
It is not known which items in the supplement combination are responsible for the combined effect. Because the study was preformed in a nutritionally deprived population with high rates of esophageal and gastric cancer, the findings might not be applicable to other populations. It is possible that the findings could be biased by a general improvement in nutrition among the participants over the postintervention period.
From the Editors
With incidence rates exceeding 100 per 10 000 person-years, the people of Linxian, China, have some of the highest rates of esophageal squamous cell carcinoma and gastric cardia adenocarcinoma in the world (1,2). Several studies in the early 1980s showed that nutritional deficiencies were common in this area, suggesting a link between these deficiencies and the high cancer rates (3). The Linxian General Population Nutrition Intervention Trial (NIT), a large-scale, randomized, double-blind, primary prevention trial, was designed to test the efficacy of four combinations of vitamins and minerals in reducing esophageal and gastric cardia cancer incidence and mortality in Linxian (4–6). The results of this trial, which started March 1, 1986, and concluded May 31, 1991, showed that supplementation with the antioxidant combination of selenium, vitamin E, and beta-carotene statistically significantly reduced total mortality, total cancer mortality, and gastric cancer mortality (5). The identification of statistically significant effects raises questions about the durability of these effects and also potential good or bad late effects related to supplementation.
Since the conclusion of active trial treatment in 1991, follow-up has continued on all participants to collect data on cancer incidence and all-cause mortality. Here we report mortality results for the total 15.25 years of the trial and posttrial follow-up (through May 31, 2001) for the original a priori trial endpoints, that is, the effects of the intervention agents on esophageal and gastric cardia cancer mortality, and for all-cause mortality.
Subjects and Methods
Design of the Trial and Posttrial Follow-up
The design and conduct of the Linxian General Population NIT and its extended follow-up have been described elsewhere (4–6). In brief, participants were recruited in 1985 from four northern communes in Linxian, a rural county in Henan Province. Residents 40–69 years of age with no history of cancer or debilitating disease were eligible for this trial and were asked to enroll. In all, 29 584 subjects, 60% of those invited, were randomly assigned in the trial. These individuals were interviewed for medical history, family history of cancer, diet, and alcohol and tobacco consumption; were given a brief medical exam; and were asked to donate 10-mL blood sample.
The nine nutrients studied in this trial and their daily doses were retinol (5000 IU, as retinol palmitate), zinc (22.5 mg, as zinc oxide), riboflavin (3.2 mg), niacin (40 mg), ascorbic acid (120 mg), molybdenum (30 μg, as molybdenum yeast complex), selenium (50 μg, as selenium yeast), alpha-tocopherol (30 mg), and beta-carotene (15 mg). Doses ranged from one to two times US Recommended Daily Allowances. An independent study of each of these nutrients and vitamins, although desirable, was not practical. Therefore, these nine nutrients were combined into four regimens or factors: retinol and zinc (factor A); riboflavin and niacin (factor B); vitamin C and molybdenum (factor C); and selenium, vitamin E, and beta-carotene (factor D).
The trial had a one-half 24 fractional factorial design, so participants were randomly assigned by a computer-generated random numbers list to take one of eight combinations of the four factors. These eight intervention groups (treatment arms) were defined by the following combinations of factors: ABCD, AB, AC, AD, BC, BD, CD, or placebo. With this design, half the subjects received and half did not receive each of the four factors (eg, half received and half did not receive factor A).
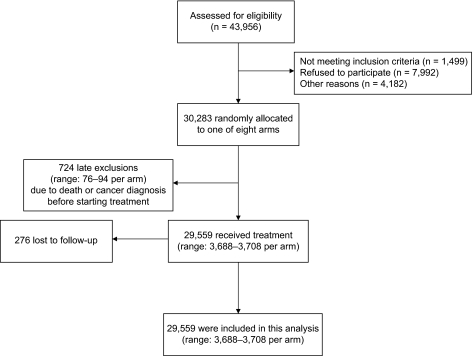
Supplements were distributed in coded pill bottles from March 1, 1986, to May 1, 1991. Throughout the trial, the pill codes were kept in a secured file at the study data management center in the United States and were available only to the study data manager. During the trial period, village health workers visited participants monthly to deliver pills, assess pill compliance, and ascertain vital and disease status. Diagnostic materials (case records, pathology slides, and X-rays) for 85% of the cancer cases in the trial period were available and were reviewed by a panel of American and Chinese experts. In the subsequent 10 years posttrial, village health workers or study interviewers continued to contact participants monthly. For new cancer diagnoses and deaths, diagnostic materials were collected and cancer diagnoses were verified by the panel of American and Chinese experts (1991 to 1996) or senior Chinese diagnosticians from Beijing (1996–2001). Throughout the trial and the posttrial follow-up, case ascertainment was considered complete and loss to follow-up minimal (n = 276, or <1%). Outcomes for this study were based on data from the full 15.25 years of follow-up, from March 1, 1986 (the start of intervention), through May 31, 2001 (10 years after the intervention ended). Due to delayed ascertainment of outcomes, the number of deaths reported here for the intervention period is higher than that in our original report.
Compliance, measured by pill disappearance rates and biochemical measurements, was excellent. The overall pill disappearance rate was 93%, with no differences by treatment arm (range = 92% to 93%). Blood was analyzed in quarterly surveys of a sample of participants for retinol, beta-carotene, ascorbic acid, and glutathione reductase activation coefficient as a measure of riboflavin status, and during the trial period all values were consistently statistically significantly different between treated and untreated individuals (all P values < .001) (4,5).
The conduct of the Linxian General Population NIT was approved by the institutional review boards of the Cancer Institute of Chinese Academy of Medical Sciences and the US National Cancer Institute, and written informed consent was obtained from all participants for participation. The trial was registered as ClinicalTrials.gov number, NCT00342654.
Statistical Methods
The main outcomes of this study were esophageal and gastric cardia cancer mortality and total mortality. Participants were censored at their last known follow-up date, date of death, or the administrative closure of follow-up for the study (May 31, 2001), whichever came first. The 15.25-year follow-up period was analyzed as a whole and in three 5-year periods: the trial period (March 1, 1986, to May 31, 1991) when the intervention agents were given and two other periods (June 1, 1991, to May 31, 1996, and June 1, 1996, to May 31, 2001) when active follow-up continued but no additional intervention was performed. Among the cancers, both esophageal squamous cell carcinoma and gastric cardia adenocarcinoma occur at epidemic rates in this population, share some etiologic risk factors, and before widespread use of endoscopy and biopsy, were diagnosed as a single disease referred to as “esophageal cancer” or “hard of swallowing disease” (7). Through 2001, esophageal adenocarcinoma was nonexistent in Linxian. We present data for the effects of the supplements on mortality of esophageal squamous cell carcinoma, gastric cardia adenocarcinoma, gastric noncardia adenocarcinoma, and the combination of esophageal and gastric cardia cancer (the original hard of swallowing disease).
