Abstract
Background
Adolescence is a transition period from childhood to adulthood that is often characterized by emotional instability. This period is also a time of increased incidence of anxiety and depression underscoring the importance of understanding biological substrates of behavioral and emotion regulation during adolescence. Developmental changes in the brain in concert with individual predispositions for anxiety may underlie the increased risk for poor outcomes reported during adolescence. We tested the hypothesis that difficulties in regulating behavior in emotional contexts in adolescents may be due to competition between heightened activity in subcortical emotional processing systems and immature top-down prefrontal systems. Individual differences in emotional reactivity may put some teens at greater risk during this sensitive transition in development.
Methods
We examined the association between emotion regulation, and frontoamygdala circuitry in 60 children, adolescents, and adults using an emotional go/nogo paradigm. We went beyond examining the magnitude of neural activity and focused on neural adaptation within this circuitry across time using fMRI.
Results
Adolescents showed exaggerated amygdala activity relative to children and adults. This age-related difference decreased with repeated exposures to the stimuli, and individual differences in self-ratings of anxiety predicted the extent of adaptation or habituation in amygdala. Individuals with higher trait anxiety showed less habituation over repeated exposures. This failure to habituate was associated with less functional connectivity between ventral prefrontal cortex and amygdala.
Conclusions
These findings suggest that exaggerated emotional reactivity during adolescence may increase the need for top-down control and put individuals with less control at greater risk for poor outcomes.
INTRODUCTION
Adolescence is a period of heightened emotional reactivity (1) and vulnerability to poor outcomes (e.g., suicide, anxiety, and depression) (2) New insights into the biological basis of emotional reactivity have been provided by human neuroimaging studies. These studies have shown heightened activity in subcortical limbic regions like the ventral striatum and amygdala in adolescents relative to adults with exposure to both positive and negative information (3, 4, 5). In addition, immature prefrontal function has been shown in adolescents relative to adults in emotional contexts (4, 5, 6). Thus, the combination of enhanced bottom-up emotional processing in subcortical regions and less effective top-down regulation from prefrontal regions may lead to an imbalance between emotion processing and control systems during adolescence. This imbalance may play a role in the increased risk for affective disorders during this period (7, 8, 9, 10).
Although increased emotional reactivity is a characteristic of adolescence in general, there is a great deal of individual variability in emotional reactivity and regulation. For example, trait anxiety has been shown to influence behavioral and neural responses involved in emotion regulation. Studies of fear potentiated startle in anxious adults have shown exaggerated emotional responses relative to less anxious individuals (11). In addition, anxious individuals are less efficient at directing attention away from irrelevant emotional information(12, 13). Adults and children with high anxiety have been shown to have elevated activity in brain regions implicated in emotion (e.g. amygdala) in response to negative or threatening information (e.g., fearful facial expressions) compared to low-anxiety individuals (14, 15, 16, 17). The amount of functional coupling (functional connectivity) between the amygdala and prefrontal regions has been shown to correlate with levels of trait anxiety in adults, such that individuals with tighter coupling have lower trait anxiety(18) emphasizing the importance of prefrontal - amygdala interactions in emotion regulation. Extending these findings to adolescence may be particularly relevant given that adolescence is a period when less proficient prefrontal regulation of hyperactive limbic circuitry may create a condition of vulnerability to pathological processes leading to the onset of affective disorders. Examination of the neurobiological basis of individual differences related to emotional reactivity may help to explain why some adolescents experience more difficulties than others in emotion regulation leading to poor outcomes (e.g., onset of anxiety disorder or depression, adolescent suicide).
In the current study, we examined the biological substrates of developmental and individual differences in emotion regulation from childhood to adulthood using fMRI. Specifically, we looked at initial reactivity and subsequent regulation/adaptation of limbic regions with repeated presentations of affective stimuli. While in the scanner, participants performed an emotional go-nogo task that required them to detect either fearful, happy, or calm emotional expressions (target expression) while ignoring non-target expressions. Anxiety levels in adults and adolescents were measured using the Spielberger state-trait anxiety inventory (19), a well validated self report. We hypothesized that adolescents would show exaggerated amygdala responses to emotional expressions compared to children and adults. In addition, we hypothesized that less habituation of the amygdala response to repeated presentations of affective stimuli and immature levels of connectivity between prefrontal control regions and the amygdala would be associated with higher trait anxiety during adolescence.
METHODS AND MATERIALS
Participants
Eighty subjects between the ages of 7 and 32 years were scanned using functional magnetic resonance imaging (fMRI). Data were excluded from six subjects due to effects of time on task in performance (i.e. steep decline in non-target accuracy after initial runs) or because the subject fell asleep; from 12 subjects (4 children, 3 adolescents, and 5 adults) due to excessive head motion (> 2mm translation or 2° of rotation) or insufficient number (<4) of correct non-target trials in a given condition; and from two subjects (1 adolescent and 1 adult) due to technical problems leaving 60 subjects (30 female) in the initial imaging analysis (Table 1).
Table 1.
