Abstract
The presence of chromosome-specific low-copy repeats (LCRs) predisposes chromosome 22 to deletions and duplications. The current diagnostic procedure for detecting aberrations at 22q11.2 is chromosomal analysis coupled with fluorescence in situ hybridization (FISH) or PCR-based multiplex ligation dependent probe amplification (MLPA). However, there are copy number variations (CNVs) in 22q11.2 that are only detected by high-resolution platforms such as array comparative genomic hybridization (aCGH). We report on development of a high-definition MLPA (MLPA-HD) 22q11 kit that detects copy number changes at 37 loci on the long arm of chromosome 22. These include the 3-Mb region commonly deleted in DiGeorge/velocardiofacial syndrome (DGS/VCFS), the cat eye syndrome (CES) region, and more distal regions in 22q11 that have recently been shown to be deleted. We have used this MLPA-HD probe set to analyze 363 previously well-characterized samples with a variety of different rearrangements at 22q11 and demonstrate that it can detect copy number alterations with high sensitivity and specificity. In addition to detection of the common recurrent deletions associated with DGS/VCFS, variant and novel chromosome 22 aberrations have been detected. These include duplications within as well as deletions distal to this region. Further, the MLPA-HD detects deletion endpoint differences between patients with the common 3-Mb deletion. The MLPA-HD kit is proposed as a cost effective alternative to the currently available detection methods for individuals with features of the 22q11 aberrations. In patients with the relevant phenotypic characteristics, this MLPA-HD probe set could replace FISH for the clinical diagnosis of 22q11.2 deletions and duplications.
Keywords: DiGeorge syndrome, velocardiofacial syndrome, cat eye syndrome, DGS, VFCS, CES, MLPA-HD, 22q11
INTRODUCTION
Genomic copy number alterations at 22q11.2 are associated with several syndromes. They are characterized by a variety of symptoms including cardiac defects, and thymic, parathyroid, craniofacial, developmental, and behavioral manifestations [Emanuel et al., 2001; Vorstman et al., 2006b; Driscoll and Emanuel, 1996]. It is thought that the presence of chromosome-specific low-copy repeats (LCRs) or segmental duplications (SDs), predisposes this region of chromosome 22 to cytogenetic rearrangements [Shaikh et al., 2001; Edelmann et al., 1999b].
To date, the majority of rearrangements identified at 22q11.2 have been deletions. It has been demonstrated that the deletion endpoints cluster, and that there is a typically deleted region of ~3 Mb in >85% of patients. It has been shown that there appear to be typical proximal and distal deletion endpoints (DEPs) as well as two recurrent variant distal DEPs occurring in the LCRs [Funke et al., 1999; Edelmann et al., 1999b; Carlson et al., 1997; Shaikh et al., 2000 and 2001]. In recent years, several deletions with variant atypical DEPs have been identified using an extended set of fluorescence in situ hybridization (FISH) probes across the region and/or microarray technologies [Urban et al., 2005; Weksberg et al., 2007; Shaikh et al., 2007; Saitta et al., 1999].
Although both deletions and duplications are expected to occur in equal proportions as a result of reciprocal LCR-mediated events, fewer duplications of 22q11.2 have been described, and the phenotype resulting from these duplications is extremely variable [Yobb et al., 2005; Vorstman et al., 2006a; Portnoi et al., 2005; Lindsay et al., 1995b; Hassed et al., 2004; Meins et al., 2003; Ensenauer et al., 2003; de La Rochebrochard et al., 2006; Mukaddes and Herguner, 2007; Alberti et al., 2007]. Further, the breakpoints implicated in generating the cat eye chromosome, a supernumerary inverted duplication of proximal 22q implicated in cat eye syndrome (CES; MIM# 115470), frequently match the proximal LCR or one of the more distal LCRs of the 22q11.2 region [McDermid et al., 1986; Mears et al., 1994]. However, detailed analysis of the extent of the duplicated material and its relationship to the ensuing phenotype has been minimal.
The diagnostic procedure most often used for detection of deletions and duplications at 22q11.2 is chromosomal analysis, which is often coupled with FISH using commercially available probes located between LCRs A and B. Previously we have shown that the DGS/VCFS multiplex ligation dependent probe amplification (MLPA) kit (MRC-Holland, Amsterdam, the Netherlands) for DiGeorge/velocardiofacial syndrome (DGS; MIM# 88400 and VCFS; MIM 192430) is a cost effective, rapid, and sensitive method for the detection of typical recurrent deletions and duplications in proximal 22q11 extending to LCR-D [Vorstman et al., 2006a].
However, we and others have identified copy number changes at 22q11.2 that would not have been detected by the current commercially available diagnostic FISH probes or the existing MLPA P023 kit [Vorstman et al., 2006a; Sivertsen et al., 2007]. The detection and analysis of these genomic copy number alterations at 22q11.2 is important because to date little information is available with regard to their prevalence and whether there are consistently associated phenotypic differences. Indeed, identification of these variant cases is of particular interest since it may provide insight into which genes or genomic regions are crucial for specific phenotypic manifestations.
Therefore, in collaboration with MRC-Holland, we have developed a high-density MLPA (MLPA-HD) probe set that incorporates probes starting proximal to LCR-A and covers the region flanked by the four LCRs distal to LCR-D, which is the distal boundary of the typical 22q11.2 deletions [Emanuel and Shaikh, 2001]. We have tested the ability of this new high-density (HD) probe set to detect copy number alterations on numerous samples with copy number changes at 22q11.2. Here, we report on the first results using this new probe set, including its reliability and cost effectiveness, as well as its capacity to detect rare, difficult to identify copy number changes in 22q11.
