Abstract
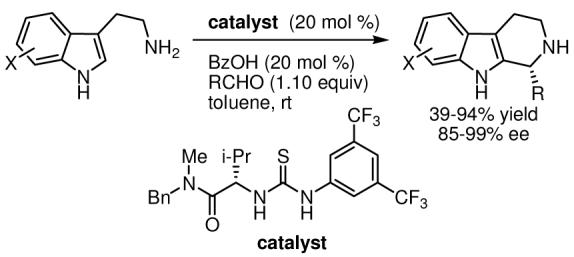 The development of one-pot imine formation and asymmetric Pictet–Spengler reactions co-catalyzed by a chiral thiourea and benzoic acid is described. Optically active tetrahydro-β-carbolines, ubiquitous structural motifs in biologically active natural products, are obtained in high ee directly from tryptamine and aldehyde precursors.
The development of one-pot imine formation and asymmetric Pictet–Spengler reactions co-catalyzed by a chiral thiourea and benzoic acid is described. Optically active tetrahydro-β-carbolines, ubiquitous structural motifs in biologically active natural products, are obtained in high ee directly from tryptamine and aldehyde precursors.
The Pictet–Spengler reaction is an important biosynthetic and laboratory method for the synthesis of tetrahydroisoquinolines and tetrahydro-β-carbolines, structural motifs in a diverse array of biologically active natural and unnatural products.1, 2 Whereas synthetically valuable diastereoselective Pictet–Spengler reactions are well known,3 catalytic, enantioselective variants have only recently been developed.4 Chiral Lewis acids have not proven to be generally useful for the Pictet–Spengler reaction, a likely result of catalyst inhibition by the Lewis basic product.5 Greater success has been achieved with Brønsted acid or hydrogen bond-donor catalysts, albeit with specialized substrates.6 Thus, asymmetric catalysis of Pictet–Spengler-type reactions has been reported with N-acyliminium ions4a or N-sulfenyliminium ions,4b or with substrates biased towards cyclization and against competing aldol pathways by gem-disubstitution adjacent to the reactive imine.4c An enantioselective, catalytic Pictet–Spengler protocol with broad substrate scope that affords unprotected tetrahydro-β-carboline products directly from simple tryptamine derivatives would represent a significant advance.
We have recently advanced a mechanistic model for asymmetric catalysis by thiourea derivatives in which substrate activation takes place via thiourea-mediated chloride abstraction to generate highly reactive N-acyliminium ions or oxocarbenium ions (Figure 1).7 This hypothesis suggests that a variety of other cationic intermediates, including protioiminium ions, could be activated towards enantioselective addition by analogous anion-binding mechanisms. In particular, we envisaged a catalytic cycle in which imine protonation is induced by a thiourea catalyst associated via H-bonding to the conjugate base of a weak Brønsted acid additive (Scheme 1).8 Cyclization of the highly reactive protioiminium ion followed by rearomatization would regenerate the Brønsted acid co-catalyst. Herein, we report that chiral thiourea derivatives in combination with benzoic acid promote catalytic asymmetric Pictet–Spengler reactions of electronically and sterically diverse imines, providing unprotected tetrahydro-β-carbolines in high ee and yield.
Figure 1.
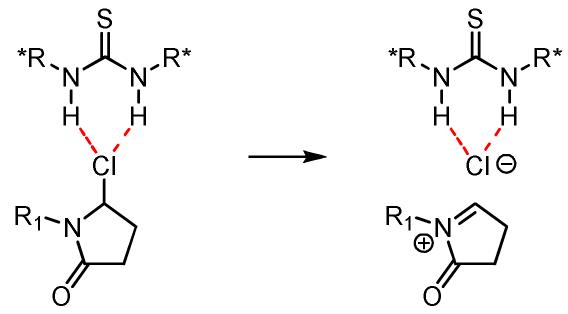
Generation of N-acyliminium ion via anion-binding by a thiourea catalyst.
Scheme 1.