Age, gender, and race description by age group
| Age range | Mean age | Male | Female | African American | Asian | Caucasian | |
|---|---|---|---|---|---|---|---|
| Children | 7–12 | 9.1 ±1.6 | 7 | 5 | 25% | 13% | 62% |
| Adolescents | 13–18 | 16.0 ± 1.5 | 14 | 10 | 25% | 21% | 54% |
| Adults | 19–32 | 23.9 ± 3.0 | 10 | 14 | 13% | 14% | 73% |
Age ranges, means, and standard deviations are given in years
Adolescents and adults completed the Spielberger state-trait anxiety inventory (STAI) (19) anxiety prior to scanning.1 Separate age appropriate norms included in the STAI manual were used to standardize anxiety scores for adolescents and adults. Trait anxiety score means and standard deviations were 46 ± 9 and 42 ± 7 for adults and adolescents respectively. Forty-six right-handed subjects2 (22 female) between the ages of 13 and 30 years were included in the analysis of anxiety and amygdala habituation. Children were not included in this portion of the analysis because the version of the STAI used is only appropriate for adolescents and adults. Subjects had no history of neurological or psychiatric disorders. Prior to participation, all subjects provided informed written consent (parental consent and subject assent for children and adolescents) approved by the Institutional Review Board of Weill Medical College of Cornell University.
Experimental Task
Subjects completed six runs of a go-nogo task using fearful, happy, and calm facial expressions as targets and non-targets (Fig. 1). All runs included only two categories of expressions, one target and one non-target that were pseudorandomized across the run to control for order of presentation. All combinations of expressions were used as both targets and non-targets.3 Before each run, subjects were given instructions to respond to a particular facial expression by pressing a button but not to respond for any other expression, and to respond as fast as possible without making mistakes. Stimuli were presented for 500 ms and the intertrial interval was varied between 2 and 14.5 sec with a mean intertrial interval of 5.2 sec. Each run lasted 307.5 seconds and consisted of 48 stimulus presentations in a pseudorandom order to ensure an equal number of targets in early, middle and late trials with targets occurring on 75% of trials for all subjects.
Figure 1.
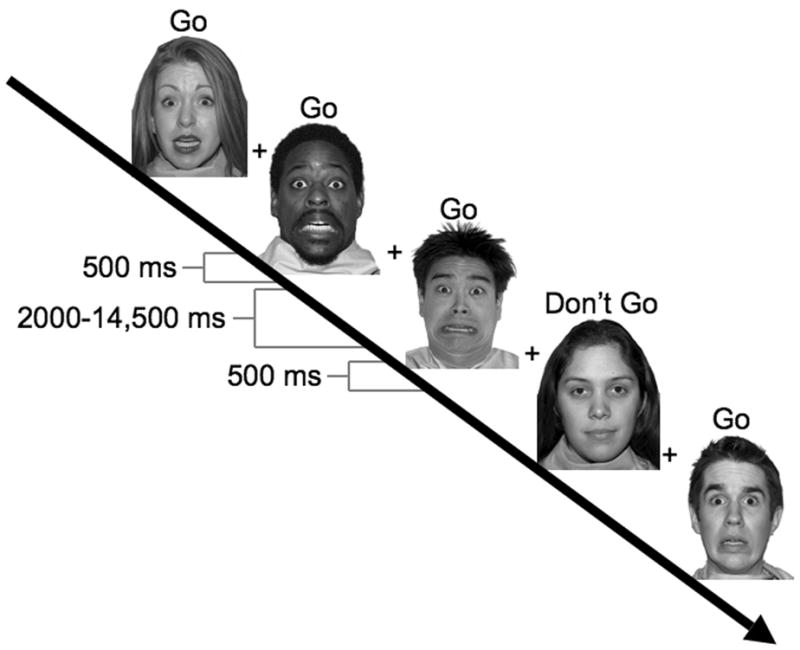
Task Design. Shown is the temporal layout of stimulus presentations within a scan where fear expressions where the target and calm expressions were the non-targets. Stimuli were presented for 500 ms and followed by a variable ISI of 2000 – 14,500 ms.
Stimuli and Apparatus
Face stimuli consisted of gray-scaled fearful, happy, and calm expressions from 12 individuals (6 female) taken from the NimStim set (20) available at www.macbrain.org . Calm expressions were used rather than neutral based on previous findings showing that pediatric populations differ from adults in their response to neutral faces (21). (see supplemental note 1). Subjects viewed images projected onto an overhead LCD panel using the IFIS-SA system (fMRI Devices Corporation, Waukesha, WI).
Image Acquisition
Subjects were scanned with a General Electric Signa 3.0 Tesla fMRI scanner (General Electric Medical Systems, Milwaukee Wisconsin) with a quadrature head coil. A high resolution, T1 weighted anatomical scan (either a 3D SPGR; 256×256 in-plane resolution, 240-mm field of view [FOV]; 124 × 1.5-mm axial slices or a 3D MPRAGE 256×256 in-plane resolution, 240-mm FOV; 124 × 1.5-mm sagittal slices) was acquired for each subject for transformation and localization of functional data into Talairach space (22). A spiral in and out sequence (23) was used to collect functional data (TR=2500, TE=30, FOV=200 mm, Flip angle=90 and 64 × 64 matrix). We obtained 34- 4mm thick coronal slices (skip 0) with a resolution of 3.125 × 3.125 mm covering the entire brain except for the posterior portion of the occipital lobe.
Behavioral data analysis
The effects of age, gender, and emotional expression on reaction time and accuracy were analyzed using repeated measures general linear models in SPSS (SPSS Inc. Chicago, IL). Anxiety was not included in this analysis but a separate model showed no effect of anxiety on reaction time or accuracy (supplemental note 2). F statistics reported for the behavioral data represent Wilks’ Lambda. Post-hoc Mann-Whitney or t-tests were performed on significant main effects and interactions.