MATERIALS AND METHODS
Over the past 20 years the Clinical Genetics Center at The Children’s Hospital of Philadelphia (CHOP) has enrolled patients with a variety of duplications, deletions, and rearrangements of 22q11 under several Institutional Review Board (IRB)-approved research protocols. Several additional samples used in this study were identified clinically after the parents had consented to genetic testing. Characterization or validation of 22q rearrangements by FISH analysis with cosmid or BAC clones was performed by our research laboratory or by clinical diagnostic laboratories. A group of 363 well-characterized samples, including typical 22q11.2 deletions, atypical deletions, duplications (trisomy and tetrasomy), and unbalanced translocations, as well as samples without a known rearrangement, were selected for the purpose of the validation study for the MLPA-HD kit. The new MLPA-HD kit was validated in a research laboratory blinded to the molecular genetic status of the samples. The test includes a group of standard 22q11.2DS deletions (64 cases), several recurrent smaller 22q11.2DS deletions (24 cases), several unique 22q11.2DS associated deletions (seven cases), a group of unbalanced translocations to 22q (four cases), several interstitial duplications of 22q11.2 (11 cases), several supernumerary der(22)t(11;22)(q23;q11) cell lines (three cases), two CES cell lines, and several balanced translocations to 22q (two cases).
Constitutional 22q11.2 Deletions Associated With Rhabdoid Tumor Samples
DNA samples from tumor tissue, cell lines, and peripheral blood samples were obtained from the four patients for INI1 analysis according to procedures approved by the IRB at The Children’s Hospital of Philadelphia. In each case the parents signed consent forms for genetic testing.
Enhanced MLPA Kit Generation and Validation
In collaboration with the group at MRC-Holland, an expanded HD-MLPA22 kit (containing 45 probes) was developed to test for copy number variation (CNV) of genomic regions within 22q11. To identify typical and atypical patients, probes are positioned in specified intervals on 22q11.2. There are nine probes between LCR-A and LCR-B (1.103 Mb); three probes between LCR-B and LCR-C (278.8 kb); three probes between LCR-C and LCR-D (272 kb); three probes between LCR-D and LCR-E (1.05 Mb); one probe between LCR-E and LCR-F (651 kb); four probes between LCR-F and LCR-G (616 kb); one probe between LCR-G and LCR-H (298 kb); and one probe placed distal to LCR H-toward the telomere. There are eight probes in the Type I CEC region proximal to LCR-A (chr22: 16,205,810–17,017,046). The location of the probes is included in Table 1. In addition, the MLPA-HD kit has one probe placed within LCR-A and four placed within LCR-D. The remaining eight loci, which serve as controls, are located at eight different chromosomal positions (chromosomes 2q, 3p, 5q [two loci], 8p, 10p, 17q, and 19p).
TABLE 1.
Probe Sets in the MLPA-HD Kit and Their Chromosomal Position Using the May 2004 Assembly
| Probe name | Chromosome Position | Chromosome Region | |
|---|---|---|---|
| 1 | IL17R | 15,954,293 | CES |
| 2 | CECR1 | 16,062,702 | CES |
| 3 | SLC25A18 | 16,417,934 | CES |
| 4 | BCL2L13 | 16,546,387 | CES |
| 5 | BID | 16,601,309 | CES |
| 6 | MICAL3 | 16,699,273 | CES |
| 7 | PEX26 | 16,935,706 | CES |
| 8 | USP18 | 17,007,539 | CES |
| 9 | LCRA-L57984 | 17,310,399 | LCR-A |
| 10 | GSCL | 17,511,823 | LCR-B |
| 11 | CLTCL1 | 17,616,215 | LCR-B |
| 12 | HIRA | 17,693,633 | LCR-B |
| 13 | CDC45(L) | 17,842,094 | LCR-B |
| 14 | CLDN5 | 17,885,934 | LCR-B |
| 15 | GP1BB | 18,086,134 | LCR-B |
| 16 | TBX1 | 18,121,732 | LCR-B |
| 17 | KIAA 1652 | 18,260,839 | LCR-B |
| 18 | ARVCF | 18,352,766 | LCR-C |
| 19 | ZNF74 | 19,073,049 | LCR-C |
| 20 | FLJ14360 | 19,167,921 | LCR-C |
| 21 | PCQAP | 19,261,367 | LCR-C |
| 22 | SNAP29 | 19,566,638 | |
| 23 | LZTR1 | 19,673,815 | |
| 24 | P2RXL1 | 19,705,448 | |
| 25 | LCR-Dr1 | 19,773,060 | LCR-D |
| 26 | LCR-Dr2 | 19,938,175 | LCR-D |
| 27 | HIC2 | 20,125,240 | LCR-D |
| 28 | LCR-Dr3 | 20,241,722 | LCR-D |
| 29 | PPIL2 | 20,241,722 | |
| 30 | TOP3B | 20,654,633 | LCR-E |
| 31 | RAB36 | 21,812,171 | LCR-F |
| 32 | SMARCB1-D01 | 22,453,988 | |
| 33 | SMARCB1-D02 | 22,500,983 | |
| 34 | MIF-D01 | 22,561,257 | |
| 35 | MIF-D03 | 22,561,884 | LCR-G |
| 36 | UPB1 | 23,215,006 | LCR-H |
| 37 | SEZ6L | 25,013,099 | |
| 38 | C1, NRXN1 | 50,060,856 | 2q14 |
| 39 | C2, APC | 112,191,603 | 5q31.1 |
| 40 | C3,SCN5A | 38,593,243 | 3p21 |
| 41 | C4, STK11 | 1,169,489 | 19p13 |
| 42 | C5, BRCA1 | 38,505,407 | 17q21 |
| 43 | C6, IL4 | 132,037,671 | 5q22 |
| 43 | C7,CUGBP2 | 11,247,596 | 2q14 |
| 45 | C8, MFHAS | 8,786,079 | 5q31.1 |
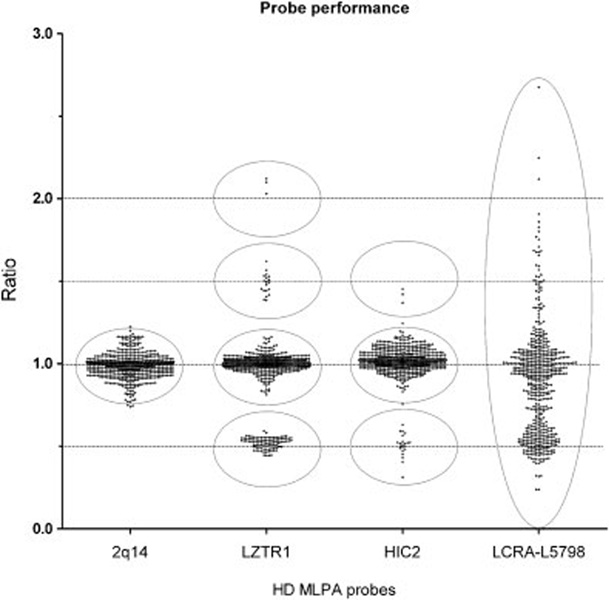
To calculate test characteristics of the MLPA-HD kit, we analyzed individual probe results of 609 runs. Results of 21 runs were excluded because of excessive variance (the standard deviation of the results of the eight control probes exceeded 0.20). We have used receiver operating characteristic (ROC) curve analysis to assess the optimal cutoff values [Vorstman et al., 2006a]. The usual cause for variance of the results was due to low quality of the DNA preparations or increased variation (> ± 10%) in DNA concentrations. In addition, in all runs, results of two probes (one in LCR-A, one in LCR-D) were also excluded from the analysis because of overall high signal variance (Fig. 1). Thus, results of a total of 588 runs, yielding more than 25,000 signals, were analyzed and compared to results obtained from previous studies and/or replication studies. Subsequently, based on these comparisons, each locus signal was individually labeled as false or true negative/positive, respectively.