Brønsted acid and H-bond donor co-catalysis
We selected 6-methoxytryptamine derivative 2a as a model substrate. Methoxy-substituted tryptamine derivatives undergo cyclization in low enantioselectivity in the previously reported acyl-Pictet–Spengler reaction,9 a significant limitation given the prevalence of methoxyand hydroxy-substituted tetrahydro-β-carbolines in natural products.10 Moreover, elevated temperatures (> −30 °C) were required for acyl-Pictet–Spengler reactions of aryl imines, conditions under which the thiourea catalyst was subject to decomposition via rapid S-acetylation. We reasoned that greater generality in the imine component would be possible in a non-acylative reaction. Imine 2a was screened against a variety of representative thiourea catalysts11 and achiral Brønsted acids. In combination with acetic acid (AcOH), catalysts 4a12 and 7a13 provided tetrahydro-β-carboline 3a in high yield and 85% and −87% ee, respectively (Scheme 2). Notably, no product was observed in the absence of AcOH.
Scheme 2.
Screen of representative (thio)urea catalysts.
Yield determined by 1H NMR on a 0.05 mmol scale. Enantioselectivity determined by chiral SFC analysis of the N-Boc derivative.
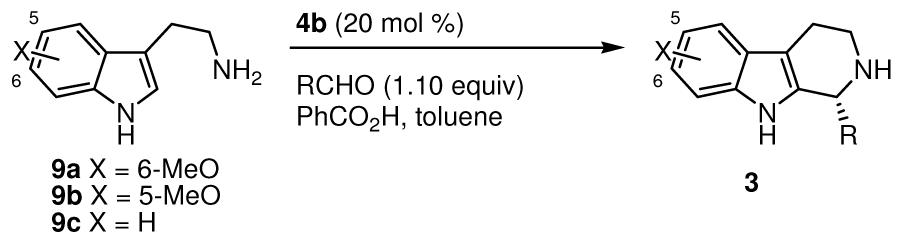
These initial results were obtained at room temperature, conditions under which imine formation is rapid. We therefore explored an operationally simpler protocol involving one-pot imine formation and thiourea-catalyzed Pictet–Spengler reaction. Treatment of tryptamine 9a and p-chlorobenzaldehyde with catalyst 4a in toluene provided product 3a in 88% ee and 54% yield (Table 1, entry 1). In contrast, sulfinamide 7a was a poor catalyst under in situ imine formation conditions, providing product in only 13% yield. A systematic evaluation of the influence of catalyst structure on both enantioselectivity and rate revealed that valine-derived catalyst 4b afforded optimal results (entry 2).14 This simple compound is prepared in 69% yield in three steps from commercially available N-methylbenzylamine and either (D)- or (L)-valine; only a single chromatographic purification is required. A screen of carboxylic acids revealed an increase in both enantioselectivity and rate with benzoic acid (PhCO2H) (entry 2 vs. 4).15 Under the in situ imine-formation conditions, highest yields and enantioselectivities were obtained in reactions carried out at lower concentration (entries 4–6).
Table 1.
Optimization studies of the one-pot Pictet–Spengler reaction.
 | |||||
|---|---|---|---|---|---|
| entry | catalyst | RCO2H | [9a] | yield (%)a | ee (%)b |
| 1 | 4a | AcOH | 0.05 M | 54 | 88 |
| 2 | 4b | AcOH | 0.05 M | 48 | 92 |
| 3 | 7a | AcOH | 0.05 M | 13 | −79 |
| 4 | 4b | PhCO2H | 0.05 M | 74 | 94 |
| 5 | 4b | PhCO2H | 0.10 M | 60 | 93 |
| 6 | 4b | PhCO2H | 0.20 M | 39 | 92 |
Yield determined by 1H NMR spectroscopy on a 0.05 mmol scale.
Determined by chiral SFC analysis of the N-Boc derivative.
A variety of substituted benzaldehyde derivatives proved to be suitable substrates in combination with 9a, providing tetrahydro-β-carboline products 3a–3g in good-to-excellent ee's and yields (Table 2, entries 1–7). Substitution is tolerated at all ring positions of the aldehyde, with the shortest reaction times observed for ortho- and meta-substituted derivatives (entries 3–5). Less electron-rich tryptamines 9b and 9c also participated effectively in the condensation/cyclization reaction (89–99% ee), although elevated acid loadings and temperatures and extended reaction times were required to achieve useful product yields (entries 8–10).
Table 2.
