Abstract
Suppression of the renin-angiotensin system has proven efficacy for mitigation and treatment of radiation nephropathy, and it has been hypothesized that this efficacy is due to suppression of radiation-induced chronic oxidative stress. It is known that radiation exposure leads to acute oxidative stress, but direct evidence for radiation-induced chronic renal oxidative stress is sparse. We looked for evidence of oxidative stress after total-body irradiation in a rat model, focusing on the period before there is physiologically significant renal damage. No statistically significant increase in urinary 8-isoprostane (a marker of lipid peroxidation) or carbonylated proteins (a marker of protein oxidation) was found over the first 42 days after irradiation, while a small but statistically significant increase in urinary 8-hydroxydeoxy-guanosine (a marker of DNA oxidation) was detected at 35-55 days. When we examined renal tissue from these animals, we found no significant increase in either DNA or protein oxidation products over the first 89 days after irradiation. Using five different standard methods for detecting oxidative stress in vivo, we found no definitive evidence for radiation-induced renal chronic oxidative stress. If chronic oxidative stress is part of the pathogenesis of radiation nephropathy, it does not leave widespread or easily detectable evidence behind.
INTRODUCTION
Suppression of the renin-angiotensin system (RAS) with angiotensin converting enzyme (ACE) inhibitors or angiotensin II (AII) receptor blockers has proven efficacy for both mitigation (1-4) and treatment (5, 6) of radiation nephropathy. Robbins and colleagues (7, 8) have hypothesized that this efficacy is due to suppression of radiation-induced chronic oxidative stress, in that AII causes production of reactive oxygen species (ROS) through activation of NADPH oxidase. If this hypothesis is correct, then antioxidants should also be effective for mitigation and treatment of radiation nephropathy. To efficiently test antioxidant therapies against radiation nephropathy, it is essential to know when the oxidative stress occurs and to monitor the efficacy of the proposed therapies for reducing or eliminating this oxidative stress. Therefore, we need an assay (preferably minimally invasive) for radiation-induced chronic renal oxidative stress.
It is well known that irradiation of biological material leads to the production of ROS, and there is some evidence that increases in ROS can persist for several days in irradiated cells in culture (9-11). However, direct evidence for chronic renal oxidative stress is sparse. Robbins et al. (12) found evidence for oxidative damage to DNA in irradiated kidneys that persisted for up to 24 weeks after irradiation; Datta et al. (13) found increased renal expression of hemeoxygenase 1 [Hmox-1, a marker of oxidative stress (14)] 7-9 weeks after irradiation. Unfortunately these data are insufficient for determining when the oxidative stress begins or for choosing a method to detect it noninvasively. In addition, it does not tell us whether oxidative stress is part of the pathogenesis of the injury or whether it is the result of the injury. We therefore sought to extend these observations, with attention to the period before there is physiologically significant renal damage.
Initial work focused on detecting oxidation products in urine after total-body irradiation (TBI) in a rat bone marrow transplantation (BMT) model (15, 16). When the urine assays failed to show definitive evidence for radiation-induced chronic oxidative stress, we shifted our focus to a direct search for oxidation products in kidney tissue from the same animals.
MATERIALS AND METHODS
Rat Syngeneic Bone Marrow Transplant (BMT) Model
TBI regimens were used to cause radiation nephropathy (15, 16). This radiation nephropathy is characterized by proteinuria, azotemia and progressive hypertension that leads to renal failure after a median time of 30 to 40 weeks (15, 16). Renal failure (uremia) is the only significant cause of illness and death in this model (15, 16). The studies were performed in syngeneic WAG/Rij/MCW rats that were bred and housed in a moderate-security barrier. The animals were free of Mycoplasma pulmonis, Pseudomonas and common rat viruses. No antibiotics or immunosuppressive drugs were used. The rats were maintained in the Biomedical Resource Center of the Medical College of Wisconsin, which is fully accredited by the American Association of Accreditation of Laboratory Animal Care. The animal protocols were approved by the Institutional Animal Care and Use Committee.
Seven- to 8-week-old male rats underwent TBI with a single dose of 10 Gy or with 18.8 Gy given in six fractions over 3 days at a dose rate of 1.95 Gy/min. Irradiation was done with a 300 kVp orthovoltage source with a half-value layer of 1.4 mm copper; the radiation dosimetry was described in detail by Cohen et al. (17). For irradiation, unanesthetized rats were immobilized in a specially constructed jig (15). Within 24 h after TBI, the rats received a BMT from a syngeneic donor (15). The center of the irradiation schedule was considered to be day zero for definition of time after irradiation.
Experimental Design
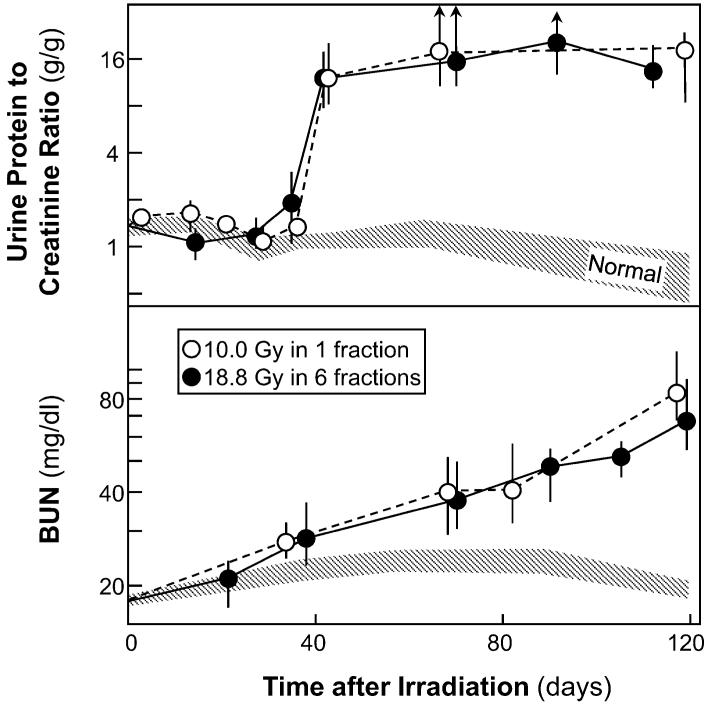
Two different TBI schedules were assessed: 18.8 Gy in six fractions (two per day on 3 consecutive days with 4 h between daily fractions) and a single 10-Gy dose. The fractionated schedule was designed to mimic the conditioning regimens used in clinical BMT (15), whereas the single-dose schedule is more relevant to radiation accident or radiological terrorism scenarios (18). The total doses in the two schedules were chosen to produce roughly equal degrees of radiation nephropathy (Fig. 1). Figure 1 also illustrates the 30-35-day latent period that is observed before the appearance of physiological evidence of radiation-induced renal injury. Assessment of evidence for chronic oxidative stress focused on the first 56 days after irradiation for several reasons. First, if the pathogenesis of late radiation-induced renal injury is driven in part by radiation-induced chronic oxidative stress, as proposed by Robbins and colleagues (7, 8), then evidence for oxidative stress should precede the appearance of physiological injury. Second, if the efficacy of renin-angiotensin blockers in the mitigation of radiation-induced renal injury is to be explained by inhibition of the generation of ROS, as also proposed by Robbins and colleagues (7, 8), then oxidative stress should be occurring during the period when these blockers are most effective, which is at 30-70 days after irradiation (19, 20). Finally, oxidative stress occurring after the animals are physiologically ill could be caused by the renal dysfunction, because renal failure itself is associated with oxidative stress (21, 22).