Imaging data analysis
Functional imaging data were preprocessed and analyzed using the AFNI software package(24). Following slice time correction images were registered to the first image volume following the high-resolution anatomical dataset using rigid body transformations and smoothed using an isotropic 6mm Gaussian kernel. Time series were normalized to percent signal change to allow comparisons across runs and individuals by dividing signal intensity at each time point by the mean intensity for that voxel and multiplying the result by 100.
Two individual models were fit for each subject. The first model encompassed all trial types and included regressors for each response type (2 responses × 3 emotional expressions = 6) by convolving the stimulus timing files with a gamma-variate hemodynamic response function. Incorrect trials were included as a separate regressor for a total of 7 regressors. The second individual model included only fearful targets paired with calm non-targets and happy targets paired with calm non-targets (supplemental note 3). Separate regressors were created for fearful targets in the early, middle, and late portions of the run (12 targets per bin) as well as calm non-targets by convolving the stimulus timing files with a gamma-variate hemodynamic response function. Regressors were created in the same manner for runs containing happy target expressions paired with calm non-targets. Incorrect trials were included as a separate regressor giving a total of 9 regressors. General linear modeling was performed to fit the percent signal change time courses to each regressor. Linear and quadratic trends were modeled in each voxel time course to control for correlated drift.
Group level analyses were conducted on the regression coefficients from the individual analysis after transformation into the standard coordinate space of Talairach and Tournoux(22) using parameters obtained from the transformation of each subjects high-resolution anatomical scan. Talairached transformed images had a resampled resolution of 3 cubic mm.
Two separate group level linear mixed effects (LME) models were conducted using the 3dLME program within AFNI. The 3dLME program uses functions from the R software package (www.R-project.org). The first LME model included the factors age group, gender, emotion, and response. Trait anxiety was not included in this model because children did not complete the STAI. The second LME model included the factors age group, trait anxiety, emotion, and trial. Gender was not included as a factor in this second analysis due to insufficient sample sizes, but a separate model showed no effect of gender on habituation (supplemental note 4). Directionality of main effects and interactions was examined using post-hoc t-tests in SPSS on the average coefficients extracted from the ROIs.
Correction for multiple comparisons was applied at the cluster level following Monte Carlo simulations conducted in the AlphaSim program within AFNI. Clusterwise false-positive rates of p< .05 corrected for multiple comparisons were determined for whole brain analyses as well as analyses restricted to the amygdala and vPFC (supplemental note 5). Corrected p-values are indicated with an asterisk (*). Coordinates presented in the text and supplemental figures represent center of mass for the ROI. Values in parentheses within the results section are means and standard errors.
A functional connectivity analysis was performed using the average time series from 16 voxels in the left amygdala where habituation was correlated with trait anxiety shown in figure 4. Linear trend removal was first conducted on the time-series in every voxel throughout the brain. The AFNI program 3dfim+ was then used to calculate correlations between the time-series for the entire run at each voxel and the mean detrended time-series from voxels within the seed point after removing potential confounds including motion parameters, average whole brain signal, signal from the ventricles, and signal from deep brain white matter and their derivatives. Correlation values were normalized using Fisher’s Z-transformation before group analysis.
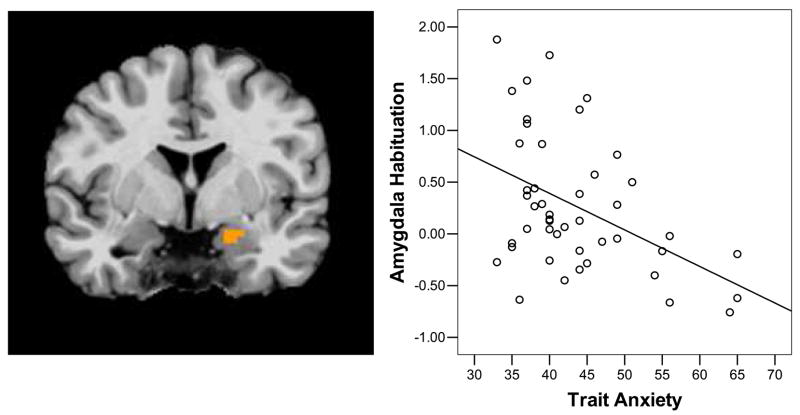
Figure 4.
Amygdala habituation and trait anxiety. Trait anxiety scores were negatively correlated with habituation (decrease from early to late trials) of amygdala activity (r = −.447; p< .001) Amygdala habituation was calculated by subtracting activity in late trials from activity in early trials a) region of the left amygdala that correlated with trait anxiety. b) Scatter plot of the correlation between trait anxiety and amygdala habituation. The y-axis represents MR Signal in the left amygdala for early minus late trials. The x-axis represents trait anxiety score.
RESULTS
Behavioral Results
The effect of emotional expression on reaction time was examined with a repeated measures GLM including age group (children, adolescents, adults) and gender (male, female) as between subjects factors and emotion (fear, happy, calm) as the within subjects factor. There were main effects of emotion (F(2,65) 42.00, p< .001) and age (F(2,66) 19.43, p< .001) on reaction time. There was also an interaction between emotion and age on reaction time (F(4,130) 6.13, p< .001). There was no effect of gender on reaction time. Post-hoc ttests showed that the main effect of emotion was due to faster reaction times for happy (592 ± 15) relative to fear (634 ± 20; t(73) = 6.43, p< .001) and calm (642 ± 19; t(73) = 7.51, p< .001). Post-hoc Mann-Whitney tests showed that adults (606 ± 16; U=123, N1=16, N2=32, p< .005) and adolescents (552 ± 17; U=67, N1=16, N2=26, p< .001) responded faster than children (787 ± 53). The interaction between age and emotion was due to relatively slower responses by adolescents (zscore = 1.03 ± .01; t(56) =2.29; p< .05) and children (zscore = 1.05 ± .01; t(46) =3.53; p< .001) for fearful target faces compared to adults (zscore = 1.00 ± .01). The repeated measures GLM examining accuracy can be found in the online supplemental material (supplemental note 6).