Substrate scope of the thiourea and benzoic acid catalyzed Pictet–Spengler reaction.
 | |||||||
|---|---|---|---|---|---|---|---|
| entry | tryptamine | Ra | product | PhCO2H (mol%) |
t (h) | yield (%)b |
ee (%)c |
| 1 | 9a | p-ClC6H4 | 3a | 20 | 66 | 78 | 94 |
| 2 | 9a | p-FC6H4 | 3b | 20 | 78 | 81 | 92 |
| 3 | 9a | p-BrC6H4 | 3c | 20 | 74 | 79 | 94 |
| 4 | 9a | m-BrC6H4 | 3d | 20 | 19 | 87 | 94 |
| 5 | 9a | o-BrC6H4 | 3e | 20 | 11 | 74 | 95 |
| 6 | 9a | p-MeOC6H4 | 3f | 20 | 91 | 78 | 85 |
| 7 | 9a | Ph | 3g | 20 | 70 | 94 | 86 |
| 8d | 9b | p-ClC6H4 | 3h | 40 | 14 d | 73 | 89 |
| 9d | 9b | o-BrC6H4 | 3i | 40 | 87 | 82 | 99 |
| 10d | 9c | o-BrC6H4 | 3j | 100 | 10 d | 45 | 95 |
| 11 | 9a | i-Pr | 3k | 20 | 4 | 60e | 88 |
| 12 | 9a | i-Pr | 3k | – | 88 | 90 | 94 |
| 13 | 9a | CH(Et)2 | 3l | – | 5 d | 84 | 95 |
| 14 | 9a | n-pentyl | 3m | – | 18 | 74 | 86 |
| 15d | 9b | i-Pr | 3n | 20 | 36 | 39 | 88 |
All aldehydes were purified immediately prior to use. See the Supporting Information for details.
Isolated yield after column chromatography for 0.50 mmol-scale reactions, unless noted otherwise.
Determined by chiral SFC analysis of the N-Boc derivative.
Reaction carried out at 35 °C.
Yield determined by 1H NMR spectroscopy on a 0.05 mmol scale.
Aliphatic aldehydes displayed unexpected reactivity with tryptamine 9a in protio-Pictet–Spengler cyclizations catalyzed by 4b. Isobutyraldehyde underwent rapid reaction under the conditions optimized for aromatic aldehydes, affording product 3k in 60% conversion and 88% ee after 4 hours at room temperature (entry 11). In contrast to aryl substrates, however, cyclization was also observed in the absence of PhCO2H. In fact, the enantioselectivity increased to 94% in the absence of acid additive, although much longer reaction times were required (entry 12). Both linear and α-branched aldehydes participated effectively in cyclizations with 9a (entries 12–14). In contrast, less nucleophilic tryptamines such as 9b and 9c were unreactive under neutral conditions. The basis for this intriguing difference in reactivity is not well understood at this stage. For substrates such as 9b, use of acidic additive was necessary (e.g., entry 15), with reactions proceeding in high ee and in modest yield due to the competitive aldol pathways.
We have identified a readily accessible chiral thiourea catalyst that promotes highly enantioselective Pictet–Spengler reactions for electronically and structurally diverse substrates under mild and operationally simple conditions. This method provides unprotected tetrahydro-β-carbolines in one step from tryptamine and aldehyde derivatives. Our current efforts are directed toward elucidation of the mechanism of co-catalysis by achiral Brønsted acids, and application of this principle to other enantioselective reactions of synthetic interest.
Supplementary Material
Acknowledgment
This work was supported by the NIH (GM-43214). We thank Mathieu Lalonde (Harvard Univ.) for crucial, helpful discussions, and Cristina Fernandez (Harvard Univ.) for experimental assistance.
Footnotes
Supporting Information Available: Complete experimental procedures and characterization data for products and isolated intermediates. This material is available free of charge from http://pubs.acs.org.
References
- 1.(a) Maresh J,J, Giddings L-A, Friedrich A, Loris EA, Panjikar S, Trout BL, Stockigt J, Peters B, O'Connor SE. J. Am. Chem. Soc. 2008;130:710–723. doi: 10.1021/ja077190z. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Luk LYP, Bunn S, Liscombe DK, Facchini PJ, Tanner ME. Biochemistry. 2007;46:10153–10161. doi: 10.1021/bi700752n. [DOI] [PubMed] [Google Scholar]
- 2.Cox ED, Cook JM. Chem. Rev. 1995;95:1797–1842. [Google Scholar]
- 3.(a) Zhou H, Liao X, Cook JM. Org. Lett. 2004;6:249–252. doi: 10.1021/ol0362212. For examples of diastereoselective Pictet–Spengler reactions of tryptophan derivatives and applications to total synthesis, see. and references therein. [DOI] [PubMed] [Google Scholar]; (b) Yamashita T, Kawai N, Tokuyama H, Fukuyama T. J. Am. Chem. Soc. 2005;127:15038–15039. doi: 10.1021/ja055832h. For an example of a diastereoselective Pictet–Spengler reaction of a tryptamine and a chiral aldehyde, see. [DOI] [PubMed] [Google Scholar]; (c) For a complete list of methods for the preparation of enantioenriched tetrahydro-β-carbolines, see the Supporting Information.
