Abstract
Provider profiling (ranking/percentiling) is prevalent in health services research. Bayesian models coupled with optimizing a loss function provide an effective framework for computing non-standard inferences such as ranks. Inferences depend on the posterior distribution and should be guided by inferential goals. However, even optimal methods might not lead to definitive results and ranks should be accompanied by valid uncertainty assessments. We outline the Bayesian approach and use estimated Standardized Mortality Ratios (SMRs) in 1998-2001 from the United States Renal Data System (USRDS) as a platform to identify issues and demonstrate approaches. Our analyses extend Liu et al. (2004) by computing estimates developed by Lin et al. (2006) that minimize errors in classifying providers above or below a percentile cut-point, by combining evidence over multiple years via a first-order, autoregressive model on log(SMR), and by use of a nonparametric prior. Results show that ranks/percentiles based on maximum likelihood estimates of the SMRs and those based on testing whether an SMR = 1 substantially under-perform the optimal estimates. Combining evidence over the four years using the autoregressive model reduces uncertainty, improving performance over percentiles based on only one year. Furthermore, percentiles based on posterior probabilities of exceeding a properly chosen SMR threshold are essentially identical to those produced by minimizing classification loss. Uncertainty measures effectively calibrate performance, showing that considerable uncertainty remains even when using optimal methods. Findings highlight the importance of using loss function guided percentiles and the necessity of accompanying estimates with uncertainty assessments.
Keywords: Provider profiling, Ranks/percentiles, Bayesian hierarchical model, Uncertainty assessment
1 Introduction
Research on and application of performance evaluation steadily increases with applications to evaluating health service providers (Christiansen and Morris 1997; Goldstein and Spiegelhalter 1996; Landrum et al. 2000; Liu et al. 2004; McClellan and Staiger 1999; Grigg et al. 2003; Zhang et al. 2006; Normand and Shahian 2007; Ohlssen et al. 2007), prioritizing environmental assessments in small areas (Conlon and Louis 1999; Louis and Shen 1999; Shen and Louis 2000) and ranking teachers and schools (Lockwood et al. 2002). Inferential goals of these studies include evaluating the population performance, such as the average performance of all health providers and comparing performance among providers. Performance evaluations include comparing unit-specific, substantive measures such as death rates, identifying the group of poorest or best performing units and overall ranking of the units, e.g., profiling or league tables (Goldstein and Spiegelhalter 1996).
The Standardized Mortality Ratio (SMR), the ratio of observed to expected deaths, is an important service quality indicator (Zaslavsky 2001). The United States Renal Data System (USRDS) produces annual estimated SMRs for several thousand dialysis centers and uses these as a quality screen (Lacson et al. 2001; ESRD 2000; USRDS 2005). Invalid estimation or inappropriate interpretation can have serious consequences for these dialysis centers and for their patients. We present an analysis of the information from the United States Renal Data System (USRDS) for 1998-2001 as a platform for demonstrating and comparing approaches to ranking health service providers. From the USRDS we obtained observed and expected deaths for the K = 3173 dialysis centers that contributed information for all four years. The approach used by USRDS to produce these values can be found in USRDS (2005).
Though estimating SMRs is a standard statistical operation (produce provider-specific expected deaths based on a statistical model, and then compute the “observed/expected” ratio), it is important and challenging to deal with complications such as the need to specify a reference population (providers included, the time period covered, attribution of events), the need to validate the model used to adjust for important patient attributes (age, gender, diabetes, type of dialysis, severity of disease), and the need to adjust for potential biases induced when attributing deaths to providers and accounting for informative censoring.
The multi-level data structure and complicated inferential goals require the use of a hierarchical Bayesian model that accounts for nesting relations and specifies both population values and random effects. Correctly specified, the model properly accounts for the sample design, variance components and other uncertainties, producing valid and efficient estimates of population parameters, variance components and unit-specific random effects (provider-, clinician-, or region-specific latent attributes), all accompanied by valid uncertainty assessments. Importantly, the Bayesian approach provides the necessary structure for developing scientific and policy-relevant inferences based on the joint posterior distribution of all unknowns.
As Shen and Louis (1998) show and Gelman and Price (1999) present in detail, no single set of estimates or assessments can effectively address multiple goals and we provide a suite of assessments. Guided by a loss function, the Bayesian approach structures non-standard inferences such as ranking (including identification of extremely poor and good performers) and estimating the histogram of unit-specific random effects. For example, as Liu et al. (2004) show, when estimation uncertainty varies over dialysis centers, ranks produced by Z-scores that test whether a provider's SMR = 1 tend to identify providers with relatively low variance as extreme because these tests have the highest power; ranks produced from the provider-specific maximum likelihood estimates (MLEs) are more likely to identify dialysis centers with relatively high variance as extreme. Effective ranks depend on striking an effective tradeoff between signal and noise.
Lin et al. (2006) present estimates that minimize errors in classifying providers above or below a percentile cut-point. Our analyses build on Liu et al. (2004) by extending the application of Lin et al. (2006)'s estimates to combine evidence over multiple years via a first-order, autoregressive model on log(SMR), and by use of a nonparametric prior. For single-year analyses we compare the results from the log-normal prior to those based on the Non-Parametric, Maximum Likelihood (NPML) prior (Laird 1978).
In following, Sect. 2 presents our models; Sect. 3 outlines several ranking methods; Sect. 4 gives uncertainty measures; Sect. 5 presents results and Sect. 6 sums up and identifies additional research. Computing code for all routines is available at, http://people.umass.edu/rlin/jhuwebhost/usrds-ranking.htm.
