Abstract
Optimal elution modes were determined for four typical two-phase solvent systems each with different physical parameters to achieve the best peak resolution and retention of the stationary phase by spiral tube high-speed countercurrent chromatography using a suitable set of test samples. Both retention of the stationary phase and partition efficiency are governed by an interplay between two forces, i.e., Archimedean Screw force and radial centrifugal force gradient of the spiral channel. In the polar solvent system represented by 1-butanol./acetic acid/water (4:1:5, v/v/v) with settling time of over 30 s, the effect by the radial centrifugal gradient force dominates giving the best separation of dipeptides either by pumping the lower phase from the inner terminal or the upper phase from the outer terminal of the spiral channel. In the moderately hydrophobic two-phase solvent system represented by hexane/ethyl acetate/methanol/0.1 M HCl (1:1:1:1) with settling time of 19 s, and two hydrophobic solvent systems of hexane/ethanol/water (5:4:1, v/v/v) and non-aqueous binary system of hexane/acetonitrile both having settling time of 9, the effect of the Archimedean screw force play a major role in hydrodynamic equilibrium, giving the best separations by pumping the lower phase from the head or the upper phase from the tail of the spiral channel.
Keywords: Spiral tube assembly, high-speed countercurrent chromatography, two-phase solvent systems, elution modes
INTRODUCTION
High-speed countercurrent chromatography (HSCCC) has been successfully performed with a multilayer coil separation column with a variety of two-phase solvent systems for separation for natural products [1]. When it was applied to highly polar solvent two-phase systems, however, it often failed to retain a sufficient amount of the stationary phase. In order to improve the stationary phase retention, the spiral disk assembly has been introduced. It consists of multiple layers of plastic disks with spiral channels, and by utilizing a radially acting centrifugal force it is able to retain a sufficient amount of the stationary phase of highly polar solvent systems such as polymer phase systems[2-5]. The system requires precise machining of each spiral disk to prevent leakage of the solvents.
More recently, a spiral tube support is introduced, which can accommodate multiple spiral layers made from a long, single piece of fluorinated plastic tubing hence avoiding a risk of leakage of solvent through the junction. The performance of this spiral tube assembly has been reported in separation of dipeptides and proteins with polar two-phase solvent systems [6,7].
In the present study, retention of the stationary phase and partition efficiency of typical two-phase solvent systems with a broad spectrum in hydrophobicity using a suitable set of test samples for each solvent system (Table 1).
Table 1.
Two-phase solvent systems and test samples
| Two-phase solvent systems* (volume ratio) | Settling time (s) | Test samples (weight) | K (UP/LP)** |
|---|---|---|---|
| 1-BuOH/AcOH/H2O (4:1:5) | 25 | Trp-Trp (1 mg) | 4.55 |
| Trp-Tyr (1 mg) | 1.69 | ||
| Leu-Tyr (4 mg) | 0.85 | ||
| Val-Tyr (4 mg) | 0.53 | ||
| Tyr-Gly (4 mg) | 0.31 | ||
| Hexane/EtOAc/MeOH/0.1M HCl (1:1:1:1) | 19 | DNP-L-Ala (0.5 mg) | 2.36 |
| DNP-β-ala (0.5 mg) | 1.18 | ||
| DNP-L-glu (0.5 mg) | 0.44 | ||
| Hexane/EtOH/H2O (5:4:1) | 9 | Sudan II (1 mg) | 2.28 |
| Sudan I (1 mg) | 1.95 | ||
| Hexane/acetonitrile | 9 | Sudan II (1 mg) | 0.77 |
| Sudan I (1 mg) | 0.39 |
BuOH: buthanol; AcOH: acetic acid; EtOAc: ethyl acetate; MeOH: methanol; EtOH: ethanol; H2O: water
K: partition coefficient; UP: upper phase; LP: lower phase
EXPERIMENTAL
Apparatus
The type-J coil planet centrifuge used in the present studies was purchased from P.C. Inc. (Model: Ito Separator/Extractor, Potomac, MD, USA). It holds a coil holder on one side and a counterweight on the other side in symmetrical positions at a distance of 10 cm from the central axis of the centrifuge. The separation column was made from a spiral tube support fabricated in the NIH machine shop, Bethesda, Maryland, USA. It is made of an aluminum disk about 16 cm in diameter and 6 cm wide. This spiral tube support contains 4 spiral grooves of 5 cm deep which are connected in series with radial grooves as described earlier [6]. A piece of PTFE (polytetrafluoroethylene) tubing of 1.6mm ID (Zeus Industrial Products, Orangeburg, SC, USA) was first pressed with pliers perpendicularly at every 1 cm intervals to interrupt laminar flow of the mobile phase which leads to longitudinal spreading of sample band [7]. This cross-pressed tubing was then inserted into the spiral grooves making 12 spiral layers each consisting of serially connected 4 identical spiral tubing with a total capacity of about 100 ml. During the column preparation, the spiral tube assembly was pressed several times with a special tool with 2 or 4 protrusions which fit into the radial grooves accommodating transfer tubing. This process improves the column efficiency by not only increasing the number of spiral layers accommodated in the tube support, but also reducing the dead space in the transfer tubes, The spiral tube assembly was finally embedded in polyethylene glycol (MW 3350) (Sigma, St. Louis, MO) which would protect tubing damage caused by vibration under fluctuating centrifugal force field, The above modified tubing configuration has been proven to improve the separation of proteins with a PEG (polyethylene glycol)/phosphate polymer phase system [8]. Each terminal of the spiral tube assembly was connected to a flow tube of 0.7 mm ID PTFE tubing (HW 22) (Zeus Industrial product). Both feed and return flow tubes were passed through the column holder shaft downward and making an arch through the hollow central shaft of the centrifuge upward to exit the apparatus at the center of the ceiling plate where they are tightly fixed with a pair of clamps. The rotation speed of the column was regulated with a speed controller (Bodine, Chicago, IL, USA).
