Abstract
We investigated the role of caveolae in the mechanism of increased pulmonary vascular permeability and edema formation induced by the activation of polymorphonuclear neutrophils (PMNs). We observed that the increase in lung vascular permeability induced by the activation of PMNs required caveolin-1, the caveolae scaffold protein. The permeability increase induced by PMN activation was blocked in caveolin-1 knockout mice and by suppressing caveolin-1 expression in rats. The response was also dependent on Src phosphorylation of caveolin-1 known to activate caveolae-mediated endocytosis in endothelial cells. To address the role of PMN interaction with endothelial cells, we used an intercellular adhesion molecule (ICAM)-1 blocking monoclonal antibody. Preventing the ICAM-1–mediated PMN binding to endothelial cells abrogated Src phosphorylation of caveolin-1, as well as the increase in endothelial permeability. Direct ICAM-1 activation by crosslinking recapitulated these responses, suggesting that ICAM-1 activates caveolin-1 signaling responsible for caveolae-mediated endothelial hyperpermeability. Our results provide support for the novel concept that a large component of pulmonary vascular hyperpermeability induced by activation of PMNs adherent to the vessel wall is dependent on signaling via caveolin-1 and increased caveolae-mediated transcytosis. Thus, it is important to consider the role of the transendothelial vesicular permeability pathway that contributes to edema formation in developing therapeutic interventions against PMN-mediated inflammatory diseases such as acute lung injury.
Keywords: caveolin-1, Src, endothelial permeability, albumin transport, lung edema
Increased lung vascular albumin permeability leads to the accumulation of protein-rich interstitial and alveolar fluid, the characteristic feature of acute lung injury.1,2 Activation of pulmonary neutrophils (PMNs) sequestered in pulmonary microvessels is an important factor in the pathogenesis of increased lung vascular permeability and tissue injury.3–5 However, the mechanisms of increased vascular permeability induced by PMNs are not completely understood. Studies have shown that activation of PMNs results in the release of mediators (eg, oxidants and proteases) that increase vascular permeability by disrupting interendothelial junctions,1–9 which normally form a restrictive barrier excluding plasma proteins of the size of albumin and greater.10,11 Other studies in lungs of sepsis-induced ARDS patients showed extensive fluid accumulation in interstitial and alveolar compartments, but in most cases, a well-preserved microvascular endothelial junctional barrier.12 An unexamined mechanism of endothelial hyperpermeability is the possibility of increased transport of albumin occurring via the transcytosis pathway involving the trafficking of caveolae across the endothelial barrier.1 It is possible that in absence of gross interendothelial junctional alterations after PMN activation, increased caveolae-mediated transport of albumin, and concentration of albumin in the lung interstitium, provides the necessary transendothelial pressure gradient to maintain a high net transcapillary fluid filtration rate. Although studies have suggested that transcytosis is a mechanism of transendothelial albumin permeability,13–16 its pathophysiological significance in PMN activation-mediated inflammatory disease has not been examined.
The continuous microvascular endothelium, the type present in lung microvessels,1 establishes a semipermeable barrier dependent on the assembly of adherens and tight junctions that anneal neighboring cells and restrict the passage of plasma proteins.1,17 Albumin, the most abundant plasma protein, traverses the endothelial barrier by a vesicular pathway.10,11 Albumin concentration of the interstitium is the primary determinant of interstitial oncotic pressure.1 We and others have described a role of caveolin-1 (Cav-1), the primary structural component of caveolae and a scaffolding protein, in regulating endothelial transcytosis.10,14–16 Cav-1 controls the formation and release of caveolae from the plasma membrane, which shuttle macromolecules across the endothelial barrier.10,11,13–16 The uptake of albumin via endocytosis and its transcytosis to tissue is the result of fission and trafficking of caveolae.13–16 Studies in Cav-1–null mice (Cav-1−/−) showed the absence of caveolae and defective transcellular transport of albumin,18,19 which could be rescued by expression of Cav-1.20 Although mechanisms of Cav-1–regulated fission and trafficking of caveolae are still unclear, phosphorylation of Cav-1 on tyrosine 14 by Src kinase is a key “switch” initiating caveolar fission from the plasma membrane.21–27
The role of Src activation on caveolae fission and trafficking and endothelial albumin transcytosis following PMN activation is not known. Activation of PMNs with the complement peptide C5a induced Src kinase activation and increased endothelial permeability.6 In addition, exposure of endothelial cells to H2O2 (a PMN-derived oxidant) increased Src activity in association with increased endothelial permeability.28 Crosslinking of endothelial cell surface ICAM-1 increased Src kinase activity,29–32 raising the possibility that PMN adhesion to endothelial cells via the CD18/ICAM-1 interaction engages the endothelial cell caveolar transport machinery. Whereas previous studies indicate that activated PMNs induce an increase in albumin permeability, the role of caveolae in the mechanism of increased vascular albumin permeability has not been addressed. Our results suggest that endothelial albumin hyperpermeability induced by transcytosis of albumin contributes significantly to the development of pulmonary edema induced by the activation of PMNs. Thus, strategies aimed at blocking vascular hyperpermeability via transcytosis may prove to be useful in preventing pulmonary edema seen in acute lung injury.
Materials and Methods
An expanded Materials and Methods section is available in the online data supplement.
Materials
Adult Sprague–Dawley rats (250 to 300 g) were purchased from Charles River Laboratories (Wilmington, Mass). Cav-1–null mice and corresponding wild-type (WT) cohorts, in the black Swiss genetic background, were obtained from The Jackson Laboratory (Bar Harbor, Me) and Taconic (Hudson, NY). All animal experiments were performed after approval from the University of Illinois Animal Care and Use Committee. Rat lung microvascular endothelial cells (RLMVECs) were obtained from Vec Technologies (Rensselaer, NY). Unless otherwise specified, all chemicals were purchased from Sigma-Aldrich (St Louis, Mo).