Imaging Results
Age and Gender Differences
To examine developmental and gender effects a linear mixed effects model including age group (children, adolescents, adults) and gender (male, female) as between subjects factors and emotion (fear, happy, calm) and response (go, nogo) as the within subjects factors was conducted on the dependant variable of blood oxygen level dependent (BOLD) signal (activity). The complete list of brain regions showing main effects and interactions is given in supplemental table 1. Within the amygdala, there were main effects of age group (xyz = 25 −6 −13; F(2,54) =4.08, p< .05*), emotion (xyz = −28 2 −17 and 24 2 −14; F(2,108) =4.98, p< .05*) and response (xyz = −26 −5 −13 and 25 −6 −13; F(1,54) =7.725, p< .05*)and an interaction between gender and response (xyz = −18 −4 −12; F(1,54) =7.725, p< .05*). Figure 2 shows that adolescents had greater amygdala activity than children (U=93, N1=14, N2=23, p< .05) and adults (U=150, N1=19, N2=34, p< .001). Post-hoc t-tests showed that there was greater amygdala activity for fear faces (.13 ± .02) than calm faces (.07 ± .02; t(59) =2.76, p< .01), but no significant difference between fear and happy (.09 ± .02). There was also greater amygdala activity for non-target than target faces (t(59) =6.29, p< .001). The interaction between gender and response was due to greater amygdala activity for non-targets in males versus females (t(58) =3.01, p< .005). There was no difference in amygdala activity between males and females for target faces.
Figure 2.
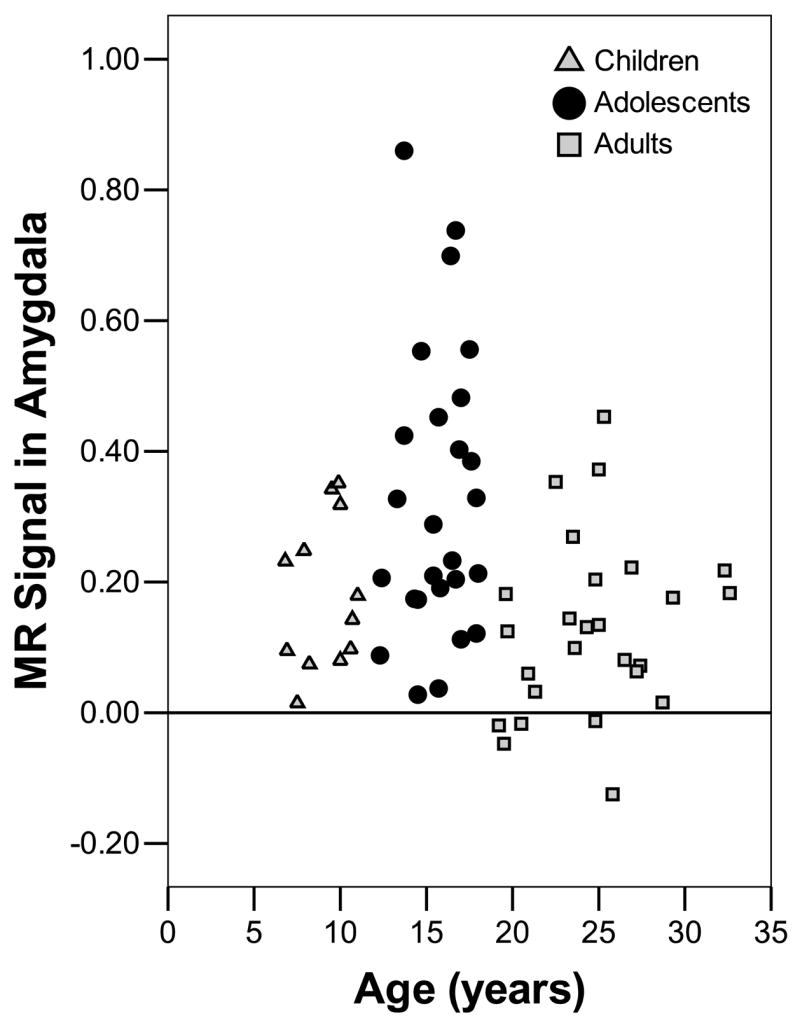
Greater amygdala reactivity in adolescents. Mean amygdala activity for both target and non-target expressions was greater for adolescents than adults and children. Scatter plot shows mean MR signal in the amygala on the y-axis. The x-axis represents age in years. Age group is coded with adults as squares, adolescents as circles and children as triangles.
Neural Correlates of Response Latency
Based on previous work (25) showing that increased activity in the amygdala is correlated with slower responses to fearful target faces, we conducted a linear regression analysis to determine if amygdala activity was correlated with reaction time in the current study. We regressed the percent difference in reaction time for fearful minus happy targets versus activity in the amygdala for fear minus happy targets. There was a significant association between reaction time and activity in the left amygdala, such that slower reaction times were associated with greater left amygdala activity (xyz = −15 −2 −16; F(1,59) =5.72; p< .05*; Fig. 3). The correlation between amygdala activity and reaction time remained significant when controlling for dprime (r(57) =.42; p< .001). To test our hypothesis that prefrontal regions were involved in modulating subcortical regions like the amygdala in the context of emotional information, we conducted another linear regression examining the percent difference in reaction time for fearful minus happy faces and prefrontal activity, controlling for left amygdala activity and dprime. This analysis showed that activity in the ventral prefrontal cortex (vPFC) was associated with faster reaction times for fear targets (xyz = 6 23 −8; F(1,56) =8.56; p< .05*; Fig. 3).