2 Statistical models
We employ both single-year and longitudinal models for observed deaths and underlying parameters, with the former a sub-model of the latter. To this end, let (Ykt, mkt) be the observed and case-mix adjusted, expected deaths for provider k in year t, k = 1,... 3173, t = 0, 1, 2, 3 and ρkt be the SMR. The USRDS computes the expecteds under the assumption that all providers give the same quality of care for patients with identical covariates, see USRDS (2005) for details. We employ the conditional Poisson model,
| (1) |
If the provider has “average performance”, ρkt = 1. For both single-year and multiple-year analyses we model θkt = log(ρkt).
2.1 Single-year analyses
For single-year analyses, we assume that for year t; θkt Gt, k = 1,…, 3173: We use a year-specific, normal prior (see the note after Eq. 2) and for the single-year analyses also use the non-parametric maximum likelihood (NPML) prior. See and Carlin and Louis (2008) and Paddock et al. (2006) for additional details and Appendix C for the estimation algorithm.
2.2 The longitudinal, AR(1) model
To model longitudinal correlation among (ρk0, ρk1, ρk2, ρk3), let ϕ = cor(θk,t, θk(t+1)), with -1 < ϕ < 1. Then, use a normal prior on the θkt and a normal prior on Z(ϕ) = 0.5 log {(1 + ϕ)/(1-ϕ)} in the hierarchical model,
| (2) |
The notation “iid” means independently and identically distributed and “ind” means independently distributed. The relation is first-order Markov, because though conditioning is on all prior θs, only ρk(t-1) appears on the right-hand side of Eq. 2.
Marginally, for year t, θkt iid N(ξt, ) and setting ϕ = 0 produces four, single-year analyses, each using the Liu et al. (2004) model with no borrowing of information over time. For ϕ > 0, we have a standard AR(1) model on the latent log(SMR)s and the posterior distribution combines evidence across dialysis centers within year and within dialysis center across years.
2.3 Posterior sampling implementation and hyper-prior parameters
We implement a Gibbs sampler for model (2) with WinBUGS via the R package R2WinBUGS, using the coda package to diagnose convergence (Spiegelhalter et al. 1999; Gelman et al. 2006; Plummer et al. 2006). We use V = 10, μ = 0.01, α = 0.05, values that stabilize the simulation while allowing sufficient adaptation to the data. With V = 10, the a priori, 95% probability interval for ξt is (-6.20, 6.20) [(0.002, 492.75) in the SMR scale]; the values for α and μ produce a distribution for τ2 with center near 100, inducing large, a priori variation for the θkt. For the AR(1) model, reported results are based on the Vϕ = 0.2. This produces an a priori 95% probability interval for ϕ of (-0.70, 0.70). In a sensitivity analysis, we also tried Vϕ = 2, which produced the a priori interval (-0.99, 0.99) and yielded results virtually identical to those based on the Vϕ = 0.2 hyper-prior. In both cases, the data likelihood dominated the priors. This can also be seen in the shrinkage of τ2 towards zero, as reported in the Sect. 5.4. There is no strong posterior correlation observed between ϕ and the τ2s.
3 Loss function based ranking methods
Two general strategies for ranking are available. The preferred strategic approach first computes the joint posterior distribution of the ranks and then uses it to produce estimates and uncertainty assessments, generally guided by a loss function that is appropriate for analytic goals. This approach ensures that estimated ranks have desired properties such as not depending on a monotone transform of the target parameters. The other approach is based on ordering estimates of target parameters (MLEs or posterior means) or on ordering statistics testing the null hypothesis that SMRk ≡ 1. If the posterior distributions of the target parameters are stochastically ordered, then for a broad class of loss functions (estimation criteria) optimally estimated ranks will not depend on the strategy. However, Lin et al. (2006) and others have shown that estimates not derived from the distribution of the ranks can perform very poorly and may not be invariant under monotone transformation of the target parameters. Producing the joint posterior distribution of the ranks is computationally intensive, but most estimates depend only on easily computable features.
We first define ranks and then specify candidate ranking methods. For clarity in defining ranks, we drop the index t and write , with the smallest ρk having rank 1. Rank-based estimates are based on the joint posterior distribution of the Rk(ρ) and are invariant under monotone transform of the ρk.
3.1 Squared-error loss
Shen and Louis (1998) and Lockwood et al. (2002) study ranks that minimize the posterior risk induced by squared error loss (SEL): . It is minimized by the posterior expected ranks,
| (3) |
where pr(·) stands for probability. The optimal mean squared error (MSE) in estimating the ranks is equal to the average posterior variance of the ranks. Generally, the are not integers; for optimal, distinct integer ranks, use .
In the notation that follows, generally we drop dependency on ρ (equivalently, on θ) and omit conditioning on Y. For example, Rk stands for Rk(θ) and stands for . We present either ranks (Rk) or, equivalently, percentiles [Pk = Rk/(K + 1)] with percentiles providing an effective normalization. For example, Lockwood et al. (2002) show that MSE for percentiles rapidly converges to a function of ranking estimator and posterior distributions of parameters that does not depend on K.
3.2 Optimizing (above γ)/(below γ) classification errors
The USRDS uses percentiles to identify the best and the worst performers. Let γ be the fraction of top performers among the total that we want to identify, 0 < γ < 1. A loss function designed to address this inferential goal was proposed by Lin et al. (2006). The loss function (Eq. 4) penalizes for misclassification and also imposes a distance penalty between estimated percentiles and the cutoff γ.
| (4) |
For ease of presentation, we have assumed that γK is an integer and so γ(K + 1) is not. It is not necessary to make the distinction between > and ≥. To minimize the posterior risk induced by (4), let and .
is minimized by:
| (5) |
Dominici et al. (1999) use this approach with γ = K/(K + 1), ordering by the probability of a unit having the largest latent attribute.