The mobile phase was pumped with a metering pump (Waters, Milford, MA, USA) and the effluent was continuously monitored with a uv detector (Uvicord SII, LKB Instruments, Stockholm, Sweden) to record the elution profile with a strip-chart recorder (Pharmacia LKB REC102, Pharmacia, Stockholm, Sweden).
Reagents
Organic solvents such as hexane, ethyl acetate, methanol, acetonitrile and 1-butanol were purchased from Fisher Scientific (Fair field, NJ, USA). Acetic acid, hydrochloric acid and ethanol were obtained from Mallinckrodt Baker (Phillipsburg, NJ, USA). Test samples including, tryptophyl-tryptophan (Trp-Trp), tryptophyl-tyrosine (Trp-Tyr), leucyl-tyrosine (Leu-Tyr), valyl-tyrosine (Val-Tyr), tyrosyl-glycine (Tyr-Gly), N-dinitrophenyl—L—glutamic acid (DNP-glu), N-dinitrophenyl-L-alanine (DNP-ala), N-dinitrophenyl-ß-alanine (DNP-ß-ala), Sudan I and Sudan II were all purchased from Sigma (St. Louis, MO, USA).
Two-Phase Solvent Systems and Sample Solutions
In the present study, four typical two-phase solvent systems covering a broad spectrum of hydrophobicity were examined each with suitable test samples. The composition of these two-phase solvent systems, their settling times, and test samples with partition coefficient values (K) are listed in Table 1.
Each solvent system was thoroughly mixed in a separatory funnel at room temperature. The sample solution was prepared by dissolving the sample mixture in the upper phase used for separation.
Partition Coefficient Measurement
Partition coefficients (KUP/LP) of each sample in the two-phase solvent system was determined using the conventional test tube method using a uv spectrophotometer (Genesis 10 uv, Thermo Spectronic, Rochester, NY, USA) at 280 nm as described elsewhere [6]. All K values are given in Table 1.
Settling Time Measurement
The settling time is an important parameter to determine the proper elution mode since it gives a high correlation with the retention of the stationary phase [9]. It is determined as follows: Thoroughly equilibrate the two-phase solvent system in a separatory funnel. Deliver 2 ml of each phase into a 5 ml-capacity graduated cylinder or a test tube (13 mm OD and 10 cm long). After capping, gently invert the container 5 times and keep it an upright position to measure the time required to form clear two phases. The measurement should be repeated 5 times to get the average value.
HSCCC Separation Procedure
In each separation, the separation column was filled with the stationary phase, either upper or lower phase, followed by injection of the sample solution. Then the column is rotated at a given revolution speed (800 or 1000 rpm) while the column was eluted with the mobile phase at a specified flow rate (2 ml, 3 ml or 5 ml/min). The effluent from the outlet of the column was monitored with a uv detector (Uvicord s, LKB Instruments, Stockholm, Sweden and the chromatogram was recorded with a strip-chart recorder. In order to improve the tracing, ethanol was mixed to the effluent at a volume ratio of 1 : 5 at the inlet of the detector using a tee connector and a fine mixing tube (PTFE 0.4 mm ID X ca 1 m). After the desired peaks were eluted, the run was stopped and the column contents were collected into a graduated cylinder by pressured air to determine the volume of the stationary phase retained in the column. The retention of the stationary phase was computed by dividing the volume of the retained stationary phase with the total column volume (100 ml).
