Abstract
A facile synthetic protocol for the production of neomycin B derivatives with various modifications at the 5″ position has been developed. Structural activity relationship (SAR) against aminoglycoside resistant bacteria equipped with various aminoglycoside-modifying enzymes (AME's) was investigated. Enzymatic and molecular modeling studies reveal that the superb substrate promiscuity of AME's allows the resistant bacteria to cope with diverse structural modifications despite the observation that several derivatives show enhanced antibacterial activity than the parent neomycin. Surprisingly, when testing synthetic neomycin derivatives against other human pathogens, two leads exhibit prominent activity against both Methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococci (VRE) that are known to exert high level of resistance against clinically used aminoglycosides. These findings can be extremely useful in developing new aminoglycoside antibiotics against resistant bacteria. Our result also suggests that new biological and antimicrobial activities can be obtained by chemical modifications of old drugs.
Introduction
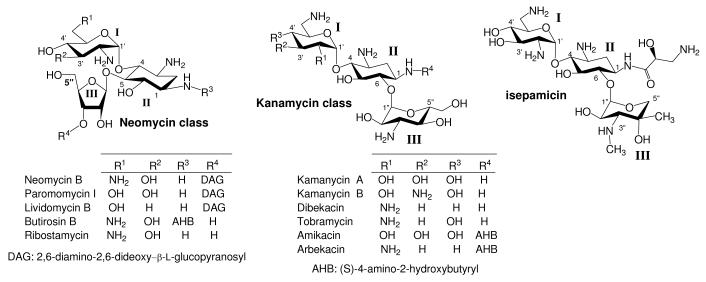
Chemical modification of aminoglycosides has been an effective way of reviving the antibacterial activity of aminoglycosides against resistant bacteria, especially against those that are equipped with aminoglycoside-modifying enzymes (AME's).1-3 For example, attachment of (S)-4-amino-2-hydroxybutanoyl (AHB) and (S)-3-amino-2-hydroxypropanoyl groups at N-1 position has led to the production of clinically useful aminoglycosides, amikacin and isepamicin, respectively (Figure 1). In general, protection of amino groups as carbamates, or transformation of amino groups into azido groups on aminoglycosides are the most commonly employed strategies for introducing desired structural motifs regiospecifically.
Figure 1.
Structures of Neomycin and Kanamycin Classes Aminoglycosides
Our group has been working on modifications of aminoglycoside antibiotics via the amine/azide transformation approach.4,5 The polyazido intermediates employed in the synthesis can be conveniently manipulated in organic media using traditional synthetic methods, which is ideal for constructing a library of aminoglycosides for identifying leads. However, the safety concern of handling azido compounds, the higher cost involved in the synthesis of azidoaminoglycosides, and the reduction of azido groups impose challenges in scale-up production of the leads for subsequent animal studies and clinical trials. By comparison, direct modification of commercially available aminoglycosides using carbamate type of protecting groups, which can be carried out in large quantity, appear to be a more practical approach for generating clinically useful novel aminoglycosides. Thus, we began to ponder the possibility of developing diversity-oriented synthesis of aminoglycoside derivatives via the use of carbamate type of protecting groups.
Design and Synthesis of 5″-Modified Neomycin B
Recently, several examples of 5″-modified neomycin class of aminoglycosides have been reported including conformational constrained aminoglycosides with intramolecular linkage between N-2′ and C-5″6,7 and between N-3 and N-6′,8 dimer of neomycin with spacing linkage via C-5″9, neomycin with 2,3-diaminopropanoyl group at 5″ position,10 and orthogonal divergent synthesis of neomycin derivatives with modifications at various amine functionalities.11 Although the constructed conformationally constrained aminoglycoside manifested much lower turn-over rate toward the AME of interest, the antibacterial activity also decreased significantly.6 The design of orthogonal divergent synthesis appears to have the potential of being employed for diversity-oriented synthesis. Nevertheless, the reported synthesis did not include the deprotection of carbamate protecting groups and the production of neomycin derivatives that can be assayed for their antibacterial activity.