3.3 Equivalence of the and ordering posterior exceedance probabilities
Given an SMR threshold t, the ranks/percentiles induced by ordering the posterior probabilities that an SMR exceeds the threshold, pr(ρk > t|Y) allow us to make a connection between the and the substantive scale (in our application, SMR). Normand et al. (1997) rank providers based on these “exceedance probabilities” and Diggle et al. (2007) use them to identify the areas with elevated disease rates. Lin et al. (2006) shows that exceedance probability based percentiles are virtually identical to the by choosing the γth percentile of the average of posterior cumulative distribution function as the threshold t, i.e., , where . We denote the percentiles based on as . In addition to providing a connection to the SMR scale, the are far easier to compute than are the . Note that the are invariant under the monotone transform of ρk.
4 Performance measures
As for all statistical procedures, estimated ranks/percentiles must be accompanied by uncertainty statement. A wide variety of univariate and multivariate performance measures are available and we propose three univariate measures of uncertainty.
4.1 Mean squared error
Using MCMC, the posterior mean squared error of percentiles produced by any method and 95% posterior intervals of dialysis center-specific percentiles can be computed. As a baseline, if the data are completely uninformative so that the percentiles (1/3174, 2/3174,…, 3173/3174) are randomly assigned to the 3173 dialysis centers, then .
4.2 Operating characteristic for (above γ)/(below γ) classification
The vector of from any ranking method can be used to classify units into (above γ)/(below γ) groups and the posterior classification performance (operating characteristic) can be computed. Following Lin et al. (2006), and suppressing dependence on Pest
| (6) |
where,ABR(γ|Y) = pr(percentile) > γ|percentile estimated < γ, Y) = pr(P > γ|Pest < γ, Y) BAR(γ|Y) = pr(percentile < γ|percentile estimated > γ, Y) = pr(P < γ|Pest > γ, Y).
The second equality in (6) results from the identity, γABR(γ|Y) = (1 - γ)BAR(γ|Y). If the goal is to identify units with the largest percentiles, then BAR(γ|Y) is similar to the False Discovery Rate (Benjamini and Hochberg 1995; Efron and Tibshirani 2002; Storey 2002; Storey 2003). ABR(γ|Y) is similar to the False non-Discovery Rate. When the data are completely uninformative, BAR(γ|Y)/γ ≐ 1 and so OC(γ|Y) produces a standardized comparison across γ values. Minimizing it produces the most informative cut point for a given Pest.
For any percentiling method, OC(γ|Y) provides a data analytic performance evaluation. The direct computation of it sums πk(γ|Y) = pr(Pk > (γ|Y) over a Pest produced set of indices.
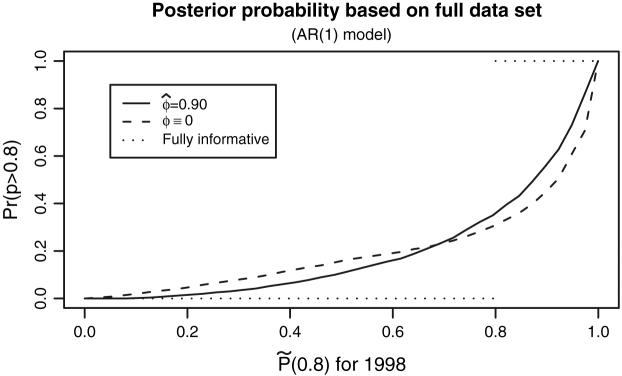
Plotting the πk(γ|Y) versus the (see Fig. 1) displays percentile-specific, classification performance. For ideal fully informative data, the exceedance probability should be 1 for those classified as above γ and 0 for those classified as below γ. OC(γ) is the area between curve and 1 for j ≥ [γK] + 1 plus the area below curve for j ≤ [γK]. Using for the X-axis produces a monotone plot and is the minimum attainable. This plot is similar to that proposed by Pepe et al. (2008)
Fig. 1.
πk (0.8|Y) versus for 1998. Optimal percentiles and posterior probabilities computed with the single year model (ϕ ≡ 0) and the AR(1) model (ϕ = 0.90) Two curves don't cross at γ = 0.8. The line for fully informative data, i.e., when there is no uncertainty associated with ranking results is given as reference
Computing the πk(γ|Y) is numerically challenging. However, the virtual equivalence between and justifies replacing these posterior probabilities by the easily computed pr(ρk > t|Y) with .
4.3 Longitudinal variation
For most of dialysis centers, we expect that percentile estimates of different years are similar to each other. To measure variation in the ranks/percentile estimates within dialysis centers over the four years, we compute Longitudinal Variation:
where is the estimated percentile for dialysis center k in year t and is the mean over the four years. A smaller LV value indicates better consistency in percentiles estimates of different years.
4.4 Subset dependency
Unlike estimating individual parameters (where there is individual shrinkage), ranks are highly correlated and so changing the posterior distribution of some target parameters or removing or adding units rearrange the order of individual parameters in a complicated manner. Ranks computed using the posterior distribution of the ranks are thus not subset invariant in that re-ranking the ranks for a subset of providers will not be the same as ranking only those providers. Section Appendix A gives a numeric illustrative example. However, if the prior distribution is known, ranks based on provider-specific summaries such as the MLEs, PMs, exceedance probabilities or single-provider hypothesis tests are subset invariant. Of course, in an empirical Bayes or fully Bayesian analysis with an unknown prior (thus, including a known hyper-prior), no method is subset invariant because the data are also used to estimate the prior or to update the hyper-prior. We investigate subset dependence by including/removing providers with small mkt (high variance MLEs). These providers are generally small dialysis centers with very few patients. Ranking procedures excluding these centers imply that the centers are first categorized according to their sizes and rankings are then generated in different categories separately. We pursue our comparison under model (2).
5 Results
5.1 Simulated performance
We conducted simulation studies comparing ranking/percentiling methods for the Poisson sampling distribution similar to those reported in Lin et al. (2006) for the Gaussian sampling distribution. Conclusions were similar with performing well over a broad class of loss functions, with MLE-based ranks performing poorly and ranks of posterior mean performing reasonably well but by no means optimally (see Louis and Shen 1999; Gelman and Price 1999). Performances of all methods improved with increasing mkt (reduced sampling variance), but generally the ranking results are quite indefinitive unless information in the sampling distribution (e.g., provided by the data) is very high relative to that in the prior.