FIG. 1.
Time course of proteinuria (top, as the urine protein to creatinine ratio) and azotemia (bottom, as blood urea nitrogen) after 18.8 Gy TBI in six fractions (plus BMT) or 10 Gy TBI in a single fraction (plus BMT). Data are shown as medians with 20-80% ranges; upward arrows show that the 80% range exceeded 20 g/g.
Monitoring the Development of Radiation Nephropathy
Animals were monitored daily in all experiments. Development of uremia was assessed for up to 52 weeks after TBI, and animals whose blood urea nitrogen (BUN) exceeded 120 mg/dl were euthanized. BUN, urine protein and urine creatinine were determined at intervals with commercial kits (23). Urine protein excretion is expressed as the ratio of urine protein to urine creatinine (UP/UC) in the same urine sample; this is done to account for the known urine-concentrating defect that occurs in renal radiation injury and to normalize for differences in animal size.
Determination of Urinary 8-Isoprostane (8-IP) Levels
To assess urinary 8-IP, 24-h urine samples were collected on ice into containers containing an antioxidant (100 μl of 2% butylated hydroxytoluene in ethanol) and were then stored at -80°C. Collection on ice with an antioxidant present is required to prevent air-induced oxidation of lipids in the urine. An aliquot of each sample was purified on an 8-IP affinity column (Cayman Chemical no. 416358, Ann Arbor, MI) and then concentrated by vacuum centrifugation and reconstituted in the Cayman enzyme immunoassay (EIA) buffer. The samples were assayed using an 8-isoprostane EIA kit (Cayman Chemical no. 516351). In brief, the samples were incubated with an 8-IP-acetylcholinesterase conjugate and a rabbit 8-IP antiserum in an EIA plate precoated with a mouse anti-rabbit IgG monoclonal antibody. The EIA plate was incubated, washed and developed with Ellman’s reagent, and the absorbance at 410 nm was measured. 8-IP levels are expressed as the ratio of 8-IP (in pg) to creatinine (in mg) in the same urine sample; this is done for the same reason as in the assessment of proteinuria (see above).
Determination of Urinary 8-Hydroxydeoxyguanosine (8-OHdG) Levels
For assessment of urinary 8-OHdG, the samples taken for the 8-IP assays were used, supplemented by additional 24-h urine samples that were collected without the ice and the antioxidant. Samples were analyzed using the New 8-OHdG Check ELISA kit (24) from the Japan Institute for the Control of Aging (no. KOG-200S/E, Genox, Baltimore, MD). The samples (or standard) and the anti-8-OHdG monoclonal antibody were added to a microtiter plate precoated with 8-OHdG. The monoclonal antibody reacts competitively with the 8-OHdG bound to the plate and in the sample. The antibody bound to the sample after incubation was washed away, leaving only the antibody, which is bound to the coated plate. An enzyme-labeled secondary antibody was added to the plate, where it bound to the monoclonal antibody bound to the 8-OHdG coated on the plate. Unbound secondary antibody was washed away, and a substrate solution was added to develop the color. The reaction was terminated with phosphoric acid solution, and the absorbance at 450 nm was measured. 8-OHdG levels are expressed as the ratio of 8-OHdG (in ng) to creatinine (in mg) in the same urine sample; this was done for the same reason as in the assessment of proteinuria (see above).
Immunohistochemistry of Renal 8-Hydroxydeoxyguanosine (8-OHdG)
For assessment of renal 8-OHdG, kidneys were perfused with 10% buffered zinc formalin, embedded in paraffin, and cut into 4-μm sections. Sections were hydrated, washed in water, incubated in citrate buffer solution (pH 6.0) in a steam bath for 20 min, and then cooled at room temperature for 20 min. Sections were blocked for endogenous avidin and biotin using the SP-2001 blocking kit from Vector Laboratories (Burlingame, CA) and then blocked for protein with 2% horse serum in PBS. They were then incubated at room temperature for 1 h with an anti-8-OHdG monoclonal antibody (no. MOG-100P, Genox). Staining was visualized using the ABC method (PK-6100, Vector Laboratories) and counterstained with Mayer’s hematoxylin. An isotype control (NC-748-R7, NeoMarkers, Fremont, CA) for the monoclonal antibody was run, and no staining was observed.
For analysis, sections were photographed at 20× with the Nuance camera system (CRi, Woburn, MA) and analyzed with the Nuance software. Three sections at the pole of the kidney and three sections at the equator of the kidney (that all contained glomeruli) were selected. To eliminate staining edge effects, these pictures were taken one viewing field in from the edge of the kidney. The camera was operated under autoexposure mode, and a blank portion of the slide was imaged and subtracted from the final picture.
When analyzing the slides for glomerular 8-OHdG staining, a single glomerulus was used to set threshold values for both the DAB and hematoxylin counterstain; these values were then checked on other slides from the same staining run and adjusted so that the best possible threshold values could be used for the entire run. All glomeruli in a particular field were then selected and analyzed.
Determination of Urinary Protein Carbonyl Levels
For assessment of urinary protein carbonyls, 24-h urine samples were collected, spun at 3500g for 10 min to remove solids, and then stored at -80°C. Protein carbonyl content was determined with a colorimetric assay according to the technique of Levine et al. (25). In brief, urine aliquots containing 1 mg total protein were incubated with 0.2% 2,4-dinitrophenylhydrazine (DNPH) in 2N hydrochloric acid (HCl) for 1 h at room temperature in the dark and mixed every 10 min. Corresponding blanks containing an equal volume of 2N HCl were treated similarly. DNPH-derivatized proteins and their blanks were precipitated with 10% trichloroacetic acid (TCA) and centrifuged at 11,000g for 4 min. The pellet was mixed with 1 ml ethanol/ethyl acetate (1:1 v/v) and centrifuged at 11,000g for 4 min; this washing step was repeated three times. The final pellet was solubilized in 0.8 ml 6 M guanidine-HCl and centrifuged for 10 min at 11,000g to remove insoluble materials. Carbonyl content in the supernatant was determined by measuring absorbance at 370 nm using a microtiter plate reader (BioTek Instruments, Winooski, VT). Protein concentrations were determined by Lowry’s method using the Bio-Rad DC protein assay kit (Bio-Rad Laboratories, Hercules, CA). Results were calculated as nmol carbonyls per mg protein using an extinction coefficient of 22,000 M-1 cm-1 (25). Absence of interference by carbonyl groups on nucleic acids was established by demonstrating that the 280- to 260-nm absorbance ratio was greater than 1.0 for all blank samples (25).