Cell culture, uptake and transendothelial transport of 125I-albumin tracer, fluorescent albumin uptake, transendothelial electric resistance (TER), dominant negative (dn) Src construct transfections, and Western blotting were performed as described.21,23,24,33,34
Isolation of Neutrophils
PMNs were isolated from rat or mouse whole blood using the hetastarch exchange transfusion and sedimentation technique.35
Cav-1 Small Interfering RNA Transfection in Endothelial Cell and Rat Lung
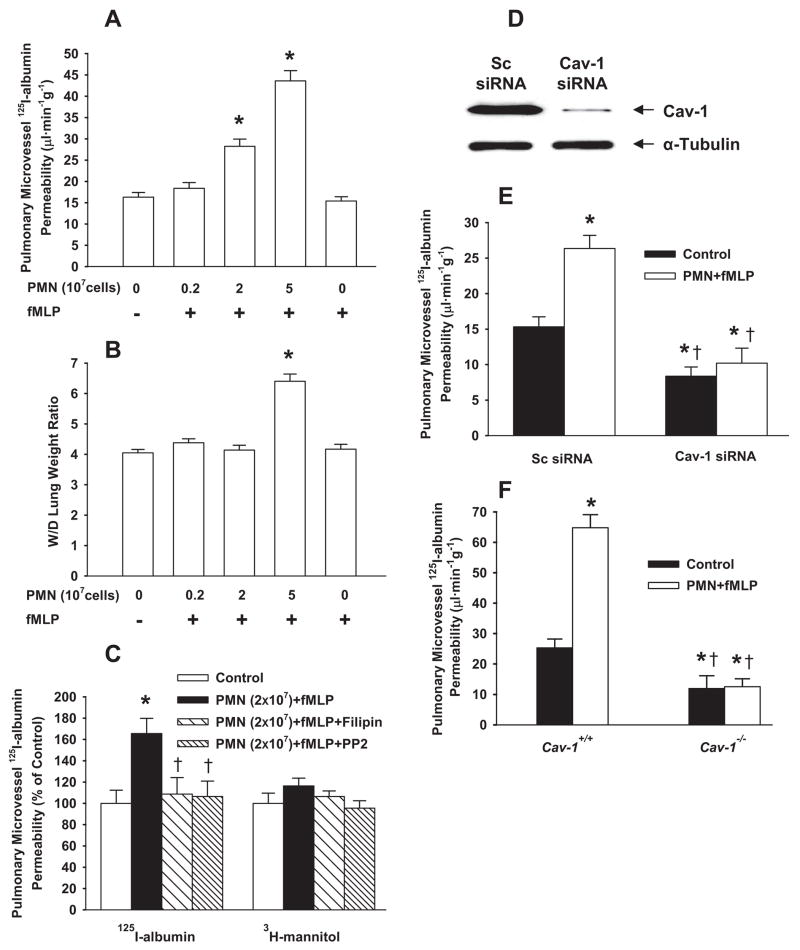
Small interfering (si)RNA duplex oligonucleotides were purchased from Dharmcon (Lafayette, Colo). The sequences of Cav-1 siRNA and negative control duplex were 5′-UCUGUGAUCCACUCUUUGAUU-3′ and 5′-UAAGGCUAUGAAGAGAUAC-3′, respectively. Preliminary experiments showed effective siRNA-mediated knockdown of Cav-1 in RLMVEC by transfecting cells with 10 nmol/L siRNA at 50% to 70% confluence using the protocol provided by the manufacturer. All experiments were performed 48 hours after transfection. In vivo cationic liposome–siRNA complexes were made as described.36 The liposome–siRNA complex was prepared by addition of 0.25 mg/kg siRNA into 500 μL of liposome suspensions. Successful transfection of Cav-1 siRNA was confirmed by Cav-1 Western blotting of lung homogenates.
Lung Preparation
Methods of isolation and perfusion of lung preparations were performed as described.37 Permeability-surface area (PS) product, an index of vascular permeability to albumin, was measured as described.38 The animals were anesthetized with pentobarbital sodium (40 mg/kg IP). The lungs were perfused with Krebs solution at a constant flow and venous pressure.
Statistic Analysis
Data were expressed as means±SEM. One-way ANOVA and the Student–Newman–Keuls test were used to compare the different groups; P<0.05 was considered significant.
Results
Caveolae-Dependent Increase in Transendothelial 125I-Albumin Transport Following PMN Activation
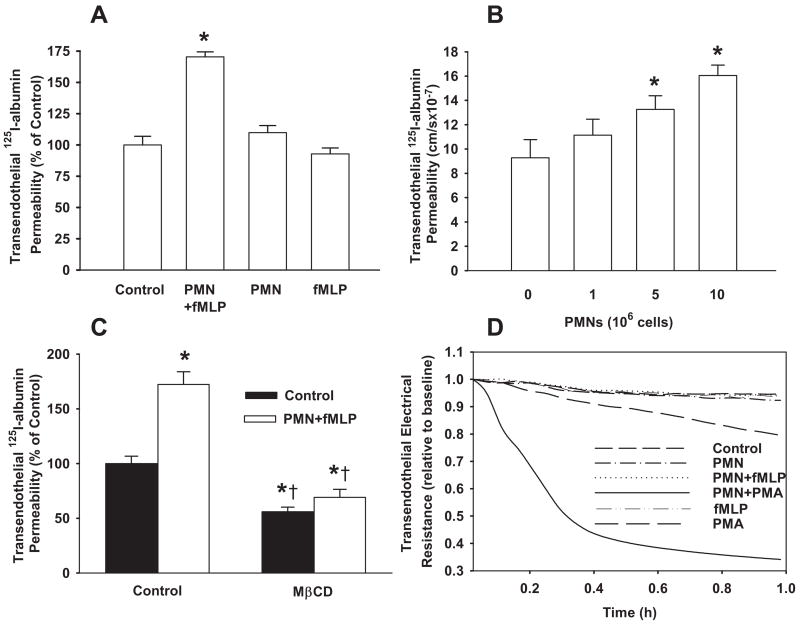
Activation of PMNs with N-formyl-methionyl-leucyl-phenyl-alanine (fMLP) resulted in a 75% increase in transendothelial transport of 125I-albumin (Figure 1A). Neither addition of unstimulated PMNs nor fMLP alone altered 125I-albumin permeability (Figure 1A). The increase in albumin permeability was dependent on the number of PMNs added (Figure 1B). To address whether intact caveolae were required for the response, endothelial caveolae were disrupted by pretreatment with cholesterol-binding agent methyl-β-cyclodextrin.33,39 Cyclodextrin prevented the PMN activation-dependent increase in 125I-albumin flux (Figure 1C). To determine whether increased transendothelial albumin flux was the result of opening of interendothelial junctions, we measured changes in TER. Activation of PMNs with fMLP did not alter TER (Figure 1D), whereas in a positive-control experiment, TER decreased by 60% from baseline (5.95±0.58 Ω · cm2) for up to 60 minutes on activation of PMNs with the phorbol ester 4-phorbol myristate 13-acetate (PMA), which is known to severely injure the endothelial barrier (Figure 1D).
Figure 1.
Activation of PMNs with fMLP increases caveolae-mediated transendothelial 125I-albumin permeability in RLMVEC monolayers. A, Effect of PMNs in the absence and presence of fMLP on transendothelial 125I-albumin permeability. B, Activation of PMNs with fMLP increased transendothelial 125I-albumin permeability in a PMN number-dependent manner. C, Effect of pretreatment of RLMVECs with MβCD on 125I-albumin permeability. The baseline permeability value for control group (without PMNs and fMLP) is 9.4±1.1 cm/sec. D, Effect of activation of PMNs (107 cells/mL) with fMLP (1.0 μmol/L) or PMA (0.1 μmol/L) on TER. Results are typical of 3 experiments (A through C). n=4 to 6 for each group. *P<0.05 compared with control group (without PMNs and fMLP), †P<0.05 compared with respective groups.