Figure 3.
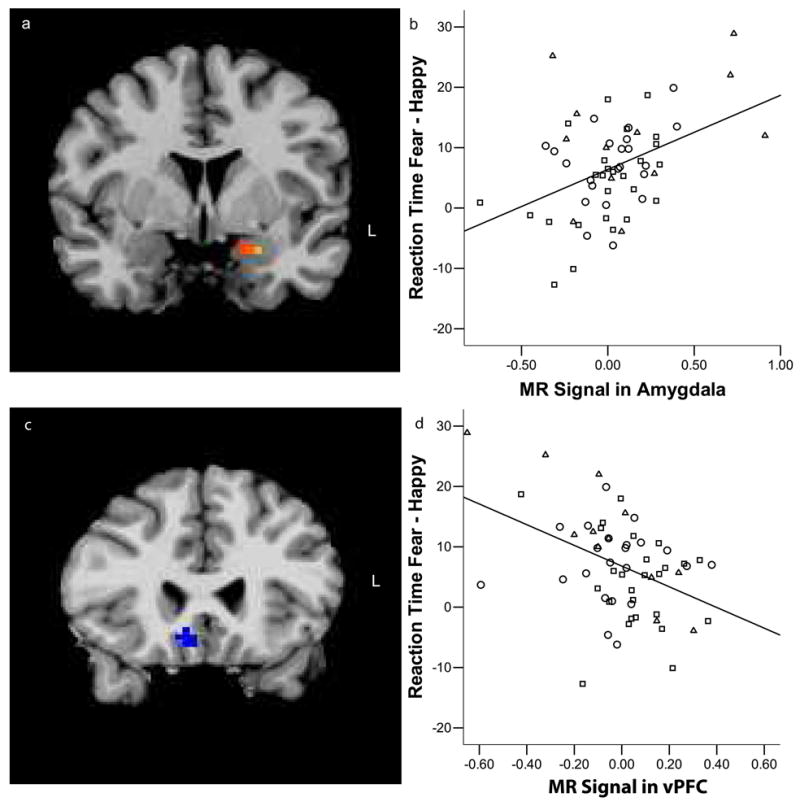
Neural correlates of reaction time for fear targets. Activity in the left amygdala was positively correlated with reaction time for fear relative to happy targets (r = .418; p< .001) while activity in the ventral prefrontal cortex vPFC was negatively correlated with reaction time (r = −.411 p< .001 a) region of the left amygdala that correlated with reaction time b) Scatter plot of the correlation between reaction time and amgdala activity. The y-axis represents the difference in reaction time between fear and happy targets as calculated by dividing the difference between mean reaction times for fear and happy by overall mean reaction time. The x-axis represents amount of activity in the amygdala for fear minus happy targets. c) region of ventral prefrontal cortex that was negatively correlated with reaction time.d) Scatter plot of the correlation between vPFC activity and reaction time. The y-axis represents the difference in reaction time between fear and happy targets as calculated by dividing the difference between mean reaction times for fear and happy by overall mean reaction time. The x-axis represents amount of activity in the vPFC for fear minus happy targets after removing the variance associated with left amygdala activity and target accuracy using linear regression. Age group is coded with adults as squares, adolescents as circles and children as triangles.
Individual Differences
To examine the association between individual differences in emotional regulation and self rated trait anxiety, a linear mixed effects model including age group (adolescent, adult) and trait anxiety (high, low) as between subjects factors and emotion (fear, happy) and trials (early, middle, late) as within subjects factors on the dependent variable of BOLD signal (activity). Within the amygdala there were main effects of emotion (xyz = −22 −5 −15 and 23 −8 −10; F(1,42) =7.29; p< .05*) and trials (xyz = −23 −5 −14 and 23 5 −14, F(2,84) =7.29; p< .05*) as well as interactions between; age and emotion (xyz= −20 −8 −20; F(1,42) =7.29; p< .05*), anxiety and emotion (xyz = 19 −4 −12; F(1,42) =7.29; p< .05*), age and trial (xyz = −26 1 −16; F(2,84) =5.50; p< .05), and age, anxiety, emotion, and trial (xyz= −28 0 −15 and 20 −9 −7; F(2,84) =7.29; p< .05*). Two regions in vPFC also showed an interaction between age, anxiety, emotion, and trial (xyz = −11 44 12 and 25 54 7, F(2,84) =11.11; p< .05*). Supplemental table 2 lists all brain regions showing main effects and interactions in this analysis. Post-hoc tests were conducted on selected interactions of interest. The age group by trial interaction was due to greater amygdala activity in adolescents (.44 ± .13) than adults (.07 ± .07) in early trials (t(44) = 2.47, p< .05), but no difference in middle (.23 ± .12 vs −.02 ± .05) or late trials (−.02 ± .10 vs −.06 ± .08). This age group by trial interaction remained significant when controlling for reaction time (supplemental notes 7 and 8). The interaction between age, anxiety, emotion, and trial was due to the fact that amygdala activity decreased from early to late trials (habituated) less for fear than happy targets in more anxious adolescents (t(9) =3.36, p< .01), but habituation did not differ as a function of emotion in less anxious teens or adults (Table 2). Greater amygdala habituation to fear targets was associated with lower trait anxiety scores across both age groups (xyz= −20 −5 −14; F(1,45) =7.86, p< .05* ; Fig. 4). The pattern of activity for fear targets in the vPFC differed as a function of age and anxiety, such that less anxious adolescents and more anxious adults showed increased activity in early trials (.19 ± .08) versus late trials (−.11 ± .08; t(25) =2.70; p< .01), while activity did not differ over time for more anxious teens and less anxious adults.