To create positive and negative controls for the protein carbonyl assay, bovine serum albumin (BSA) was first oxidized with hydrogen peroxide (H2O2) in the presence of ferrous sulfate (FeSO4), and then an aliquot was reduced with sodium borohydrate (NaBH4). BSA was dissolved in 20 mM pH 7.4 Tris buffer and incubated with 1 mM H2O2 and 1 mM FeSO4 for 10 min at room temperature. The oxidized BSA sample was precipitated with 10% TCA and centrifuged at 1000g for 10 min, and then the BSA pellet was washed three times with TCA. One aliquot was resuspended in pH 8.5 Tris-EDTA buffer (85.7 mM Tris, 0.857 mM EDTA), and the second was resuspended in the Tris-EDTA buffer plus 20 mM NaBH4 at 37°C for 30 min. The third aliquot of BSA was neither oxidized nor reduced but was precipitated, washed with TCA, and resuspended in the Tris-EDTA buffer.
Determination of Renal Protein Carbonyl Levels
Carbonylated proteins were assayed in total renal tissue using an adaptation of the DNPH method used to assess urinary protein carbonyls. Rats were anesthetized with isoflurane and perfused with saline before the kidneys were removed. The kidneys were frozen on dry ice immediately and stored at -80°C until assayed. Whole tissue extracts were prepared by homogenizing 100 mg of renal tissue (cortex unless indicted otherwise) on ice in 0.5 ml of ice-cold RIPA lysis buffer (Santa Cruz Biotechnology Inc., Santa Cruz, CA). Homogenization was done with a Tissue Tearor (Model 985-370, Biospec, Racine, WI) using three 10-15-s sessions. Samples (1 mg each) of the whole tissue extract were then processed as described for the urinary colorimetric protein carbonylation assay.
Oxidized renal proteins were also studied by immunoblotting using a modification of the methods described by Levine et al. (26). In brief, 10-50 μg of protein from the whole tissue extract was electrophoresed using SDS-PAGE on precast 4-20% gradient gels (Bio-Rad Laboratories) at constant voltage for ∼2 h. The gel was then equilibrated in electrotransfer Tris/glycine buffer (25 mM Tris, 191 mM glycine, 20% methanol, Bio-Rad) and electrotransferred onto PVDF membrane (Immun-Blot, Bio-Rad). The membrane was equilibrated in 100% methanol, then in 20% (v/v) methanol in TBS buffer (Bio-Rad), and incubated with 0.02% DNPH in 0.2N HCl. The membrane was washed with 0.2N HCl and then with 100% methanol. The membrane was blocked with 5% nonfat milk in TBS-Tween 20 (TBS-T) buffer and then incubated overnight at 4°C with rabbit polyclonal anti-DNP antibody (Molecular Probes, Eugene, OR) diluted 1:5,000. The membrane was then washed with TBS-T buffer and incubated with a secondary horseradish peroxidase-conjugated polyclonal anti-rabbit IgG antibody (Molecular Probes). Proteins were detected on Kodak BioMax X-ray film using ECL reagent (Amersham Life Science, Piscataway, NJ).
Statistical Methods
BUN, BP, UP/UC and measures of oxidative stress are usually shown as medians with 20-80% ranges; medians and ranges were used because physiological parameters in the groups showing abnormal renal function were often neither normally nor log-normally distributed. Two-group physiological data were compared by the Mann-Whitney test, while three or more groups were compared with the Kruskal-Wallis test. Trends in values with time were analyzed using the Kendall rank correlation test. Where multiple comparison issues are relevant, they are discussed.
RESULTS
Development of Radiation Nephropathy
The 18.8-Gy fractionated schedule and the 10-Gy single-dose schedule produced roughly equal degrees of radiation nephropathy (Fig. 1). As in previous studies (16), proteinuria first appears at 30-35 days after irradiation followed soon after by azotemia and several weeks later by hypertension. In both schedules, BUN levels exceeded 120 mg/dl at 140-170 days after irradiation (data not shown).
Urinary Lipid Peroxidation Products after TBI
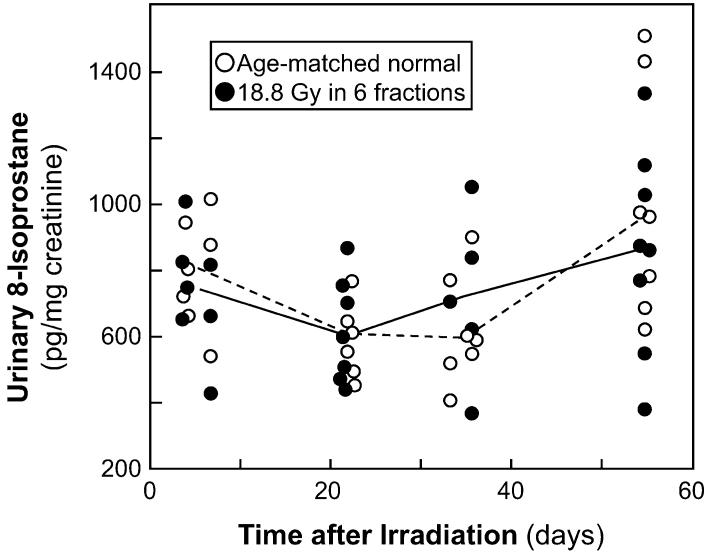
Urinary 8-IP is a commonly used marker for renal oxidative stress (27-29). Urinary 8-IP was measured in 14 animals after 18.8 Gy fractionated TBI plus BMT and in 16 normal age-matched animals (Fig. 2). Most animals were measured at only one time, but six irradiated and six age-matched animals were assessed at multiple times. There was no significant trend of 8-IP levels with time in either irradiated or age-matched normal animals (P > 0.20). In the irradiated animals, the median urinary 8-IP level was 768 (20-80% range 492-934) pg per mg creatinine, whereas in the age-matched controls the levels were 679 (557-948) pg/mg; the 13% increase in the irradiated animals is not statistically significant (P > 0.20). If the individual times are evaluated separately, the ratio of 8-IP levels in irradiated compared to age-matched normal animals ranges from 0.9 at 7 days to 1.2 at 35 days (all P > 0.20). There is no significant evidence for an increase in 8-IP levels after irradiation, although an increase of 20-30% at 35 days after irradiation cannot be excluded.
FIG. 2.
Time course of urinary excretion of 8-isoprostane (8-IP), a marker of lipid oxidation, after 18.8 Gy fractionated TBI plus BMT. Data are shown for animals receiving TBI and age-matched controls. The lines trace the median values.
Urinary DNA Oxidation Products after TBI
Urinary 8-OHdG is a commonly used marker for renal oxidative stress (29-31). Urinary 8-OHdG was measured in all the samples for which urinary 8-IP was measured after irradiation and in 21 of 26 samples from the age-matched normal animals in that set (Fig. 3). Urinary 8-OHdG was also measured in six additional irradiated animals (18.8 Gy in six fractions) and six age-matched controls; these 12 animals were each assessed at three or four different times (Fig. 3). For irradiated and age-matched animals, there were significant decreasing trends of 8-OHdG levels with time (both P ≤ 0.002). In the irradiated animals, the median urinary 8-OHdG level was 71 (20-80% range 50-84) ng per mg creatinine, whereas in the age-matched controls the levels were 52 (37-70) ng/mg; the overall increase is statistically significant (P < 0.001). If the individual times are evaluated separately, the ratio of 8-OHdG levels in irradiated compared to age-matched normal animals increased with time after irradiation, being 1.0 at 4-7 days, 1.1 at 20-22 days (P = 0.049), and 1.3 at 34-56 days (P = 0.013 at 34-36 days and P = 0.036 at 55-56 days).