In addition, it has also been suggested that aminoglycosides bearing deoxygenation at 3′ position, such as in the case of tobramycin, display relatively higher cytotoxicity than those that still contain 3′-OH.12 Neomycin B, which contains 3′-OH, exerts broad spectrum antibacterial activity and can be available in large quantity at relatively low cost. Thus, we reason that derivatizing neomycin with 5″-modifications could be valuable in investigating the structure-activity relationship (SAR) against resistant bacteria, the efficacy of avoiding enzyme-catalyzed modifications from aminoglycoside phosphotransferases (APH) that targets 3′-OH or, perhaps, aminoglycoside nucleotidyltransferases (ANT) that targets 4′-OH, and even the structure-cytotoxicity relationship. Neomycin derivatives bearing carbohydrate moieties at 5″-OH have also been shown to have good activity against a panel of bacteria.13,14
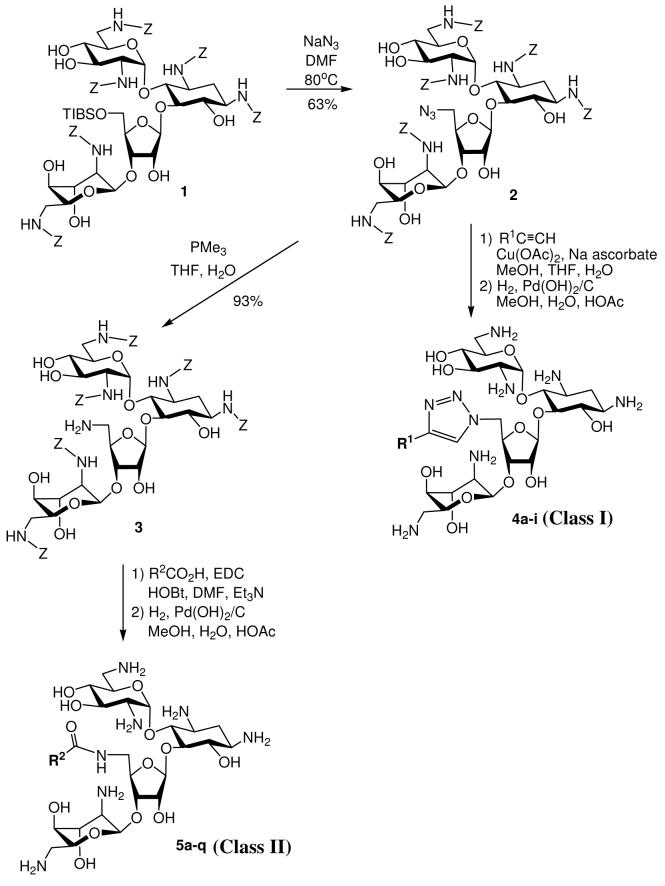
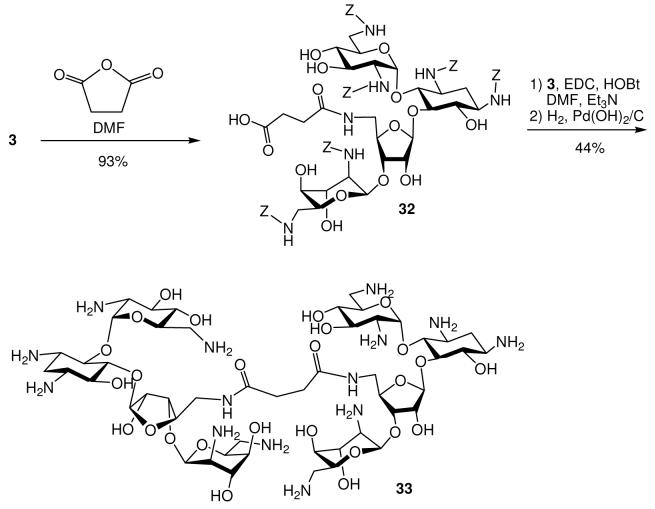
We prefer to employ the carbobenzyloxy (Cbz or Z) group as the protecting group of amines on neomycin since it can be tracelessly removed using hydrogenolysis. The synthetic strategy should be simple, which is suitable in producing a library of such derivatives for expedient screening and subsequent gram-scale synthesis of the leads. From our molecular modeling studies and SAR of others,13,14 we have also noticed that diverse functional groups can be attached at C-5″ without significantly obstructing the antibacterial activity. Finally, 5″-OH is the only primary hydroxyl group on neomycin B which can be regioselectively converted to azido group and serve as the site of diversity-oriented modifications via “Click” chemistry or amide linkage. Our initial design was to randomly introduce structural moieties containing alkyl, aryl, cationic and anionic substitutents for probing the SAR. The diversity-oriented synthesis started from a known Cbz-protected neomycin B derivative, 18 followed by regioselective substitution of 5″-OH with azide (Scheme 1). The 5″-azido group can undergo 1,3-dipolar cyclization with alkynes (class I design).15 Alternatively, the 5″-azido group of 1 can be reduced to an amino group and coupled with various carboxylic acids or amino acids (class II design). Global deprotection of the Cbz groups of these derivatives from both routes provided 5″-modified neomycin derivatives ready for antibacterial assay. The yields and structural designs are summarized in Tables 1 and 2. With the goal to expand the structural diversity, a dimeric neomycin derivative, 33 can be prepared by reacting the 5″-NH2 group with succinic anhydride providing 32, and then coupled to another molecule of 3 (Scheme 2).
Scheme 1.
Synthesis of Neomycin Derivatives
Table 1.
Yields and structural designs of class I 5″-modified neomycin derivatives
| Compounds | Alkynes | Yield (%) | Source of alkynes |
|---|---|---|---|
| 4a | N-propargyl-5-acetamidomethyl-2-oxazolidinone (6) | 86% | Ref. 35 |
| 4b | 1-octyne (7) | 69% | Commercially available |
| 4c | N,N-dimethyl propargylamine (8) | 66% | Commercially available |
| 4d | N-propargyl-2-phenyl-4-quinolinecarboxamide (9) | 69% | Prepared in this work |
| 4e | N-dimethyl propargylamine (10) | 61% | Commercially available |
| 4f | 2-ethynylpyridine (11) | 68% | Commercially available |
| 4g | N-carbobenzyloxypropargylamine (12) | 77% | Ref. 36 |
| 4h | N-propargylisonicotinamide (13) | 40% | Prepared in this work |
| 4i | N-carbobenzyloxy-L-proline N′-propargylamine (14) | 49% | Prepared in this work |
Table 2.