We tabulated baseline frequencies and percentages of participants by demographics. We compared risk between those who received each factor and those who did not. In the analysis of factorial trials, this kind of analysis is known as “at the margins analysis” and has the most power to examine the effect of each factor (8). Cox proportional hazards models were used to calculate hazard ratios (HRs) and 95% confidence intervals (95% CIs) for each factor, adjusting for the other three treatment factors, sex, age (continuous), and commune (four communes). These analyses were conducted on 29 559 of 29 584 initial study participants; 25 were lost to follow-up before the intervention began (Figure 1). Interactions between each of the factors and age group or sex were examined by including appropriate terms in the Cox models. This testing of interactions between each of the treatments (factors) and age and sex was planned a priori (9,10) The 55-year age cut point was chosen because it is the midpoint in the 40- to 69-year age distribution of the trial population. Kaplan–Meier estimates of cumulative event rates were plotted to compare time to death for each factor, for all subjects, and by sex and age group (11). In addition to “pure” risks determined from Cox models and Kaplan–Meier estimates, we also estimated selected “crude” risks without adjustment for covariates (ie, cumulative incidence), analogous to the approach of Fine and Gray (12). The pure cumulative risks slightly exceeded the crude cumulative risks, as expected (data not shown). Indeed, the estimated pure risks exceeded the crude risk by no more than 0.01 for total mortality, total cancer mortality, gastric cancer mortality, and esophageal cancer mortality. To estimate the calendar time–specific hazard ratios for the figures, we calculated smoothed (moving) hazard ratio estimates using a generalized additive model with a local regression (loess) smoother with span 0.4 and a test-based confidence band.
Figure 1.
CONSORT flow diagram of the Linxian General Population Trial.
The assumption of a constant treatment hazard ratio across the three study periods was verified for each of the analyses by examining treatment by time period interactions. All P values are two-sided and P values less than .05 were considered statistically significant. Analyses were conducted using SAS version 9.1.3 service pack 4 (SAS Institute, Inc, Cary, NC); figures were produced using S-Plus 6.2 for Windows and Sigma Plot 8.0 (Systat Software, Inc, San Jose, CA).
Results
Through May 31, 2001, there were 381 954 total person-years of follow-up over a median of 15.25 years of observation. The baseline demographic characteristics, smoking and alcohol use, and family history of esophageal cancer for all subjects are shown in Table 1. As expected, there were no statistically significant differences between any of these baseline characteristics by treatment group assignments. Smoking prevalence was comparable and alcohol consumption was much less common than in the United States. A family history of esophageal cancer was substantially more common than in the West.
Table 1.
Baseline demographic characteristics, smoking and alcohol consumption, and family history of cancer
| Characteristic | All participants (% of total) | Range for the eight treatment arms |
| No. of participants | 29 559 | 3688–3708 |
| Age group (y) | ||
| <50 | 12 365 (42%) | 42% |
| 50–59 | 10 258 (35%) | 34%–35% |
| ≥60 | 6936 (23%) | 23%–24% |
| Sex | ||
| Female | 16 378 (55%) | 55%–56% |
| Male | 13 181 (45%) | 44%–45% |
| Smoking*,† | ||
| Nonsmoker | 20 613 (70%) | 70%–71% |
| Smoker | 8842 (30%) | 29%–30% |
| Alcohol drinking†,‡ | ||
| Nondrinker | 22 539 (77%) | 76%–77% |
| Drinker | 6915 (23%) | 23%–24% |
| Family history of esophageal cancer† | ||
| No | 20 800 (71%) | 70%–72% |
| Yes | 8651 (29%) | 28%–30% |
Ever smoking cigarettes for 6 or more months.
Data on smoking (n = 104), drinking (n = 105), and family history (n = 108) were not available for some subjects.
Ever drinking any alcoholic beverage in the last 12 months.
Total and cause-specific numbers of deaths for the entire study period and for the three 5-year periods are shown in Table 2. A total of 9727 deaths were reported during the 15.25 years of follow-up. Deaths due to cancer and cerebrovascular diseases each accounted for approximately one-third of the total deaths. The most common specific causes of death were cerebrovascular events (n = 2984), esophageal cancer (n = 1515), and gastric cancer (n = 1199). Total numbers of deaths were 2528 in the trial period (follow-up period 1), 3555 in follow-up period 2, and 3644 in follow-up period 3.
Table 2.
Hazard ratios and 95% confidence intervals for death by cause and vitamin and mineral treatment factors*
| Vitamin and mineral treatment factor†,‡ | |||||
| Cause of death by study period | N | Factor A (vs no factor A), HR (95% CI) | Factor B (vs no factor B), HR (95% CI) | Factor C (vs no factor C), HR (95% CI) | Factor D (vs no factor D), HR (95% CI) |
| Total study period (1986–2001) | |||||
| Total deaths | 9727 | 1.04 (1.00 to 1.09)‡ | 0.98 (0.94 to 1.02) | 0.97 (0.94 to 1.01) | 0.95 (0.91 to 0.99)‡ |
| Cancer | 3242 | 1.00 (0.93 to 1.07) | 0.96 (0.90 to 1.03) | 1.04 (0.97 to 1.11) | 0.95 (0.89 to 1.02) |
| Esophageal | 1515 | 1.07 (0.97 to 1.19) | 0.93 (0.84 to 1.02) | 1.09 (0.98 to 1.20) | 1.01 (0.91 to 1.11) |
| Gastric | 1199 | 0.97 (0.87 to 1.09) | 0.99 (0.88 to 1.10) | 1.05 (0.94 to 1.18) | 0.89 (0.79 to 1.00)‡ |
| Cardia | 873 | 0.95 (0.84 to 1.09) | 1.00 (0.87 to 1.14) | 1.07 (0.94 to 1.23) | 0.89 (0.78 to 1.01) |
| Noncardia | 326 | 1.02 (0.82 to 1.27) | 0.95 (0.88 to 1.03) | 0.99 (0.80 to 1.24) | 0.90 (0.72 to 1.12) |
| Esophageal and cardia | 2388 | 1.03 (0.95 to 1.11) | 0.95 (0.88 to 1.03) | 1.08 (1.00 to 1.17) | 0.96 (0.89 to 1.04) |
| Other cancer | 528 | 0.87 (0.73 to 1.03) | 1.02 (0.86 to 1.21) | 0.89 (0.75 to 1.05) | 0.94 (0.79 to 1.11) |
| Cerebrovascular | 2984 | 1.08 (1.00 to 1.16)‡ | 1.02 (0.95 to 1.10) | 0.92 (0.86 to 0.99)‡ | 0.98 (0.91 to 1.05) |
| Other | 3501 | 1.06 (0.99 to 1.13) | 0.96 (0.90 to 1.03) | 0.96 (0.90 to 1.03) | 0.92 (0.86 to 0.98)‡ |