Table 2.
Amygdala habituation to target faces
| Age Group | Trait Anxiety | Fear | Happy |
|---|---|---|---|
| Adolescents | Less Anxious | 0.45 ± 0.14 | 0.24 ± 0.13 |
| More Anxious | −0.09 ± 0.19 | 0.96 ± 0.25 | |
| Adults | Less Anxious | 0.19 ± 0.08 | 0.55 ± 0.19 |
| More Anxious | 0.06 ± 0.13 | 0.11 ± 0.10 |
Values represent the mean difference and standard error in MR signal between early and late trials in blocks with fear and happy targets. One outlier was removed from the mean for happy targets in more anxious adolescents.
Functional Connectivity
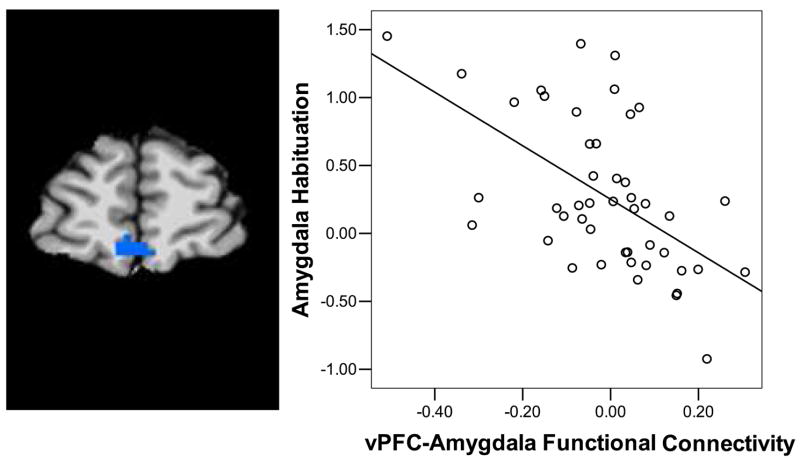
To examine the relationship between activity in the amygdala and vPFC we conducted a functional connectivity analysis. There was a negative correlation between activity in the amygdala and vPFC for fear targets (t(45) = 3.51, p< .05*; supplemental table 4). A linear regression analysis showed that stronger connectivity between the vPFC (xyz= 2 55 −5, 21 30 3, and −17 58 4) and the amygdala was associated with greater habituation of amygdala activity (early – late) (F(1,45) =8.72, p< .05*). A conjunction analysis showed that there was overlap between the areas of vPFC showing an interaction between age, anxiety, emotion, and trial and vPFC regions where functional connectivity with the amygdala was associated with greater amygdala habituation (Fig 5).
Figure 5.
Ventral prefrontal – amygdala connectivity and habituation. There was negative functional connectivity between the amygdala and the vPFC. The magnitude of activity in vPFC and the strength of the connectivity between vPFC and the amygdala were negatively correlated with amygdala habituation (r = −.559 p< .001). Amygdala habituation was calculated by subtracting activity in late trials from activity in early trials.a) shows the conjunction analysis for a vPFC region displaying a significant interaction between age group, emotion, trial, and anxiety and where stronger functional connectivity predicted greater amygdala habituation d) Scatterplot of vPFC-amygdala connectivity values versus amygdala habituation. The y-axis represents MR Signal in the leftamygdala for early minus late trials. The x-axis represents z-scored vPFC-amygdala connectivity values.
DISSCUSION
A neural basis for difficulties in regulating behavior in emotional contexts in adolescents was tested. The findings are consistent with a neurobiological model (20, 26) of competition between enhanced activity in subcortical emotional processing systems and less mature top-down prefrontal systems. The ability to engage in top-down regulation of emotional centers such as the amygdala is likely to be important during adolescence in guiding behavior in highly emotional contexts. Our findings suggest elevated amygdala activity in such situations in adolescents relative to children and adults. Differences in the strength of connectivity between top-down control and bottom-up emotion processing regions may underlie individual differences in emotion regulation especially during adolescence when these bottom up systems appear to be elevated in activity. Anatomical studies of brain development have shown protracted development of prefrontal regions in terms of both local decreases in gray matter density and increases in the myelination of fibers linking prefrontal cortex to other brain regions (27). Both local refinements and increased connectivity are likely to improve the efficiency of emotion regulation based on our findings showing that the strength of coupling between ventral prefrontal cortex and the amygdala is correlated with greater habituation of amygdala activity during adolescence. Despite the relative immaturity of prefrontal cortex during adolescence, amygdala activity decreased to near or even below baseline with repeated exposure to empty threat (fearful faces) in both adults and adolescents. These data are consistent with previous neuroimaging studies of cognitive control showing that adolescents can suppress a competing response, but must recruit prefrontal regions more than adults to do so (28). The fact that adolescents respond more slowly to fear targets and show less prefrontal relative to amygdala activity for these trials than adults suggests that adolescents might be more susceptible to emotional interference relative to adults. Greater initial reactivity in subcortical limbic regions in adolescents relative to adults may explain why poor decisions may be made in the heat of the moment even though adolescents know better. Given the role of prefrontal regions in guiding appropriate actions, immature prefrontal activity might hinder decisions within an emotional context (i.e., heat of the moment).