FIG. 3.
Time course of urinary excretion of 8-hydroxydeoxyguanosine (8-OHdG), a marker of DNA oxidation, after 18.8 Gy fractionated TBI plus BMT. Data are shown for animals receiving TBI and age-matched controls. The lines trace the median values.
Urinary Protein Oxidation Products after TBI
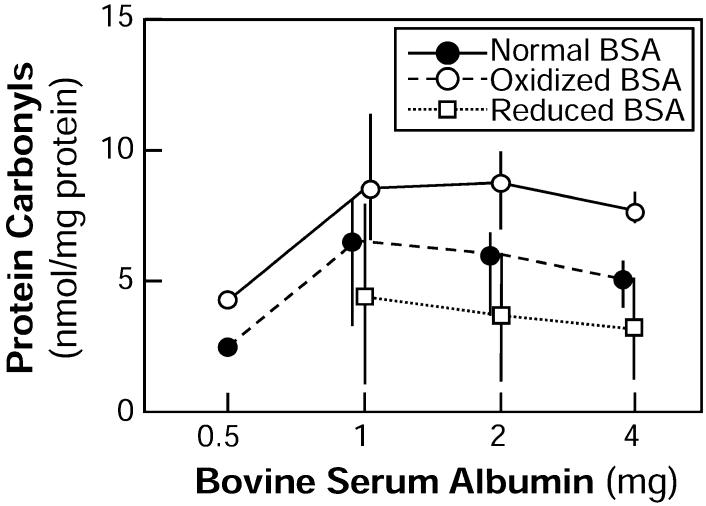
Urinary protein carbonyl content has been used as a marker for oxidative stress, although it is not used as frequently as serum protein carbonyls, urinary 8-IP and urinary 8-OHdG (29, 32, 33). As part of the development of the assay, normal BSA was assayed at different concentrations, and the protein carbonyl levels were shown to be proportional to the amount of protein assayed (Fig. 4). As a further test, the BSA was oxidized in vitro (a positive control), and it was shown that levels of carbonyls increased; it was further shown that the oxidized BSA could be reduced and that this decreased the level of protein carbonyls (Fig. 4). This experiment demonstrated that we could detect and quantify oxidative injury to albumin at levels similar to those found in normal unirradiated rats (i.e., 1.5-3.5 nmol per mg protein; Fig. 6).
FIG. 4.
Assessment of protein carbonyl levels in normal, oxidized and reduced bovine serum albumin as a function of the amount of protein assayed. Data are shown as medians with ranges. There were only single samples analyzed at 0.5 mg; at 1-4 mg, three samples were analyzed for normal and oxidized BSA and two samples for reduced BSA.
FIG. 6.
Time course of urinary excretion of carbonylated proteins, a marker of protein oxidation, after 18.8 Gy fractionated TBI plus BMT. Data are shown for animals receiving TBI and age-matched controls. The lines trace the median values.
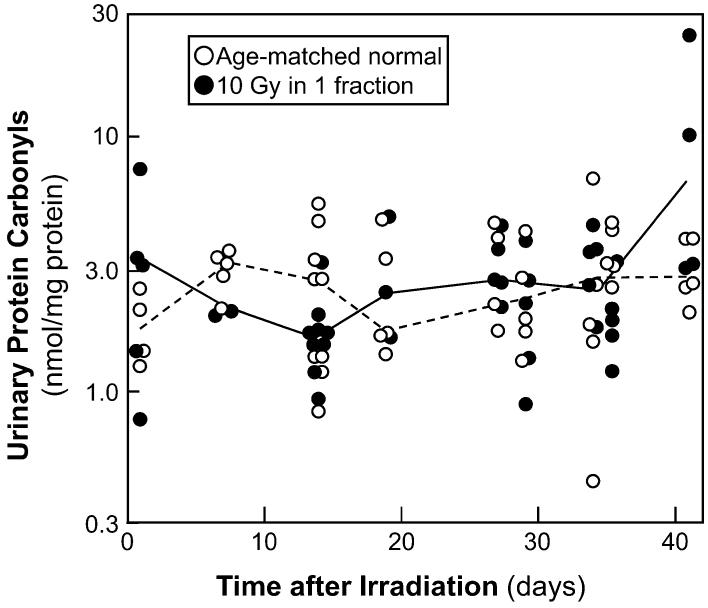
Urinary protein carbonyls were measured in 10 rats (three to seven different times each) between 1 and 42 days after a single 10-Gy dose of radiation and in 10 age-matched normal animals (Fig. 5). For normal animals, there was no significant trend of protein carbonyl levels with time (P = 0.18), but in irradiated animals there were slightly increased levels with time (P = 0.03). In the irradiated animals the median urinary protein carbonyl level was 2.2 (20-80% range 1.6-3.5) nmol per mg protein, whereas in the age-matched controls the levels were 2.6 (1.6-3.9) nmol/mg; the decrease is not statistically significant (P > 0.20). If the individual times are evaluated separately, the ratio of protein carbonyl levels in irradiated compared to age-matched normal animals ranges from 0.9 at 14-35 days to 1.1 at 41 days (all P ≥ 0.14). Note that while overt proteinuria does not begin until 45-50 days after irradiation (Fig. 1), low-level proteinuria is detectable in these animals from 14 to 41 days after irradiation, with UP/UC in the irradiated animals being 1.50 (1.30-2.10) g/g compared to 1.25 (1.10-1.65) g/g in age-matched normal animals (P < 0.005). Western blots (data not shown) indicate that this excess protein is albumin.
FIG. 5.
Time course of urinary excretion of carbonylated proteins, a marker of protein oxidation, after 10 Gy single-fraction TBI plus BMT. Data are shown for animals receiving TBI and age-matched controls. The lines trace the median values.
Renal DNA Oxidation Products after TBI
Immunohistochemical detection of 8-OHdG is a commonly used marker for oxidative stress (12, 29, 34). Renal 8-OHdG staining was analyzed in 20 of the irradiated animals used for the urinary 8-IP and 8-OHdG assays and in 20 age-matched normal animals from those studies; this was supplemented with an additional 16 irradiated and 12 normal animals. All irradiated animals received 18.8 Gy in six fractions, and they and their age-matched controls were analyzed 7 to 89 days later. Essentially all tubular cells from all animals, irradiated and control, stained positive for 8-OHdG, so tubular staining differences could not be analyzed. In glomeruli, cytoplasmic staining was largely absent in all animals. The median fraction of glomerular nuclei that were positive for 8-OHdG was 0.68 (20-80% range 0.39-0.83) in irradiated animals and 0.73 (0.52-0.91) in age-matched controls; this difference is not statistically significant (P < 0.20). If the individual times are evaluated separately, the ratio of glomerular 8-OHdG staining in irradiated compared to age-matched normal animals ranges from 0.6 at 7 days (P = 0.07) to 1.2 at 89 days (P > 0.20).