Measurement of Partition Efficiency
The partition efficiency of separation column in each run was evaluated by computing theoretical plate number (TP or N) for each peak and peak resolution (Rs) between the peaks using the following conventional equations:
| (1) |
| (2) |
Where R denotes the elution time and W, the peak width at the base. The subscript number in Eq 2 indicates specified peak.
RESULTS AND DISCUSSION
A series of experiments was performed to evaluate the performance of the spiral tube assembly by stationary phase retention and partition efficiency in terms of theoretical plate number (TP or N) and peak resolution (RS) in 4 different two-phase solvent systems with a suitable set of test samples listed in Table 1.
In the spiral column, there are 4 different elution modes for each mobile phase, and therefore a total of 8 different modes as follows;
LIH (Lower phase introduced from Inner Head terminal of the spiral channel) ;
LIT (Lower phase introduced from Inner Tail terminal of the spiral channel);
LOH (Lower phase introduced from Outer Head terminal of the spiral channel);
LOT ((Lower phase introduced from Outer Tail terminal of the spiral channel);
UOT (Upper phase introduced from Outer Tail terminal of the spiral channel:;
UOH (Upper phase introduced from Outer Head terminal of the spiral channel:;
UIT (Upper phase introduced from Inner Tail terminal of the spiral channel);
UIH (Upper phase introduced from inner Head terminal of the spiral channel).
Among those LOH, LOT, UIT and UIH are the elution modes in which the mobile phase flows against the centrifugal force gradient effect formed by the spiral channel configuration, and therefore they are expected to retain much less stationary phase than their counterparts, LIH, LIT, UOT and UOH, which have a beneficial centrifugal gradient effect for the retention of the stationary phase. For example, LIH and LIT would produce higher retention of the stationary phase than LOH and LOT, respectively.
On the other hand, the Archimedean screw effect expressed by Head and Tail elution modes also can play an important role in the retention of the stationary phase. As described earlier [9], this effect on stationary phase retention is dependent on the physical parameter of the two-phase solvent system which is represented by its settling time of the two phases in a test tube. When the settling time is less than 25 sec, upper phase is distributed toward the head of the coil, and therefore lower phase should be introduced from the head of the coil, and the upper phase from the tail of the coil. However, if the settling time exceeds 25 sec, this effect is reversed and the lower phase should be introduced from the tail and the upper phase from the head of the coil.
In the spiral tube assembly column, the retention of the stationary phase is determined by the interplay between these forces. Consequently, for the best retention and peak resolution, the suitable elution mode of mobile phase should be chosen according to the physical properties of the solvent system.
Below, the results of experiments for performance of the spiral tube assembly may be presented in the order of the polarity of the two-phase solvent system.
1-Butanol/Acetic Acid/Water (4:1:5)
This solvent system has a high polarity represented by low interfacial tension (< 1 dyne/cm), relatively high viscosity (1.63/1.40 c.p.), and small difference in density between two phases (0.05 g/cm3) with settling time of over 30 seconds, and is not well retained in the conventional multilayer separation coil in type-J coil planet centrifuge. However, it is very useful for separation of polar compounds such as peptides and glycosides. The retention and partition efficiency obtained from this solvent system has been reported using a spiral tube assembly made from the same size of tubing but without cross-pressed treatment [6].
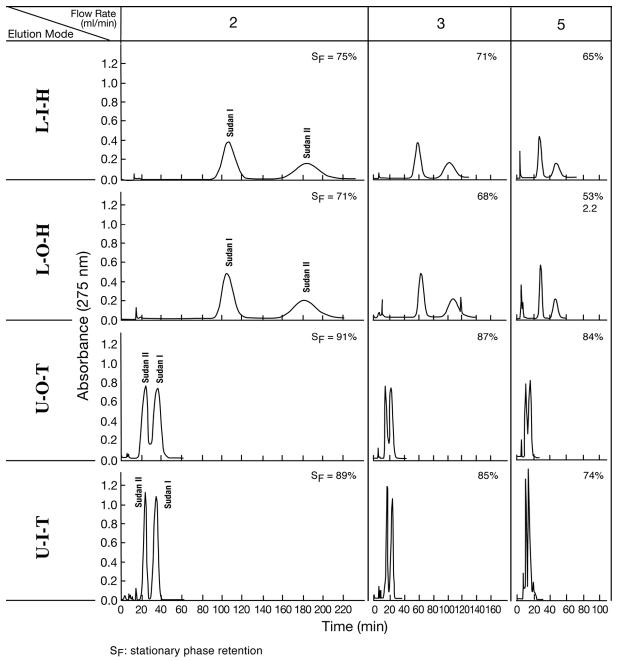
The results of the experiments obtained from this solvent system using the present spiral tube assembly (cross-pressed, radial groove compressed and embedded in PEG 3350) is summarized in Fig. 1 where a set of chromatograms are arranged according to the elution mode (column) and the flow rates (row). The data from elution modes including LOH, LOT, UIH, and UIT are eliminated due to low stationary phase retention and poor peak resolution. Five dipeptide samples are separated while in LIH and LIT, the 5th sample (Trp-Trp) were still retained in the column. The names of test samples and their K values are listed in Table 1.