Yields and structural designs of class II 5″-modified neomycin derivatives
| Compounds | Carboxylic acids or amino acids | Yield (%) | Source of carboxylic acids or amino acids |
|---|---|---|---|
| 5a | heptanoic acid (15) | 88% | Commercially available |
| 5b | palmitic acid (16)- | 63% | Commercially available |
| 5c | stearic acid (17)- | 54% | Commercially available |
| 5d | 2-phenyl-4-quinolinecarboxylic acid (18) | 30% | Commercially available |
| 5e | (S)-Z-4-amino-2-benzyloxybutyric acid (19) | 90% | Ref. 37 |
| 5f | Z-Gly (20) | 27% | Commercially available |
| 5g | Z-l-Ala (21) | 36% | Commercially available |
| 5h | Z-l-Pro (22) | 50% | Commercially available |
| 5i | Z-l-Trp (23) | 67% | Commercially available |
| 5j | Z-l-Ser (24) | 52% | Commercially available |
| 5k | Z-l-Lys (25) | 61% | Commercially available |
| 5l | succinic anhydride (26) | 85% | Commercially available |
| 5m | Z-d-Ala (27) | 69% | Commercially available |
| 5n | Z-d-Ser (28) | 57% | Commercially available |
| 5o | Z-d-Pro (29) | 53% | Commercially available |
| 5p | Z-d-Lys (30) | 25% | Commercially available |
| 5q | Z-Gly-Gly (31) | 42% | Commercially available |
Scheme 2.
Synthesis of Neomycin Dimer
Antibacterial Activity of 5″-Modified Neomycin B
The synthesized aminoglycosides were assayed against susceptible and resistant bacterial strains using neomycin B as the control. Aminoglycoside susceptible Escherichia coli (ATCC 25922) and Staphylococcus aureus (ATCC 25923) were used as standard reference strains. E. coli (pTZ19U-3) and E. coli (pSF815) are laboratory resistant strains using E. coli (TG1) as the host. The first one is equipped with the pTZ19U-3 plasmid encoded for APH(3′)-I, which catalyzes phosphorylation at the 3′-OH of both neomycin and kanamycin classes of aminoglycosides. The second one is equipped with the pSF815 plasmid encoded for a bifunctional enzyme, AAC(6′)/APH(″), which catalyzes acetylation of the amino group at 6′-NH2 and phosphorylation of the hydroxyl group at 2″-OH. These enzymes are among the most prevalent modes of resistance found in aminoglycoside resistant strains. The minimum inhibitory concentrations (MIC's) are summarized in Table 3.
Table 3.
MIC of the 5″-Modified Neomycin Derivatives f
| Compounds | E. colia | S. aureusb | E. coli (TG1) c | E. coli (pSF815) d | E. coli (pTZ19U-3) e |
|---|---|---|---|---|---|
| Neomycin B | 4 | 1 | 4-8 | 4-8 | ≥2000 |
| 4a | 32 | 4-8 | 16 | 32 | ≥2000 |
| 4b | 32 | 4 | 8-16 | 16 | 500 |
| 4c | 8 | 2 | 8-16 | 16 | ≥2000 |
| 4d | 32 | 8 | 8-16 | 32 | 500-1000 |
| 4e | 8 | 1-2 | 16 | 16 | ≥2000 |
| 4f | 8-16 | 2 | 16 | 16 | ≥2000 |
| 4g | 8-16 | 1-2 | 16 | 16 | 1000 |
| 4h | 16-32 | 4 | 16 | 32 | ≥2000 |
| 4i | 16 | 2 | 16 | 16 | ≥2000 |
| 5a | 16-32 | 4 | 8 | 16-32 | ≥2000 |
| 5b | 4 | 2 | 2-4 | 16 | 8 |
| 5c | 4-8 | 4 | 2-4 | 32 | 8 |
| 5d | 8-16 | 4-8 | 8-16 | 16 | ≥2000 |
| 5e | 8-16 | 1-2 | 16 | 16-32 | ≥2000 |
| 5f | 16 | 1 | 8-16 | 8-16 | 500-1000 |
| 5g | 8-16 | 0.5 | 8-16 | 16 | ≥2000 |
| 5h | 16-32 | 0.5-1 | 16 | 16-32 | ≥2000 |
| 5i | 16 | 2 | 8-16 | 16 | ≥2000 |
| 5j | 8 | 1 | 8-16 | 16 | ≥2000 |
| 5k | 16-32 | 2 | 16 | 32 | 125-250 |
| 5l | 64 | 8 | 32 | 64 | ≥2000 |
| 5m | 16-32 | 1 | 16 | 16-32 | ≥2000 |
| 5n | 8-16 | 1 | 8-16 | 16 | ≥2000 |
| 5o | 8-16 | 0.5-1 | 8 | 16 | ≥2000 |
| 5p | 32 | 1-2 | 16 | 64 | ≥2000 |
| 5q | 16-32 | 2-4 | 16-32 | 16 | 125-250 |
| 33 | 32 | 2-4 | 8-16 | 16-32 | 500 |
Escherichia coli (ATCC 25922),
Staphylococcus aureus (ATCC 25923),
E. coli (TG1) (aminoglycoside susceptible strain),
E. coli (TG1) (pSF815 plasmid encoded for (AAC(6′)/APH(2″)),
E. coli (TG1) (pTZ19U-3 plasmid encoded for APH(3′)-I),
Unit: μg/mL.