While the procedures used for the 8-OHdG immunohistochemistry were standard, the nearly ubiquitous staining suggested that the staining might be nonspecific. We have tested a number of modifications of the protocol to address this possibility. In brief, we have tested a different antibody (AB5830, Millipore, Billerica, MA), we have tried a serumfree protein block (X0909, DakoCytomation, Carpenteria, CA), we have verified that we do not have endogenous peroxidase, and we have tried the protocol without blocking avidin and biotin. None of these changes affected the ubiquitous staining pattern. At this point, we do not know whether the 8-OHdG immunohistochemistry results suggest the absence of radiation-induced renal oxidative stress or nonspecificity of the antibody.
Renal Protein Carbonylation after TBI
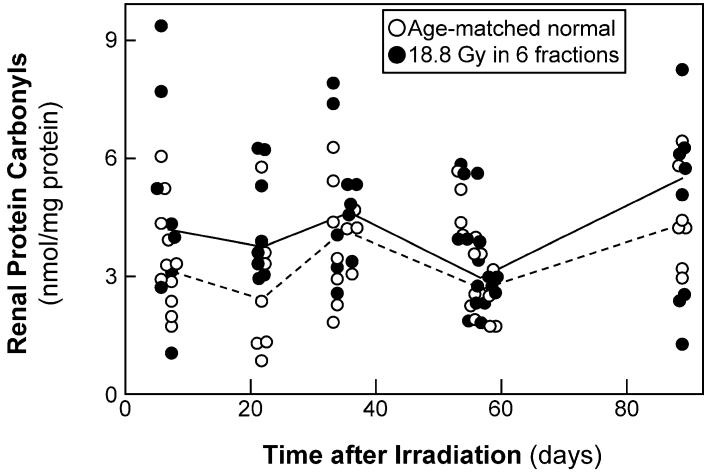
The carbonyl content of renal proteins is a commonly used marker for oxidative stress (29, 35, 36). Renal carbonyl content was analyzed in 24 of the irradiated animals used for the urinary 8-IP and 8-OHdG assays and in 25 age-matched normal animals from those studies; this was supplemented with an additional 26 irradiated and 26 age-matched normal animals. All irradiated animals received 18.8 Gy in six fractions, and they and their age-matched controls were analyzed 6 to 89 days later. There was no trend of renal protein carbonyl levels with time in either irradiated animals or age-matched controls (Fig. 6, both P > 0.20). In the irradiated animals the median renal protein carbonyl level was 3.9 (20-80% range 2.7-5.7) nmol per mg protein, whereas in the age-matched controls the levels were 3.2 (2.3-4.4) nmol/mg; the increase approaches statistical significance (P = 0.06). If the individual times are evaluated separately, the ratio of renal protein carbonyl levels in irradiated compared to age-matched normal animals ranges from 1.6 at 21-22 days (P = 0.06) to 1.1 at 54-57 days P > 0.20). If the data from days 6-22 are pooled, the ratio of renal protein carbonyl levels in irradiated compared to age-matched normal animals is 1.4 (P = 0.038); however, this is a post hoc comparison, and by any method of multiple comparison correction, it is not a statistically significant increase.
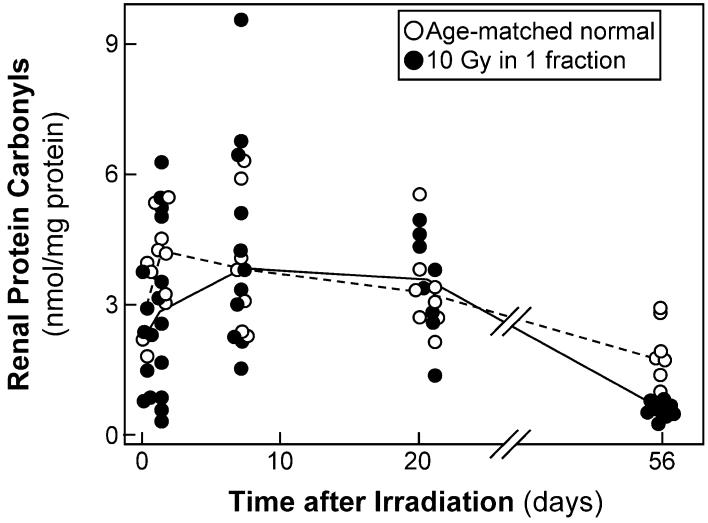
Because these studies suggested that renal protein carbonyl levels might be elevated at short times after irradiation, the study was repeated using a single 10-Gy dose and times ranging from 1 h to 56 days (Fig. 7). Renal carbonyl content was analyzed in 37 irradiated animals and in 33 age-matched normal animals; this included animals used in the urinary protein carbonylation study shown in Fig. 5. In the irradiated animals the median renal protein carbonyl level was 3.3 (20-80% range 1.7-4.9) nmol per mg protein, whereas in the age-matched controls the levels were 3.7 (2.9-4.7) nmol/mg; the difference is not statistically significant (P = 0.10). If the individual times are evaluated separately, the ratio of renal protein carbonyl levels in irradiated compared to age-matched normal animals is never greater than 1.1, and there are no significant differences except at 56 days, where the ratio is 0.50 (P < 0.01). When the two sets are pooled, there is no significant evidence for an increase in renal protein carbonylation after irradiation, although an increase of 30-40% at 7-35 days after irradiation cannot be excluded.
FIG. 7.
Time course of renal carbonylated proteins, a marker of protein oxidation, after 10 Gy single-fraction TBI plus BMT. Data are shown for animals receiving TBI and age-matched controls. The lines trace the median values.
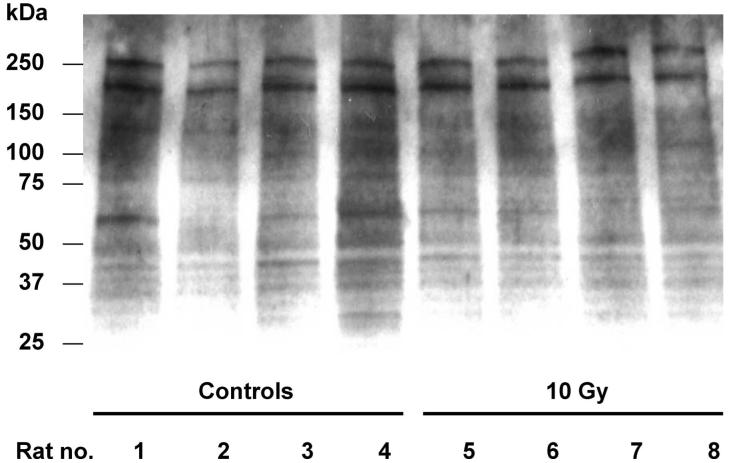
Several hypotheses were developed to explain why no evidence of renal protein oxidation was found in the studies shown in Figs. 6 and 7. First, the freezing of the tissue might have either destroyed carbonyls or created them. Second, the focus on cortex could have missed radiation-induced carbonylation in the medulla, because superoxide production is known to be higher in medulla than in cortex (37, 38), and because increased renal medullary oxidative stress produces hypertension (39). Finally, radiation-induced carbonylation could be restricted to a small subset of proteins. To address these issues, we separately assessed cortex and medulla of fresh kidneys (four animals per group) at 21 days after a single total-body dose of 10 Gy and analyzed the proteins by both the spectrophotometric method and immunoblotting (Fig. 8). As expected, carbonylation levels were higher in the medulla (median of 3.2 nmol per mg protein) than in the cortex (median of 1.5 nmol/mg), but the levels in irradiated medulla (median of 3.4 nmol/mg) were not significantly different from those in the medulla of age-matched controls (median of 2.8 nmol/mg). Carbonylation levels from the fresh cortex (median of 1.9 nmol/mg) were lower than in frozen cortex (3.4 nmol/mg), but the levels in fresh irradiated cortex (median of 1.5 nmol/mg) were not significantly different from those in the fresh cortex of age-matched controls (median of 1.9 nmol/mg). The immunoblots (Fig. 8) showed no reproducible differences between the irradiated animals and their age-matched controls. Additional immunoblots were done on a subset of the samples shown in Fig. 6 (8 to 89 days, n = 14) and Fig. 7 (4 h to 56 days, n = 8); again, no reproducible differences were seen between the irradiated animals and their age-matched controls (these immunoblots not shown).