- 4.(a) Taylor MS, Jacobsen EN. J. Am. Chem. Soc. 2004;126:10558–10559. doi: 10.1021/ja046259p. [DOI] [PubMed] [Google Scholar]; (b) Wanner MJ, van der Haas RNS, de Cuba KR, van Maarseveen JH, Hiemstra H. Angew. Chem., Int. Ed. 2007;46:7485–7487. doi: 10.1002/anie.200701808. [DOI] [PubMed] [Google Scholar]; (c) Seayad J, Seayad AM, List B. J. Am. Chem. Soc. 2006;128:1086–1087. doi: 10.1021/ja057444l. [DOI] [PubMed] [Google Scholar]; (d) Sewgobind NV, Wanner MJ, Ingemann S, de Gelder R, van Maarseveen JH, Hiemstra H. J. Org. Chem. 2008;73:6405–6408. doi: 10.1021/jo8010478. [DOI] [PubMed] [Google Scholar]
- 5.Superstoichiometric amounts of diisopinocampheylchloroborane (Ipc2BCl) promote Pictet–Spengler reactions of N-β-hydroxytryptamines. Yamada H, Kawate T, Matsumizu M, Nishida A, Yamaguchi K, Nakagwawa M. J. Org. Chem. 1998;63:6348–6354. doi: 10.1021/jo980810h.
- 6.(a) Taylor MS, Jacobsen EN. Angew. Chem., Int. Ed. 2006;45:1520–1543. doi: 10.1002/anie.200503132. [DOI] [PubMed] [Google Scholar]; (b) Doyle AG, Jacobsen EN. Chem. Rev. 2007;107:5713–5743. doi: 10.1021/cr068373r. [DOI] [PubMed] [Google Scholar]
- 7.(a) Raheem IT, Thiara PS, Peterson EA, Jacobsen EN. J. Am. Chem. Soc. 2007;129:13404–13405. doi: 10.1021/ja076179w. [DOI] [PubMed] [Google Scholar]; (b) Reisman SE, Doyle AG, Jacobsen EN. J. Am. Chem. Soc. 2008;130:7198–7199. doi: 10.1021/ja801514m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.For an example of a non-stereoselctive application of this principle employing mandelic acid and achiral thiourea in the catalytic alcoholysis of styrene oxides, see: Weil T, Kotke M, Kleiner CM, Schreiner PR. Org. Lett. 2008;10:1513–1516. doi: 10.1021/ol800149y.
- 9.4 substrates: 44-86% ee. Taylor MS. Harvard University; 2005. Dissertation.
- 10.Examples of methoxy and hydroxy substituted indole alkaloids include reserpine, tubulosine, and the eudimistin family of natural products. Osorio EJ, Robledo SM, Bastida J. In: The Alkaloids. 1st ed. Cordell GA, editor. Vol. 66. Academic Press; San Diego, CA: 2008. pp. 149–159. Chapter 2.
- 11.The catalysts shown in Scheme 2 are available from Sigma-Aldrich.
- 12.Catalysts analogous to 4a were first developed in the context of enantioselective Mannich-type reactions. Wenzel AG, Lalonde MP, Jacobsen EN. Synlett. 2003;12:1919–1922.
- 13.Catalyst 7a promotes enantioselective allylation of acylhydrazones. Tan KL, Jacobsen EN. Angew. Chem., Int. Ed. 2007;46:1315–1317. doi: 10.1002/anie.200603354.
- 14.For a complete description of catalyst screening and reaction optimization, see the Supporting Information.
- 15.Stronger acids, such as hydrogen chloride and methanesulfonic acid, provided product in low yield and ee, potentially due to catalyst inhibition due to protonation of the Lewis basic product.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