| Trial period (1986–1991) | |||||
| Total deaths | 2528 | 1.03 (0.95 to 1.11) | 0.99 (0.91 to 1.07) | 1.00 (0.93 to 1.08) | 0.91 (0.84 to 0.99)‡ |
| Cancer | 992 | 0.99 (0.87 to 1.12) | 1.03 (0.91 to 1.16) | 1.04 (0.92 to 1.18) | 0.91 (0.81 to 1.04) |
| Esophageal | 448 | 0.97 (0.81 to 1.17) | 0.90 (0.75 to 1.08) | 1.06 (0.88 to 1.28) | 1.00 (0.84 to 1.21) |
| Gastric | 406 | 1.05 (0.86 to 1.27) | 1.08 (0.89 to 1.31) | 1.06 (0.87 to 1.28) | 0.81 (0.66 to 0.98)‡ |
| Cardia | 297 | 1.15 (0.92 to 1.45) | 1.07 (0.85 to 1.34) | 1.10 (0.88 to 1.39) | 0.83 (0.66 to 1.04) |
| Noncardia | 109 | 0.81 (0.56 to 1.18) | 1.09 (0.75 to 1.59) | 0.94 (0.65 to 1.37) | 0.75 (0.51 to 1.10) |
| Esophageal and cardia | 745 | 1.04 (0.90 to 1.20) | 0.96 (0.84 to 1.11) | 1.08 (0.93 to 1.25) | 0.93 (0.81 to 1.07) |
| Other cancer | 138 | 0.86 (0.62 to 1.20) | 1.37 (0.98 to 1.93) | 0.91 (0.65 to 1.28) | 0.96 (0.69 to 1.35) |
| Cerebrovascular | 643 | 1.06 (0.91 to 1.24) | 0.93 (0.80 to 1.09) | 0.99 (0.85 to 1.16) | 0.90 (0.77 to 1.05) |
| Other | 893 | 1.05 (0.92 to 1.20) | 0.98 (0.86 to 1.12) | 0.97 (0.85 to 1.10) | 0.92 (0.81 to 1.05) |
| Follow-up period 2 (1991–1996) | |||||
| Total deaths | 3555 | 1.05 (0.98 to 1.12) | 0.97 (0.91 to 1.03) | 0.95 (0.89 to 1.02) | 0.99 (0.93 to 1.06) |
| Cancer | 1245 | 1.02 (0.91 to 1.14) | 0.89 (0.80 to 1.00)‡ | 1.04 (0.93 to 1.16) | 0.99 (0.89 to 1.11) |
| Esophageal | 594 | 1.15 (0.98 to 1.35) | 0.91 (0.77 to 1.07) | 1.04 (0.89 to 1.22) | 1.05 (0.89 to 1.23) |
| Gastric | 436 | 1.00 (0.83 to 1.21) | 0.91 (0.75 to 1.10) | 1.11 (0.92 to 1.34) | 0.95 (0.79 to 1.14) |
| Cardia | 321 | 0.99 (0.80 to 1.24) | 0.90 (0.72 to 1.12) | 1.11 (0.89 to 1.38) | 0.96 (0.77 to 1.20) |
| Noncardia | 115 | 1.02 (0.71 to 1.47) | 0.94 (0.65 to 1.35) | 1.13 (0.78 to 1.63) | 0.91 (0.63 to 1.31) |
| Esophageal and cardia | 915 | 1.09 (0.96 to 1.24) | 0.90 (0.79 to 1.03) | 1.06 (0.94 to 1.21) | 1.02 (0.89 to 1.16) |
| Other cancer | 215 | 0.76 (0.58 to 1.00) | 0.81 (0.62 to 1.07) | 0.90 (0.69 to 1.18) | 0.93 (0.71 to 1.22) |
| Cerebrovascular | 1037 | 1.07 (0.95 to 1.21) | 1.11 (0.98 to 1.26) | 0.89 (0.79 to 1.01) | 1.10 (0.97 to 1.24) |
| Other | 1273 | 1.05 (0.94 to 1.17) | 0.94 (0.84 to 1.05) | 0.92 (0.83 to 1.03) | 0.90 (0.81 to 1.01) |
| Follow-up period 3 (1996–2001) | |||||
| Total deaths | 3644 | 1.05 (0.99 to 1.12) | 0.99 (0.92 to 1.05) | 0.97 (0.91 to 1.04) | 0.94 (0.88 to 1.00)‡ |
| Cancer | 1005 | 0.98 (0.87 to 1.11) | 0.99 (0.86 to 1.13) | 1.04 (0.92 to 1.18) | 0.94 (0.83 to 1.06) |
| Esophageal | 473 | 1.08 (0.90 to 1.29) | 0.97 (0.81 to 1.16) | 1.17 (0.98 to 1.40) | 0.95 (0.80 to 1.14) |
| Gastric | 357 | 0.86 (0.70 to 1.06) | 0.99 (0.80 to 1.21) | 0.98 (0.79 to 1.20) | 0.91 (0.74 to 1.13) |
| Cardia | 255 | 0.73 (0.57 to 0.93)‡ | 1.05 (0.82 to 1.34) | 1.00 (0.78 to 1.28) | 0.86 (0.67 to 1.10) |
| Noncardia | 102 | 1.30 (0.88 to 1.93) | 0.84 (0.57 to 1.24) | 0.91 (0.62 to 1.35) | 1.06 (0.72 to 1.57) |
| Esophageal and cardia | 728 | 0.94 (0.81 to 1.09) | 1.00 (0.86 to 1.15) | 1.11 (0.96 to 1.28) | 0.92 (0.80 to 1.06) |
| Other cancer | 175 | 1.02 (0.75 to 1.37) | 1.08 (0.80 to 1.45) | 0.85 (0.63 to 1.15) | 0.93 (0.69 to 1.25) |
| Cerebrovascular | 1304 | 1.08 (0.97 to 1.20) | 1.00 (0.90 to 1.12) | 0.90 (0.81 to 1.01) | 0.94 (0.84 to 1.05) |
| Other | 1335 | 1.08 (0.97 to 1.20) | 0.96 (0.86 to 1.07) | 1.00 (0.89 to 1.11) | 0.93 (0.84 to 1.04) |
HR = hazard ratio; CI = confidence interval.
Factor A = vitamin A (5000 IU/d) + zinc (22.5 mg/d); factor B = riboflavin (3.2 mg/d) + niacin (40 mg/d); factor C = ascorbic acid (120 mg/d) + molybdenum (30 μg/d); factor D = selenium (50 μg/d) + vitamin E (30 mg/d) + beta-carotene (15 mg/d). HRs (95% CIs) adjusted for the other three treatments factors, age (continuous), sex, and commune (four communes).
P < .05.
Adjusted hazard ratios (95% confidence intervals) for the associations between each intervention factor and total and cause-specific deaths for the entire study period and for each of the 5-year follow-up periods are also shown in Table 2. Subjects given factor A (retinol and zinc) had a marginally increased risk of total mortality for the total follow-up period (HR = 1.04, 95% CI = 1.00 to 1.09; P = .035) compared with subjects not given factor A. In the factor A group, the hazard ratio point estimate for total deaths remained consistently higher than unity in all three follow-up periods, with period-specific estimates of 1.03, 1.05, and 1.05, respectively, although none of the period-specific estimates was statistically significant. This increased risk was mainly due to the effect of factor A on noncancer deaths: The hazard ratio for cerebrovascular deaths was 1.08 (95% CI = 1.00 to 1.16; P = .045) and for other causes of death was 1.06 (95% CI = 0.99 to 1.13; P = .088). The hazard ratio point estimates associated with these causes of death remained higher than unity (although not statistically significant) for all three follow-up periods. Neither sex (interaction P = .372) nor age group (interaction P = .757) statistically significantly modified the effect of factor A on total mortality. Factor A was also associated with period-specific protective effects for other cancer deaths in period 2 (HR = 0.76, 95% CI = 0.58 to 1.00; P = .050) and for cardia cancer deaths in period 3 (HR = 0.73, 95% CI = 0.57 to 0.93; P = .012), although neither effect was consistent in the other time periods.
Intervention with factor B (riboflavin and niacin) was not associated with total deaths in the overall (HR = 0.98, 95% CI = 0.94 to 1.02; P = .318) or period-specific analyses when compared with lack of factor B, and hazard ratio point estimates remained close to 1 in all three follow-up periods (Table 2). This factor was not associated with cause-specific deaths overall or in any follow-up period, except for a marginally statistically significant protective effect on total cancer deaths in follow-up period 2 (HR = 0.89, 95% CI = 0.80 to 1.00; P = .043). The effect of factor B on total deaths was not statistically significantly modified by sex (interaction P = .177) or age group (interaction P = .109).