From the MIC results, it appears that the 5″ position can tolerate the incorporation of diverse structural components, which is consistent with previous literature results.13,14 Derivatives with 1,2,3-triazole linkage manifested modest antibacterial activities comparable to neomycin B with 4e and 4g as the most active derivatives. Most of the derivatives bearing amide-based linkages were less active than the parent neomycin B. Nevertheless, derivatives incorporated with long linear alkyl chains (5b and 5c) showed better antibacterial activity than neomycin B, and the one with shorter linear alkyl chain (5a), even against resistant strains. Among the derivatives attached with amino acids, glycine (5f), alanine (5g and 5m) and proline (5h and 5o) manifested similar levels of antibacterial activity with no particular difference between d and l amino acids. On average, it appears that these 5″-modified neomycin derivatives are more active against G+ bacteria (S. aureus) than G− bacteria (E. coli). For example, there is only 4-fold MIC difference for neomycin B between G+ and G− bacteria while, in most of the synthetic derivatives, the differences are in the range of 4 to 16 fold.
As expected, compound 5l incorporated with a negatively-charged carboxylate group showed much lower antibacterial activity as compared to other derivatives. With the exception of 5b and 5c, all the derivatives are inactive against resistant strain equipped with APH(3′)-I while retaining moderate activities against the susceptible control strain. This finding implies that APH(3′)-I has much broader substrate promiscuity than the RNA binding site for 5″-modified neomycin derivatives, which, again, exemplifies the challenges in developing aminoglycosides against drug-resistant bacteria. The activity of 5b and 5c against resistant bacteria is of particular interest, which prompts further enzymatic and molecular modeling investigations.
Enzymatic and Antibacterial Studies of 5″-Modified Neomycin B against APH(3′)-IIIa
Although no significant activity against bacteria equipped with APH(3′)-I was observed for most of the derivatives, several derivatives did appear to have improved or similar activity as compared to the parent neomycin B. Thus, effort was devoted to investigation of the relationship between enzyme kinetic studies and whole-cell based assay. Our initial strategy is to introduce structural variants at the 5″-position, which is close to the 3′-OH targeted by many APH(3′). There are as many as seven isoforms of APH(3′) (I-VII) which have been identified with various substrate specificity among gram-negative and gram-positive bacteria.16-19 We selected APH(3′)-IIIa for enzymatic study due to consideration of its availability and clinical significance. For example, studies of the epidemiology of AME's have revealed the prevalence of the APH(3′)-IIIa in Methicillin-resistant Staphylococcus aureus (MRSA), Enterococcus faecalis and Enterococcus faecium.20-23
The enzyme APH(3′)-IIIa was expressed and purified according to the literature procedure.24 Enzyme kinetic studies for 5″-modified neomycin derivatives, kanamycin A, amikacin and neomycin B were performed as reported.25 The laboratory resistant strain was generated using E. coli (TG1) as the host harboring pET28a plasmid encoded for APH(3′)-IIIa. Neomycin B, kanamycin B (Kan B) and amikacin were used as controls in the antibacterial assay. The results from enzymatic studies and the MIC are summarized in Table 4.
Table 4.
Kinetic Parameters and MIC of Aminoglycosides for APH(3′)-IIIa
| Compound | KM (μM) | Vmax (μmol/mg/min) |
kcat (S−1) | kcat/KM/104 (S−1M−1) |
MIC against bacterium with APH(3′)-IIIa a |
MIC ratio b |
|---|---|---|---|---|---|---|
| Kanamycin A | 8.2±0.7 | 6.96±0.12 | 3.48±0.06 | 42.4 | 125 (Kan B) | 32 (Kan B) |
| Amikacin | 57.4±5.7 | 2.75±0.13 | 1.38±0.07 | 24.0 | 1 | 1 |
| Neomycin B | 6.0±0.8 | 3.22±0.17 | 1.61±0.09 | 26.8 | 64 | 8 |
| 4a | 160±15 | 0.41±0.03 | 0.21±0.02 | 0.13 | 125 | 8 |
| 4b | 127±13 | 0.75±0.04 | 0.38±0.02 | 0.30 | 32-64 | 4 |
| 4c | 120±14 | 0.65±0.05 | 0.33±0.03 | 0.28 | 64-125 | 8 |
| 4d | 410±38 | 0.30±0.03 | 0.15±0.02 | 0.037 | 16-32 | 2 |
| 4e | 100±9 | 0.72±0.07 | 0.36±0.04 | 0.36 | 125 | 8 |
| 4f | 150±16 | 0.54±0.05 | 0.27±0.03 | 0.18 | 125 | 8 |
| 4g | 111±12 | 0.68±0.04 | 0.34±0.02 | 0.3 | 64 | 4 |
| 4h | 171±18 | 0.41±0.04 | 0.21±0.02 | 0.12 | 64-125 | 8 |
| 4i | 175±18 | 0.40±0.04 | 0.20±0.02 | 0.14 | 64-125 | 8 |
| 5a | 48±6 | 0.95±0.06 | 0.48±0.05 | 1.0 | 64 | 8 |
| 5b | 58±4 | 0.85±0.08 | 0.42±0.05 | 0.72 | 1-2 | 0.5 |
| 5c | 60±7 | 0.84±0.05 | 0.42±0.03 | 0.7 | 2 | 1-2 |
| 5d | 400±35 | 0.38±0.04 | 0.19±0.02 | 0.048 | 32-64 | 4 |
| 5e | 50±4 | 0.93±0.07 | 0.47±0.05 | 0.94 | 125 | 8 |
| 5f | 75±8 | 0.88±0.06 | 0.44±0.03 | 0.6 | 32 | 2 |
| 5g | 60±5 | 0.90±0.09 | 0.45±0.05 | 0.75 | 64 | 4 |
| 5h | 80±9 | 0.63±0.07 | 0.32±0.03 | 0.4 | 64 | 4 |
| 5i | 322±31 | 0.57±0.06 | 0.29±0.03 | 0.1 | 64 | 4 |