5.2 Subset dependency and the effect of unstable SMR estimates
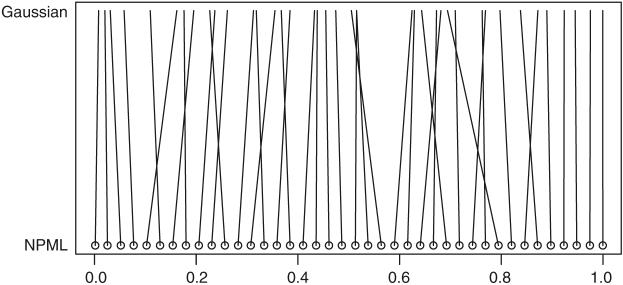
We studied the effect including or excluding providers with small mkt (high-variance MLE estimates) by running both single-year and multiple-year analysis with and without the 68 providers with expected deaths <0.1 in 1998. Comparisons based on in a graph similar to Fig. 2 shows that, there is almost no change in percentiles for providers ranked either high or low, but noticeable re-ordering happens in the middle range. This is not surprising in that the ranks for high-variance providers are shrunken considerably towards the midrank (K + 1)/2 and are not ranked at the extremes. The high variance providers “mix up” with the ranks from more stably estimated, central region providers, but are not contenders for extreme ranks/percentiles. Also, there are more providers in a given interval length in the middle of the distribution of parameters than in the tails. The ranks of these large providers in the middle range will be more sensitive to the change of the joint posterior distribution caused by including small providers. Performance measures MSE and OC(γ) were very similar for the two datasets.
Fig. 2.
Comparison of 1998 with NPML and Gaussian prior. Circles represent 40 dialysis centers evenly spread across percentiles estimated with NPML prior. The percentiles of the same center are connected
5.3 Comparisons using the 1998 data
We computed ranks (formula (7)) based on the MLE and hypothesis testing statistics Z-scores (testing the hypothesis H0: ρ = 1 for 1998); we computed the Bayesian estimates and percentiles based on the posterior means () using model (2) with ϕ ≡ 0.
| (7) |
Globally, if we regard a dialysis center with greater than 1.5 as “flagged,” then 379 (12%) of dialysis centers will be identified; if we regard a dialysis center with Z-score greater than 1.645 as “flagged,” then 647 (20.4%) of dialysis centers are identified.
To compare methods, we select the 634 (20%) worst dialysis centers by ranking and selecting the largest MLEs and Z-scores and compare to those identified by (0.8). The 80th percentiles of the ρmle and Z-score are 1.44 and 1.67, respectively, whereas the 80th percentile of the ρpm is 1.10 (these PMs are closer to 1 than their respective MLEs).
We calculate the kappa statistics between the (above γ)/(below γ) classifications based on and other estimators. The classifications based on and ρpm have high agreement with those based on with respective kappa statistics 0.90 and 0.94. The kappa statistics between the MLE and is 0.78, between the Z-score and is 0.83, and between MLE and Z-score is the lowest 0.68.
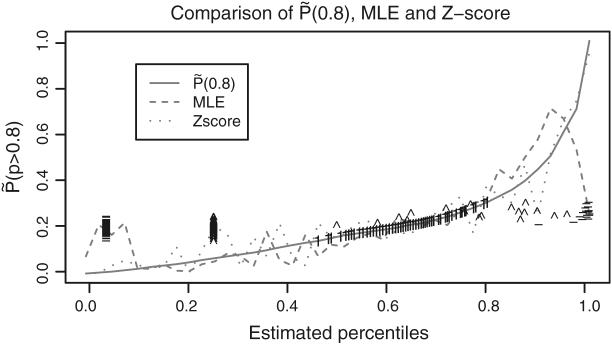
Figure 3 compares different methods based on their posterior probability of correct classification pr(Pk > 0.8|Y) The curve for is monotone and optimal because we construct by ranking these probabilities. The curves for percentiles based on ρpm and on are very close to that for (not plotted). The curves for MLE-based and Z-score-based percentiles are far from monotone.
Fig. 3.
πk(0.8) versus estimated percentiles by three ranking methods using the 1998 data: , MLE-based and Z-score-based. For small dialysis centers (fewer than 5 patients in 1998), the symbol “-” represents the MLE-based percentiles, the symbol “1” the Z-score-based percentiles and the symbol “ ^ ” the
The MLE SMRs for centers with relatively small expected deaths have relatively large variances. To study the impact of large and small variances on estimated percentiles, Fig. 3 identifies those for the 147 dialysis centers that treated fewer than 5 patients in 1998. These constitute 4.5% of all centers. Generally, MLE-based percentiles for these centers are at the extremes whereas Z-score based percentiles tend to be near 0.5. However, because the posterior distribution of ρk for the high variance centers is concentrated around 1, the for these centers are near 0.5 and similarly for and percentiles based on ρpm. For dialysis centers with a large number of patients and thereby a small variance, the optimally estimated percentiles, and spread out to cover full range from 0 to 1. There is better agreement between MLE-based, Z-score-based and optimal percentiles when the small centers are removed from the dataset and estimates are recomputed.