Fig. 1.
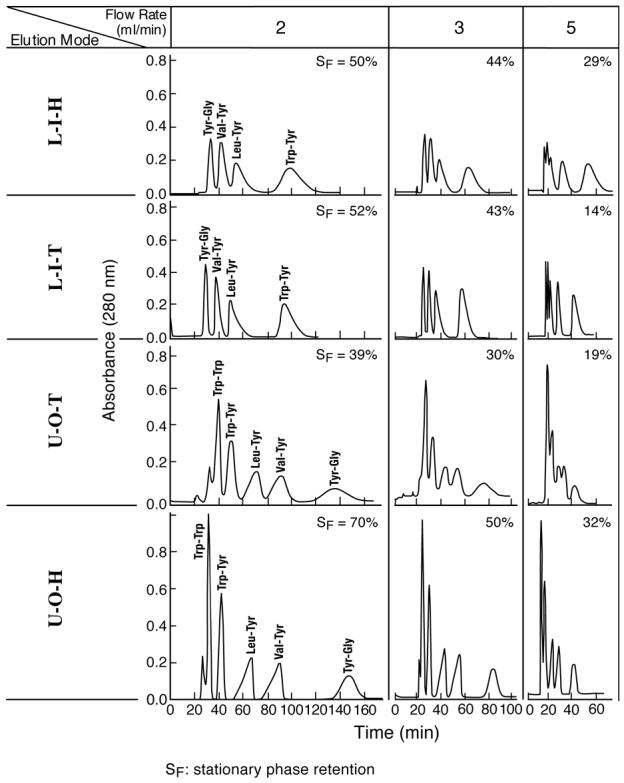
Dipeptide separation with a polar two-phase solvent system by improved spiral tube assembly. Apparatus: Type-J coil planet centrifuge with 10 cm revolution radius; spiral tube assembly: cross-pressed, radial-groove compressed, and embedded in PEG3500 with 12 spiral layers of 1.6 mm I.D. PTFE (polytetrafluoroethylene) tubing with a total capacity of 100 ml. Each spiral layer consists of 4 spiral channels with a 1.6 cm spiral pitch; solvent system: 1-butanol/acetic acid/water (4:1:5, v/v/v); samples: Trp-Trp (1 mg), Trp-Tyr (1 mg), Leu-tyr (4 mg), Val-Tyr (4 mg) and Gly-Tyr (4 mg) in l ml of upper phase; Revolution: 1000 rpm; detection: 280 nm; SF: retention of stationary phase. Symbols for elution modes are defined in the text.
The results clearly show that the elution modes of LIT and UOH yields higher peak resolution than their respective counterparts, LIH and UOT. The partition efficiency in terms of theoretical plate number (TP) and peak resolution (Rs) are listed in Table 2 together with the % retention of the stationary phase. The RS value between Trp-Tyr and Val-Tyr obtained by the UOH elution mode (Rs = 3.98) exceeds that of the spiral disk assembly (Rs = 3.00) and of spiral tube assembly with an untreated tube under otherwise same experimental condition (Rs = 3.12). Both LIT and UOH elution modes can retain over 50 % of the stationary phase at a flow rate of 2 ml/min. These elution modes can also be effectively applied to more polar solvent systems such as polymer phase systems for separation of proteins [7].
Table 2.