Factor C (vitamin C and molybdenum) was not associated with total deaths in overall (HR = 0.97, 95% CI = 0.94 to 1.01; P = .177) or period-specific analyses when compared with lack of factor C (Table 2). However, this factor was inversely associated with overall risk of cerebrovascular deaths (HR = 0.92, 95% CI = 0.86 to 0.99; P = .023), an effect not seen during the trial period but which became apparent during later follow-up. There were no statistically significant associations between factor C and total cancer mortality, esophageal or gastric cancer mortality, or other causes of mortality in the overall or period-specific analyses, although a marginally statistically insignificant increase in the combined esophageal and cardia cancer mortality endpoint was noted in the overall analysis (HR = 1.08, 95% CI = 1.00 to 1.17; P = .052). Sex did not modify the effect of factor C on total mortality (interaction P = .290), but there was a statistically significant interaction with age (interaction P = .003) such that younger (<55 years at random assignment) participants had reduced risk (HR = 0.89, 95% CI = 0.83 to 0.96; P = .001) and older (≥55 years at random assignment) participants were unaffected (HR = 1.01, 95% CI = 0.97 to 1.06; P = .607). Statistically significant age group interactions were also seen for factor C with total cancer mortality and esophageal cancer mortality; younger patients were unaffected but older participants had elevated hazard ratios (Table 3).
Table 3.
Hazard ratios for total and cause-specific mortality by age group and vitamin and mineral treatment factor for the total 15¼ year follow-up for endpoints and factors with statistically significant or close to statistically significant interactions*
| Age group‡ | P for age group interaction | ||
| Cause of death and treatment factor† | <55 years at random assignment, HR (95% CI) | ≥55 years at random assignment, HR (95% CI) | |
| Total mortality (n = 9727) | |||
| Factor C (vs no factor C) | 0.89 (0.83 to 0.96) | 1.01 (0.97 to 1.06) | .002 |
| Factor D (vs no factor D) | 0.88 (0.82 to 0.95) | 0.98 (0.93 to 1.03) | .023 |
| Total cancer mortality (n = 3242) | |||
| Factor C (vs no factor C) | 0.93 (0.83 to 1.03) | 1.12 (1.02 to 1.22) | .016 |
| Factor D (vs no factor D) | 0.85 (0.76 to 0.95) | 1.02 (0.94 to 1.12) | .019 |
| Esophageal cancer mortality (n = 1515) | |||
| Factor C (vs no factor C) | 0.96 (0.81 to 1.12) | 1.18 (1.04 to 1.35) | .058 |
| Factor D (vs no factor D) | 0.83 (0.71 to 0.98) | 1.14 (1.00 to 1.30) | .003 |
HR = hazard ratio; CI = confidence interval.
Factor C = ascorbic acid (120 mg/d) + molybdenum (30 μg/d); factor D = selenium (50 μg/d) + vitamin E (30 mg/d) + beta-carotene (15 mg/d).
HRs (95% CIs) adjusted for the other three treatments factors, age (continuous), sex, and commune (four communes).
Factor D (selenium, vitamin E, and beta-carotene) reduced total mortality (HR = 0.95, 95% CI = 0.91 to 0.99; P = .009; reduction in cumulative mortality from 33.62% in the no–factor D group to 32.19% in the factor D group). Moving hazard ratio curves (Figure 2) show the smoothed hazard ratios remained less than 1.0 for most of the 15.25 years of observation, indicating a protective effect during most of this period. The estimated hazard ratios for the three follow-up periods were 0.91 (95% CI = 0.84 to 0.99; P = .023), 0.99 (95% CI = 0.93 to 1.06; P = .723), and 0.94 (95% CI = 0.88 to 1.00; P = .046), respectively (Table 2). The hazard ratios for the three major group causes of mortality for the full 15.25 years of follow-up were also less than 1.0 but were not uniformly statistically significant: 0.95 (95% CI = 0.89 to 1.02; P = .148) for cancer, 0.98 (95% CI = 0.98 to 1.05; P = .613) for cerebrovascular events, and 0.92 (95% CI = 0.86 to 0.98; P = .013) for other causes. The majority of the overall effect was attributed to a reduced risk of death from gastric cancer and causes of death other than cancer or cerebrovascular diseases.
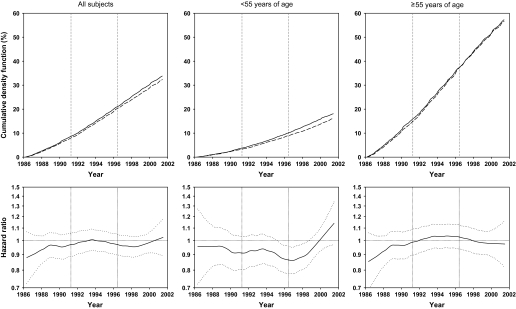
Figure 2.
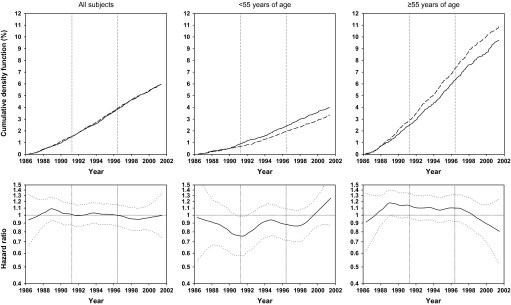
Effects of factor D (a combination of 50 μg selenium, 30 mg vitamin E, and 1.5 mg beta-carotene daily) on total mortality for all subjects, subjects younger than 55 years at random assignment, and subjects 55 years and older at random assignment, as shown by cumulative event rates (cumulative density function, as percentages) from Kaplan–Meier estimates and smoothed (moving) hazard ratio curves. In Kaplan-Meier–based curves, dashed lines represent participants who received factor D; solid lines represent participants who did not receive factor D. In smoothed hazard ratio curves, dotted lines represent 95% confidence intervals around the hazard ratios.
The effect of factor D on total mortality was not modified by sex (interaction P = .843), but was modified by age group (interaction P = .024). Total and age-specific cumulative event and moving hazard ratio curves presented in Figure 2 show cumulative mortality by factor D. The hazard ratios were 0.95 (95% CI = 0.91 to 0.99; P = .009) for all study subjects, 0.88 (95% CI = 0.82 to 0.95; P < .001) for subjects younger than 55 years old at study entry, and 0.98 (95% CI = 0.94 to 1.03; P = .367) for subjects 55 or older at study entry (Table 3). Therefore, virtually the entire effect of factor D on total mortality was due to effects in individuals younger than 55 years.
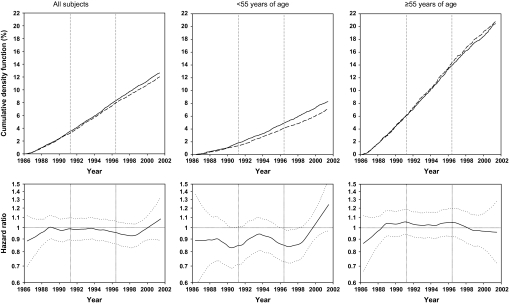
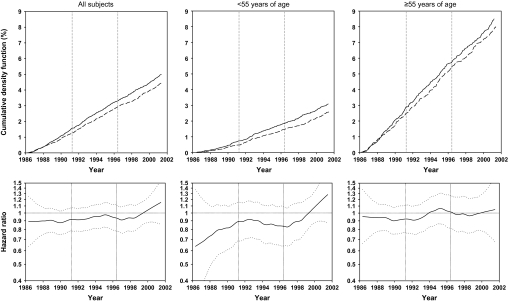
Similar cumulative event rate curves and moving hazard ratio curves for total cancer mortality (Figure 3), total gastric cancer mortality (Figure 4), and esophageal cancer mortality (Figure 5) all show that the effects of factor D were predominantly or exclusively in subjects younger than 55 years. The hazard ratios for total cancer mortality associated with factor D were 0.95 (95% CI = 0.89 to 1.02; P = .148) for all subjects, 0.85 (95% CI = 0.76 to 0.95; P = .003) for those younger than 55, and 1.02 (95% CI = −0.94 to 1.12; P = .976) for those 55 or older (Table 3). Corresponding hazard ratios for gastric cancer mortality were 0.89 (95% CI = 0.79 to 1.00; P = .043), 0.83 (95% CI = 0.69 to 1.00; P = .046), and 0.93 (95% CI = 0.80 to 1.07; P = .307). Cumulative crude gastric cancer mortality for all subjects was 4.28% in the no–factor D group compared with 3.84% in the factor D group, an overall reduction of 0.44%. For esophageal cancer mortality, effect modification by age was even more pronounced. There was no overall association between factor D and esophageal cancer mortality for all subjects (HR = 1.01, 95% CI = .91 to 1.11; P = .905); however, in subjects younger than 55 years, factor D esophageal cancer mortality decreased (HR = 0.83, 95% CI = 0.71 to 0.98; P = .025), whereas in individuals aged 55 years or older, it increased (HR = 1.14, 95% CI = 1.00 to 1.30; P = .047) (Table 3).