PMN Activation With fMLP Induces Albumin Endocytosis in Endothelial Cells
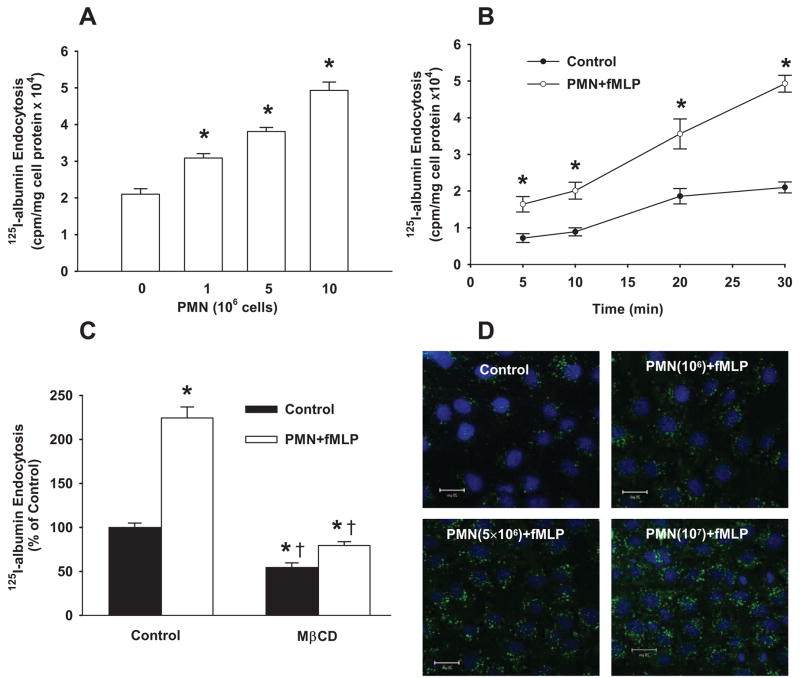
We next addressed the possibility that PMN activation stimulated caveolae-mediated endocytosis of albumin in endothelial cells, the initial step in albumin transport via transcytosis.1,13–16 As shown in Figure 2A and 2B, fMLP activation of PMNs increased 125I-albumin endocytosis by endothelial cells within minutes. The response was dependent on the number of PMNs added to endothelial cell monolayers. Neither PMNs nor fMLP alone affected the uptake of 125I-albumin (data not shown). The increase in albumin endocytosis was abolished by pretreatment with cyclodextrin (Figure 2C). Fluorescence quantified as pixel intensity per cell using confocal microscopy showed that fMLP stimulation of 106, 5×106, or 10×106 PMNs induced significant increases in Alexa 488 albumin uptake (Figure 2D), paralleling the responses observed with 125I-albumin tracer.
Figure 2.
Activation of PMNs with fMLP increases caveolae-mediated endocytosis of albumin in endothelial cells. A, Activation of PMNs with fMLP increased 125I-albumin endocytosis in a PMN number-dependent manner. B, Time course of effects of fMLP activation of PMNs (107 cells/mL) on 125I-albumin endocytosis. C, Effect of pretreatment of RLMVECs with MβCD on PMN-induced (107 cells/mL) 125I-albumin endocytosis. Results are representative of 3 experiments. Scale bars=10 μm. n=4 to 6 for each group (A and C) and time point (B). *P<0.05 compared with control group (without PMNs and fMLP), †P<0.05 compared with respective groups. D, Confocal images showing effect of fMLP activation of PMNs induced a PMN number-dependent increase in uptake of Alexa 488–labeled albumin (green). The nucleus (blue) was stained with DAPI. Results are representative of 3 experiments.
Cav-1 Is Required for PMN Activation–Induced Endocytosis and Transendothelial Flux of Albumin
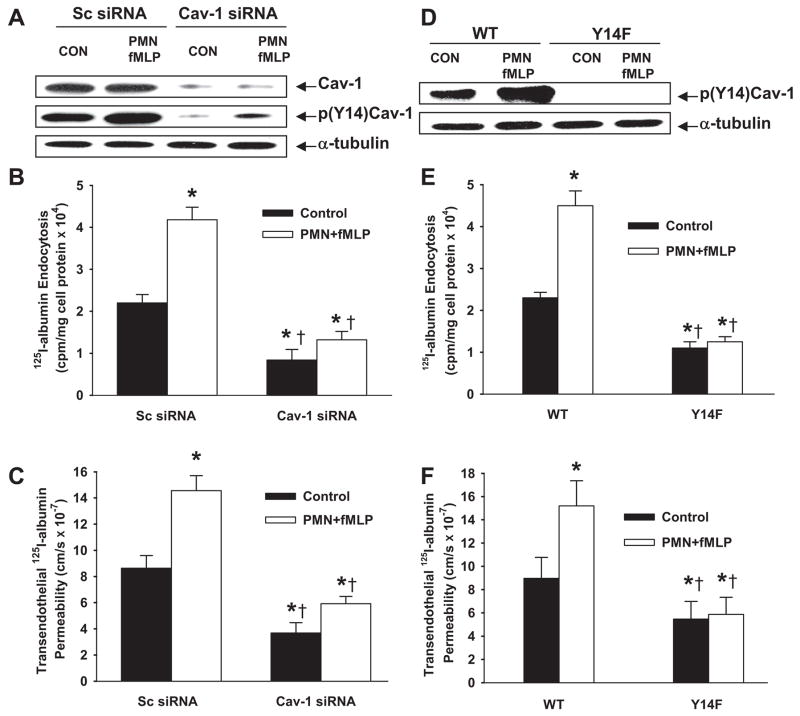
fMLP activation of PMNs increased the phosphorylation of Cav-1 in endothelial cells (Figure 3A and Figure IA in the online data supplement), whereas PMNs or fMLP alone had no effect (data not shown). Endothelial cells transduced with Cav-1 siRNA showed 90% decrease in caveoilin-1 protein after 48 hours (Figure 3A and supplemental Figure IA). These cells were used to address the role of Cav-1 in mediating endothelial transcytosis. As shown in Figure 3B and 3C, fMLP activation of PMNs increased 125I-albumin endocytosis and flux 2-fold in endothelial cells transduced with scrambled siRNA, whereas Cav-1 knockdown abolished this effect. To address further the role of Cav-1 in the PMN activation–induced increase in albumin permeability, we compared the effects of activated PMNs on endothelial endocytosis and transendothelial transport of 125I-albumin in WT and Y14F-Cav-1 mutant (nonphosphorylatable Cav-1)– expressing RLMVECs (Figure 3D and supplemental Figure IB).26 PMN activation-induced increases in endocytosis and transcytosis of 125I-albumin were abolished in Y14F-Cav-1 mutant–expressing cells (Figure 3E and 3F).
Figure 3.
Activation of PMNs with fMLP increases 125I-albumin endocytosis and transendothelial permeability through Cav-1 phosphorylation. RLMVECs were coincubated with PMNs in the presence or absence of fMLP (1.0 μmol/L) for 30 minutes at 37°C. A through C, Effects of activation of PMNs with fMLP on Cav-1 expression and phosphorylation (A and supplemental Figure IA) and 125I-albumin endocytosis (B) and transendothelial albumin permeability (C) in cells transfected with Cav-1 siRNA or scrambled (Sc) siRNA. D through F, Effects of activation of PMNs with fMLP on Cav-1 phosphorylation (D and supplemental Figure IB) and 125I-albumin endocytosis (E) and transendothelial albumin permeability (F) in cells stably expressing phosphorylation-defective Y14F-Cav-1 mutant. Exogenous Cav-1 is myc-tagged (D). Results are typical of 3 experiments (A and D). n=4 to 6 for each group (B, C, E, and F). *P<0.05 compared with control group, †P<0.05 compared with respective groups.