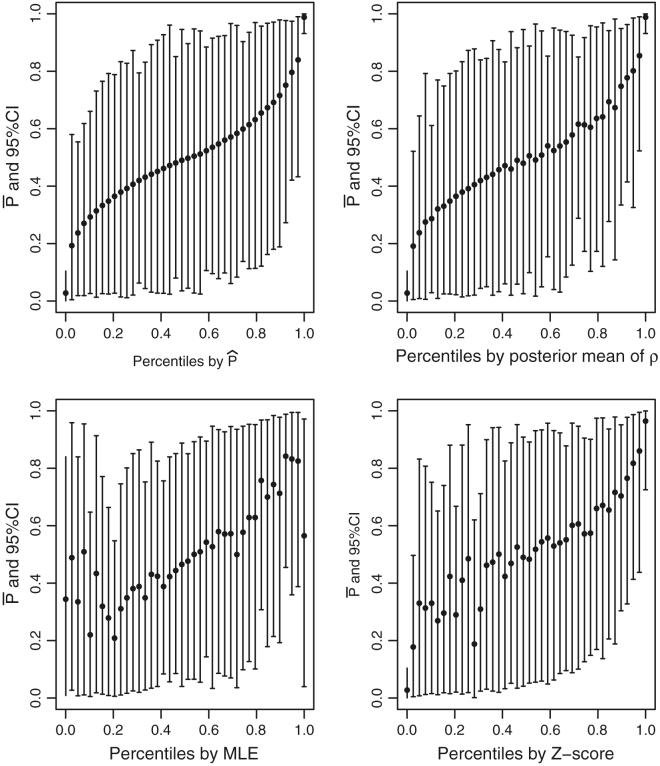
Figure 4 displays estimates for the 40 providers at the 1/3174, 82/3174, 163/3174,…, 3173/3174 percentiles as determined by . For each display, the Y-axis is 100 × with its 95% posterior interval. The X-axis for the upper left panel is , for the upper right is percentiles based on ρpm, for the lower left is percentiles based on ρmle, and for the lower right is percentiles based on Z-scores testing ρk = 1. To deal with cases where Ykt = 0, for the hypothesis test statistic we use
Fig. 4.
SEL-based percentiles for 1998. For each display, the Y-axis is 100 × with its 95% probability interval. The X-axis for the upper left panel is , for the upper right is percentiles based on ρpm, for the lower left is percentiles based on the ρmle and for the lower right is percentiles based on Z-scores testing ρk = 1
See Conlon and Louis (1999) for a similar plot based on SMRs of disease rates in small areas.
Note that in the upper left display the do not fill out the (0, 1.0) percentile range; they are shrunken toward 0.50 by an amount that reflects estimation uncertainty. Also, the posterior probability intervals are very wide, indicating considerable uncertainty in estimating ranks/percentiles. The plotted points in the upper left display are monotone because the X-axis is the percentile based on ranking of Y-axis values. Plotted points in the upper right display, which are based on posterior mean, are almost monotone and close to the best attainable. The lower left and lower right panels show considerable departure from monotonicity, indicating that MLE-based ranks and hypothesis test-based ranks are very far from optimal. Note also that the pattern of departures is quite different in the two panels, showing that these methods produce quite different ranks. Similar comparisons for SMRs estimated from the pooled 1998-2001 data would be qualitatively similar, but the departures from monotonicity would be less extreme.
We divide MSE for different ranking methods by the MSE of randomly assigned ranks (Sect. 4.1) for standardization. The methods based on posterior distributions, Ppm, , and P☆ (0.8) perform pretty close to each other with standardized MSEs 44.5%, 44.4%, 46.2% and 46.2%, respectively. Rankings based on MLE and Z-score have less improvement (52.3% and 47.4%) over randomly assigned ranks. The differences in are less substantial and the wide 95% intervals presented in Fig. 4 indicate that none of methods can give a conclusive ranking result.
5.4 Single year and multi-year analyses
Using model (2) we estimated single-year based and AR(1) model based percentiles. Table 1 reports that the ξ are near 0, as should be the case since we have used internal standardization (the typical log(SMR) = 0). The within year, between provider variation in 100 × log(SMR) is essentially constant at approximately 100 × τ = 24, producing a 95% a priori interval for the ρkt (0.62, 1.60). While we have a prior centering around 1000 for 100 × τ, the data likelihood dominates the prior information and the posterior 95% credible interval of 100 × τt for all 4 years is (22.8, 26.8). Use of the AR(1) model to combine evidence over years (with the posterior distribution for ϕ concentrated around 0.90) reduces 100 × from around 61 to around 48, a twenty percent decrease. Classification performance comparison using the is very close to that for the optimal 100 × .
Table 1.
Results for and . In the multi-year section, 100 × OC(0.8) is for the indicated year as estimated from the multi-year model and 889092 is a notation for posterior median 90 and 95% credible interval (88, 92) (Louis and Zeger 2008)
| Single year: (ϕ ≡ 0) |
Multi-year: 100 × ϕ ∼ 889092) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | 1998 | 1999 | 2000 | 2001 | 1998 | 1999 | 2000 | 2001 |
| 100 × ζ | -2.8 | -1.3 | -2.3 | -0.7 | -3.1 | -0.8 | -1.7 | -0.3 |
| 100 × τ | 24.1 | 23.5 | 23.1 | 22.2 | 25.8 | 25.0 | 24.9 | 24.1 |
| 100 × | 62 | 61 | 60 | 62 | 49 | 47 | 46 | 50 |
| LV() | 62 | 4 | ||||||
Figure 1 displays the details behind the improvement of classification performance. In the upper range of , the curve for the AR(1) model lies above that for the single year, in the lower range it lies below. For the AR(1) model to dominate the single year at all values of , the curves would need to cross at = 0.8, but the curves cross at about 0.7. Appendix B provides some discussion on this phenomenon.
Longitudinal variation in ranks/percentiles (LVPest is dramatically reduced for the AR(1) model going from 62 for the year-by-year analysis to 4 for the multi-year. As a basis for comparison, if ϕ → 1, → 0 and if the data provide no information on the SMRs (the τ → ∞), then = 83.