FIGURE 1.
A visualization of probe performance. The ratio for copy number values is expected to cluster around 0.0, 0.5, 1.0, 1.5, or 2.0, representing homozygous deletion; hemizygous deletion; disomic, two copies; trisomic, three copies; and tetrasomic, four copies, respectively. The above dot plot shows the performance of a control probe and three test probes based on the analysis of 609 runs. The results of control probe; CNT-2q14 clearly shows a cluster around 1.0 representing two copies. Test probes LZTR1 and HIC-2 display clustering closely around 0.5, 1.0, 1.5, and 2.0, representing deletion, and disomic, trisomic, and tetrasomic, representing good performance, respectively. In comparison, the results of LCRA-L5798 lack clear clustering around expected values of 0.5, 1, or 1.5 respectively, indicating poor performance. The gray circles shows areas of clustering.
Laboratory Methods
The MLPA-HD test was performed on genomic DNA according the manufacturer’s method (MRC-Holland; MLPA-HD Kit; www.mrc-holland.com). Briefly, 500 ng of genomic DNA was denatured (5 min at 98°C) and subsequently hybridized to the new HR-MLPA22 probe set. PCR amplification was carried out on an ABI 9700 thermocycler (Applied Biosystems, Foster City, CA), and electrophoresis was performed using the ABI 3700 DNA analyzer (Applied Biosystems) with Rox 500 size standards [Schouten et al., 2002; Slater et al., 2003].
Data Analysis
We have used a commercially available software, Gene Marker from SoftGenetics (State College, PA) to analyze our data. Gene Marker has developed a completely integrated application for MLPA analysis. The Gene Marker program has integrated functions that are specific for the analysis of data derived from the MLPA reactions. The software exploits two selectable normalization methods. The first normalization method is the traditional method based upon the control probes. The second method normalizes peak intensities based upon the statistically most probable median intensities and was the method used for this study (see Supplementary Material, which includes Supplementary Fig. 1FIGS1; available online at http://www.interscience.wiley.com/jpages/1059-7794/suppmat).
RESULTS
The MLPA-HD kit was designed to contain a total of 45 loci. Of these, there are 37 loci located on the long arm of chromosome 22. Most of the probe sets are located within genes, of which 15 loci are located between LCR-A and LCR-D. We chose five unique loci located within the LCR regions, one probe positioned within LCR-A and four placed within LCR-D for detecting differences in deletion endpoints. Two of these LCR probes, one in LCR-A and one in LCR-D did not pass quality control and were removed from our analysis (Supplementary Material). The MLPA-HD kit provides more extensive coverage of the typically deleted region (TDR) within 22q11.2, allowing for the identification and characterization of atypical deletions. It also includes 10 probe sets that are located distal to LCR-D in the interval between LCR-D and LCR-H. The remaining eight loci, which serve as controls, are located at eight different chromosomal positions (chromosomes 2q, 3p, 5q [two loci], 8p, 10p, 17q, and 19p). Table 1 lists the probe sets in the MLPA-HD kit and their location in the sequence of chromosome 22 based upon the NCBI reference sequence (May 2004 assembly, hg17). Utilizing a delta value of 0.22, which implicates a deletion at signals below 0.78 and a duplication at signals exceeding 1.22, the MLPA-HD kit demonstrated very robust test characteristics; sensitivity and specificity were 0.997 (95% confidence interval [CI] = 0.994–0.999) and 0.989 (95% CI = 0.987–0.990). These values were not essentially altered when analyzing test results utilizing the more commonly applied delta value of 0.3; the associated sensitivity and specificity values were 0.992 and 0.995, respectively.
Detecting Atypical 22q11 Deletions
Figure 2, Patients 1–10, shows a panel of patients with typical proximal, atypical proximal and distal 22q11 deletions that were studied under several different protocols. Patients 1 and 2 are from a cohort of VCFS patients with known typical 3-Mb deletions. Patient 3 was a member of a cohort of patients with conotruncal defects studied in a Specialized Center of Clinical Research funded by the National Heart, Lung, and Blood Institute (NHLBI) (HL74731). In this study the MLPA-HD kit was utilized in an attempt to streamline screening patients with conotruncal defects for the presence of a 22q11.2 deletion. Individual 4 is the parent of Patient 3. Patients 5, 6, 7, 8, and 10 were patients with multiple congenital anomalies that did not fit a specific syndrome who were evaluated by clinical geneticists. Their samples were sent for array comparative genomic hybridization (aCGH) studies (Signature Genomics, Spokane, WA). After a deletion of one or more chromosome 22 BACs was detected by the array, the MLPA-HD kit analysis was able to more precisely assess the extent of the deletion in each of these individuals. Patient 9 has been reported previously [Saitta et al., 1999; Shaikh et al., 2007]. The MLPA-HD kit results agreed with any prior and subsequent confirmatory FISH and molecular analyses.
FIGURE 2.