Figure 3.
Effects of factor D on total cancer mortality for all subjects, subjects younger than 55 years at random assignment, and subjects 55 years and older at random assignment, as shown by cumulative event rates (as percentages) from Kaplan–Meier estimates and smoothed (moving) hazard ratio curves. In Kaplan-Meier–based curves, dashed lines represent participants who received factor D; solid lines represent participants who did not receive factor D. In smoothed hazard ratio curves, dotted lines represent 95% confidence intervals around the hazard ratios.
Figure 4.
Effects of factor D on total gastric cancer mortality for all subjects, subjects younger than 55 years at random assignment, and subjects 55 years and older at random assignment, as shown by cumulative event rates (cumulative density function, as percentages) from Kaplan–Meier estimates and smoothed (moving) hazard ratio curves. In Kaplan-Meier–based curves, dashed lines represent participants who received factor D; solid lines represent participants who did not receive factor D. In smoothed hazard ratio curves, dotted lines represent 95% confidence intervals around the hazard ratios.
Figure 5.
Effects of factor D on esophageal cancer mortality for all subjects, subjects younger than 55 years old at random assignment, and subjects 55 years and older at random assignment, as shown by cumulative event rates (cumulative density function, as percentages) from Kaplan–Meier estimates and smoothed (moving) hazard ratio curves. In Kaplan-Meier–based curves, dashed lines represent participants who received factor D; solid lines represent participants who did not receive factor D. In smoothed hazard ratio curves, dotted lines represent 95% confidence intervals around the hazard ratios.
Discussion
The initial results of the Linxian General Population NIT, published in 1993, showed no association between factors A, B, or C and overall mortality, total cancer mortality, or mortality from esophageal or gastric cancers (5). However, factor D, which included selenium, vitamin E, and beta-carotene, statistically significantly reduced total mortality, total cancer mortality, and mortality from gastric cancer (5). An important question remained, however, whether the preventive effects of factor D would last beyond the trial period. The results of the continued follow-up show that hazard ratios, as indicated by moving hazard ratio curves, remained less than 1.0 for each of these endpoints for the majority of the follow-up period; 10 years after completion of the trial, the group that received factor D still showed a 5% reduction in total mortality and an 11% reduction in gastric cancer mortality. Overall, one in 70 people who took factor D was spared death from all causes, and one in 227 was spared death from gastric cancer.
Stratification of results by sex and age was planned a priori. There were no statistically significant interactions with sex. However, when stratified by age, factor D had a strong protective effect in individuals younger than 55 years but almost no effect on subjects aged 55 years or older. This pattern was seen consistently for total mortality, total cancer mortality, gastric cancer mortality, and esophageal cancer mortality. Indeed, the effect of factor D on esophageal cancer was reversed by age, showing a protective effect for younger but a harmful effect for older individuals.
Because this trial provided selenium, vitamin E, and beta-carotene as one factor, it was not possible to disentangle the effects of these three supplements. However, observational case–cohort studies using subjects in this cohort and patients with upper gastrointestinal tract cancers that developed during the intervention period showed inverse associations between risk of esophageal cancer and baseline serum levels of selenium and alpha-tocopherol, but not beta-carotene (13–15). Higher baseline serum selenium also was associated with reduced risk of gastric cardia cancer (13). These results suggest that the protective effects seen in the randomized trial were due to the selenium and vitamin E components. In a subcohort of 1103 subjects from this trial followed through May 31, 2001, higher baseline serum selenium levels also were associated with statistically significant reductions in esophageal and gastric cardia cancer mortality (16). A separate randomized controlled trial in Linxian (17) gave further support for a preventive effect of selenium in subjects with preexisting esophageal squamous dysplasia, the precursor lesion of esophageal squamous cell carcinoma. Compared with control subjects, those with mild dysplasia who received 10 months of daily supplementation with 200 μg of selenomethionine were more likely to have regression and less likely to have progression of their esophageal squamous dysplasia.
In addition to evaluating the durability of the beneficial effects observed during the trial period for factor D, we also evaluated other postintervention events in this trial to look for late effects, and several were noted. When the full 15.25 years of follow-up were considered, nutritional supplementation with factor A (vitamin A and zinc) was associated with increased total mortality, mainly due to an increase in stroke deaths among subjects given factor A compared with those who were not given factor A, whereas supplementation with factor C (vitamin C and molybdenum) was associated with a decrease in stroke deaths and with a slight increase in esophageal/cardia cancer deaths.
Increased mortality among factor A recipients was not expected, given the low retinol levels of the population at the start of the trial, the modest doses of both vitamin A and zinc, and the generally null or protective results from previous observational studies in this cohort relating high serum retinol and tissue zinc concentrations to various cancer endpoints (15,18). In fact, a previous analysis of stroke (the main contributor to the observed increase in total mortality) in this trial showed a protective effect for persons who took the combination of factor A and factor D (HR = 0.71, 95% CI = 0.50 to 1.00, for group AD vs placebo) (19).
We were surprised that factor C, a combination of vitamin C and molybdenum, appeared to be associated with increased risk for the combined esophageal and cardia cancer endpoint, given the well-known role of vitamin C as an antioxidant and inhibitor of carcinogenic N-nitroso compound production in the stomach (20). Furthermore, many epidemiological studies have shown reduced risk of these cancers in persons with high consumption of fruits and vegetables and rich sources of vitamin C, and the only prospective study of plasma vitamin C and gastric cancer showed a protective effect for high concentrations (21). No similar prospective studies of plasma vitamin C values in esophageal cancer are known.
The decreased risk of stroke among trial participants who received factor C was not wholly unexpected. Higher plasma vitamin C levels have been associated with reduced risk of stroke in prospective epidemiological studies (22–24), although randomized trials that have included vitamin C as part of an antioxidant vitamin treatment arm have not shown any effect (25,26). There are no data on molybdenum and stroke.
The effects of vitamin and mineral supplements on reduction of total mortality and cancer mortality have been heavily debated over the past 25 years. The results of this study need to be interpreted in the context of other trials of vitamin and mineral supplementation. By the 1980s, it was established that antioxidants could quench free oxygen radicals and potentially reduce the risk of cancer by preventing DNA damage by these radicals. Observational epidemiological studies showed inverse associations between cancer incidence and dietary intake of several vitamins and minerals, but more definitive evidence awaited the completion of randomized trials (27). It was generally assumed that prescribing pills would be a more convenient and acceptable way to prevent cancer than proscribing carcinogens. These facts and assumptions motivated the design and conduct of the first generation of randomized controlled cancer prevention trials, including the Linxian General Population NIT, to reduce cancer risk using vitamins and minerals. The results of the largest and most informative of these trials (ie, those with more than 10 000 participants) were often contrary to the initial expectations. Beta-carotene supplementation increased total mortality in both the Alpha-Tocopherol, Beta-Carotene (ATBC) Cancer Prevention Study (28) and the beta-CArotene and Retinol Efficacy Trial (CARET) Study (29), whereas no mortality benefit for beta-carotene was seen in either The Physicians’ Health Study (PHS) (30) or the Women’s Health Study (31). The most likely explanation for the unexpected findings from these four large trials conducted in the West is a mismatch of the design of the trials with the population attributes: each of these trials tested pharmacological doses of micronutrients in already well-nourished populations (32).