We have not compared fit of the AR(1) model to other correlation structures such as compound symmetry (constant correlation rather than exponential damping). With only 4 years of data per center, the power to compare different correlation structures will be low. With ϕ's posterior mean 0.90 and 95% credible interval (0.88, 0.92), the AR(1) model is well supported by the data relative to independence. Note that the AR(1) model operates on the θkt = log(ρkt) and not on the observed estimates . The induced model for these is approximately ARMA(1, 1), a hidden Markov model.
5.5 Parametric and non-parametric priors
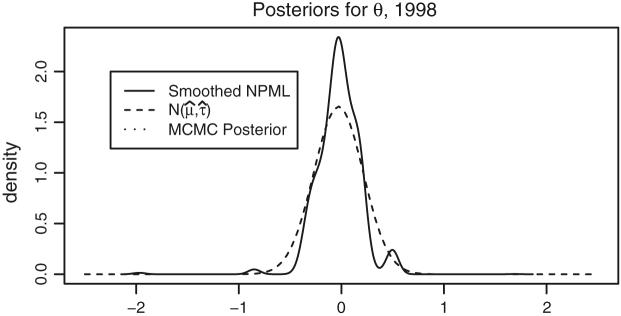
We compare percentiles based on posterior distributions under the parametric and NPML priors using 1998 data. Figure 5 displays Gaussian, posterior expected and smoothed NPML estimated priors for θ = log(ρ). The Gaussian is produced by plugging in the posterior medians for (μ0, τ0). The posterior expected is a mixture of Gaussians using the posterior distribution of (μ0, τ0). The posterior distribution of (μ0, τ0) has close to 0 variance, so the two parametric curves superimpose. The NPML is discrete and was smoothed using the “density” function in R with adjustment parameter 10 (i.e., the Gaussian kernel bandwidth is ten times of the default value, see Silverman (1986)). We smooth the NPML to graph a smooth curve, but use the NPML itself to produce ranks/percentiles. Note that the smoothed NPML has at least two modes with a considerable mass at approximately θ = 0.5; ρ = 1.65. However, this departure from the Gaussian distribution has little effect on classification performance. Using 1998 data, for the NPML 100 × OC(0.8) ≈ 67 while for the Gaussian prior the value is 62 (see Table 1). For performance evaluations of the NPML, see Paddock et al. (2006). Fig. 2 compares under the two priors. The centers at the top or the bottom have less uncertainty in percentiles (strong signal), and their percentiles are generally same under two priors. For the dialysis centers with larger variance, the percentiles depend on the prior.
Fig. 5.
Estimated priors for θ = log(ρ) using the 1998 data. The solid curve is a smoothed NPML using the “density” function in R with adjustment parameter = 10. The dashed curve is Gaussian using posterior medians for (μ, τ); the dotted curve is a mixture of Gaussians with (μ, τ) sampled from their MCMC computed joint posterior distribution
5.6 Ranks based on exceedance probabilities
We compute P*(0.8) (see Sect. 3.3) using the Gaussian prior for θ and 1998 data. The θ-threshold, (ρ-threshold = 1.184). Lin et al. (2006) prove the near equivalence of P*(γ) and and Fig. 1 displays this equivalence in that the curve based on P*(0.8) is nearly identical to that based on for ϕ = 0.
6 Discussion
Ranks and percentiles are computed to address specific policy or management goals. It is important to use a procedure that performs well for the primary goals. A structured approach guided by a Bayesian hierarchical model and a loss function helps clarify goals and produces ranks/percentiles that outperform other contenders, such as those based on MLEs and Z-scores. When the uncertainties of the direct estimate vary considerably over providers, the estimates are very sensitive to the method used. In that situation, a structured approach is especially important.
Our data-analytic assessments support the Lin et al. (2006) finding that the (general purpose percentiles) perform well over inferential goals addressed by a range of loss functions, but that if a specific percentile cut-point, γ, is identified, (or P*(γ)) should be used. Unless the substantive application dictates otherwise, we recommend the use of these. Cost or other considerations can be incorporated to select γ.
Though the loss function guided estimates are the best possible, the ranking results might not be conclusive, partially indicated by the wide confidence interval as shown in the Fig. 4. Therefore, data-analytic performance evaluations are a necessary companion to estimated ranks. Uncertainty assessments include standard errors and tabulation or display of the probabilities of correct classification in a (above γ)/(below γ) assessment (our πk(γ|Y)). These probabilities can be used to temper penalties or rewards. When available, data of multiple years can be combined to reduce the uncertainty in ranking results, as shown in Table 1 and Fig. 1.
Robustness of efficiency and validity are important attributes of any statistical procedure. For sufficiently large K, using a smoothed non-parametric prior is highly efficient relative to a correct, parametric approach and confers considerable robustness (see Paddock et al. 2006). Additional study of this strategy is needed.
Percentiles are prima facie relative comparisons in that it is possible that all providers are doing well or that all are doing poorly; percentiles will not pick this up. Indeed, the SMR is, itself, a relative measure and so percentiles produced from it are twice removed from a normative context! In situations where normative values are available (e.g., death rates), percentiles that have a normative interpretation are attractive and those based on posterior probabilities of exceeding some threshold (P*(γ)) are essentially identical to a loss function based approach and so provide an excellent link to a substantively relevant scale. And, they confer a considerable computing advantage over using the posterior distribution of the ranks to find the .
Finally, because percentiles can be very sensitive to the estimation methods and because there is considerable uncertainty associated with all percentiling methods, stakeholders need to be informed of the issues in producing percentiles, in interpreting them, in their role in science and policy, and in insisting on uncertainty assessments.
Acknowledgements
Supported by grant R01-DK61662 from U.S NIH, National Institute of Diabetes, Digestive and Kidney Diseases., We thank Chris Forrest for his advice and comments.