Requirement for Src Phosphorylation of Cav-1 in Signaling Endocytosis and Transendothelial Albumin Transport Induced by PMN Activation
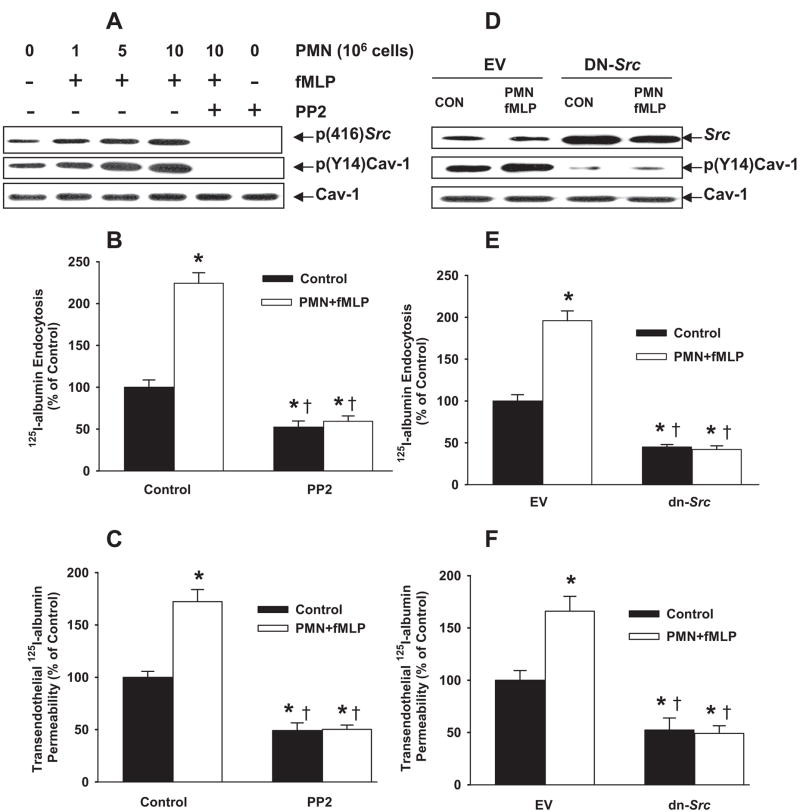
fMLP activation of PMNs significantly increased Src activity in endothelial cells and pretreatment with the Src inhibitor PP2 (4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4,d] pyrimidine prevented this response (Figure 4A and supplemental Figure IC). Activation of increasing numbers of PMNs with fMLP also increased Cav-1 phosphorylation (Figure 4A and supplemental Figure IC), 125I-albumin endocytosis (Figure 4B), and transendothelial 125I-albumin flux; in all cases, these responses were inhibited by PP2. Neither PMNs alone nor fMLP alone elicited these effects (data not shown).
Figure 4.
Activation of PMNs with fMLP stimulates 125I-albumin endocytosis and transendothelial permeability through Src signaling. RLMVECs were coincubated with PMNs in the presence or absence of fMLP (1.0 μmol/L) for 30 minutes at 37°C. A, Effects of fMLP activation of PMNs and PP2 (15 μmol/L) pretreatment on Cav-1 protein expression and phosphorylation of Src and Cav-1. B, C, and supplemental Figure IC, Effects of PP2 pretreatment on PMN activation-induced change in 125I-albumin endocytosis (B) and transendothelial albumin permeability (C). D through F, Effects of activation of PMNs with fMLP on Src protein expression and Cav-1 phosphorylation (D and supplemental Figure ID) and 125I-albumin endocytosis (E) and transendothelial albumin permeability (F) in RLMVECs infected with dn-Src pFB adenovirus or empty vector (EV). Results are typical of 3 experiments (A and D). n=4 to 6 for each group (B, C, E, and F). The baseline permeability values for control group (without PMNs and fMLP) are 9.2±1.4 cm/sec (C) and 9.1±1.3 cm/sec (F), respectively. *P<0.05 compared with control group, †P<0.05 compared with respective groups.
To address further the role of Src phosphorylation of Cav-1 in signaling endocytosis and transendothelial albumin transport induced by PMN activation, we transfected endothelial cells with the pFB retroviral vector encoding dn Src. Total Src protein in dn-Src–transfected cells was significantly greater than endogenous Src protein (Figure 4D and supplemental Figure ID); ie, the exogenously introduced dn-Src kinase represented most of the total Src protein. dn-Src expression blocked basal Cav-1 phosphorylation, indicating that inhibiting the activity of endogenous Src kinase prevented Cav-1 phosphorylation (Figure 4D and supplemental Figure ID). Expression of dn-Src also prevented 125I-albumin endocytosis (Figure 4E) and transendothelial 125I-albumin flux stimulated by activated PMNs (Figure 4F).
PMN Activation With fMLP Increases Vascular Albumin Permeability and Tissue Water Content via Cav-1-Dependent Pathway in Rat Lungs
Administration of 2×107 PMNs or 5×107 PMNs followed by 1 μmol/L fMLP increased 125I-albumin PS product by 74% and 140%, respectively, whereas PMN (2×107 or 5×107 cells) or fMLP alone (1 μmol/L) did not alter 125I-albumin PS (Figure 5A). To address whether the caveolae-mediated transcytosis pathway was involved, we pretreated lungs with the cholesterol-binding agent filipin to ablate caveolae40 before injection of the 125I-albumin tracer. As shown in Figure 5B, filipin blocked the stimulatory effect of fMLP-activated PMNs (2×107 cells) on 125I-albumin PS product. However, transport of 3H-mannitol, a low-molecular-weight (182-Da) tracer that traverses the endothelial barrier via the paracellular (junctional) pathway,33 was not affected by fMLP activation of PMNs. Also, Src inhibitor PP2 prevented the increase in 125I-albumin PS product but did not alter the 3H-mannitol PS product (Figure 5B).
Figure 5.
Activation of PMNs with fMLP induces caveolae-mediated albumin hyperpermeability in rat and mouse lung vessels. After lung perfusion stabilization of 20 minutes, PMNs and fMLP were separately infused in the pulmonary circulation for 30 minutes. A and B, Effect of fMLP-activated rat PMNs (2×106, 2×107, or 5×107 cells) on 125I-albumin PS product (A) and W/D lung weight ratio (B). C, Effects of filipin and PP2 (1 μg/kg) on PMN (2×107 cells) activation-induced changes in 125I-albumin and 3H-mannitol PS products. D and E, Rats were injected with liposomes containing scrambled (Sc) or Cav-1 siRNA in the tail vein. After 48 hours, Western blot analyses of Cav-1 expression (D and supplemental Figure IIA) and pulmonary vascular 125I-albumin PS (E) were assessed. F, Effects of activation of mouse PMNs (2×106 cells) on pulmonary vascular 125I-albumin PS product in Cav-1+/+ and Cav-1−/− mouse lungs. *P<0.05 compared with control groups (without PMNs and fMLP) (A through C and F) or with scrambled siRNA control (E). †P<0.05 compared with PMN (2×107 cells)+fMLP group (C) or with respective groups (E and F).
Because measurement of 125I-albumin PS product provides an assessment of the transvascular flux of albumin, we also addressed whether increase in PS value resulted in alterations in the final wet-to-dry (W/D) lung weight ratio, a measure of pulmonary edema formation (Figure 5C). Activation of 2×107 PMNs in lung microvessels with fMLP (1 μmol/L) did not alter W/D lung weight ratio (Figure 5C) in the presence of 74% increase in 125I-albumin PS product (Figure 5A), whereas activation of 5×107 PMNs significantly increased lung W/D ratio (Figure 5C) concomitant with a 140% increase in 125I-albumin PS product (Figure 5A). Neither PMNs nor fMLP alone affected 125I-albumin PS product or W/D ratio values. Thus, 2×107 PMNs resulted in an increase in 125I-albumin PS product but without pulmonary edema formation, whereas 5×107 PMNs resulted in a 2-fold greater increase in 125I-albumin PS product and edema formation.
We next assessed the effects of siRNA depletion of Cav-1 in rat lung microvessels on vascular permeability. As shown in Figure 5D and supplemental Figure IIA, Cav-1 siRNA significantly decreased total rat lung Cav-1 expression compared to scrambled siRNA-treated lungs. In lungs treated with Cav-1 siRNA, we observed a significant decrease in basal, as well as PMN-stimulated, 125I-albumin PS relative to scrambled siRNA-treated rat lungs (Figure 5E), consistent with the filipin study shown above (Figure 5B).