FIG. 8.
A representative Western blot showing detection of carbonylated renal proteins. The samples used to prepare the homogenates were collected at 21 days after 10 Gy single-dose TBI plus BMT (Lanes 5-8). Age-matched sham-irradiated rats served as controls (Lanes 1-4). Proteins (10 μg/lane) were resolved by SDS-PAGE (4-20%) and transferred to PVDF membrane, derivatized with DNPH on the membrane, and probed with anti-DNP antibodies.
DISCUSSION
It has been hypothesized that chronic oxidative stress plays a role in the pathogenesis of late radiation injuries (7, 8, 12, 13, 40). More specifically, it has been proposed that the benefit of RAS antagonism in mitigation and treatment of radiation nephropathy (1-6) occurred because ACE inhibitors or AII blockers reduced oxidative injury (7, 8). However, the present data do not make a strong case for a role for chronic oxidative stress in experimental radiation nephropathy. Of five standard methods (29, 32, 41) for detecting oxidative stress in vivo that were assessed, four (urinary lipid oxidation products, urinary and renal protein oxidation products, and renal DNA oxidation products) found no evidence for radiation-induced oxidative stress, and one (urinary DNA oxidation products) found only slight evidence.
In testing the hypothesis that chronic oxidative injury plays a causative role in radiation nephropathy, we considered what to test for and when to do that testing. For the former, there are in vitro and ex vivo techniques for measuring ROS directly (e.g., using spin traps), but these are problematic because their use might perturb the tissue under study or in themselves might act against damage (29, 32). One could test for reactive changes in antioxidant defense compounds [(e.g., the dichlorofluorescein assay (42)], but either increases or decreases in such compounds could occur with chronic oxidative injury, with a true mechanism being obscured by countervailing phenomena. Finally, one could test for the products of oxidative injury, namely oxidatively modified lipids (27-29, 32, 41, 43), proteins (25, 26, 29, 32, 33, 35, 36, 41), or DNA (12, 29-32, 34, 41, 43). We chose the latter approach and chose to assess all three classes of oxidized products because there are reported circumstances where some classes of oxidized products have been elevated and others not (29, 33, 43).
Oxidatively modified lipids and proteins were not significantly increased in the urine in the first 42 days after TBI (Figs. 2 and 5). It is possible that longer follow-up would have found excess levels of oxidatively modified lipids and proteins, but since the animals were already displaying physiological evidence of renal injury by 42 days (Fig. 1), such late increases could be caused by the renal injury (21, 22) rather than being part of the pathogenesis of the injury.
The renal carbonylated protein assay showed a baseline amount of carbonylated protein that is consistent with levels of carbonylated proteins reported by others in vitro (44) and in tissue specimens (45). These levels did not change in the first 84 days after TBI (Figs. 6-8). It is possible that protein oxidation would occur at higher radiation doses, but since the radiation doses used here are sufficient to produce renal failure, effects occurring only at higher doses would not imply a role in the pathogenesis of the injury. It is also possible that oxidative modification of proteins would lead to an increase in the rate of catabolism of these proteins (46), and this might then obscure enhanced production of oxidized proteins; however, such a mechanism remains speculative.
The small increase of urinary 8-OHdG that we found (Fig. 3) was not accompanied by an increase in the immunohistochemical staining for 8-OHdG in the irradiated kidney. It is possible that the urinary 8-OHdG derives from oxidation products produced in organs other than the kidneys, and that these are filtered by the kidneys and thus appear in the urine. It is also possible that the OHdG immunostaining is nonspecific or that the small increase in urinary OHdG (by a factor of 1.3) is a multiple-comparison artifact. Direct assessment of oxidation of renal mitochondrial and nuclear DNA will be required to resolve these issues, and such studies are in progress.
In a cisplatinum model of acute renal failure (47), the urinary markers of lipid and DNA oxidative stress were elevated, but the elevations occurred at non-overlapping times. By analogy, it is possible that testing for markers of radiation-induced oxidative stress may yield negative results if testing is done at the wrong time. In the present studies, however, the markers of oxidative stress were assessed at frequent intervals between the time of irradiation and the development of clear-cut renal injury, which is the “interval” time during which causally relevant oxidative stress should be occurring. It is possible that earlier times (i.e., minutes after irradiation) could be tested, but any evidence for oxidative stress immediately after irradiation could be the residue of the irradiation itself rather than evidence for persistent chronic oxidative stress.
It is worth noting that even if there were enhanced oxidative stress in this model, antioxidant treatments may not be beneficial (48). For example, Prabhakar et al. (49) have recently reported that in experimental diabetic nephropathy, where there is both intrarenal and urinary evidence for oxidative stress, the antioxidant alpha-lipoic acid was not successful in slowing the progression of renal failure.
Taken together, our data do not show direct evidence for chronic oxidative stress in radiation nephropathy and little evidence for chronic oxidative stress after TBI. In fact, standard assays for oxidative stress yielded good evidence for the absence of significant excess levels of oxidatively modified protein, DNA and lipids after irradiation. We cannot, of course, prove that radiation-induced chronic oxidative stress has no role in the pathogenesis of renal radiation injury, because there is no “gold standard” for measuring such injury (29, 41) and because a negative cannot be proven. What we have shown is that if chronic oxidative stress is part of the pathogenesis of chronic radiation-induced renal injury, it does not leave widespread or easily detectable evidence behind.
ACKNOWLEDGMENTS
These studies were supported by an NIH grant (CA-24652) and an NIH cooperative agreement (AI-67734). Yvonne A. Morauski assisted with preparation of the manuscript. Marylou Mäder, Jaya Bhojok and Ruchi Singhal provided expert technical assistance. David Ramenofsky and Marcus Crosby, MCW medical students, assisted with the initial development of the 8-IP assay and protein carbonylation assays, respectively.