Appendices
Appendix A: An illustrative example of subset dependency
In general, ranks depend on the framework for comparison and the list of contenders; they are not necessarily subset invariant. For illustration, consider ranking 3 dialysis centers by as defined in Eq. 3. We have,
Let , i = 1, 2, 3. Let μ1 = 0, μ2 = -0.15, μ3 = 0.05, , then pr(ρ1 > ρ2) = 0.51 > 0.49 = pr(ρ2 > ρ1); pr(ρ1 > ρ2) + pr(ρ1 > ρ3) = 0.51 < 0.98 = pr(ρ2 > ρ1) + pr(ρ2 > ρ3). Thus if we rank only dialysis centers 1 and 2, center 2 has better rank (smaller θ) than center 1; if we rank all 3 centers, center 2 has worse rank than center 1.
Appendix B: Crossing points of exceedance probability curves
We start from a simplified scenario where θk's share a common posterior variance. Assume θk ~ N(μk, ν) a posteriori, Φ(t; μk, ν) = pr(θ > t; μk, ν). Let ν0 and be two possible values of ν, ν0 > . Without loss of generality, let μ1 < μ2 < …< μK.
For any given t,
If ;
If ;
By the common variance assumption and μ1 < μ2 <…μK, the posterior distributions of θk are stochastically ordered and the rank of θk is k. The curves and are both monotone increasing and cross each other between and if μi < t < μi+1. The value of y-coordinate of the crossing point is around 0.5 due to . We denote the x-coordinate of the crossing point as ignoring at most 1/K difference.
If t1 and t2 satisfy
then the curves and cross each other at ; the curves and cross each other at ; And the crossing-over of curves and happens between and . When γ is greater or smaller than both of and , which depend on γ, ν0, and vector (μ1, μ2,…, μK), the x-coordinate of the crossing point can not be at γ.
Denote the posterior distributions of θk as N(μk, νk) and N in single year and multiple years analyses. If the dialysis centers perform consistently over years, inference uncertainty of θk should be reduced (assuming < νk) by accumulating data over years while the means do not change much (assuming = μk). Assuming N(μk, νk)'s are stochastically ordered, N's are stochastically ordered, the above discussion applies to the crossing point of the curves and .
In Fig. 1, two curves and are plotted without the stochastically ordered assumption and the location crossing point is more complicated. In general, it is not necessary that the x-coordinate of the crossing point will be at γ.
Appendix C: The NPML algorithm
Assume ρk ~ G, k = 1,…, K. G is discrete having at most J mass points u1,…,uJ with probabilities p1,…, pJ. We use EM algorithm (Dempster et al. 1977) to estimate the u's and p's. Start with and , for each recursion,
This recursion converges to a fixed point and, if unique, to the NPML. The recursion is stopped when the maximum relative change in each step for both the and the , j = 1, 2,…,K is smaller than 0.001. At convergence, is both prior and the Shen and Louis (1998) histogram estimate .
Care is needed in programming the recursion. The w-recursion is:
Since can be extremely small ( > can be extremely large), to stabilize the computations we define,
and write
The w-recursion becomes:
Contributor Information
Rongheng Lin, Department of Public Health, University of Massachusetts Amherst, Rm 411 Arnold House, 715 N. Pleasant Rd., Amherst, MA 01003, USA.
Thomas A. Louis, Department of Biostatistics, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD 21205, USA e-mail: tlouis@jhsph.edu
Susan M. Paddock, RAND Corporation, Santa Monica, CA 90407, USA e-mail: paddock@rand.org
Greg Ridgeway, e-mail: gregr@rand.org.
References
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. C Stat. Methodol. 1995;57:289–300. [Google Scholar]
- Carlin BP, Louis TA. Bayesian Methods for Data Analysis. 3rd edn. Chapman and Hall/CRC Press; Boca Raton FL: 2008. [Google Scholar]
- Christiansen C, Morris C. Improving the statistical approach to health care provider profiling. Ann. Intern. Med. 1997;127:764–768. doi: 10.7326/0003-4819-127-8_part_2-199710151-00065. [DOI] [PubMed] [Google Scholar]
- Conlon EM, Louis TA. Addressing multiple goals in evaluating region-specific risk using Bayesian methods. In: Lawson A, Biggeri A, Böhning D, Lesaffre E, Viel J-F, Bertollini R, editors. Disease Mapping and Risk Assessment for Public Health. Wiley; 1999. pp. 31–47. chap. 3. [Google Scholar]
- Dempster AP, Laird NM, Rubin DB. Maximum likelihood from incomplete data via the EM algorithm (C/R: P 22-37) J. R. Stat. Soc. Ser. C Stat. Methodol. 1977;39:1–22. [Google Scholar]
- Diggle PJ, Thomson MC, Christensen OF, Rowlingson B, Obsomer V, Gardon J, Wanji S, Takougang I, Enyong P, Kamgno J, Remme JH, Boussinesq M, Molyneux DH. Spatial modelling and the prediction of Loa loa risk: decision making under uncertainty. Ann. Trop. Med. Parasitol. 2007;101(6):499–509. doi: 10.1179/136485913X13789813917463. [DOI] [PubMed] [Google Scholar]
- Dominici F, Parmigiani G, Wolpert RL, Hasselblad V. Meta-analysis of migraine headache treatments: combining information from heterogeneous designs. J. Am. Stat. Assoc. 1999;94:16–28. [Google Scholar]
- Efron B, Tibshirani R. Empirical Bayes methods and false discovery rates for microarrays. Genet. Epidemiol. 2002;23:70–86. doi: 10.1002/gepi.1124. [DOI] [PubMed] [Google Scholar]
- ESRD . 1999 Annual Report: ESRD Clinical Performance Measures Project. Health Care Financing Administration; 2000. Technical Report. [Google Scholar]
- Gelman A, Price P. All maps of parameter estimates are misleading. Stat. Med. 1999;18:3221–3234. doi: 10.1002/(sici)1097-0258(19991215)18:23<3221::aid-sim312>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Gelman A, Sturtz S, Ligges U, Gorjanc G, Kerman J. 2006 The R2WinBUGS package. http://cran.r-project.org/doc/packages/R2WinBUGS.pdf.