We also carried out studies in lungs from mice with targeted deletion of Cav-1 (Cav-1−/−). Figure 5F compares the effect of fMLP-activated WT mouse PMNs on 125I-albumin PS product in WT and Cav-1−/− mouse lungs. Infusion of PMNs (2×106 cells) followed by fMLP (1 μmol/L) induced 1.6-fold increase in pulmonary vascular 125I-albumin PS in WT mouse lungs. In Cav-1−/− mouse lungs, we observed a decrease in basal 125I-albumin PS, as evidenced by the role of caveolae in transporting albumin across the endothelial barrier. In contrast to WT, activation of PMNs did not result in increased 125I-albumin PS in Cav-1−/− mouse lungs.
Requirement for ICAM-1 Signaling Increased Pulmonary Vascular Albumin Permeability Induced by Activation of PMN
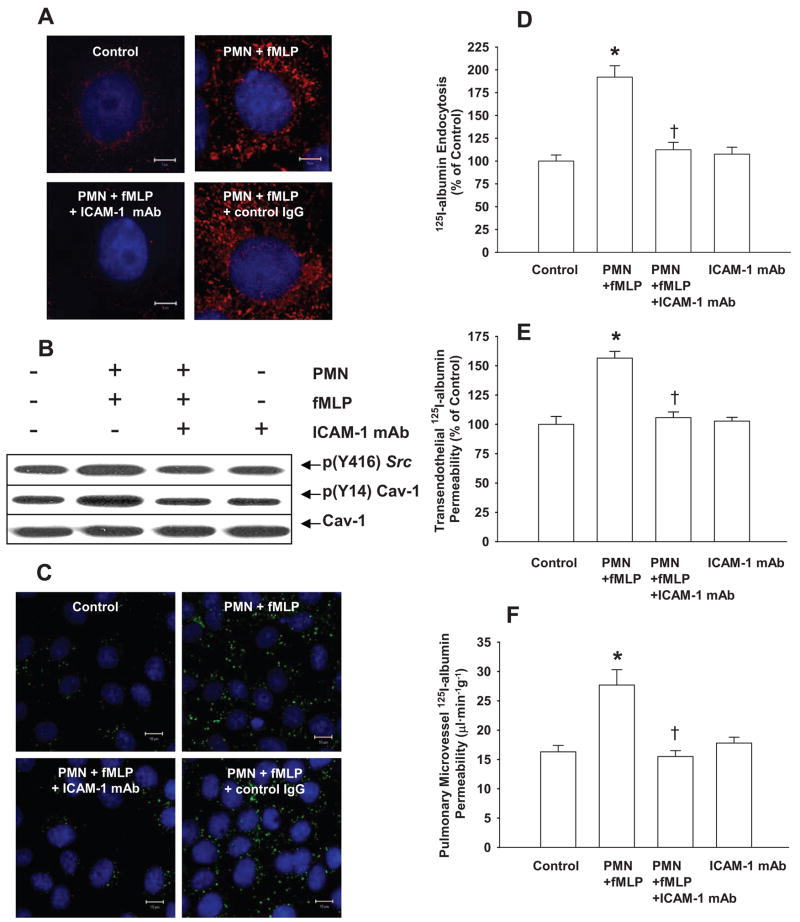
Confocal imaging showed clustering of endothelial cell surface ICAM-1 within 30 minutes following the addition of fMLP to PMN–endothelial cell cocultures (Figure 6A). Neither PMNs nor fMLP alone altered ICAM-1 surface distribution (data not shown). fMLP activation of PMNs also induced Src activation and Src-dependent phosphorylation of Cav-1 in RLMVEC monolayers, and these effects were blocked by anti–ICAM-1 monoclonal antibody (mAb) (Figure 6B and supplemental Figure IIB). Compared with control IgG group, treatment of endothelial cells with anti–ICAM-1 blocking mAb also prevented the increased endocytosis of fluorescent-albumin and 125I-albumin tracer, as well as increased transendothelial 125I-albumin flux (Figure 6C through 6E) induced by fMLP activation of PMNs. Neither anti–ICAM-1 mAb nor control IgG alone affected ICAM-1 surface distribution and endocytosis of fluorescent-albumin (data not shown).
Figure 6.
Role of ICAM-1 in mediating Src activation and Cav-1 phosphorylation in endothelial cells and its consequence in increasing caveolae-mediated endothelial hyperpermeability. A, Effects of fMLP activation of PMNs with and without anti–ICAM-1 mAb pretreatment on cell surface ICAM-1 distribution. Each image shows merged ICAM-1 immunostaining (red) and nucleus (blue). Scale bars=5 μm. B and supplemental Figure IIB, Effects of fMLP activation of PMNs with and without anti–ICAM-1 Ab pretreatment on Src and Cav-1 phosphorylation. C, Effects of activation of PMNs with fMLP or combined with anti–ICAM-1 Ab pretreatment on Alexa 488–labeled albumin (green) endocytosis. Cells were stained with 4′,6-diamidino-2-phenylindole (DAPI) to label nuclei (blue). Results are typical of 3 experiments. Scales bar=10 μm. D and E, Effects of anti–ICAM-1 Ab on PMN activation-induced increase in 125I-albumin endocytosis (D) and transcytosis (E). F, Effects of anti–ICAM-1 Ab on PMN activation-induced increase in pulmonary trans-vascular 125I-albumin PS product in rats. The baseline permeability value for control group (without PMNs and fMLP) was 9.1±1.1 cm/sec (E) (n=4 to 6 for each group). *P<0.05 compared with control group, †P<0.05 compared with PMN+fMLP groups.
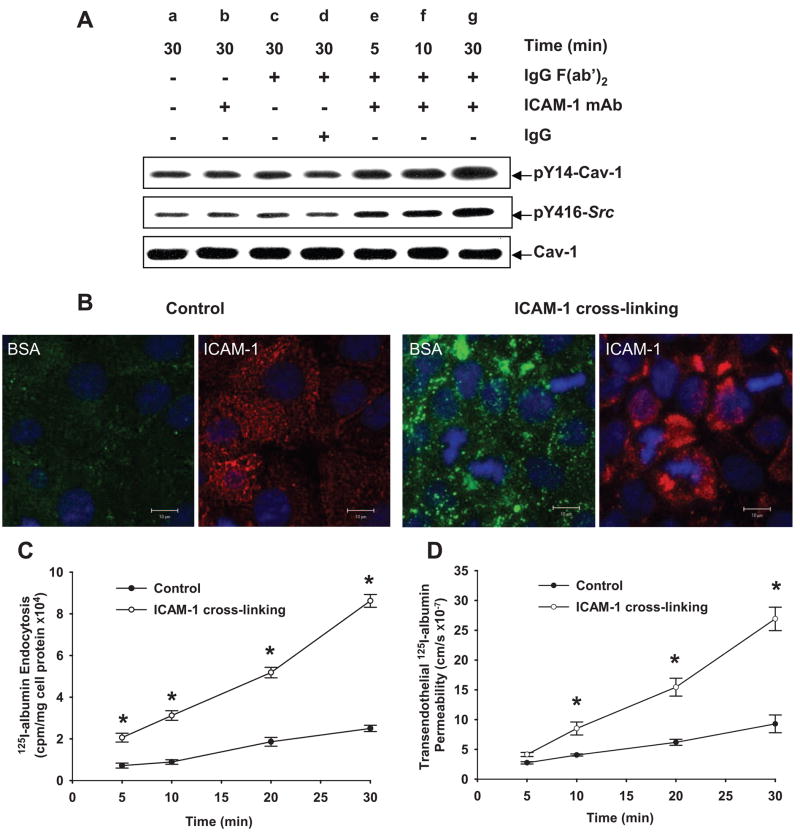
In the rat lung preparation, we observed that administration of anti–ICAM-1 blocking mAb (1A29), but not the isotype-matched control mAb, significantly reduced PMN activation-dependent increase in pulmonary vascular 125I-albumin PS product (Figure 6F). Using the ICAM-1 crosslinking method in endothelial cells,29,41 we observed activation of Src and Cav-1 phosphorylation (Figure 7A and supplemental Figure IIC). Neither Src activation nor Cav-1 phosphorylation was seen following treatment with anti–ICAM-1 mAb, control mouse IgG plus antimouse IgG (Fab′)2 (secondary Ab), or secondary Ab IgG (Fab′)2 alone (Figure 7A and supplemental Figure IIC). Confocal imaging showed ICAM-1 clustering on the endothelial cell surface and increased Alexa 488–albumin endocytosis (Figure 7B). ICAM-1 crosslinking also increased 125I-albumin endocytosis in endothelial cells (Figure 7C) and transendothelial albumin permeability (Figure 7D).