Graphical representation of MLPA-HD data. Shown above the representation of chromosome 22 are the FISH probes (cosmids) used in the laboratory designated either by cosmid address or locus name. The MLPA probes (orange rectangles, existing MLPA PO23 loci; yellow rectangles, new MLPA loci) are shown in map order based upon data derived from the UCSC genome browser. All MLPA loci were tested in all samples. Copy number for MLPA probes is indicated by color (purple = nullisomic, homozygous deletion; gray = disomic, two copies; red = hemizygous deletion, one copy; green = trisomic, three copies; blue = tetrasomic, four copies). All results are confirmed in two independent runs. 22q11 deletions (Patients 1–10): 1,2) CH99-023 and CH02-125 with 3-Mb LCR-A to LCR-D deletions. Patient 2 has an extended deletion; Patients 3 and 4) CH06-198, DO6-231 with LCR-B to LCR-D deletions; Patients 5 and 6) 06-006997 and 07000363 with a distal variant of LCR-B to LCR-D deletion; Patient 7) 06-006837 an atypical deletion; Patient 8) 06-004115, an LCR-C to LCR-D deletion; Patient 9) CH98-018, an LCR-D to LCR-E deletion; Patient 10) 07-0000553, an LCR-D to LCR-F deletion. 22q11 duplications (Patients 11–16): Patients 11 and 12 are individuals with cat eye syndrome; Patient 11) CH00-120 shows tetrasomy within the CES region proximal to LCR-A; Patient 12) CH06-196 tetrasomy extending from the CES region through LCR-D; Patients 13–15) duplication of the region from LCR-A to LCR-D. Patient 13 is the proband, Patient 14 the mother, and Patient 15 the grandmother; Patient 16) CH00-178 is from a supernumerary der(22)t(8;22)(q24.13;q11.21)pat [Gotter et al., 2007]. 22q11.2 deletions associated with rhabdoid tumor (Patients 17–24): MLPA-HD results on individuals with a constitutional 22q11.2 deletion and rhabdoid tumor. Samples with the notation B are DNA from whole blood and samples with the T notation represent DNA from tumor samples. Patients 17–20) T05-53 B and T and T05-47 B and T demonstrate a deletion of probes from LCR-D to LCR-G; Samples 21 and 22) T05-11B and D are deleted for the probes located between LCR-E to LCR-G; Patients 23 and 24) T05-216 B and T are deleted for one copy of the probes located between LCR-F to LCR-G; Samples 22 and 24) show homozygous deletions in the tumor samples.
In the patients with known LCR-A to LCR-D deletions (Patients 1 and 2), the MLPA-HD not only confirmed the FISH and existing MLPA kit (P0-23) results but also revealed that the extent of the deletion was different in these two cases. Patient 1 showed a hemizygous loss of one copy of 16 probes covering the region located between LCR-A and LCR-D while Patient 2 showed loss of one copy of an additional probe (HIC2), clearly showing differences in the extent of the deletion between the two patients as a result of different distal deletion endpoints.
Patients 3 and 4 (CH06-198 and D06-231) showed loss of one copy of seven probes located between LCR-B and LCR-D, indicating an atypical LCR-B to LCR-D deletion. For patient CH06-198, FISH screening with a probe (N25) in the DGS/VCFS region had been normal, suggesting that there was no 22q11.2 deletion. Patients 5 and 6 (06-006997 and 07-000363) have similar LCR-B to LCR-D deletions. Interestingly, the distal deletion breakpoints are at a different location for these latter two cases when compared with the previous two cases. In these latter cases there is the loss of the HIC2 probe, suggesting a more extensive deleted area. In Patient 7 (06-006837), there are nine loci deleted, expanding the deleted region proximal to the typical LCR located breakpoints. Patient 8 (06-004115) is deleted between LCR-C and LCR-D, such that five probes show a hemizygous loss. Patient 9 (CH98-018) is deleted for one copy of the four probes within the region between LCR-D and LCR-E, while Patient 10 (07-00053) has a deletion that extends farther, such that it also includes the region between LCR-E and LCR-F.
It is of interest that none of these atypical cases (Patients 3–10) would have been detected by the N25 or TUPLE/HIRA probes which are the routine FISH probes used for detecting deletions of 22q11. Although patients with deletions between LCR-B and LCR-D (Patients 3–8) would have been detected using the existing MLPA kit; neither of the cases with distal deletions that extend beyond LCR-D (Patients 9 and 10) would have been detected. Differences in deletion endpoints within LCR-D would not be detected utilizing the existing kit. Further, only the MLPA-HD kit is capable of detecting copy number changes within and distal to LCR-D.
22q11 Duplications
Duplications involving chromosome 22 can be difficult to identify using standard technology. Figure 2 shows a schematic diagram of several cases with a variety of duplications that have been studied with the MLPA-HD kit (Patients 11–16). The bisatellited supernumerary marker chromosomes associated with the cat eye syndrome (CEC) are variable in size and can be asymmetric with regard to the region duplicated [Mears et al., 1994]. There are no commercially available, unique diagnostic probes for identifying the CEC when identified pre- or postnatally. We have previously determined that the breakpoints of the symmetrical CES chromosome cluster in two intervals such that there are small and large cat eye chromosomes. In Patient 11 (CH00-120) MLPA detected tetrasomy for the eight most proximal probes distributed throughout the cat eye syndrome chromosomal region (CECR). In Patient 12 (CH06-160), all the probes in the CECR as well as the 16 probes between LCR-A and LCR-D are present in four copies, confirming the presence of the Cat Eye chromosome as well as determining the extent of the duplicated material. Thus, Patient 11 has a smaller CES chromosome that is symmetrical, classified as a Type I CES chromosome and has both breakpoints located within LCR-A. Patient 12 has a larger type II CES chromosome that is symmetrical with both breakpoints located in the distal (LCR-D) interval. The MLPA-HD kit provided a rapid assessment of these two supernumerary marker chromosomes.
Individuals with 22q11 interstitial duplications have been identified less frequently than anticipated and some seem to share some phenotypic similarities with 22q11.2 deletion syndrome patients [Lindsay et al., 1995a; Edelmann et al., 1999a; Ensenauer et al., 2003; Meins et al., 2003; Hassed et al., 2004; Portnoi et al., 2005; Yobb et al., 2005]. The detection of these individuals has been problematic utilizing FISH because it requires interphase analysis. We have detected a three-generation familial microduplication. Patient 13 (CH06-290; proband) showed a duplication of all of the probes located between LCR-A and LCR-D. Patients 13 (CH06-288) and 14 (CH07-003) are the mother and maternal grandmother of the patient, who both exhibit the same pattern of duplication. Thus, the MLPA-HD kit provided a rapid screening test for the presence of the duplication.