- Goldstein H, Spiegelhalter D. League tables and their limitations: statistical issues in comparisons of institutional performance (with discussion) J. R. Stat. Soc. Ser. A. 1996;159:385–443. [Google Scholar]
- Grigg OA, Farewell VT, Spiegelhalter DJ. Use of risk-adjusted CUSUM and RSPRT charts for monitoring in medical contexts. Stat. Methods Med. Res. 2003;12(2):147–170. doi: 10.1177/096228020301200205. [DOI] [PubMed] [Google Scholar]
- Lacson E, Teng M, Lazarus J, Lew N, Lowrie E, Owen W. Limitations of the facility-specific standardized mortality ratio for profiling health care quality in dialysis. Am. J. Kidney Dis. 2001;37:267–275. doi: 10.1053/ajkd.2001.21288. [DOI] [PubMed] [Google Scholar]
- Laird NM. Nonparametric maximum likelihood estimation of a mixing distribution. J. Am. Stat. Assoc. 1978;78:805–811. [Google Scholar]
- Landrum M, Bronskill S, Normand S-L. Analytic methods for constructing cross-sectional profiles of health care providers. Health. Serv. Outcomes Res. Method. 2000;1:23–48. [Google Scholar]
- Lin R, Louis TA, Paddock SM, Ridgeway G. Loss function based ranking in two-stages, hierarchical models. Bayesian Anal. 2006;1(4):915–946. doi: 10.1214/06-BA130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Louis TA, Pan W, Ma J, Collins A. Methods for estimating and interpreting provider-specific, standardized mortality ratios. Health. Serv. Outcomes Res. Method. 2004;4:135–149. doi: 10.1023/B:HSOR.0000031400.77979.b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood J, Louis TA, McCaffrey DF. Uncertainty in rank estimation: implications for value-added modeling accountability systems. J. Edu. Behav. Stat. 2002;27(3):255–270. doi: 10.3102/10769986027003255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis TA, Shen W. Innovations in Bayes and empirical Bayes methods: estimating parameters, populations and ranks. Stat. Med. 1999;18:2493–2505. doi: 10.1002/(sici)1097-0258(19990915/30)18:17/18<2493::aid-sim271>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Louis TA, Zeger SL. Effective communication of standard errors and confidence intervals. Biostatistics. 2008 doi: 10.1093/biostatistics/kxn014. http://dx.doi.org/10.1093/biostatistics/kxn014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClellan M, Staiger D. The Quality of Health Care Providers. National Bureau of Economic Research; 1999. Technical Report 7327. Working Paper. [Google Scholar]
- Normand S-LT, Glickman ME, Gatsonis CA. Statistical methods for profiling providers of medical care: issues and applications. J. Am. Stat. Assoc. 1997;92:803–814. [Google Scholar]
- Normand S-LT, Shahian DM. Statistical and clinical aspects of hospital outcomes profiling. Stat. Sci. 2007;22:206–226. [Google Scholar]
- Ohlssen DI, Sharples LD, Spiegelhalter DJ. A hierarchical modelling framework for identifying unusual performance in health care providers. J. R. Stat. Soc. Ser. A Stat. Soc. 2007;170(4):865–890. [Google Scholar]
- Paddock S, Ridgeway G, Lin R, Louis TA. Flexible distributions for triple-goal estimates in two-stage hierarchical models. Comput. Stat. Data Anal. 2006;50(11):3243–3262. doi: 10.1016/j.csda.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepe MS, Feng Z, Huang Y, Longton G, Prentice R, Thompson IM, Zheng Y. Integrating the predictiveness of a marker with its performance as a classifier. Am. J. Epidemiol. 2008;167(3):362–368. doi: 10.1093/aje/kwm305. http://dx.doi.org/10.1093/aje/kwm305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer M, Best N, Cowles K, Vines K. The CODA Package. 2006. http://cran.r-project.org/doc/packages/coda.pdf [Google Scholar]
- Shen W, Louis TA. Triple-goal estimates in two-stage, hierarchical models. J. R. Stat. Soc. Ser. B. 1998;60:455–471. [Google Scholar]
- Shen W, Louis TA. Triple-goal estimates for disease mapping. Stat. Med. 2000;19:2295–2308. doi: 10.1002/1097-0258(20000915/30)19:17/18<2295::aid-sim570>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Silverman BW. Density Estimation for Statistics and Data Analysis. Chapman & Hall Ltd; 1986. [Google Scholar]
- Spiegelhalter D, Thomas A, Best N, Gilks W. BUGS: Bayesian Inference Using Gibbs Sampling. Version 0.60. Medical Research Council Biostatistics Unit, Institute of Public Health; Cambridge University: 1999. Technical Report. [Google Scholar]
- Storey JD. A direct approach to false discovery rates. J. R. Stat. Soc. Ser. B Methodol. 2002;64(3):479–498. [Google Scholar]
- Storey JD. The positive false discovery rate: a Bayesian Interpretation and the q-value. Ann. Stat. 2003;31(6):2013–2035. [Google Scholar]
- USRDS . 2005 Annual Data Report: Atlas of end-stage renal disease in the United States. Health Care Financing Administration; 2005. Technical report. [Google Scholar]
- Zaslavsky AM. Statistical issues in reporting quality data: small samples and casemix variation. Int. J. Qual. Health Care. 2001;13(6):481–488. doi: 10.1093/intqhc/13.6.481. [DOI] [PubMed] [Google Scholar]
- Zhang M, Strawderman RL, Cowen ME, Wells MT. Bayesian inference for a two-part hierarchical model: an application to profiling providers in managed health care. J. Am. Stat. Assoc. 2006;101(475):934–945. [Google Scholar]