Differences in the efficiency of prefrontal regulation may also explain the lower levels of vPFC activity in less anxious adults in the current study. Whereas less anxious teens showed greater vPFC activity in early versus late trials mirroring the decrease in amygdala activity, less anxious adults showed little activity in vPFC regions for fear targets. However, less anxious adults also showed rapid habituation (decrease from early to late trials) of amygdala activity to levels below baseline. Less anxious adults may be more efficient in regulating amygdala activity and, therefore require less vPFC activity.
Theories of the neurobiological basis of affective disorders emphasize the role of circuits including the amygdala and ventral prefrontal cortex(29, 30, 31, 32). The current study showed differences in the amygdala and ventral prefrontal cortex as a function of variance in trait anxiety within the normal range and these differences may be even greater in clinically anxious populations. Functional magnetic resonance imaging studies have found greater amygdala activity in response to negatively valenced information (often fearful faces) and diminished activation of ventral prefrontal cortex(16, 33, 34) in clinically anxious children and adults relative to controls. We, and others(18), have shown that less functional connectivity between amygdala and ventral prefrontal cortex is associated with higher anxiety. Functional coupling between the amygdala and ventral prefrontal cortex is influenced by emotional context (35, 36). This association may be especially important during adolescence when transitions from childhood to adulthood result in increased independence (separation from care-givers) and require more self-regulation of emotion.
Increased amygdala activity has been shown during initial fear conditioning in healthy controls that diminishes with extinction (37, 38). There is evidence for less habituation of amygdala activity (ie., diminished activity with repeated exposures to empty threat) in clinically anxious populations than in healthy controls. A recent meta-analysis of studies utilizing fear conditioning paradigms in participants with anxiety disorders revealed consistent deficits in extinction following simple fear conditioning relative to controls (39). A similar study directly examining amygdala habituation to signals of threat (fearful faces) found a trend for less habituation in patients with PTSD compared to healthy controls (40). These results are consistent with our data showing a correlation between amygdala habituation and trait anxiety in healthy adolescents and adults.
The current study required subjects to make a response that was in opposition to an affective signal (i.e. approach fearful expressions that are associated with threat), and to do so as fast as they could. Thus, optimal performance required emotion regulation and allowed us to examine individual and age-related differences in sensitivity to affective interference. Adolescents and children were relatively slower than adults when responding to fearful target faces suggesting that adolescents and children were less efficient at overriding affective interference compared to adults. We have now shown in two separate experiments that, in the context of a go-nogo task, mean reaction times for fearful facial expressions as targets are positively correlated with amygdala activity(25). Ventral PFC activity was associated with both faster reaction times to fear targets and greater amygdala habituation. Together these findings suggest that ventral prefrontal regions are engaged to regulate affective processing and facilitate appropriate responses in the presence of affective interference. Prefrontal regulation may be especially important during adolescence due to increased reactivity of affective processing systems like the amygdala in response to emotional information compared to children and adults. Furthermore, adolescents must deal with dramatic changes in their social environment and interactions that may serve as stressors driving activity in hypersensitive affective systems that immature prefrontal control circuitry cannot effectively regulate. Therefore, the combination of biological susceptibility and environmental context may underlie the prevalence of affective disorder onset during adolescence.
Supplementary Material
Acknowledgments
This work was supported by National Institute of Drug abuse grant R01 DA 18879 to BJC and National Institute of Mental Health grant F31 MH 073265 to TAH.
Footnotes
Two adults and one adolescent completed the STAI ratings immediately following the scanning session
Two adults were removed do the presence of outlying values (> 3 SD from the mean) when the blocks were divided into early, middle. & late trials.
Four adolescents successfully completed only 4 runs of the task, but all four completed the fear target with calm non-target and happy target with calm non-target runs on which the habituation analysis was based. These four subjects were not included in the behavioral analysis due to failing to complete the experiment.
Disclosure of Biomedical Financial Interests and Potential Conflicts of Interest
Drs. Hare, Tottenham, Galvan, Voss, Glover. & Casey report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting. & review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content. & all legal disclaimers that apply to the journal pertain.