| 5j | 42±4 | 1.01±0.09 | 0.51±0.05 | 1.2 | 32-64 | 4 |
| 5k | 78±8 | 0.79±0.08 | 0.40±0.03 | 0.51 | 32-64 | 4 |
| 5l | 53±6 | 0.86±0.09 | 0.43±0.04 | 0.81 | 250 | 8 |
| 5m | 63±7 | 0.82±0.09 | 0.41±0.04 | 0.65 | 64-125 | 8 |
| 5n | 57±6 | 0.93±0.10 | 0.47±0.05 | 0.82 | 64 | 4 |
| 5o | 70±8 | 0.72±0.07 | 0.36±0.04 | 0.51 | 64 | 8 |
| 5p | 75±8 | 0.70±0.07 | 0.35±0.04 | 0.47 | 64-125 | 8 |
| 33 | >500 | <0.1 | <0.05 | <0.01 | 64-125 | 8 |
Unit: μg/mL,
MIC ratio = (MIC against E. coli (TG1))/(MIC against E. coli (TG1) with APH(3′)-IIIa )
By comparing the kinetic parameters from the commercially available aminoglycosides (kanamycin A, amikacin and neomycin B), the presence of AHB group increases KM but lowers Vmax and kcat indicating that amikacin has lower binding affinity and turnover rate toward APH(3′)-IIIa. Interestingly, most of the 5″-modified neomycin derivatives manifest much higher KM and lower Vmax and kcat implying that these derivatives can better evade the enzyme-catalyzed modification as well. The high KM indicates that these 5″-modified neomycin derivatives are poor substrates for APH(3′)-IIIa. The low kcat suggests that the added functionalities at 5″ position could disrupt the rate of phosphorylation at 3′-OH even in the case of 5f where a relatively smaller glycine is incorporated. From the kinetic data, no obvious correlation between the kcat/KM and MIC can be deduced. For example, 4a has a relatively low kcat/KM but still exerts no significant activity in the whole-cell based antibacterial assay against the resistant strain. Several possible reasons can account for these observations: 1) binding of the 5″-modified neomycin derivatives to RNA targeted site could have been disrupted by the structural modifications leading to lower antibacterial activity; 2) APH(3′)-IIIa can still inactivate the 5″-modified neomycin derivatives. Although inactivation occurs at a much slower rate, the higher concentration of APH(3′)-IIIa as compared to the targeted rRNA site renders the lower kcat/KM values insignificant for most of the neomycin derivatives in regaining their antibacterial activity; and 3) the uptake of the 5″-modified neomycin derivatives by bacteria could have also been hampered by the structural modifications.
To better compare the changes of antibacterial efficiency, we calculated the MIC ratio by dividing the MIC against susceptible strain with the MIC against resistant one (right column, Table 4). Amikacin, which has excellent activity against resistant strain has MIC ratio of 1, which means there is no loss of activity against the resistant strain. Several interesting findings were noted. While neomycin and most of the neomycin derivatives have MIC ratio range from 4 to 8, two compounds, 5b and 5c have ratios of 0.5 and 1, respectively. The kinetic studies also suggested that these derivatives should be “poor substrates” for the enzyme. Thus, these results point to an interesting question: why are these two compounds still active against resistant strains while other derivatives with similar or low kcat/KM values are inactive or less active?
Two possible reasons could account for the observed activity of 5b and 5c having similar antibacterial efficiency as compared to amikacin and superior efficiency as compared to neomycin. First: the structural modifications at N-5″ of 5b and 5c may have similar effects as the AHB group at N-1 of amikacin. From the X-ray crystallography study,26 it has been demonstrated that AHB side chain can be accommodated by the binding site of rRNA while rendering amikacin a “poor substrate” for APH(3′)-IIIa. Second: these two derivatives may have different modes of antibacterial action. We favor the second postulate. Since the enzyme-catalyzed modifications occur within bacteria, neomycin derivatives with similar kcat/KM values should still undergo phosphorylation leading to the inactivation of these modified aminoglycosides. Therefore, 5b and 5c may exert their antibacterial activity not by binding to the rRNA which occurs within bacteria, but by a different mode of antibacterial action.
Compounds 5f and 4d exhibit better activity against resistant strains than does neomycin without suffering significant activity loss against susceptible strains. Since protected glycine can be readily prepared in large-scale and, in particular, 5f has excellent activity against S. aureus, we decided to treat compound 5f as a lead for further chemical modifications to be discussed later. Finally, despite having very low kcat/KM value, the dimer, 33 displayed only modest activities against susceptible strains and low activity against resistant strains. Once again, this result implies the broader substrate promiscuity of APH(3′)-IIIa than the binding pocket of targeted rRNA site. Overall, our results from enzymatic studies suggest that a low kcat/KM value may not be applicable to predict the antibacterial activity of aminoglycoside constructs against resistant bacteria equipped with AME's.