Partition efficiency and stationary phase retention of polar two-phase solvent system in spiral HSCCC
Two-phase solvent system: 1-BuOH/AcOH/H20 (4:1:5)
Sample: trp-trp (1 mg), trp-tyr (1 mg), leu-tyr (4 mg), val-tyr (4 mg), tyr-gly (4 mg) in 1 ml solvent (UP 0.4 ml + LP 0.6 ml)
Column: Sprial tube assembly, 1.6 mm ID, cross-pressed, 4 radial grooves compressed, 12 layers, 100 ml capacity, embedded in PEG3350
| Elution mode | Flow rate (ml/min) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 5 | |||||||
| TP | RS | Sf% | TP | RS | Sf% | TP | RS | SF% | |
| LIH | 414/292/195/194/- | 2.93 | 50 | 676/415/198/210/- | 2.48 | 44 | -/-/-/189/145 | - | 29 |
| LIT | 784/442/299/392/- | 4.23 | 52 | 1810/661/360/391/- | 3.61 | 43 | -/-/1079/406/344 | - | 14 |
| UOT | 522/331/252/290/191 | 2.66 | 39 | 345/333/210/256/166 | 2.12 | 30 | -/-/-/-/169 | - | 19 |
| UOH | 1024/784/351/398/623 | 3.98 | 70 | 900/816/492/622/442 | 3.69 | 50 | -/1764/1239/917/375 | 3.48 | 32 |
TP: theoretical plate number (1st/2nd/3rd/4th/5th peaks); Rs: peak resolution between the 2nd and the 4th peaks; Sf: stationary phase retention - : measurement was not made.
The above results clearly indicate that in this polar two-phase solvent system with long settling time the radially acting centrifugal force gradient effect plays a major role for retention of the stationary phase.
Hexane/Ethyl Acetate/Methanol/0.1 M HCl (1:1:1:1)
This moderately hydrophobic solvent system with different volume ratios is extremely useful for separation of a variety of compounds from natural products [10]. It has a moderate interfacial tension, low viscosity, and a medium degree of density difference with settling time of 19 seconds. In the present study It was tested with three test samples, DNP-L-glutamic acid (DNP-glu), DNP-β-alanine (DNP- β-ala), and DNP-L-alanine with suitable K values as listed in Table 1. Hydrochloric acid was added to normalize the skewed peaks where molar concentrations between 0.1 and 0.01 do not significantly change the partition coefficients of these test samples.
Quite different from the 1-butanol/acetic acid/water (4:1:5) system described above, Archimedean screw force plays an important role in the retention of the stationary phase, and among 8 possible elution modes LIH, LOH, UOT, and UIT give high retention of the stationary phase while the stationary phase retention in upper phase mobile substantially exceeds that of the lower phase mobile under a given flow rate as seen in Fig. 2 and Table 3. Among these 4 elution modes LIH and UOT give the highest stationary phase retention due to the combined effects of Archimedean screw force and the radial gradient centrifugal force yielding higher RS compared to their counterparts, i.e., LOH and UIT, respectively. When one has a choice of the mobile phase, the lower mobile phase can yield higher RS values than the upper mobile phase. It is interesting to note that UIT (upper phase pumped from the internal terminal of the spiral channel) gives substantially higher peak resolution (Rs) than UOT (upper phase pumped from the outer terminal of the spiral channel) under a low flow rate (2 ml/min) of the mobile phase. The effect of the radial centrifugal gradient effect is evident in UOH which yields some separation against the effect of Archimedean screw force.
Fig. 2.
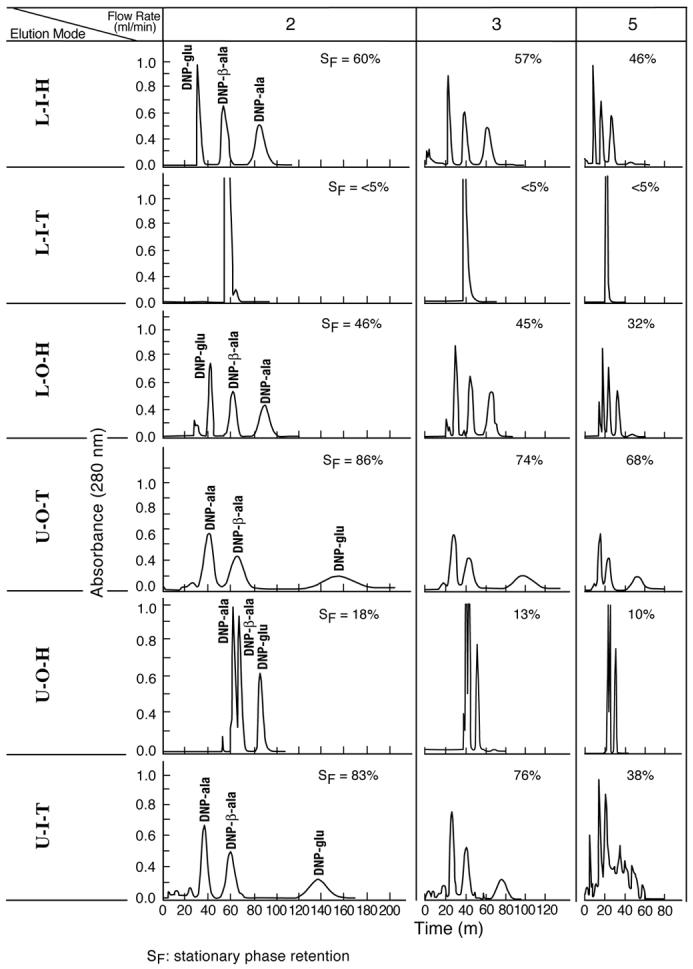
DNP-amino acid separation with a moderately hydrophobic two-phase solvent system by improved spiral tube assembly Experimental conditions: Apparatus: Type-J coil planet centrifuge with 10 cm revolution radius; spiral tube assembly: cross-pressed, radial-groove compressed, and embedded in PEG3500 with 12 spiral layers of 1.6 mm I.D. PTFE (polytetrafluoroethylene) tubing with a total capacity of 100 ml. Each spiral layer consists of 4 spiral channels with a 1.6 cm spiral pitch; solvent system: hexane/ethyl acetate/methanol/0.1M HCl (1:1:1:1, v/v/v/v); samples: DNP-L-glutamic acid, DNP-β-alanine, and DNP-L-alanine each 0.1 mg in 0.1 ml upper phase; Revolution: 800 rpm; detection: 280 nm. SF: retention of stationary phase. Symbols for elution modes are defined in the text.