A recent meta-analysis of randomized trials of antioxidant supplements for prevention of cancer and other diseases (33) combined the results of these large-scale studies with many smaller studies and concluded that treatment with beta-carotene, vitamin A, or vitamin E likely increased total mortality, and the effect of vitamin C or selenium on total mortality needed further study. The results of this study agree in part with the meta-analysis conclusions. In this trial, factor A (which included vitamin A) increased mortality, factor C (which included vitamin C) was not associated with overall mortality, and factor D (which included selenium) decreased mortality. However, beta-carotene and vitamin E, two supplements that were associated with increased mortality risk in the meta-analysis, were in factor D, which reduced mortality in this trial. Several potential explanations exist for these apparently discrepant results. First, the protective effect of selenium may have been stronger than the possible deleterious effects of beta-carotene and vitamin E in this trial, so the overall effect of factor D was beneficial. This hypothesis is supported by medium-sized trials that have shown beneficial effects for selenium in reducing mortality and cancer risk (34–36).
A second possible explanation for these discrepancies is that baseline nutritional status of the populations studied influenced the supplementation effects. The people of Linxian are nutritionally deficient (3,37), so vitamin and mineral supplements may be more beneficial to them than to other populations that have been studied. The results of the Dysplasia NIT (38), a medium-sized randomized nutrition intervention trial that was conducted among subjects with cytologically diagnosed esophageal squamous dysplasia in Linxian at the same time as the General Population NIT, showed that supplementation with 26 minerals and vitamins was associated with a non–statistically significant 7% reduction in mortality risk. Results from the Nutritional Prevention of Cancer Trial showed that the benefits for selenium supplementation on total cancer mortality (39) and the development of prostate cancer (36) were essentially limited to participants with lower selenium levels at the start of the trial. Further support for this hypothesis comes from the data of the meta-analysis of antioxidant supplement trials and total mortality itself (33). We classified the 68 study populations included in this analysis as Western (n = 58), East Asian (n = 8), or other (n = 2) and found statistically significant heterogeneity between the results of the studies performed in Western and East Asian populations. The Western studies had a combined odds ratio (OR) of 1.04 (95% CI = 1.01 to 1.06) and the East Asian studies had a combined OR of 0.92 (95% CI = 0.84 to 1.02) (χ2 for heterogeneity P = .02). Because nearly all of the events in the East Asian group came from Linxian, which we know has borderline or deficient nutrition, this difference in meta-analysis results may well reflect differences in the baseline nutritional status of the populations evaluated.
A third possible explanation for the heterogeneity of results observed among studies that have evaluated the association of vitamin supplements and total mortality or gastrointestinal cancer risk is effect modification by the stage of disease at study entry. Our results show that only individuals younger than 55 years benefited from factor D. This result may indicate greater benefit earlier in the course of carcinogenesis and is consistent with a “point of no return,” beyond which supplementation with vitamins is not useful and may be harmful, preferentially benefiting the developing tumor more than the host. This hypothesis may help explain why observational studies, which reflect long-term intake of vitamins and vitamin-containing fruits and vegetables, have usually shown beneficial associations, whereas trials, which have largely been conducted in older patients, have sometimes shown harmful effects from vitamin interventions.
Participants in the ATBC and CARET studies, in addition to being older than those in the Linxian general population trial (ages 50–69 years for ATBC and 45–74 years for CARET), were heavy smokers and some were exposed to asbestos, both powerful carcinogenic exposures that may have put them beyond the point in the disease process that they could benefit from supplements. Detailed analyses of both of these studies have shown that the increased risk associated with vitamin use was almost exclusively seen in current (as opposed to former) smokers (40) and in those who smoked most (41). In contrast, the PHS study, which included fewer than 10% smokers, showed no adverse effect of beta-carotene (30).
In addition to this report, three other cancer prevention nutrition intervention trials have reported results from continued follow-up after the termination of intervention. Follow-up of the participants of the ATBC Study for up to 8 years after the end of the intervention showed that both the harmful effects of beta-carotene (ie, increased total mortality and lung cancer incidence) and the beneficial effect of vitamin E (ie, decreased prostate cancer incidence) disappeared, albeit slowly (42). However, analyses of cerebral infarcts (80% of all strokes) among vitamin E recipients in the ATBC Study showed reversed effects during the trial (relative risk [RR] = 0.86, 95% CI = 0.75 to 0.99) (43) and the 6 years posttrial (RR = 1.13, 95% CI = 1.00 to 1.27) (44). After 6 years of postintervention follow-up in the CARET study, the relative risk of total mortality remained greater than 1.0, but this elevated risk diminished and was no longer statistically significant (45). Lung cancer mortality, however, was still statistically significantly increased. The Calcium Polyp Prevention Trial reported that the protective effect of calcium supplementation on colorectal adenoma recurrence found during the trial period (RR = 0.81, 95% CI = 0.74 to 0.98) (46) continued up to 5 years after supplementation ended and was, if anything, stronger after than during the intervention itself (RR = 0.63, 95% CI = 0.46 to 0.87) (47).
Durability of cancer prevention effects after cessation of intervention has also been observed with nonnutritional agents. In fact, the most consistent example of a sustained cancer prevention effect reported to date from any cancer prevention agent tested in trials is for tamoxifen in the primary prevention of breast cancer. Posttrial follow-up from three tamoxifen trials (48–50) consistently found benefit after the conclusion of active treatment, and in one trial (50), statistically significant reduction in risk (among patients with estrogen receptor-positive tumors) was seen only after treatment had ceased.
This study has several strengths. It was a randomized double-blind design and had excellent compliance and long-term follow-up with virtually complete ascertainment of cases in a well-defined population.
This study also has limitations. Interventions with factors containing multiple agents do not allow evaluation of the effects of individual agents alone, nor were we able to evaluate more than one dose for each of the agents supplemented. The people of Linxian are deficient in many micronutrients, which may limit the generalizability of these results to well-nourished populations. If the protective effects of this study are due to replacement of essential nutrients in a nutritionally deprived population, then similar interventions might be useful in similarly deprived populations in the West, including the United States, although populations with low rates of esophageal and gastric cancer mortality are unlikely to avert as many deaths as high-rate populations such as that in Linxian. Finally, the smoothed hazard ratios that we presented were intended to provide an alternative visual representation of the effects at specific points in time and to complement the cumulative view offered by the Kaplan–Meier curves. These smoothed hazard ratios should be interpreted with caution, however, because the confidence intervals around these curves nearly always include 1.0. Thus, such curves are affected by the play of chance and may be biased by choice of smoothing parameters, edge effects, and other factors.
It should be noted that the follow-up period occurred during a time of dramatic economic progress in China as a whole. Although documented improvements in dietary intakes in Linxian during follow-up were modest (37), more substantial undocumented changes almost certainly occurred. Effects of dietary improvements should have been evenly distributed across all participants in the various randomized treatment groups in the trial. Thus, if the effects of the supplements and the dietary micronutrient intake are additive, any dietary changes that might have occurred should not bias the treatment group effects in the postintervention period. If instead the benefit from supplementation was to correct a deficiency state to exceed some minimum required threshold, then, if all people started to become less deficient because of dietary improvements over time, the observed treatment effects would be expected to weaken. It is all the more remarkable then that benefits persisted despite this likely improved nutrition and its attendant attenuation of treatment effects. It is also possible that improved diet may have modified effects in the postintervention period, including the enhanced benefit widely observed in younger participants and the emergence of late effects, most notably the benefit for factor C on cerebrovascular deaths.