Patient 16 (CH00-178) carries a complex supernumerary der(22)t(8;22)(q24.1;q11.2)pat chromosome. We previously mapped the breakpoints as well as the translocation junction to the LCRs found on chromosome 22 [Gotter et al., 2007]. Trisomic signal was observed in Patient 16 (CH00-178) for the eight most proximal MLPA probes distributed throughout the CECR including the USP18 probe at the proximal edge of LCR-A. All six of the MLPA probes located between LCRs B and D also exhibited trisomic signals, indicating that these sequences are also present on the supernumerary der(22). The derivative chromosome is the result of an inversion of the region between LCR-A and LCR-D prior to the occurrence of the translocation. The nine probes corresponding to loci located between LCR-A and B, however, are disomic. These MLPA-HD results are entirely consistent with FISH and molecular mapping results and confirm the previously reported mechanism presumed responsible for the rearrangement.
Constitutional 22q11.2 Deletions Associated With Rhabdoid Tumor
In the third group of samples we screened four patients with constitutional 22q11.2 deletions associated with rhabdoid tumor. Of these, Patients 17 and 18 had phenotypic findings that were suggestive but not diagnostic for the 22q11.2 deletion (DGS/VCFS). The initial genetic analysis did not reveal an underlying diagnosis, as the chromosomal analysis and FISH studies using a diagnostic probe (TUPLE1) in the DGS/VCFS region were normal. However, the probands were subsequently diagnosed with malignant rhabdoid tumors.
We were fortunate to have access to blood and tumor samples for these four cases. MLPA-HD confirmed the findings of FISH with probes from the rhabdoid tumor region and microarray analysis [Jackson et al., 2007]. Figure 2, Patients 17–24, represent the findings on these patients. DNA from blood (B) and tumor (T) sample of Patients 17–20 (T05-53 and T05-47) showed the loss of one copy of nine probes, confirming a LCR-D to LCR-G deletion. Genomic DNA from blood from Patient 21 (T05-11 B) revealed the hemizygous loss of five probes spanning the region between LCR-E to LCR-G. The tumor sample from this patient (Sample 22; T05-11T) not only confirms the same extent of the deletion but also exhibits homozygous deletion for the five probes contained within the deleted region. Patient 23 demonstrated a deletion of the region between LCR-F and LCR-G, revealed by the loss of one copy of four loci covering this region. The DNA from the tumor sample from Sample 24 showed a homozygous deletion for the same four probes. In all of these patients, the MLPA-HD analysis confirmed the deletion of SMARCB1 (INI1), the tumor-suppressor gene inactivated in rhabdoid tumors. Thus, in Patients 21 and 23, MLPA-HD confirmed homozygous deletions of the INI1 gene observed in the majority of rhabdoid tumor samples and also validated the copy number change in one of these cases (Sample 22; T0511T).
DISCUSSION
Aberrations of the 22q11 region are amongst the most common constitutional chromosomal abnormalities. The resulting varied and complex phenotypes make these disorders a significant health problem [Botto et al., 2003; du Montcel et al., 1996; Oskarsdottir et al., 2004; Burn et al., 1993]. We have previously utilized a DGS/VCFS MLPA kit (P023) to assess copy number changes of 22q11.2 [Vorstman et al., 2006a]. MLPA proved to be a relatively inexpensive assay with high sensitivity and specificity. However, there have been reports from several laboratories about variant deletions and duplications that are neither detected by chromosomal analysis with FISH using the N25 or TUPLE1/HIRA diagnostic probes nor the currently available MLPA kit for DGS/VCFS [Shaikh et al., 2007; Rauch et al., 1998; O’Donnell et al., 1997; Kurahashi et al., 1997]. Therefore, in collaboration with MRC Holland we have developed a MLPA-HD kit for the 22q11 region. The new MLPA probe set detects copy number changes at 37 loci on the long arm of chromosome 22. The MLPA-HD kit not only has a greater density of probe coverage within the typically deleted region LCR-A to LCR-D (18 probes), but also includes probes covering other important regions, including the CES region (eight probes) and the region located between LCR-D and LCR-H (nine probes). There are also eight control probes located in regions not associated with DGS/VCFS.
In the current study, results are presented from this new high density MLPA probe set for 22q11. Based on a sizeable number of verified probe signals, we show that this new assay is highly accurate with excellent sensitivity and specificity values. Further, using a unique sample set with a variety of different rearrangements at 22q11, we demonstrate the ability of the new MLPA probe set to correctly detect gain or loss of genomic material, to accurately indicate the number of allelic copies (from n = 0 to n = 4) and to delineate the extent of the region involved in the rearrangement. Of note, several abnormalities that were detected by the new MLPA probe set would have remained undetected by either FISH using the standard probe at 22q11.2 (N25 or TUPLE1) or the currently available MLPA kit.
The majority of the DGS/VCFS patients have a 3-Mb deletion (86.3%) extending from LCR-A to LCR-D. However, the presence of CNVs and sequence similarity within the LCRs (>96%) has proved to be an obstacle in interrogating the extent of the deletion in this group of patients [Wong et al., 2007; Sharp et al., 2005; Conrad et al., 2006]. Thus, we have addressed this by designing probes for the unique regions interspersed within the LCRs. Five probes were designed within the LCR regions, one within LCR-A and four within the LCR-D (Table 1). Two of the probes, LCR-A and LCR-D2, proved to be problematic; however, the performance of the other three, two probes flanking LCR-D and the HIC2 probe inside the unique region of LCR-D, gave consistent results.
Investigators have been looking for modifiers of the phenotype in patients with typical LCR-A to LCR-D deletions that reside outside the typical 3-Mb deletion. The variations in DEPs in patient with LCR-A to LCR-D has been reported, using isothermal HR-CGH array [Urban et al., 2005]. Here, for the first time using a PCR-based method, we demonstrate that this group of patients is not a homogeneous group, confirming variation of DEPS in patients with the TDR. Patients 1 and 2 clearly show a difference in deletion size (Fig. 2). We have used samples from 77 patients with typical deletions of LCR-A to LCR-D, of whom five patients had extended deletions including the HIC2 locus. The signals of the HIC2 probe in normal control samples were found to cluster closely around 1.0 (n = 2), which indicates good performance for this particular probe (Fig.1). It is of interest that although the proximal part of the HIC2 region is within a reported CNV region, the MLPA probe covering HIC2 is outside the CNV region [Wong et al., 2007] (http://projects.tcag.ca/variation). This variation in DEP might eventually be useful in explaining differences in the mechanism of the rearrangement in affected patients as well as finding explanations for the extreme variability in the spectrum of the phenotype.