Table 3.
Partition efficiency and stationary phase retention of moderately hydrophobic two-phase solvent system in spiral HSCCC
Two-phase solvent system: Hexane/EtOAc/MeOH/0.1M HCl (1:1:1:1)
Sample: DNP-glu, DNP-β-ala, DNP-ala, 0.5 mg each
Column: Spiral tube assembly, 1.6 mm ID, cross-pressed, 4 radial grooves compressed, 12 layers, 100 ml capacity, embedded in PEG3350
| Elution mode | Flow rate (ml/min) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 5 | |||||||
| TP | Rs | Sf % | TP | Rs | Sf % | TP | Rs | Sf % | |
| LIH | 757/614/423 | 3.18/2.38 | 60 | 816/480/400 | 2.78/2.23 | 57 | 798/621/506 | 2.33/1.91 | 46 |
| LIT | - | - | ≈ 0 | - | - | ≈ 0 | - | - | ≈ 0 |
| LOH | 666/576/391 | 2.47/2.07 | 46 | 667/625/463 | 2.85/2.00 | 45 | 576/529/457 | 1.43/1.63 | 32 |
| UOT | 111/145/154 | 1.36/2.51 | 86 | 108/126/130 | 1.22/2.24 | 74 | 176/135/136 | 1.23/2.14 | 68 |
| UOH | 4225/3136/1965 | 1.11/2.77 | 18 | 9526/6864/3095 | 1.00/2.40 | 13 | 5476/3184/2500 | 1.04/2.42 | 10 |
| UIT | 253/294/324 | 1.93/3.48 | 83 | 204/171/138 | 1.18/2.28 | 76 | 164/247/300 | 1.13/1.24 | 38 |
TP: theoretical plate number (1st peak/2nd peak/3rd peak); Rs: peak resolution (1st & 2nd/2nd & 3rd peaks); Sf: stationary phase retention
The choice of the elution mode in this two-phase solvent system may also be well applied to other hydrophobic solvent systems with moderate hydrophobicity including methyl t-butyl ether/acetonitrile/water, chloroform/acetic acid or methanol/water at various volume ratios.
Hexane/Ethanol/Water (5:4:1)
This two-phase solvent system is characterized by low viscosity, high interfacial tension and moderately high density difference between the two phases with a short settling time of about 9 seconds. It represents the most hydrophobic solvent group for high-speed CCC for separation of non-polar compounds. In this solvent system, Archimedean screw effects overpower the radial force gradient effect and the high stationary phase retention is obtained from the elution modes of LIH, LOH, UOT, and UIT while the rest of the elution modes gives only few milliliters of the stationary phase which were apparently retained within the radial transfer tubes between the spiral channels.
It should be noted that the LIH and UOT (utilizing both force effects) can yield higher retention of the stationary phase, but substantially less peak resolution (RS) compared to their respective counterparts (LOH and UIT) in which the mobile phase was pumped against the centrifugal force gradient through the spiral channel. Chromatographic data including TP, RS and SF are given in Fig. 3 and Table 4.
Fig. 3.