References
- 1.Arnett JJ. Adolescent storm and stress, reconsidered. Am Psychol. 1999;54:317–26. doi: 10.1037//0003-066x.54.5.317. [DOI] [PubMed] [Google Scholar]
- 2.Eaton DK, et al. Youth risk behavior surveillance--United States, 2005. J Sch Health. 2006;76:353–72. doi: 10.1111/j.1746-1561.2006.00127.x. [DOI] [PubMed] [Google Scholar]
- 3.Ernst M, et al. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25:1279–91. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 4.Galvan A, et al. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J Neurosci. 2006;26:6885–92. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monk CS, et al. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. Neuroimage. 2003;20:420–8. doi: 10.1016/s1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- 6.Eshel N, et al. Neural substrates of choice selection in adults and adolescents: development of the ventrolateral prefrontal and anterior cingulate cortices. Neuropsychologia. 2007;45:1270–9. doi: 10.1016/j.neuropsychologia.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steinberg L. Cognitive and affective development in adolescence. Trends Cogn Sci. 2005;9:69–74. doi: 10.1016/j.tics.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychol Med. 2006;36:299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 10.Dahl RE. Adolescent brain development: a period of vulnerabilities and opportunities. Keynote address. Ann N Y Acad Sci. 2004;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- 11.Grillon C, Baas J. A review of the modulation of the startle reflex by affective states and its application in psychiatry. Clin Neurophysiol. 2003;114:1557–79. doi: 10.1016/s1388-2457(03)00202-5. [DOI] [PubMed] [Google Scholar]
- 12.Mogg K, et al. Selective attention to threat: A test of two cognitive models of anxiety. Cognition & Emotion. 2000;14:375–399. [Google Scholar]
- 13.Bishop SJ, Jenkins R, Lawrence AD. Neural processing of fearful faces: effects of anxiety are gated by perceptual capacity limitations. Cereb Cortex. 2007;17:1595–603. doi: 10.1093/cercor/bhl070. [DOI] [PubMed] [Google Scholar]
- 14.Bishop SJ, Duncan J, Lawrence AD. State anxiety modulation of the amygdala response to unattended threat-related stimuli. J Neurosci. 2004;24:10364–8. doi: 10.1523/JNEUROSCI.2550-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Somerville LH, et al. Human amygdala responses during presentation of happy and neutral faces: correlations with state anxiety. Biol Psychiatry. 2004;55:897–903. doi: 10.1016/j.biopsych.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Thomas KM, et al. Amygdala response to fearful faces in anxious and depressed children. Arch Gen Psychiatry. 2001;58:1057–63. doi: 10.1001/archpsyc.58.11.1057. [DOI] [PubMed] [Google Scholar]
- 17.Etkin A, et al. Individual differences in trait anxiety predict the response of the basolateral amygdala to unconsciously processed fearful faces. Neuron. 2004;44:1043–55. doi: 10.1016/j.neuron.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Pezawas L, et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8:828–34. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- 19.Spielberger CD. Manual for the State-Trait Anxiety Inventory (STAI) Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 20.Tottenham N, et al. The NimStim Set of Facial Expressions: Judgments from Untrained Research Participants. Psychiatry Res. doi: 10.1016/j.psychres.2008.05.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomas KM, et al. Amygdala response to facial expressions in children and adults. Biol Psychiatry. 2001;49:309–16. doi: 10.1016/s0006-3223(00)01066-0. [DOI] [PubMed] [Google Scholar]
- 22.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme; 1988. [Google Scholar]
- 23.Glover GH, Thomason ME. Improved combination of spiral-in/out images for BOLD fMRI. Magn Reson Med. 2004;51:863–8. doi: 10.1002/mrm.20016. [DOI] [PubMed] [Google Scholar]
- 24.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computations in Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 25.Hare TA, et al. Contributions of amygdala and striatal activity in emotion regulation. Biol Psychiatry. 2005;57:624–32. doi: 10.1016/j.biopsych.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 26.Casey BJ, Galvan A, Getz S. The Adolescent Brain. Developmental Review. doi: 10.1016/j.dr.2007.08.003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Casey BJ, Galvan A, Hare TA. Changes in cerebral functional organization during cognitive development. Curr Opin Neurobiol. 2005;15:239–44. doi: 10.1016/j.conb.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 28.Luna B, Sweeney JA. The emergence of collaborative brain function: FMRI studies of the development of response inhibition. Ann N Y Acad Sci. 2004;1021:296–309. doi: 10.1196/annals.1308.035. [DOI] [PubMed] [Google Scholar]
- 29.Drevets WC. Neuroimaging abnormalities in the amygdala in mood disorders. Ann N Y Acad Sci. 2003;985:420–44. doi: 10.1111/j.1749-6632.2003.tb07098.x. [DOI] [PubMed] [Google Scholar]
- 30.Mayberg HS. Limbic-cortical dysregulation: a proposed model of depression. J Neuropsychiatry Clin Neurosci. 1997;9:471–81. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- 31.Mayberg HS. Defining the neural circuitry of depression: toward a new nosology with therapeutic implications. Biol Psychiatry. 2007;61:729–30. doi: 10.1016/j.biopsych.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 32.Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research--past, present, and future. Biol Psychiatry. 2006;60:376–82. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 33.McClure EB, et al. Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Arch Gen Psychiatry. 2007;64:97–106. doi: 10.1001/archpsyc.64.1.97. [DOI] [PubMed] [Google Scholar]
- 34.Shin LM, et al. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch Gen Psychiatry. 2004;61:168–76. doi: 10.1001/archpsyc.61.2.168. [DOI] [PubMed] [Google Scholar]
- 35.Kim H, et al. Contextual modulation of amygdala responsivity to surprised faces. J Cogn Neurosci. 2004;16:1730–45. doi: 10.1162/0898929042947865. [DOI] [PubMed] [Google Scholar]
- 36.Kim H, et al. Inverse amygdala and medial prefrontal cortex responses to surprised faces. Neuroreport. 2003;14:2317–22. doi: 10.1097/00001756-200312190-00006. [DOI] [PubMed] [Google Scholar]
- 37.LaBar KS, et al. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20:937–45. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- 38.Phelps EA, et al. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 39.Lissek S, et al. Classical fear conditioning in the anxiety disorders: a meta-analysis. Behav Res Ther. 2005;43:1391–424. doi: 10.1016/j.brat.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 40.Wright CI, et al. Differential prefrontal cortex and amygdala habituation to repeatedly presented emotional stimuli. Neuroreport. 2001;12:379–83. doi: 10.1097/00001756-200102120-00039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.