The MLPA-HD kit has eight probes located in the CES region of chromosome 22. We were able to detect variation in the size of the CECs in two individuals carrying a supernumerary chromosome comprised of different duplications of chromosome 22. The availability of the MLPA-HD kit will permit a better understanding of genotype–phenotype correlations in cat eye syndrome patients. Further, the kit will fill a major void in diagnostics for CES, as there are no chromosome 22–specific FISH probes currently available for this purpose.
Based on these findings, we suggest that the new MLPA probe set represents an appealing alternative for the currently available detection methods. Naturally, oligonucleotide-based microarray-based methods have a greater resolution than MLPA. In addition, they have the potential of genome-wide screening of CNVs. However, the high expense associated with array-based techniques discourages the use of these methods for routine screening of patients. This is an important limitation since screening of large numbers of individuals with phenotypic characteristics of the 22q11.2 deletion syndrome is likely to be an important approach to unraveling the enigma of the high level of phenotypic variations observed in patients with 22q11 rearrangements. This MLPA-HD kit will allow for a cost-effective and inexpensive screening method for individuals with specific features of the deletion such as the typical associated conotruncal cardiac defects. Further, while several duplications at 22q11 have been reported, to date the total number of identified patients with this condition is too small to accurately describe the associated phenotypic characteristics. A perfect example is the familial duplication found by the MLPA-HD kit. In this family the phenotypes are quite varied (unpublished data, BSE) and clinical findings will be the subject of future studies.
The recent identification of small deletions within or close to the typically deleted portion of 22q11.2 may prove to be relevant with regard to the identification of candidate genes in the region. It is likely that the identification of additional individuals with atypical or small deletions or duplications will greatly contribute to our understanding of the genes involved in the etiology of different aspects of the 22q11.2 deletion syndrome phenotype. We strongly believe that with the new MLPA probe set screening of high numbers of individuals will be feasible for most medical centers, and therefore the identification of many cases such as those described in this report can be anticipated.
Apart from the implications for research, we propose that the new MLPA probe set could replace FISH with N25 or TUPLE1 for the clinical testing of 22q11.2 deletions in patients with the phenotypic characteristics. In comparison to standard FISH, MLPA is easier to carry out, less expensive, and equally reliable in the detection of typical deletions. In addition, with MLPA smaller deletions outside the typically deleted region are identified with precise identification of the extent of the region involved. While duplications can be easily missed using standard FISH on metaphase spreads, with MLPA any copy number change, either a loss or gain of material, can be accurately assessed. In conclusion, given the many advantages of MLPA over standard FISH methodology for the detection of rearrangements at 22q11, it is likely that it will replace 22q FISH in the very near future.
Supplementary Material
The Supplementary Material referred to in this article can be accessed at http://www.interscience.wiley.com/jpages/1059-7794/suppmat.
ACKNOWLEDGMENTS
We acknowledge the excellent technical assistance of April Hacker, Julia Brown, David Tomkins, and Kathryn Pickering. We are grateful to several colleagues who were instrumental in obtaining samples from the study subjects, including Drs. Elizabeth Goldmuntz, Paige Kaplan, Sulagna Saitta, and Elaine Zackai, as well as several genetic counselors who assisted in these efforts, Livija Medne and Donna McDonald-McGinn. Authors Errami and Vijzelaar are employees of MRC Holland.
Grant sponsor: National Organization for Rare Disorders (NORD); Grant sponsor: National Heart, Lung, and Blood Institute (NHLBI); Grant number: HL 74731; Grant sponsor: National Cancer Institute (NCI); Grant number: CA 39926.
REFERENCES
- Alberti A, Romano C, Falco M, Cali F, Schinocca P, Galesi O, Spalletta A, Di BD, Fichera M. 1.5 Mb de novo 22q11.21 microduplication in a patient with cognitive deficits and dysmorphic facial features. Clin Genet. 2007;71:177–182. doi: 10.1111/j.1399-0004.2007.00750.x. [DOI] [PubMed] [Google Scholar]
- Botto LD, May K, Fernhoff PM, Correa A, Coleman K, Rasmussen SA, Merritt RK, O’Leary LA, Wong LY, Elixson EM, Mahle WT, Campbell RM. A population-based study of the 22q11.2 deletion: phenotype, incidence, and contribution to major birth defects in the population. Pediatrics. 2003;112:101–107. doi: 10.1542/peds.112.1.101. [DOI] [PubMed] [Google Scholar]
- Burn J, Takao A, Wilson D, Cross I, Momma K, Wadey R, Scambler P, Goodship J. Conotruncal anomaly face syndrome is associated with a deletion within chromosome 22q11. J Med Genet. 1993;30:822–824. doi: 10.1136/jmg.30.10.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson C, Papolos D, Pandita RK, Faedda GL, Veit S, Goldberg R, Shprintzen R, Kucherlapati R, Morrow B. Molecular analysis of velo-cardio-facial syndrome patients with psychiatric disorders. Am J Hum Genet. 1997;60:851–859. [PMC free article] [PubMed] [Google Scholar]