REFERENCES
- 1.Cohen EP, Irving AA, Drobyski WR, Klein JP, Passweg J, Talano J, Juckett M, Moulder JE. Captopril to mitigate chronic renal failure after hematopoietic stem cell transplantation: a randomized controlled trial. Int. J. Radiat. Oncol. Biol. Phys. 2008;70:1546–1551. doi: 10.1016/j.ijrobp.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oikawa T, Freeman M, Lo W, Vaughan DE, Fogo A. Modulation of plasminogen activator inhibitor-1 in vivo: A new mechanism for the anti-fibrotic effect of renin-angiotensin inhibition. Kidney Int. 1997;51:164–172. doi: 10.1038/ki.1997.20. [DOI] [PubMed] [Google Scholar]
- 3.Juncos LI, Carrasco Dueñas S, Cornejo JC, Broglia CA, Cejas H. Long-term enalapril and hydrochlorothiazide in radiation nephritis. Nephron. 1993;64:249–255. doi: 10.1159/000187322. [DOI] [PubMed] [Google Scholar]
- 4.Moulder JE, Fish BL, Cohen EP. ACE inhibitors and AII receptor antagonists in the treatment and prevention of bone marrow transplant nephropathy. Curr. Pharm. Design. 2003;9:737–749. doi: 10.2174/1381612033455422. [DOI] [PubMed] [Google Scholar]
- 5.Moulder JE, Fish BL, Cohen EP. Treatment of radiation nephropathy with ACE inhibitors and AII type-1 and type-2 receptor antagonists. Curr. Pharm. Design. 2007;13:1317–1325. doi: 10.2174/138161207780618821. [DOI] [PubMed] [Google Scholar]
- 6.Moulder JE, Cohen EP. Future strategies for mitigation and treatment of chronic radiation-induced normal tissue injury. Semin. Radiat. Oncol. 2007;17:141–148. doi: 10.1016/j.semradonc.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Robbins ME, Diz DI. Pathogenic role of the renin-angiotensin system in modulating radiation-induced late effects. Int. J. Radiat. Oncol. Biol. Phys. 2006;64:6–12. doi: 10.1016/j.ijrobp.2005.08.033. [DOI] [PubMed] [Google Scholar]
- 8.Zhao W, Diz DI, Robbins ME. Oxidative damage pathways in relation to normal tissue injury. Br. J. Radiol. 2007;80:S23–S31. doi: 10.1259/bjr/18237646. [DOI] [PubMed] [Google Scholar]
- 9.Kumagai J, Masui K, Itagaki Y, Shiotani M, Kodama S, Watanabe M, Miyazaki T. Long-lived mutagenic radicals induced in mammalian cells by ionizing radiation are mainly localized to proteins. Radiat. Res. 2003;160:95–102. doi: 10.1667/rr3015. [DOI] [PubMed] [Google Scholar]
- 10.Tominaga H, Kodama S, Matsuda N, Suzuki K, Watanabe M. Involvement of reactive oxygen species (ROS) in the induction of genetic instability by radiation. J. Radiat. Res. (Tokyo) 2004;45:181–188. doi: 10.1269/jrr.45.181. [DOI] [PubMed] [Google Scholar]
- 11.Wang ZB, Liu YQ, Zhang Y, Li Y, An XX, Xu H, Guo Y, Jin W, Jiang ZJ, Cui YF. Reactive oxygen species, but not mitochondrial membrane potential, is associated with radiation-induced apoptosis of AHH-1 human lymphoblastoid cells. Cell Biol. Int. 2007;31:1353–1358. doi: 10.1016/j.cellbi.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 12.Robbins MEC, Zhao WL, Davis CS, Toyokuni S, Bonsib SM. Radiation-induced kidney injury: a role for chronic oxidative stress? Micron. 2002;33:133–141. doi: 10.1016/s0968-4328(01)00006-3. [DOI] [PubMed] [Google Scholar]
- 13.Datta PK, Moulder JE, Fish BL, Cohen EP, Lianos EA. Induction of heme oxygenase-1 in radiation nephropathy: Role of angiotensin. Radiat. Res. 2001;155:734–739. doi: 10.1667/0033-7587(2001)155[0734:iohoir]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 14.Abraham NG, Kappas A. Heme oxygenase and the cardiovascular-renal system. Free Radic. Biol. Med. 2005;39:1–25. doi: 10.1016/j.freeradbiomed.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Moulder JE, Fish BL. Late toxicity of total body irradiation with bone marrow transplantation in a rat model. Int. J. Radiat. Oncol. Biol. Phys. 1989;16:1501–1509. doi: 10.1016/0360-3016(89)90955-3. [DOI] [PubMed] [Google Scholar]
- 16.Cohen EP, Fish BL, Moulder JE. The renin-angiotensin system in experimental radiation nephropathy. J. Lab. Clin. Med. 2002;139:251–257. doi: 10.1067/mlc.2002.122279. [DOI] [PubMed] [Google Scholar]
- 17.Cohen EP, Fish BL, Sharma M, Li XA, Moulder JE. The role of the angiotensin II type-2 receptor in radiation nephropathy. Transl. Res. 2007;150:106–115. doi: 10.1016/j.trsl.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moulder JE. Post-irradiation approaches to treatment of radiation injuries in the context of radiological terrorism and radiation accidents: a review. Int. J. Radiat. Biol. 2004;80:3–10. doi: 10.1080/09553000310001642920. [DOI] [PubMed] [Google Scholar]
- 19.Moulder JE, Fish BL, Cohen EP. Brief pharmacologic intervention in experimental radiation nephropathy. Radiat. Res. 1998;150:535–541. [PubMed] [Google Scholar]
- 20.Cohen EP, Fish BL, Moulder JE. Successful brief captopril treatment in radiation nephropathy. J. Lab. Clin. Med. 1997;129:536–547. doi: 10.1016/s0022-2143(97)90008-1. [DOI] [PubMed] [Google Scholar]
- 21.Witko-Sarsat V, Friedlander M, Capeillère-Blandin C, Nguyen-Khoa T, Nguyen AT, Zingraff J, Jungers P, Descamps-Latscha B. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996;49:1304–1313. doi: 10.1038/ki.1996.186. [DOI] [PubMed] [Google Scholar]
- 22.Lim PS, Ma YS, Cheng YM, Chai H, Lee CF, Chen TL, Wei YH. Mitochondrial DNA mutations and oxidative damage in skeletal muscle of patients with chronic uremia. J. Biomed. Sci. 2002;9:549–560. doi: 10.1159/000064728. [DOI] [PubMed] [Google Scholar]
- 23.Cohen EP, Moulder JE, Fish BL, Hill P. Prophylaxis of experimental bone marrow transplant nephropathy. J. Lab. Clin. Med. 1994;124:371–380. [PubMed] [Google Scholar]
- 24.Shimoike T, Inoguchi T, Umeda F, Nawata H, Kawano K, Ochi H. The meaning of serum levels of advanced glycosylation end products in diabetic nephropathy. Metab. Clin. Exp. 2000;49:1030–1035. doi: 10.1053/meta.2000.7738. [DOI] [PubMed] [Google Scholar]
- 25.Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn BW, Shaltiel S, Stadtman ER. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–478. doi: 10.1016/0076-6879(90)86141-h. [DOI] [PubMed] [Google Scholar]