Molecular Modeling of 5″-Modified Neomycin B
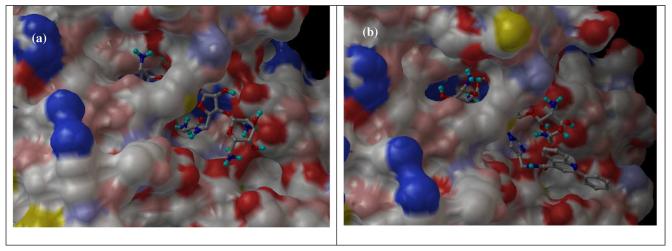
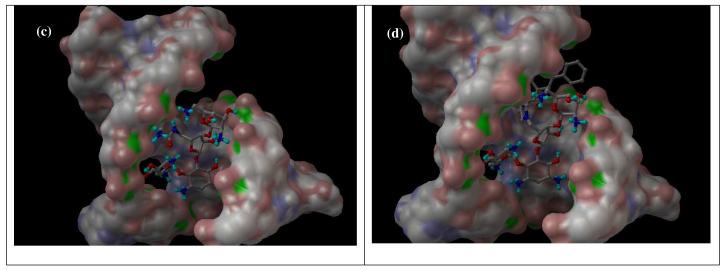
The bactericidal activity of neomycin and kanamycin arises from the binding of aminoglycosides to the A-site decoding region of 16S ribosomal RNA. To examine whether the targeted site of rRNA can accommodate the added functional groups at 5″ position, molecular modeling27 was carried out using the reported X-ray structures of APH(3′)-IIIa and neomycin-bound rRNA as the templates.28,29 Two particularly active compounds: 5f and 4d, representative of classes I and II, were selected for the molecular modeling analysis (Figure 2). While the part of the APH (3′)-IIIa enzyme responsible for the catalysis and the tight binding of the neamine portion of the molecule (rings I and II) is shaped in the form of a deep cleft, other fragments of the aminoglycoside molecule are bound to the rather sterically unhindered surface of the enzyme. This allows for the enzyme's unique substrate promiscuity, which is facilitated also by the substrate's conformational flexibility. Due to the steric consideration, the 5″-sidechain of 4d is pointed away from the enzyme implying poor recognition and turnover from APH(3′)-IIIa, which is supported by the high KM and low kcat/KM.
Figure 2.
(a) APH(3′)-IIIa with 5f, (b) APH(3′)-IIIa with 4d, (c) RNA with 5f, (d) RNA with 4d.
Both 5f and 4d can also bind to the targeted rRNA site although the 5″-sidechain appears to have significant flexibility by adopting different conformations in both cases. In contrast to the binding of neomycin derivatives to APH(3′)-IIIa, all four rings from neomycin scaffold interact with the RNA suggesting a lesser tolerance to structural modifications. It is expected that the larger 5″-sidechain of 4d will lower the binding affinity thus accounting for the lower antibacterial activity. Nevertheless, as mentioned previously, the superior substrate promiscuity and higher concentration of APH(3′) will render most of the structural modifications at rings III and IV less effective. Unfortunately, the 5″-sidechain of 5b and 5c exhibit too much freedom to be properly predicted by molecular modeling.
Although scoring was attempted, the scores obtained do not correlate well with the experimental MICs. We have noticed earlier that theoretical scores are better correlated with experiment for less active compounds.4 The correlation deteriorates for more active compounds. This suggests that the predictions are accurate when the ligand-macromolecule fit is the limiting factor. When it is not, other factors, such as solubility, transport or degradation of the aminoglycosides, cannot be neglected when constructing a quantitative, linear SAR.
Antibacterial Assay of Selected Derivatives against Other Pathogens
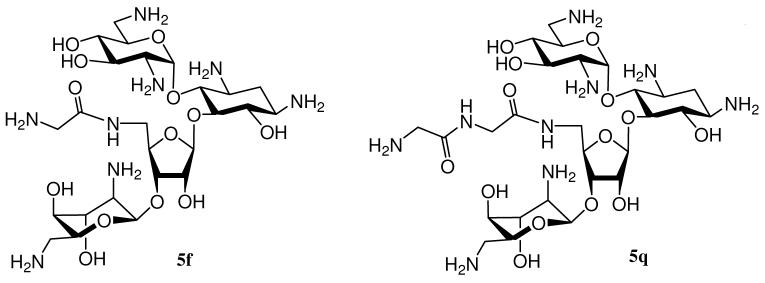
As mentioned previously, 5″-modified neomycin derivatives seem to be more active against G+ bacteria (S. aureus) than G− bacteria (E. coli), and 5f appears to be one of the most prominent leads having similar MIC ratio as amikacin. We decided to further derivatize 5f by preparing 5q by extending one glycidal unit (Figure 3). In addition, we also intend to explore the spectrum of antibacterial activity of 5b and 5c against other clinically significant pathogens including Klebsiella pneumoniae (Gram negative), Methicillin-resistant S. aureus (MRSA), Pseudomonas aeruginosa (Gram negative) and Enterococcus faecalis (Gram positive).
Figure 3.