- Conrad DF, Andrews TD, Carter NP, Hurles ME, Pritchard JK. A high-resolution survey of deletion polymorphism in the human genome. Nat Genet. 2006;38:75–81. doi: 10.1038/ng1697. [DOI] [PubMed] [Google Scholar]
- de La Rochebrochard C, Joly-Hélas G, Goldenberg A, Durand I, Laquerriére A, Ickowicz V, Saugier-Veber P, Eurin D, Moirot H, Diguet A, de Kergal F, Tiercin C, Mace B, Marpeau L, Frebourg T. The intrafamilial variability of the 22q11.2 microduplication encompasses a spectrum from minor cognitive deficits to severe congenital anomalies. Am J Med Genet A. 2006;140:1608–1613. doi: 10.1002/ajmg.a.31227. [DOI] [PubMed] [Google Scholar]
- Driscoll DA, Emanuel BS. DiGeorge and velocardiofacial syndromes: the 22q11 deletion syndrome. Ment Retard Dev Disabil Res Rev. 1996;2:130–138. [Google Scholar]
- du Montcel ST, Mendizabal H, Ayme S, Levy A, Philip N. Prevalence of 22q11 microdeletion. J Med Genet. 1996;33:719. doi: 10.1136/jmg.33.8.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelmann L, Pandita RK, Morrow BE. Low-copy repeats mediate the common 3-Mb deletion in patients with velo-cardio-facial syndrome. Am J Hum Genet. 1999a;64:1076–1086. doi: 10.1086/302343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelmann L, Pandita RK, Spiteri E, Funke B, Goldberg R, Palanisamy N, Chaganti RS, Magenis E, Shprintzen RJ, Morrow BE. A common molecular basis for rearrangement disorders on chromosome 22q11. Hum Mol Genet. 1999b;8:1157–1167. doi: 10.1093/hmg/8.7.1157. [DOI] [PubMed] [Google Scholar]
- Emanuel BS, McDonald-McGinn D, Saitta SC, Zackai EH. The 22q11.2 deletion syndrome. Adv Pediatr. 2001;48:39–73. [PubMed] [Google Scholar]
- Emanuel BS, Shaikh TH. Segmental duplications: an ‘expanding’ role in genomic instability and disease. Nat Rev Genet. 2001;2:791–800. doi: 10.1038/35093500. [DOI] [PubMed] [Google Scholar]
- Ensenauer RE, Adeyinka A, Flynn HC, Michels VV, Lindor NM, Dawson DB, Thorland EC, Lorentz CP, Goldstein JL, McDonald MT, Smith WE, Simon-Fayard E, Alexander AA, Kulharya AS, Ketterling RP, Clark RD, Jalal SM. Microduplication 22q11.2, an emerging syndrome: clinical, cytogenetic, and molecular analysis of thirteen patients. Am J Hum Genet. 2003;73:1027–1040. doi: 10.1086/378818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funke B, Edelmann L, McCain N, Pandita RK, Ferreira J, Merscher S, Zohouri M, Cannizzaro L, Shanske A, Morrow BE. Der(22) syndrome and velo-cardio-facial syndrome/DiGeorge syndrome share a 1.5-Mb region of overlap on chromosome 22q11. Am J Hum Genet. 1999;64:747–758. doi: 10.1086/302284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotter AL, Nimmakayalu MA, Jalali GR, Hacker AM, Vorstman J, Conforto DD, Medne L, Emanuel BS. A palindrome-driven complex rearrangement of 22q11.2 and 8q24.1 elucidated using novel technologies. Genome Res. 2007;17:470–481. doi: 10.1101/gr.6130907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassed SJ, Hopcus-Niccum D, Zhang L, Li S, Mulvihill JJ. A new genomic duplication syndrome complementary to the velocardiofacial (22q11 deletion) syndrome. Clin Genet. 2004;65:400–404. doi: 10.1111/j.0009-9163.2004.0212.x. [DOI] [PubMed] [Google Scholar]
- Jackson EM, Shaikh TH, Gururangan S, Jones MC, Malkin D, Nikkel SM, Zuppan CW, Wainwright LM, Zhang F, Biegel JA. High-density single nucleotide polymorphism array analysis in patients with germline deletions of 22q11.2 and malignant rhabdoid tumor. Hum Genet. 2007;122:117–127. doi: 10.1007/s00439-007-0386-3. [DOI] [PubMed] [Google Scholar]
- Kurahashi H, Tsuda E, Kohama R, Nakayama T, Masuno M, Imaizumi K, Kamiya T, Sano T, Okada S, Nishisho I. Another critical region for deletion of 22q11: a study of 100 patients. Am J Med Genet. 1997;72:180–185. doi: 10.1002/(sici)1096-8628(19971017)72:2<180::aid-ajmg10>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Lindsay EA, Goldberg R, Jurecic V, Morrow B, Carlson C, Kucherlapati RS, Shprintzen RJ, Baldini A. Velo-cardio-facial syndrome: frequency and extent of 22q11 deletions. Am J Med Genet. 1995a;57:514–522. doi: 10.1002/ajmg.1320570339. [DOI] [PubMed] [Google Scholar]
- Lindsay EA, Shaffer LG, Carrozzo R, Greenberg F, Baldini A. De novo tandem duplication of chromosome segment 22q11–q12: clinical, cytogenetic, and molecular characterization. Am J Med Genet. 1995b;56:296–299. doi: 10.1002/ajmg.1320560316. [DOI] [PubMed] [Google Scholar]
- McDermid HE, Duncan AM, Brasch KR, Holden JJ, Magenis E, Sheehy R, Burn J, Kardon N, Noel B, Schinzel A. Characterization of the supernumerary chromosome in cat eye syndrome. Science. 1986;232:646–648. doi: 10.1126/science.3961499. [DOI] [PubMed] [Google Scholar]
- Mears AJ, Duncan AM, Budarf ML, Emanuel BS, Sellinger B, Siegel-Bartelt J, Greenberg CR, McDermid HE. Molecular characterization of the marker chromosome associated with cat eye syndrome. Am J Hum Genet. 1994;55:134–142. [PMC free article] [PubMed] [Google Scholar]
- Meins M, Burfeind P, Motsch S, Trappe R, Bartmus D, Langer S, Speicher MR, Muhlendyck H, Bartels I, Zoll B. Partial trisomy of chromosome 22 resulting from an interstitial duplication of 22q11.2 in a child with typical cat eye syndrome. J Med Genet. 2003;40:e62. doi: 10.1136/jmg.40.5.e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukaddes NM, Herguner S. Autistic disorder and 22q11.2 duplication. World J Biol Psychiatry. 2007;8:127–130. doi: 10.1080/15622970601026701. [DOI] [PubMed] [Google Scholar]