Figure 7.
ICAM-1 crosslinking induces Src activation and Cav-1 phosphorylation and activates albumin endocytosis and transcytosis. RLMVECs were incubated with 10 μg/mL anti–ICAM-1 Ab at 37°C for 30 minutes and washed and incubated with 0.1 μg/mL goat antimouse IgG F(ab′)2 at 37°C for 5, 10, and 30 minutes. A and supplemental Figure IIC, Effects of ICAM-1 crosslinking on Src activation and Cav-1 phosphorylation. B, Effects of ICAM-1 crosslinking on Alexa 488 –labeled albumin uptake (green) and anti–ICAM-1 IgG+anti-mouse Alexa 568 immunostaining (red). Confocal images show ICAM-1 clustering and increased tracer albumin uptake following ICAM-1 crosslinking. Scales bar=10 μm. C and D, Time course of ICAM-1 crosslinking effect on 125I-albumin endocytosis (C) and transcytosis (D). Results are typical of 3 experiments (A and B). n=4 to 6 for each time point (C and D). *P<0.05 compared with control group.
Discussion
The present study demonstrates a novel role of caveolae-mediated transcytosis of albumin in microvascular endothelial cells in the mechanism of PMN activation–induced increase in lung vascular permeability. Our results show that ICAM-1 signaling activated by the binding of PMNs to the endothelial cell surface promotes Src phosphorylation of Cav-1, a requirement for activation of the caveolar transport machinery.21–27 The increase in albumin permeability by means of caveolae was also shown to be an important mechanism of pulmonary edema formation. These results suggest that caveolae-mediated transport of albumin following PMN activation is an important determinant of the formation of pulmonary edema.
In our studies, we induced PMN adhesion to endothelial cells and activated PMNs with fMLP, a secretagogue specific to PMNs (versus endothelial cells).42 We observed that activation of PMNs increased endothelial permeability to albumin dependent on Src phosphorylation of Cav-1. This finding is consistent with the evidence that Src signaling is required for the engagement of the caveolae-mediated transcytosis machinery.21–27 Because endocytosis is the required initial step in transcytosis, we used several approaches to address the effects of activation of PMNs on albumin endocytosis. Using 125I-albumin and Alexa 488–albumin tracers, we observed that PMN activation with fMLP induced albumin endocytosis. The cholesterol-depleting agent cyclodextrin or siRNA-induced depletion of Cav-1 prevented the endocytosis of albumin, indicative of a caveolae-dependent mechanism. Using a number of PMNs sufficient to activate caveolae-mediated albumin transcytosis in endothelial monolayers, we did not observe a decrease in TER, a measure of loss of endothelial junctional integrity. Finally, data showed the inability of fMLP-activated PMNs (at a PMN:endothelial cell ratio of 10:1) to disrupt interendothelial junctions consistent with previous findings,43 whereas PMN activation with PMA, a potent PMN stimulating agent, resulted in decreased TER, a characteristic of severe endothelial junctional injury. Thus, activation of transcytosis under these conditions was not directly coupled to a disruption of endothelial junctional integrity.
The present studies were also carried out in rat and mouse lungs to buttress the endothelial monolayer studies and to address in vivo significance of PMN activation-induced increase in albumin transcytosis in endothelial cells. These results also showed that the PMN activation-induced increase in lung vascular permeability was dependent on caveolae-mediated transcytosis of albumin. Pulmonary edema developed only in isolated rat lungs in which 5×107 PMNs were added to the perfusate. Interestingly, a lower number of PMNs (2×107 PMNs) added to the perfusate and then activated with fMLP increased albumin permeability but produced no pulmonary edema, whereas a higher number of PMNs (5×107 cells) induced a greater increase in albumin permeability, as well as pulmonary edema. These results in lungs, which are consistent with the effects of addition of different PMN numbers to endothelial monolayers, suggest that PMNs activated with fMLP increase lung microvessel permeability via a caveolae-mediated pathway in a PMN number-dependent manner. The more severe edema observed with the higher PMN number may be the result of a greater increase in vascular permeability to albumin because of increases in both junctional permeability and transcytosis.
Our finding of reduced basal pulmonary microvascular albumin permeability in Cav-1−/− mice is consistent with other studies in these mice.18 Using siRNA to decrease Cav-1 expression in mouse lungs, we have shown an increase in the number of open interendothelial junctions and reduction in caveolae-mediated albumin transcytosis.44 The present studies are different because we have defined for the first time the important role for activation of caveolae-mediated transport induced by activated PMNs in pulmonary edema formation. Although a constitutive level of transcytosis via caveolae may be important in maintaining tissue fluid balance,1,10,13 the present studies reveal the potentially crucial pathogenic role of caveolae trafficking in the mechanism of edema formation.
There are several pathways available for the transport of plasma protein the size of albumin and greater across the continuous endothelium: caveolae, clathrin-coated vesicles, interendothelial junctions, and micropinocytosis involving caveolin- and clathrin-independent mechanisms.1 Caveolae-mediated transcytosis is thought to be the primary mechanism of basal albumin permeability across continuous endothelial cell barrier.1 Transcytosis was reduced by disruption of caveolae and inhibition of Src-dependent Cav-1 phosphorylation.21–25 Our findings suggest that binding of activated PMNs increases vascular permeability through the augmentation of this mechanism. Thus, caveolae trafficking represents the primary albumin transport pathway under basal conditions in continuous endothelial cells, and, as we have shown, it also contributes to the mechanism of increased albumin permeability after PMN activation. Caveolae are not detectable in Cav-1–null mice.19,20 However, a few vesicles of the same size or slightly greater than caveolae were detected19,20 but whose molecular identity has not been characterized. These structures were noted in the original Cav-1 mouse knockout studies by Drab et al19 and Razani et al20 but do not appear to compensate sufficiently for the loss of caveolae.
Transcytosis of albumin in endothelial cells requires the binding of albumin to the albumin binding protein gp60 on the cell surface,23,33 interaction of gp60 with Cav-1,23 and Src activation.22 We have shown that Src phosphorylates Cav-1, gp60, and dynamin-2 to initiate caveolae fission from the plasma membrane.21,22,24 In the present study, we showed that PMN activation also induced Src phosphorylation of Cav-1, whereas expression dn-Src prevented the phosphorylation, and, importantly, it inhibited endocytosis and transcytosis of albumin. Thus, Src phosphorylation of Cav-1 is a key determinant of PMN activation-mediated increase in albumin transcytosis in endothelial cells.