In summary, 10 years of postintervention follow-up of participants in this cancer prevention trial demonstrated the durability of previously observed beneficial effects on mortality from supplementation with selenium, vitamin E, and beta-carotene. The persistence of risk reduction for up to 10 years after treatment in this trial reinforces the validity of the original trial findings and is consistent with an emerging new paradigm in cancer prevention, namely, that prevention may be achievable with short-term as opposed to life-long treatment. Striking age interactions were seen, suggesting that supplements may be more beneficial in younger age groups. Late beneficial and harmful effects on mortality not observed during the trial period of supplementation were also seen for other supplementation groups. Durability and late effects should be examined in other prevention trials.
Funding
National Cancer Institute contracts (N01-SC-91030 and N01-RC-47701 to the Cancer Institute, Chinese Academy of Medical Sciences); the Intramural Research Program of the Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health; the Cancer Institute, Chinese Academy of Medical Sciences.
Footnotes
The authors declare that they have no conflict of interest. The authors are solely responsible for the design of the study; the collection and analysis of data and the interpretation of the results; the preparation of the manuscript; and the decision to submit the manuscript for publication. The authors wish to thank the many citizens of Linxian who have faithfully participated in these studies over the past 20 years.
References
- 1.Li JY, Liu BQ, Li GY, Chen ZJ, Sun XI, Rong SD. Atlas of cancer mortality in the People's Republic of China. An aid for cancer control and research. Int J Epidemiol. 1981;10(2):127–133. doi: 10.1093/ije/10.2.127. [DOI] [PubMed] [Google Scholar]
- 2.Li JY. Epidemiology of esophageal cancer in China. J Natl Cancer Inst Monogr. 1982;62:113–120. [PubMed] [Google Scholar]
- 3.Yang CS, Sun Y, Yang QU, et al. Vitamin A and other deficiencies in Linxian, a high esophageal cancer incidence area in northern China. J Natl Cancer Inst. 1984;73(6):1449–1153. [PubMed] [Google Scholar]
- 4.Li B, Taylor PR, Li JY, et al. Linxian nutrition intervention trials. Design, methods, participant characteristics, and compliance. Ann Epidemiol. 1993;3(6):577–585. doi: 10.1016/1047-2797(93)90078-i. [DOI] [PubMed] [Google Scholar]
- 5.Blot WJ, Li JY, Taylor PR, et al. Nutrition intervention trials in Linxian, China: supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. J Natl Cancer Inst. 1993;85(18):1483–1492. doi: 10.1093/jnci/85.18.1483. [DOI] [PubMed] [Google Scholar]
- 6.Tran GD, Sun XD, Abnet CC, et al. Prospective study of risk factors for esophageal and gastric cancers in the Linxian general population trial cohort in China. Int J Cancer. 2005;113(3):456–463. doi: 10.1002/ijc.20616. [DOI] [PubMed] [Google Scholar]
- 7.Liu SF, Shen Q, Dawsey SM, et al. Esophageal balloon cytology and subsequent risk of esophageal and gastric-cardia cancer in a high-risk Chinese population. Int J Cancer. 1994;57(6):775–780. doi: 10.1002/ijc.2910570603. [DOI] [PubMed] [Google Scholar]
- 8.McAlister FA, Straus SE, Sackett DL, Altman DG. Analysis and reporting of factorial trials: a systematic review. JAMA. 2003;289(19):2545–2553. doi: 10.1001/jama.289.19.2545. [DOI] [PubMed] [Google Scholar]
- 9.Blot WJ, Li JY, Taylor PR, Guo W, Dawsey SM, Li B. The Linxian trials: mortality rates by vitamin-mineral intervention group. Am J Clin Nutr. 1995;62:1424S–1426S. doi: 10.1093/ajcn/62.6.1424S. [DOI] [PubMed] [Google Scholar]
- 10.Qu CX, Kamangar F, Fan JH, et al. Chemoprevention of primary liver cancer: a randomized, double-blind trial in Linxian, China. J Natl Cancer Inst. 2007;99(16):1240–1247. doi: 10.1093/jnci/djm084. [DOI] [PubMed] [Google Scholar]
- 11.Gray RJ. A class of K-sample test for comparing cumulative incidence of competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 12.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 13.Mark SD, Qiao YL, Dawsey SM, et al. Prospective study of serum selenium levels and incident esophageal and gastric cancers. J Natl Cancer Inst. 2000;92(21):1753–1763. doi: 10.1093/jnci/92.21.1753. [DOI] [PubMed] [Google Scholar]
- 14.Taylor PR, Qiao YL, Abnet CC, et al. Prospective study of serum vitamin E levels and esophageal and gastric cancers. J Natl Cancer Inst. 2003;95(18):1414–1416. doi: 10.1093/jnci/djg044. [DOI] [PubMed] [Google Scholar]
- 15.Abnet CC, Qiao YL, Dawsey SM, et al. Prospective study of serum retinol, beta-carotene, beta-cryptoxanthin, and lutein/zeaxanthin and esophageal and gastric cancers in China. Cancer Causes Control. 2003;14(7):645–655. doi: 10.1023/a:1025619608851. [DOI] [PubMed] [Google Scholar]
- 16.Wei W, Abnet CC, Qiao YL, et al. Prospective study of serum selenium concentration and esophageal and gastric cardia cancer, heart disease, stroke, and total death. Am J Clin Nutr. 2004;79(1):80–85. doi: 10.1093/ajcn/79.1.80. [DOI] [PubMed] [Google Scholar]
- 17.Limburg PJ, Wei W, Ahnen DJ, et al. Randomized, placebo-controlled, esophageal squamous cell cancer chemoprevention trial of selenomethionine and celecoxib. Gastroenterology. 2005;129(3):863–873. doi: 10.1053/j.gastro.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 18.Abnet CC, Lai B, Qiao YL, et al. Zinc concentration in esophageal biopsy specimens measured by x-ray fluorescence and esophageal cancer risk. J Natl Cancer Inst. 2005;97(4):301–306. doi: 10.1093/jnci/dji042. [DOI] [PubMed] [Google Scholar]
- 19.Mark SD, Wang W, Fraumeni JF, Jr, et al. Do nutritional supplements lower the risk of stroke or hypertension? Epidemiology. 1998;9(1):9–15. [PubMed] [Google Scholar]
- 20.Mirvish SS. Role of N-nitroso compounds (NOC) and N-nitrosation in etiology of gastric, esophageal, nasopharyngeal and bladder cancer and contribution to cancer of known exposures to NOC. Cancer Lett. 1995;93(1):17–48. doi: 10.1016/0304-3835(95)03786-V. [DOI] [PubMed] [Google Scholar]
- 21.Jenab M, Riboli E, Ferrari P, et al. Plasma and dietary vitamin C levels and risk of gastric cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST) Carcinogenesis. 2006;27(11):2250–2257. doi: 10.1093/carcin/bgl096. [DOI] [PubMed] [Google Scholar]
- 22.Simon JA, Hudes ES, Browner WS. Serum ascorbic acid and cardiovascular disease prevalence in U.S. adults. Epidemiology. 1998;9(3):316–321. [PubMed] [Google Scholar]
- 23.Yokoyama T, Date C, Kokubo Y, Yoshiike N, Matsumura Y, Tanaka H. Serum vitamin C concentration was inversely associated with subsequent 20-year incidence of stroke in a Japanese rural community. The Shibata study. Stroke. 2000;31(10):2287–2294. doi: 10.1161/01.str.31.10.2287. [DOI] [PubMed] [Google Scholar]