- O’Donnell H, McKeown C, Gould C, Morrow B, Scambler P. Detection of an atypical 22q11 deletion that has no overlap with the DiGeorge syndrome critical region. Am J Hum Genet. 1997;60:1544–1548. doi: 10.1016/S0002-9297(07)64250-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oskarsdottir S, Vujic M, Fasth A. Incidence and prevalence of the 22q11 deletion syndrome: a population-based study in Western Sweden. Arch Dis Child. 2004;89:148–151. doi: 10.1136/adc.2003.026880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoi MF, Lebas F, Gruchy N, Ardalan A, Biran-Mucignat V, Malan V, Finkel L, Roger G, Ducrocq S, Gold F, Taillemite JL, Marlin S. 22q11.2 Duplication syndrome: two new familial cases with some overlapping features with DiGeorge/velocardiofacial syndromes. Am J Med Genet A. 2005;137A:47–51. doi: 10.1002/ajmg.a.30847. [DOI] [PubMed] [Google Scholar]
- Rauch A, Hofbeck M, Leipold G, Klinge J, Trautmann U, Kirsch M, Singer H, Pfeiffer RA. Incidence and significance of 22q11.2 hemizygosity in patients with interrupted aortic arch. Am J Med Genet. 1998;78:322–331. [PubMed] [Google Scholar]
- Saitta SC, McGrath JM, Mensch H, Shaikh TH, Zackai EH, Emanuel BS. A 22q11.2 deletion that excludes UFD1L and CDC45L in a patient with conotruncal and craniofacial defects. Am J Hum Genet. 1999;65:562–566. doi: 10.1086/302514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schouten JP, McElgunn CJ, Waaijer R, Zwijnenburg D, Diepvens F, Pals G. Relative quantification of 40 nucleic acid sequences by multiplex ligation-dependent probe amplification. Nucleic Acids Res. 2002;30:e57. doi: 10.1093/nar/gnf056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh TH, Kurahashi H, Saitta SC, O’Hare AM, Hu P, Roe BA, Driscoll DA, McDonald-McGinn DM, Zackai EH, Budarf ML, Emanuel BS. Chromosome 22-specific low copy repeats and the 22q11.2 deletion syndrome: genomic organization and deletion endpoint analysis. Hum Mol Genet. 2000;9:489–501. doi: 10.1093/hmg/9.4.489. [DOI] [PubMed] [Google Scholar]
- Shaikh TH, Kurahashi H, Emanuel BS. Evolutionarily conserved low copy repeats (LCRs) in 22q11 mediate deletions, duplications, translocations, and genomic instability: an update and literature review. Genet Med. 2001;3:6–13. doi: 10.1097/00125817-200101000-00003. [DOI] [PubMed] [Google Scholar]
- Shaikh TH, O’Connor RJ, Pierpont ME, McGrath J, Hacker AM, Nimmakayalu M, Geiger E, Emanuel BS, Saitta SC. Low copy repeats mediate distal chromosome 22q11.2 deletions: sequence analysis predicts breakpoint mechanisms. Genome Res. 2007;17:482–491. doi: 10.1101/gr.5986507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp AJ, Locke DP, McGrath SD, Cheng Z, Bailey JA, Vallente RU, Pertz LM, Clark RA, Schwartz S, Segraves R, Oseroff VV, Albertson DG, Pinkel D, Eichler EE. Segmental duplications and copy-number variation in the human genome. Am J Hum Genet. 2005;77:78–88. doi: 10.1086/431652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivertsen A, Lie RT, Wilcox AJ, Abyholm F, Vindenes H, Haukanes BI, Houge G. Prevalence of duplications and deletions of the 22q11 DiGeorge syndrome region in a population-based sample of infants with cleft palate. Am J Med Genet A. 2007;143A:129–134. doi: 10.1002/ajmg.a.31445. [DOI] [PubMed] [Google Scholar]
- Slater HR, Bruno DL, Ren H, Pertile M, Schouten JP, Choo KH. Rapid, high throughput prenatal detection of aneuploidy using a novel quantitative method (MLPA) J Med Genet. 2003;40:907–912. doi: 10.1136/jmg.40.12.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban A, Selzer R, Richmond T, Popescu G, Cubells JF, Green R, Emanuel B, Weissman SM, Snyder M. Application of ultra-high resolution fine-tiling array CGH (FT-CGH) to the analysis of 22q11 deletion syndrome. Am J Med Genet B Neuropsychiatr Genet. 2005;138B:138. [Google Scholar]
- Vorstman JA, Jalali GR, Rappaport EF, Hacker AM, Scott C, Emanuel BS. MLPA: a rapid, reliable, and sensitive method for detection and analysis of abnormalities of 22q. Hum Mutat. 2006a;27:814–821. doi: 10.1002/humu.20330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorstman JAS, Morcus MEJ, Duijff SN, Klaassen PWJ, Heineman-de Boer JA, Beemer FA, Swaab H, Kahn RS, van Engeland H. The 22q11.2 deletion in children: high rate of autistic disorders and early onset of psychotic symptoms. J Am Acad Child Adolesc Psychiatry. 2006b;45:1104–1113. doi: 10.1097/01.chi.0000228131.56956.c1. [DOI] [PubMed] [Google Scholar]
- Weksberg R, Stachon AC, Squire JA, Moldovan L, Bayani J, Meyn S, Chow E, Bassett AS. Molecular characterization of deletion breakpoints in adults with 22q11 deletion syndrome. Hum Genet. 2007;120:837–845. doi: 10.1007/s00439-006-0242-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KK, deLeeuw RJ, Dosanjh NS, Kimm LR, Cheng Z, Horsman DE, MacAulay C, Ng RT, Brown CJ, Eichler EE, Lam WL. A comprehensive analysis of common copy-number variations in the human genome. Am J Hum Genet. 2007;80:91–104. doi: 10.1086/510560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yobb TM, Somerville MJ, Willatt L, Firth HV, Harrison K, MacKenzie J, Gallo N, Morrow BE, Shaffer LG, Babcock M, Chernos J, Bernier F, Sprysak K, Christiansen J, Haase S, Elyas B, Lilley M, Bamforth S, McDermid HE. Microduplication and triplication of 22q11.2: a highly variable syndrome. Am J Hum Genet. 2005;76:865–876. doi: 10.1086/429841. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The Supplementary Material referred to in this article can be accessed at http://www.interscience.wiley.com/jpages/1059-7794/suppmat.