To address how Src kinase may be activated by PMNs, we focused on the role of ICAM-1 localized on the endothelial cell plasma membrane. Previous studies have shown that ICAM-1 binding to CD18 on PMNs mediates “outside-in” signaling that can activate Src.30 We observed here that activation of PMNs with fMLP caused endothelial cell surface clustering of ICAM-1 and that anti–ICAM-1 mAb blocked Src activation and Cav-1 phosphorylation, consistent with a role of ICAM-1–induced signaling. We also observed that anti–ICAM-1 blocking mAb prevented the PMN activation-induced increase in 125I-albumin endocytosis and transcytosis in endothelial cells. To address whether ICAM-1 may be directly responsible for signaling, we crosslinked cell surface ICAM-129,41 to induce clustering and observed activation of Src and Cav-1 phosphorylation. Moreover, ICAM-1 crosslinking increased albumin endocytosis and transendothelial albumin permeability, thus mimicking the effects of PMN activation of endothelial cells. Recent studies have shown a role for engagement of ICAM-1 by leukocytes in mediating Src activation and tyrosine phosphorylation of VE-cadherin as a requirement for transendothelial migration of PMNs.43 Thus, our results are consistent with the emerging concept that ICAM-1 engagement transmits signals into endothelial cells that promote PMN migration45 and, as shown in the present study, the activation of caveolae-mediated transcytosis of albumin contributing to pulmonary edema formation.
In summary, fMLP activation of PMNs increased caveolae-mediated transendothelial albumin permeability and induced pulmonary edema formation in rats and mice. The response was initiated by ICAM-1–dependent Src activation and Src phosphorylation of Cav-1 following the binding of PMN to endothelial cells, which resulted in caveolae-mediated transcytosis of albumin. Therefore, pulmonary vascular transcytosis of albumin is a potentially important mechanism contributing to pulmonary edema formation and needs to be considered as a crucial factor in the pathogenesis of acute lung injury.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by NIH grants R01 HL71626 (to R.D.M.), P01 HL60678 (to A.B.M. and R.D.M.) and American Heart Association Grant 0730331N (to G.H.).
Footnotes
Disclosures
None.
References
- 1.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86:279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- 2.Matthay MA, Wiener-Kronish JP. Intact epithelial barrier function is critical for the resolution of alveolar edema in humans. Am Rev Respir Dis. 1990;142:1250–1257. doi: 10.1164/ajrccm/142.6_Pt_1.1250. [DOI] [PubMed] [Google Scholar]
- 3.Matthay MA, Zimmerman GA. Acute lung injury and the acute respiratory distress syndrome: four decades of inquiry into pathogenesis and rational management. Am J Respir Cell Mol Biol. 2005;33:319–327. doi: 10.1165/rcmb.F305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lo SK, Everitt J, Gu J, Malik AB. Tumor necrosis factor mediates experimental pulmonary edema by ICAM-1 and CD18-dependent mechanisms. J Clin Invest. 1992;89:981–988. doi: 10.1172/JCI115681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lindbom L. Regulation of vascular permeability by neutrophils in acute inflammation. Chem Immunol Allergy. 2003;83:146–166. doi: 10.1159/000071559. [DOI] [PubMed] [Google Scholar]
- 6.Tinsley JH, Ustinova EE, Xu W, Yuan SY. Src-dependent, neutrophil-mediated vascular hyperpermeability and β-catenin modification. Am J Physiol. 2002;283:C1745–C1751. doi: 10.1152/ajpcell.00230.2002. [DOI] [PubMed] [Google Scholar]
- 7.Cepinskas G, Noseworthy R, Kvietys PR. Transendothelial neutrophil migration. Role of neutrophil-derived proteases and relationship to transendothelial protein movement. Circ Res. 1997;81:618–626. doi: 10.1161/01.res.81.4.618. [DOI] [PubMed] [Google Scholar]
- 8.Lum H, Malik AB. Mechanisms of increased endothelial permeability. Can J Physiol Pharmacol. 1996;74:787–800. doi: 10.1139/y96-081. [DOI] [PubMed] [Google Scholar]
- 9.Gibbs LS, Lai L, Malik AB. Tumor necrosis factor enhances the neutrophil-dependent increase in endothelial permeability. J Cell Physiol. 1990;145:496–500. doi: 10.1002/jcp.1041450315. [DOI] [PubMed] [Google Scholar]
- 10.Minshall RD, Tiruppathi C, Vogel SM, Malik AB. Vesicle formation and trafficking in endothelial cells and regulation of endothelial barrier function. Histochem Cell Biol. 2002;117:105–112. doi: 10.1007/s00418-001-0367-x. [DOI] [PubMed] [Google Scholar]
- 11.Predescu SA, Predescu DN, Palade GE. Plasmalemmal vesicles function as transcytotic carriers for small proteins in the continuous endothelium. Am J Physiol. 1997;272:H937–H949. doi: 10.1152/ajpheart.1997.272.2.H937. [DOI] [PubMed] [Google Scholar]
- 12.Bachofen H, Bachofen M, Weibel ER. Ultrastructural aspects of pulmonary edema. J Thorac Imaging. 1988;3:1–7. doi: 10.1097/00005382-198807000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Minshall RD, Sessa WC, Stan RV, Anderson RG, Malik AB. Caveolin regulation of endothelial function. Am J Physiol. 2003;285:L1179–L1183. doi: 10.1152/ajplung.00242.2003. [DOI] [PubMed] [Google Scholar]
- 14.Rothberg KG, Heuser JE, Donzell WC, Ying Y-S, Glenney JR, Anderson RG. Caveolin, a protein component of caveolae membrane coats. Cell. 1992;68:673–682. doi: 10.1016/0092-8674(92)90143-z. [DOI] [PubMed] [Google Scholar]
- 15.Pelkmans L, Helenius A. Endocytosis via caveolae. Traffic. 2002;3:311–320. doi: 10.1034/j.1600-0854.2002.30501.x. [DOI] [PubMed] [Google Scholar]
- 16.Stan RV. Structure and function of endothelial caveolae. Microsc Res Tech. 2002;57:350–364. doi: 10.1002/jemt.10089. [DOI] [PubMed] [Google Scholar]
- 17.Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev. 2004;84:869–901. doi: 10.1152/physrev.00035.2003. [DOI] [PubMed] [Google Scholar]
- 18.Schubert W, Frank PG, Razani B, Park DS, Chow CW, Lisanti MP. Caveolae-deficient endothelial cells show defects in the uptake and transport of albumin in vivo. J Biol Chem. 2001;276:48619–48622. doi: 10.1074/jbc.C100613200. [DOI] [PubMed] [Google Scholar]
- 19.Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, Menne J, Lindschau C, Mende F, Luft FC, Schedl A, Haller H, Kurzchalia TV. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293:2449–2452. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- 20.Razani B, Engelman JA, Wang XB, Schubert W, Zhang XL, Marks CB, Macaluso F, Russell RG, Li M, Pestell RG, Di Vizio D, Hou H, Jr, Kneitz B, Lagaud G, Christ GJ, Edelmann W, Lisanti MP. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem. 2001;276:38121–38138. doi: 10.1074/jbc.M105408200. [DOI] [PubMed] [Google Scholar]