Separation of Sudan I and Sudan II with a hydrophobic two-phase solvent system by improved spiral tube assembly Experimental conditions: Apparatus: Type-J coil planet centrifuge with 10 cm revolution radius; spiral tube assembly: cross-pressed, radial-groove compressed, and embedded in PEG3500 with 12 spiral layers of 1.6 mm I.D. PTFE (polytetrafluoroethylene) tubing with a total capacity of 100 ml. Each spiral layer consists of 4 spiral channels with a 1.6 cm spiral pitch; solvent system: hexane/ethanol/water (5:4:1, v/v/v) samples:Sudan I and Sudan II each 0.3 mg in 0.3 ml of upper phase; Revolution: 800 rpm; detection: 280 nm. SF: retention of stationary phase. Symbols for elution modes are defined in the text.
Table 4.
Partition efficiency and stationary phase retention for hydrophobic two-phase solvent system in spiral HSCCC
Two-phase solvent system: hexane/ethanol/water (5:4:1)
Sample: Sudan I & Sudan II each 0.3 mg in 0.3 ml upper phase
Column: Sprial tube assembly, 1.6 mm ID, cross-pressed, 4 radial grooves compressed, 12 layers, 100 ml capacity, embedded in PEG3350
| Elution mode | Flow rate (ml/min) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 5 | |||||||
| TP | Rs | Sf % | TP | Rs | Sf % | TP | Rs | Sf % | |
| LIH | 299/256 | 2.21 | 75 | 288/248 | 2.12 | 71 | 228/201 | 1.88 | 65 |
| LOH | 434/297 | 2.44 | 71 | 426/290 | 2.31 | 68 | 421/324 | 2.22 | 53 |
| UOT | 110/99 | 1.07 | 91 | 75/111 | 0.95 | 87 | 182/118 | 1.01 | 84 |
| UIT | 252/297 | 1.55 | 89 | 351/256 | 1.27 | 85 | 196/177 | 1.05 | 74 |
TP: theoretical plate number (1st peak/2 nd peak); Rs: peak resolution; Sf: stationary phase retention
Hexane/Acetonitrile
This non-aqueous binary system is characterized by low viscosity, low interfacial tension, and small difference in density between the two phases. It is useful when the sample is affected by the presence of water in the solvent system. The performance of this solvent system was evaluated by separation of Sudan I and Sudan II as in the previous system. As shown in Table 5, both stationary phase retention and partition efficiency are similar to those obtained from the Hexane/EtOH/H2O above described, i.e. LOH and UOT yield better RS than LIH and UIT, the counterparts eluted against the centrifugal force gradient effect. The reason of this strange phenomenon is unknown, but it may be due to the Coriolis force which have shown considerable effects on partition effficiency of proteins in toroidal coil countercurrent chromatography[11].
Table 5.
Partition efficiency and stationary phase retention of non-aqueous two-phase solvent system in spiral HSCCC
Two-phase solvent system: hexane/acetonitrile (6:5, v/v for ca equal volume of each phase)
Sample: Sudan I & Sudan II each 0.3 mg in 0.3 ml upper phase
Column: Sprial tube assembly, 1.6 mm ID, cross-pressed, 4 radial grooves compressed, 12 layers, 100 ml capacity, embedded in PEG3350
| Elution mode | Flow rate (ml/min) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 5 | |||||||
| TP | Rs | Sf % | TP | Rs | Sf % | TP | Rs | Sf % | |
| LIH | 351/300 | 2.02 | 79 | 310/339 | 2.06 | 74 | 217/309 | 1.45 | 68 |
| LOH | 711/625 | 2.81 | 76 | 653/590 | 2.39 | 71 | 625/461 | 1.74 | 59 |
| UOT | 214/474 | 2.11 | 88 | 191/247 | 2.15 | 87 | 196/220 | 1.92 | 84 |
| UIT | 425/443 | 3.01 | 86 | 520/524 | 3.06 | 80 | 377/512 | 2.58 | 68 |
TP: theoretical plate number (1st peak/2 nd peak); Rs: peak resolution; Sf: stationary phase retention
CONCLUSIONS
In the spiral tube assembly the retention of the stationary phase and partition efficiency are determined by an interplay between two forces, i.e. the Archimedean screw and radial centrifugal force gradient through the spiral channel. These effects were further modified by the physical property of the two-phase solvent system which is represented by the settling time.
In the polar solvent system of 1-butanol/acetic acid/water (4:1:5) with settling time of over 30 s, the centrifugal force effect plays a dominant role in stationary phase retention where the high retention is attained by eluting the lower phase from the internal terminal (LIH and LIH) and upper phase from the external terminal of the spiral channel (UOH or UOT). Among those the highest peak resolution is given by LIT (lower phase pumped from internal tail terminal of the spiral channel) or UOH (upper phase pumped from external head terminal of the spiral channel).