- 24.Kurl S, Tuomainen TP, Laukkanen JA, et al. Plasma vitamin C modifies the association between hypertension and risk of stroke. Stroke. 2002;33(6):1568–1573. doi: 10.1161/01.str.0000017220.78722.d7. [DOI] [PubMed] [Google Scholar]
- 25.Waters DD, Alderman EL, Hsia J, et al. Effects of hormone replacement therapy and antioxidant vitamin supplements on coronary atherosclerosis in postmenopausal women: a randomized controlled trial. JAMA. 2002;288(19):2432–2440. doi: 10.1001/jama.288.19.2432. [DOI] [PubMed] [Google Scholar]
- 26.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individualsa randomised placebo-controlled trial. Lancet. 2002;360(9325):23–33. [Google Scholar]
- 27.Peto R, Doll R, Buckley JD, Sporn MB. Can dietary beta-carotene materially reduce human cancer rates? Nature. 1981;290(5803):201–208. doi: 10.1038/290201a0. [DOI] [PubMed] [Google Scholar]
- 28.The ATBC Cancer Prevention Study Group. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. The Alpha-TocopherolBeta Carotene Cancer Prevention Study Group. N Engl J Med. 1994;330(15):1029–1035. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 29.Omenn GS, Goodman GE, Thornquist MD, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334(18):1150–1155. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- 30.Hennekens CH, Buring JE, Manson JE, et al. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N Engl J Med. 1996;334(18):1145–1149. doi: 10.1056/NEJM199605023341801. [DOI] [PubMed] [Google Scholar]
- 31.Lee IM, Cook NR, Gaziano JM, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women's Health Study: a randomized controlled trial. JAMA. 2005;294(1):56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 32.Taylor PR. Prevention of gastric cancer: a miss. J Natl Cancer Inst. 2007;99(2):101–103. doi: 10.1093/jnci/djk026. [DOI] [PubMed] [Google Scholar]
- 33.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297(8):842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 34.Yu SY, Zhu YJ, Li WG, et al. A preliminary report on the intervention trials of primary liver cancer in high-risk populations with nutritional supplementation of selenium in China. Biol Trace Elem Res. 1991;29(3):289–294. doi: 10.1007/BF03032685. [DOI] [PubMed] [Google Scholar]
- 35.Yu SY, Zhu YJ, Li WG. Protective role of selenium against hepatitis B virus and primary liver cancer in Qidong. Biol Trace Elem Res. 1997;56(1):117–124. doi: 10.1007/BF02778987. [DOI] [PubMed] [Google Scholar]
- 36.Duffield-Lillico AJ, Dalkin BL, Reid ME, et al. Selenium supplementation, baseline plasma selenium status and incidence of prostate cancer: an analysis of the complete treatment period of the Nutritional Prevention of Cancer Trial. BJU Int. 2003;91(7):608–612. doi: 10.1046/j.1464-410x.2003.04167.x. [DOI] [PubMed] [Google Scholar]
- 37.Zou XN, Taylor PR, Mark SD, et al. Seasonal variation of food consumption and selected nutrient intake in Linxian, a high risk area for esophageal cancer in China. Int J Vitam Nutr Res. 2002;72(6):375–382. doi: 10.1024/0300-9831.72.6.375. [DOI] [PubMed] [Google Scholar]
- 38.Li JY, Taylor PR, Li B, et al. Nutrition intervention trials in Linxian, China: multiple vitamin/mineral supplementation, cancer incidence, and disease-specific mortality among adults with esophageal dysplasia. J Natl Cancer Inst. 1993;85(18):1492–1498. doi: 10.1093/jnci/85.18.1492. [DOI] [PubMed] [Google Scholar]
- 39.Duffield-Lillico AJ, Reid ME, Turnbull BW, et al. Baseline characteristics and the effect of selenium supplementation on cancer incidence in a randomized clinical trial: a summary report of the Nutritional Prevention of Cancer Trial. Cancer Epidemiol Biomarkers Prev. 2002;11(7):630–639. [PubMed] [Google Scholar]
- 40.Omenn GS, Goodman GE, Thornquist MD, et al. Risk factors for lung cancer and for intervention effects in CARET, the Beta-Carotene and Retinol Efficacy Trial. J Natl Cancer Inst. 1996;88(21):1550–1559. doi: 10.1093/jnci/88.21.1550. [DOI] [PubMed] [Google Scholar]
- 41.Albanes D, Heinonen OP, Taylor PR, et al. Alpha-tocopherol and beta-carotene supplements and lung cancer incidence in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study: effects of base-line characteristics and study compliance. J Natl Cancer Inst. 1996;88(21):1560–1570. doi: 10.1093/jnci/88.21.1560. [DOI] [PubMed] [Google Scholar]
- 42.Virtamo J, Pietinen P, Huttunen JK, et al. Incidence of cancer and mortality following alpha-tocopherol and beta-carotene supplementation: a postintervention follow-up. JAMA. 2003;290(4):476–485. doi: 10.1001/jama.290.4.476. [DOI] [PubMed] [Google Scholar]
- 43.Leppala JM, Virtamo J, Fogelholm R, et al. Controlled trial of alpha-tocopherol and beta-carotene supplements on stroke incidence and mortality in male smokers. Arterioscler Thromb Vasc Biol. 2000;20(1):230–235. doi: 10.1161/01.atv.20.1.230. [DOI] [PubMed] [Google Scholar]
- 44.Tornwall ME, Virtamo J, Korhonen PA, Virtanen MJ, Albanes D, Huttunen JK. Postintervention effect of alpha tocopherol and beta carotene on different strokes: a 6-year follow-up of the Alpha Tocopherol, Beta Carotene Cancer Prevention Study. Stroke. 2004;35(8):1908–1913. doi: 10.1161/01.STR.0000131750.60270.42. [DOI] [PubMed] [Google Scholar]
- 45.Goodman GE, Thornquist MD, Balmes J, et al. The Beta-Carotene and Retinol Efficacy Trial: incidence of lung cancer and cardiovascular disease mortality during 6-year follow-up after stopping beta-carotene and retinol supplements. J Natl Cancer Inst. 2004;96(23):1743–1750. doi: 10.1093/jnci/djh320. [DOI] [PubMed] [Google Scholar]
- 46.Baron JA, Beach M, Mandel JS, et al. Calcium supplements for the prevention of colorectal adenomas. Calcium Polyp Prevention Study Group. N Engl J Med. 1999;340(2):101–107. doi: 10.1056/NEJM199901143400204. [DOI] [PubMed] [Google Scholar]
- 47.Grau MV, Baron JA, Sandler RS, et al. Prolonged effect of calcium supplementation on risk of colorectal adenomas in a randomized trial. J Natl Cancer Inst. 2007;99(2):129–136. doi: 10.1093/jnci/djk016. [DOI] [PubMed] [Google Scholar]
- 48.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97(22):1652–1662. doi: 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- 49.Cuzick J, Forbes JF, Sestak I, et al. Long-term results of tamoxifen prophylaxis for breast cancer—96-month follow-up of the randomized IBIS-I trial. J Natl Cancer Inst. 2007;99(4):272–282. doi: 10.1093/jnci/djk049. [DOI] [PubMed] [Google Scholar]
- 50.Powles TJ, Ashley S, Tidy A, Smith IE, Dowsett M. Twenty-year follow-up of the Royal Marsden randomized, double-blinded tamoxifen breast cancer prevention trial. J Natl Cancer Inst. 2007;99(4):283–290. doi: 10.1093/jnci/djk050. [DOI] [PubMed] [Google Scholar]