- 21.Shajahan AN, Timblin BK, Sandoval R, Tiruppathi C, Malik AB, Minshall RD. Role of Src-induced dynamin-2 phosphorylation in caveolae-mediated endocytosis in endothelial cells. J Biol Chem. 2004;279:20392–20400. doi: 10.1074/jbc.M308710200. [DOI] [PubMed] [Google Scholar]
- 22.Tiruppathi C, Song W, Bergenfeldt M, Sass P, Malik AB. Gp60 activation mediates albumin transcytosis in endothelial cells by a tyrosine kinase-dependent pathway. J Biol Chem. 1997;272:25968–25975. doi: 10.1074/jbc.272.41.25968. [DOI] [PubMed] [Google Scholar]
- 23.Minshall RD, Tiruppathi C, Vogel SM, Niles WD, Gilchrist A, Hamm HE, Malik AB. Endothelial cell-surface gp60 activates vesicle formation and trafficking via Gi-coupled Src kinase signaling pathway. J Cell Biol. 2000;150:1057–1070. doi: 10.1083/jcb.150.5.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shajahan AN, Tiruppathi C, Smrcka AV, Malik AB, Minshall RD. Gβγ activation of Src induces caveolae-mediated endocytosis in endothelial cells. J Biol Chem. 2004;279:48055–48062. doi: 10.1074/jbc.M405837200. [DOI] [PubMed] [Google Scholar]
- 25.Parton RG, Joggerst B, Simons K. Regulated internalization of caveolae. J Cell Biol. 1994;127:1199–1215. doi: 10.1083/jcb.127.5.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li S, Seitz R, Lisanti MP. Phosphorylation of caveolin by Src tyrosine kinases. The α-isoform of caveolin is selectively phosphorylated by v-Src in vivo. J Biol Chem. 1996;271:3863–3868. [PubMed] [Google Scholar]
- 27.Glenney JR., Jr Tyrosine phosphorylation of a 22-kDa protein is correlated with transformation by Rous sarcoma virus. J Biol Chem. 1989;264:20163–20166. [PubMed] [Google Scholar]
- 28.Kevil CG, Okayama N, Alexander JS. H2O2-mediated permeability II: importance of tyrosine phosphatase and kinase activity. Am J Physiol. 2001;281:C1940–C1947. doi: 10.1152/ajpcell.2001.281.6.C1940. [DOI] [PubMed] [Google Scholar]
- 29.Wang Q, Pfeiffer GR, II, Gaarde WA. Activation of Src tyrosine kinases in response to ICAM-1 ligation in pulmonary microvascular endothelial cells. J Biol Chem. 2003;278:47731–47743. doi: 10.1074/jbc.M308466200. [DOI] [PubMed] [Google Scholar]
- 30.Tilghman RW, Hoover RL. The Src-cortactin pathway is required for clustering of E-selectin and ICAM-1 in endothelial cells. FASEB J. 2002;16:1257–1259. doi: 10.1096/fj.01-0969fje. [DOI] [PubMed] [Google Scholar]
- 31.Brumell JH, Burkhardt AL, Bolen JB, Grinstein S. Endogenous reactive oxygen intermediates activate tyrosine kinases in human neutrophils. J Biol Chem. 1996;271:1455–1461. doi: 10.1074/jbc.271.3.1455. [DOI] [PubMed] [Google Scholar]
- 32.Volonté D, Galbiati F, Pestell RG, Lisanti MP. Cellular stress induces the tyrosine phosphorylation of caveolin-1 (Tyr-14) via activation of p38 mitogen-activated protein kinase and c-Src kinase. Evidence for caveolae, the actin cytoskeleton, and focal adhesions as mechanical sensors of osmotic stress. J Biol Chem. 2001;276:8094–8103. doi: 10.1074/jbc.M009245200. [DOI] [PubMed] [Google Scholar]
- 33.John TA, Vogel SM, Tiruppathi C, Malik AB, Minshall RD. Quantitative analysis of albumin uptake and transport in the rat microvessel endothelial monolayer. Am J Physiol. 2003;284:L187–L196. doi: 10.1152/ajplung.00152.2002. [DOI] [PubMed] [Google Scholar]
- 34.Tiruppathi C, Malik AB, Del Vecchio PJ, Keese CR, Giaever I. Electrical method for detection of endothelial cell shape change in real time: assessment of endothelial barrier function. Proc Natl Acad Sci U S A. 1992;89:7919–7923. doi: 10.1073/pnas.89.17.7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams JH, Jr, Moser KM, Ulich T, Cairo MS. Harvesting the noncirculating pool of polymorphonuclear leukocytes in rats by hetastarch exchange transfusion (HET): yield and functional assessment. J Leukoc Biol. 1987;42:455–462. doi: 10.1002/jlb.42.5.455. [DOI] [PubMed] [Google Scholar]
- 36.Xu N, Rahman A, Minshall RD, Tiruppathi C, Malik AB. β2-Integrin blockade driven by E-selectin promoter prevents neutrophil sequestration and lung injury in mice. Circ Res. 2000;87:254–260. doi: 10.1161/01.res.87.3.254. [DOI] [PubMed] [Google Scholar]
- 37.Vogel SM, Minshall RD, Pilipovic M, Tiruppathi C, Malik AB. Albumin uptake and transcytosis in endothelial cells in vivo induced by albumin-binding protein. Am J Physiol. 2001;281:L1512–L1522. doi: 10.1152/ajplung.2001.281.6.L1512. [DOI] [PubMed] [Google Scholar]
- 38.Parker JC, Townsley MI. Evaluation of lung injury in rats and mice. Am J Physiol. 2004;286:L231–L246. doi: 10.1152/ajplung.00049.2003. [DOI] [PubMed] [Google Scholar]
- 39.Keller P, Simons K. Cholesterol is required for surface transport of influenza virus hemagglutinin. J Cell Biol. 1998;140:1357–1367. doi: 10.1083/jcb.140.6.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schnitzer JE, Oh P, Pinney E, Allard JJ. Filipin-sensitive caveolae-mediated transport in endothelium: reduced transcytosis, scavenger endocytosis, and capillary permeability of select macromolecules. J Cell Biol. 1994;127:1217–1232. doi: 10.1083/jcb.127.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sano H, Nakagawa N, Chiba R, Kurasawa K, Saito Y, Iwamoto I. Cross-linking of intercellular adhesion molecule-1 induces interleukin-8 and RANTES production through the activation of MAP kinases in human vascular endothelial cells. Biochem Biophys Res Commun. 1998;250:694–698. doi: 10.1006/bbrc.1998.9385. [DOI] [PubMed] [Google Scholar]
- 42.Harlan JM, Schwartz BR, Reidy MA, Schwartz SM, Ochs HD, Harker LA. Activated neutrophils disrupt endothelial monolayer integrity by an oxygen radical-independent mechanism. Lab Invest. 1985;52:141–150. [PubMed] [Google Scholar]
- 43.Burns AR, Bowden RA, MacDonell SD, Walker DC, Odebunmi TO, Donnachie EM, Simon SI, Entman ML, Smith CW. Analysis of tight junctions during neutrophil transendothelial migration. J Cell Sci. 2000;113:45–57. doi: 10.1242/jcs.113.1.45. [DOI] [PubMed] [Google Scholar]
- 44.Miyawaki-Shimizu K, Predescu D, Shimizu J, Broman M, Predescu S, Malik AB. siRNA-induced caveolin-1 knockdown in mice increases lung vascular permeability via the junctional pathway. Am J Physiol. 2006;290:L405–L413. doi: 10.1152/ajplung.00292.2005. [DOI] [PubMed] [Google Scholar]
- 45.Allingham MJ, van Buul JD, Burridge K. ICAM-1-mediated, Src- and Pyk2-dependent vascular endothelial cadherin tyrosine phosphorylation is required for leukocyte transendothelial migration. J Immunol. 2007;179:4053–4064. doi: 10.4049/jimmunol.179.6.4053. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.