In the moderately hydrophobic two-phase solvent system represented by hexane/ethyl acetate/methanol/0.1 M HCl with settling time of 19 s, the effect of the Archimedean screw force which distributes the upper phase toward the head and the lower phase toward the tail starts to dominate the retention of the stationary phase, and the highest retention is produced by LIH and LOH or UIT and UOT. Among those LIH produced better peak resolution (and higher retention of the stationary phase) than LOH under all flow rates of the mobile phase tested. However, when the upper phase is used as the mobile phase, the higher peak resolution (RS) is obtained by UIH than UOH at a low flow rate of 2 ml/min. In this solvent system, the lower mobile phase gives higher RS values than the upper mobile phase
In the hydrophobic two-phase solvent system composed of hexane/ethanol/water (5:4:1) and hexane/acetonitrile binary system both having short settling time of 9 s, the Archimedean screw force effect completely overpowers the radial centrifugal gradient effect in peak resolution (RS), where LOH and UIT yield the highest RS despite slightly less stationary phase retention than their respective counterparts, LIH and IOT.
The choice of the elution modes for the two-phase solvent systems in the spiral tube assembly are summarised in Table 6.
Table 6.
Choise of the elution mdoe for 4 typical two-phase solvent systems in spiral HSCCC
| Two-phase solvent system* (volume ratio) |
settling time (second) |
choice of the elution mode** | ||
|---|---|---|---|---|
| Lower mobile phase | Upper mobile phase | U vs.L | ||
| 1-BuOH-AcOH-H2O (4:1:5) | >30 | LIT>>LIH | UOH>>UOT | LIT≈UOH |
| Hexane-EtOAc-MeOH-0.1M HCl (1:1:1:1) |
19 | LIH>>LOH | UIT>UOT>UOH | LIH>UIT |
| Hexane-EtOH-H2O (5:4:1) | 9 | LOH≤LIH | UIT>UOT | LOH>>UIT |
| Hexane-acetonitrile | 9 | LOH>LIH | UIT>>UOT | LOH<UIT |
BuOH: butanol; AcOH: acetic acid; EtOAc: ethyl acetate; MeOH: methanol; EtOH: ethanol
L: lower mobile phase; U: upper mobile phase; I: internal terminal of the spiral channel; O: outer terminal of the spiral channel; H: head of the spiral channel; T: tail of the spiral channel.
ACKNOWLEDGMENT
The authors are indebted to Messrs, Frank Sharpnak and Howard Metger in the NIH Machine Instrumentation Facility for their help in repairing the separation column for high-speed countercurrent chromatography instrument.
REFERENCES
- 1.Ito Y. CRC Crit. Rev. Anal. Chem. 1986;17(1):65–143. [Google Scholar]
- 2.Ito Y, Yang F-Q, Fitze PE, Sullivan JV. J. Liq. Chromatogr. Rel. Technol. 2003;26(910):1355–1372. [Google Scholar]
- 3.Ito Y, Yang F-Q, Fitze PE, Powell J, Ide DJ. J. Chromatogr. A. 2003;1017:71–81. doi: 10.1016/j.chroma.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 4.Ito Y, Qi L, Powell J, sharpnack F, Metger H, Yost J, Cao X-L, Dong Y-M, Huo L-S, Zhu X, Li T. J. Chromatogr. A. 2007:108–114. doi: 10.1016/j.chroma.2006.11.078. [DOI] [PubMed] [Google Scholar]
- 5.Ito Y, Clary R, Sharpnack F, Metger H, Powell J. J Chromatogr. A. 2007;1172:151–159. doi: 10.1016/j.chroma.2007.09.078. [DOI] [PubMed] [Google Scholar]
- 6.Ito Y, Clary R, Powell J, Knight M, Finn TM. J. Liq. Chromatgr. Rel. Technol. 2008;31:1346–1357. doi: 10.1080/10826070802019913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ito Y, Clary R, Powell J, Knight M, Finn TM. J. Chromatogr. A. doi: 10.1016/j.chroma.2008.10.126. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albertsson P. Å Partition of Cell Particles and Macromolecules. Wiley-Interscience; New York: 1984. [Google Scholar]
- 9.Ito Y, Conway WD. J. Chromatogr. 1984;301:405–414. doi: 10.1016/s0021-9673(01)89214-1. [DOI] [PubMed] [Google Scholar]
- 10.Ito Y. J. Chromatogr. A. 2005;1065:145–168. doi: 10.1016/j.chroma.2004.12.044. [DOI] [PubMed] [Google Scholar]
- 11.Ito Y, Ma Y. J. Liq. Chromatogr. Rel. Technol. 1998;21(12):1–17. [Google Scholar]