- 26.Levine RL, Wehr N, Williams JA, Stadtman ER, Shacter E. Determination of carbonyl groups in oxidized proteins. Methods Mol. Biol. 2000;99:15–24. doi: 10.1385/1-59259-054-3:15. [DOI] [PubMed] [Google Scholar]
- 27.Suzaki Y, Ozawa Y, Kobori H. Intrarenal oxidative stress and augmented angiotensinogen are precedent to renal injury in Zucker diabetic fatty rats. Int. J. Biol. Sci. 2007;3:40–46. doi: 10.7150/ijbs.3.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang YF, Chen AF, Wang DH. Enhanced oxidative stress in kidneys of salt-sensitive hypertension: Role of sensory nerves. Am. J. Physiol. 2006;291:H3136–H3143. doi: 10.1152/ajpheart.00529.2006. [DOI] [PubMed] [Google Scholar]
- 29.Dalle-Donne I, Rossi R, Colombo R, Giustarini D, Milzani A. Biomarkers of oxidative damage in human disease. Clin. Chem. 2006;52:601–623. doi: 10.1373/clinchem.2005.061408. [DOI] [PubMed] [Google Scholar]
- 30.Hirai T, Okumura K, Nishimoto Y, Shumiya T, Murakami R, Takahashi R, Asai T, Murakami H, Numaguchi Y, Murohara T. Upregulation of renal eNOS by high-sodium diet facilitates hypertension in doxorubicin-treated rats through enhanced oxidative stress. Toxicology. 2006;225:81–89. doi: 10.1016/j.tox.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 31.Tsukahara H, Hiraoka M, Kobata R, Hata I, Ohshima Y, Jiang MZ, Noiri E, Mayumi M. Increased oxidative stress in rats with chronic nitric oxide depletion: measurement of urinary 8-hydroxy-2′-deoxyguanosine excretion. Redox Rep. 2000;5:23–28. doi: 10.1179/rer.2000.5.1.23. [DOI] [PubMed] [Google Scholar]
- 32.Halliwell B, Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br. J. Pharmacol. 2004;142:231–255. doi: 10.1038/sj.bjp.0705776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kadiiska MB, Gladen BC, Baird DD, Germolec D, Graham LB, Parker CE, Nyska A, Wachsman JT, Ames BN, Barrett JC. Biomarkers of oxidative stress study II: are oxidation products of lipids, proteins, and DNA markers of CCl4 poisoning? Free Radic. Biol. Med. 2005;38:698–710. doi: 10.1016/j.freeradbiomed.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 34.Frank J, Biesalski HK, Dominici S, Pompella A. The visualization of oxidant stress in tissues and isolated cells. Histol. Histopathol. 2000;15:173–184. doi: 10.14670/HH-15.173. [DOI] [PubMed] [Google Scholar]
- 35.Haugen EN, Croatt AJ, Nath KA. Angiotensin II induces renal oxidant stress in vivo and heme oxygenase-1 in vivo and in vitro. Kidney Int. 2000;58:144–152. doi: 10.1046/j.1523-1755.2000.00150.x. [DOI] [PubMed] [Google Scholar]
- 36.Santos NAG, Catão CS, Martins NM, Curti C, Bianchi MLP, Santos AC. Cisplatin-induced nephrotoxicity is associated with oxidative stress, redox state unbalance, impairment of energetic metabolism and apoptosis in rat kidney mitochondria. Arch. Toxicol. 2007;81:495–504. doi: 10.1007/s00204-006-0173-2. [DOI] [PubMed] [Google Scholar]
- 37.Zou AP, Li N, Cowley AW. Production and actions of superoxide in the renal medulla. Hypertension. 2001;37:547–553. doi: 10.1161/01.hyp.37.2.547. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Z, Dmitrieva NI, Park JH, Levine RL, Burg MB. High urea and NaCl carbonylate proteins in renal cells in culture and in vivo, and high urea causes 8-oxoguanine lesions in their DNA. Proc. Natl. Acad. Sci. USA. 2004;101:9491–9496. doi: 10.1073/pnas.0402961101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Makino A, Skelton MM, Zou AP, Roman RJ, Cowley AW. Increased renal medullary oxidative stress produces hypertension. Hypertension. 2002;39:667–672. doi: 10.1161/hy0202.103469. [DOI] [PubMed] [Google Scholar]
- 40.Robbins MEC, Zhao W. Chronic oxidative stress and radiation-induced late normal tissue injury: A review. Int. J. Radiat. Biol. 2004;80:251–259. doi: 10.1080/09553000410001692726. [DOI] [PubMed] [Google Scholar]
- 41.Grune T, Berger MM. Markers of oxidative stress in ICU clinical settings: present and future. Cur. Opin. Clin. Nutr. Metab. Care. 2007;10:712–717. doi: 10.1097/MCO.0b013e3282f0c97c. [DOI] [PubMed] [Google Scholar]
- 42.Wan XS, Zhou Z, Ware JH, Kennedy AR. Standardization of a fluorometric assay for measuring oxidative stress in irradiated cells. Radiat. Res. 2005;163:232–240. doi: 10.1667/rr3299. [DOI] [PubMed] [Google Scholar]
- 43.England T, Beatty E, Rehman A, Nourooz-Zadeh J, Pereira P, O’Reilly J, Wiseman H, Geissler C, Halliwell B. The steady-state levels of oxidative DNA damage and of lipid peroxidation (F-2-isoprostanes) are not correlated in healthy human subjects. Free Radic. Res. 2000;32:355–362. doi: 10.1080/10715760000300351. [DOI] [PubMed] [Google Scholar]
- 44.Lee MH, Hyun DH, Jenner P, Halliwell B. Effect of proteasome inhibition on cellular oxidative damage, antioxidant defences and nitric oxide production. J. Neurochem. 2001;78:32–41. doi: 10.1046/j.1471-4159.2001.00416.x. [DOI] [PubMed] [Google Scholar]
- 45.Mecocci P, Fanó G, Fulle S, MacGarvey U, Shinobu L, Polidori MC, Cherubini A, Vecchiet J, Senin U, Beal MF. Age-dependent increases in oxidative damage to DNA, lipids, and proteins in human skeletal muscle. Free Radic. Biol. Med. 1999;26:303–308. doi: 10.1016/s0891-5849(98)00208-1. [DOI] [PubMed] [Google Scholar]
- 46.Grune T, Reinheckel T, Davies KJ. Degradation of oxidized proteins in mammalian cells. FASEB J. 1997;11:526–534. [PubMed] [Google Scholar]
- 47.Zhou H, Kato A, Miyaji T, Yasuda H, Fujigaki Y, Yamamoto T, Yonemura K, Takebayashi S, Mineta H, Hishida A. Urinary marker for oxidative stress in kidneys in cisplatin-induced acute renal failure in rats. Nephrol. Dial. Transplant. 2006;21:616–623. doi: 10.1093/ndt/gfi314. [DOI] [PubMed] [Google Scholar]
- 48.Aldini G, Dalle-Donne I, Facino RM, Milzani A, Carini M. Intervention strategies to inhibit protein carbonylation by lipoxidation-derived reactive carbonyls. Med. Res. Rev. 2007;27:817–868. doi: 10.1002/med.20073. [DOI] [PubMed] [Google Scholar]
- 49.Prabhakar S, Starnes J, Shi S, Lonis B, Tran R. Diabetic nephropathy is associated with oxidative stress and decreased renal nitric oxide production. J. Am. Soc. Nephrol. 2007;18:2945–2952. doi: 10.1681/ASN.2006080895. [DOI] [PubMed] [Google Scholar]