Structures of 5f and 5q
K. pneumoniae (ATCC 700603) is a clinical isolate that is resistant to ceftazidime, other β-lactams, and several aminoglycosides (ANT(2″).30 K. pneumoniae (ATCC 13883) is resistant to ampicillin but susceptible to aminoglycosides. P. aeruginosa (ATCC 27853) that expresses APH(3′)-IIb manifests modest resistance toward aminoglycosides.31 Methicillin-resistant S. aureus (ATCC 33591) (MRSA) is the leading cause of bacterial infections and many MRSA strains harbor genes encoded for APH(3′), ANT(4′), and AAC(6′)/APH(2″), which render the bacteria resistant to various aminoglycosides.20 It has been reported that microorganisms like enterococci and other anaerobes are intrinsically resistant to aminoglycosides due to the dull uptake of aminoglycosides as a result of the deficiency in their membrane-associated electron transport systems.19 Like MRSA, vancomycin-resistant enterococci (VRE) that cause various types of nosocomial infections also represent a great threat to the public health.32 Enterococcus faecalis (ATCC51299) which is one of the vancomycin-resistant enterococci (VRE), contains vanB, ant(6)-I, and aac(6′)-aph(2″) resistance genes and exerts high level of resistance to aminoglycosides and vancomycin.33 E. faecalis (ATCC 29212), which is susceptible to vancomycin but manifests moderate resistance to aminoglycosides, is used as comparison.
The results from the antibacterial assay are summarized in Table 5. As expected, the derivatives with relatively smaller structural modifications (5f and 5q) display similar antibacterial profile as neomycin suggesting unaltered modes of bactericidal and resistant mechanisms. 5f and 5q are more active against P. aeruginosa as compared to neomycin but are less active as compared to amikacin and gentamicin. Overall, 5f is slightly more active than neomycin implies that the structural modification with glycidyl group is beneficial. Nonetheless, such a modification is insufficient in reviving the antibacterial activity against aminoglycoside resistant bacteria.
Table 5.
MIC of the 5″-Modified Neomycin Derivatives against Other Strains of Bacteria a
| entry | Compound |
K. pneumoniae b |
K. pneumoniae c |
S. aureus d |
P. aeruginosa e |
E. faecalisf | E. faecalisg |
|---|---|---|---|---|---|---|---|
| 1 | 5b | 8-16 | 16-32 | 2-4 | 4 | 2-4 | 4-8 |
| 2 | 5c | 8-16 | 32 | 4-8 | 8-16 | 4-8 | 8-16 |
| 3 | 5f | 4 | 8 | 16-32 | 8-16 | 32-64 | ≥ 250 |
| 4 | 5q | 4 | 8 | 32 | 16-32 | 64-125 | ≥ 250 |
| 5 | Neomycin B | 4 | 16-32 | 125 | 64 | 64-125 | ≥ 250 |
| 6 | Amikacin | 1 | 0.5 | 8-16 | 0.5-1 | 32-64 | ≥ 250 |
| 7 | Gentamicin | 1 | 8 | 2-4 | 0.5-1 | 8-16 | ≥ 250 |
| 8 | Vancomycin | ND | ND | ND | ND | ND | 125 |
Unit: μg/mL, ND: Not Determined,
Klebsiella pneumoniae (ATCC 13883),
K. pneumoniae (ATCC 700603),
Staphylococcus aureus (ATCC 33591) (MRSA),
Pseudomonas aeruginosa (ATCC 27853),
Enterococcus faecalis (ATCC 29212),
E. faecalis (ATCC51299) (VRE).
Intriguing results were obtained from the screening of 5b and 5c that have drastic difference in their antibacterial activity profile from other aminoglycosides. In general, 5b and 5c are also more active against G+ bacteria than G− bacteria as indicated in the MIC values. However, 5b and 5c exhibit prominent activity against E. faecalis (ATCC29212) while other aminoglycosides, including clinically used amikacin and gentamicin, are much less active. More impressively, 5b and 5c show excellent activity against VRE as compared to amikacin, gentamicin and even vancomycin. As mentioned previously, enterococci have intrinsic resistance against aminoglycosides. This result strongly supports our speculation that 5b and 5c have different modes of antibacterial action. We have also introduced similar structural modifications on kanamycin class aminoglycosides.34 However, from the preliminary screening, such modifications are ineffective in reviving the activity of these kanamycin analogs against aminoglycoside resistant bacteria. Thus, to our knowledge, the modifications on 5b and 5c are one of the very few examples of aminoglycoside derivatives with clinically useful activity against MRSA, VRE and other resistant strains.
Discussion and Conclusion
We have developed a facile and cost-effective method for constructing neomycin derivatives. Although the original strategy was to avoid the inactivation from APH(3′), unexpected and useful outcomes were obtained. First of all, structural modifications, which can be accommodated by the binding site of rRNA, can be readily accommodated by AME's as well. Results from our enzymatic studies show that low kcat/KM values for the structurally modified aminoglycosides may not applicable to suggest that these aminoglycosides could avoid the enzyme-catalyzed inactivation and regain their antibacterial activity. Molecular modeling results indicate that AME's recognition on neamine ring is sufficient for enzymes to inactivate aminoglycoside derivatives bearing diverse modifications at rings III and IV of neomycin or ring III of kanamycin. Structural modifications targeting on neamine, such as the attachment of AHB group at N-1 and 3′,4′-dideoxygenation, remain to be the few effective approaches. Although several examples from our work show enhanced activity is possible, it could be challenging to reproduce the effectiveness of N-1 AHB group on kanamycin or ribostamycin. In summary, it may not be easy to develop structurally modified aminoglycosides active against resistant bacteria while maintaining the same mode of antibacterial action of traditional aminoglycosides.
Nonetheless, our results also reveal another strategy to combat the problem of resistance. It is possible to revive the antibacterial activity of aminoglycoside using structural modifications that can alter the original mode of action. For example MRSA and VRE are known to exert high level resistance to aminoglycosides. The discovery of two leads active against both MRSA and VRE is particularly significant since the transfer of resistance from enterococci to opportunistic S. aureus has been recognized as one of the stringent threats to public health.33 Ongoing efforts have been devoted into the investigation of possible antibacterial modes of action for 5b and 5c, and further structural optimization from these two leads.
Experimental Section
General Procedure for Coupling of Compound 5 with Carboxylic Acids
To a solution of compound 3 (0.20 g, 0.14 mmol) and carboxylic acids (0.28 mmol) in DMF (10 mL) and Et3N (0.04 mL, 0.28 mmol), HOBt (0.030 g, 0.21 mmol) and EDC (0.040 g, 0.21 mmol) were added. The reaction was stirred at room temperature overnight. After completion of the reaction, the reaction was concentrated and diluted with EtOAc. The organic solution was washed with water, saturated NaHCO3(aq), brine and dried over anhydrous Na2SO4. After removal of the solvent followed by a fast gradient column chromatography (eluted from hexane/EtOAc = 1/1 to EtOAc/MeOH = 9:1), the product was usually obtained as a solid, which was characterized with 1H and/or 13C NMR and subjected to hydrogenation without further purification.
General Procedure for the 1,3-Dipolar Cycloaddition
A solution of compound 3 (0.028 mmol), alkyne (0.05 mmol), Cu(OAc)2 (0.05 mmol) and sodium ascorbate (0.05 mmol) in a mixed solution of MeOH (1.7 mL), THF (0.46 mL) and water (0.3 mL) was sonicated at ambient temperature for 14 minutes (7 min.×2). After completion of the reaction, the reaction mixture was diluted with CH2Cl2 then filtered through Celite. After removal of the solvent followed by a fast gradient column chromatography purification (CH2Cl2:MeOH = 100:0 to 60:40), the product was typically obtained as a solid, which was characterized with 1H and/or 13C NMR and subjected to hydrogenation without further purification.
General Procedure for Hydrogenation and Purification
The solids from 1,3-dipolar cycloaddition or acid/amine coupling reaction (0.1 – 0.2 mmol) were dissolved in degassed MeOH (9 mL) followed by the addition of 1 mL HOAc : H2O (1/4 ratio) solution. Catalytic amount of Pd(OH)2/C powder was added and the system was well sealed and further degassed. The system was stirred under atmospheric H2 at room temperature for 10 hours. The reaction was then quenched by filtering through Celite and the residue washed with H2O and the combined solutions were concentrated. The crude product was purified with Amberlite CG50 (NH4+) eluted with a gradient of NH4OH solution (0% – 20%). After collection of the desired fractions and removal of solvent, the product was re-dissolved in water and loaded to an ion-exchange column packed with Dowex 1X8-200 (Cl− form), and eluted with water. After removal of solvent, the product was obtained as white solid.
N-Propargyl-2-phenyl-4-quinolinecarboxamide (9)
To a solution of 2-phenyl-4-quinolinecarboxylic acid (2.09 g, 8.02 mmol) DMF (30 mL), Et3N (2.3 mL), EDC (2.31 g, 12.0 mmol) and HOBt (1.63 g, 12.0 mmol) were added. The reaction was stirred at room temperature for 2 days. After completion of the reaction, reaction was concentrated and then diluted with EtOAc. The organic solution was washed with 1N HCl, H2O, NaHCO3 and brine, and then dried over Na2SO4. After removal of solvent and recrystallization from ether, the desired product was obtained as a crystal (1.69 g, 5.90 mmol, 74%). 1H NMR (CDCl3, 400MHz) δ 8.17 (d, J = 9.5 Hz, 1H), 8.1 (m, 2H), 7.81 (s, 1H), 7.72 (td, J = 9.2 Hz, J = 1.1 Hz, 1H), 7.4 – 7.5 (m, 5H), 6.54 (s, 1H), 4.36 (dd, J = 5.9 Hz, J = 2.9 Hz, 2H), 2.35 (t, J = 2.9Hz, 1H); 13C (CDCl3, 100MHz) δ166.6, 156.1, 148.0, 141.1, 138.1, 129.8, 129.5, 129.3, 128.4 (2 carbons), 127.0, 126.9(2 carbons), 124.3, 122.7, 116.0, 78.3, 72.0, 29.3; ESI/APCI Calcd for C19H15N2O ([M+H]+ m/e 287.1179; found m/e 287.1176.
N-Propargylisonicotinamide (13)
The product was synthesized similarly as compound 9 except the purification was accomplished using gradient column chromatography (eluted from Hexane/EtOAc = 60/40 to Hexane/EtOAc = 0/100, 92%). 1H NMR (CDCl3, 400 MHz) δ 8.71 (dd, J = 4.5 Hz, J = 1.7 Hz, 2H), 7.61 (dd, J = 4.5 Hz, J = 1.6 Hz, 2H), 6.81 (s, 1H), 4.23 (dd, J = 5.3 Hz, J = 2.6 Hz, 2H), 2.27 (dd, J = 2.4 Hz, J = 1.1 Hz, 1H); 13C NMR (CDCl3, 100 MHz) δ 165.5, 150.8 (2 carbons), 136.0, 121.2 (2 carbons), 79.0, 72.5, 30.1; ESI/APCI Calcd for C9H9N2O ([M+H]+) m/e 161.0709; found m/e 161.0713.
N-propargylcarbobenzyloxy-L-prolinamide (14)
The product was synthesized similarly as compound 9 except the purification was accomplished using gradient column chromatography (eluted from Hexane/EtOAc = 90/10 to Hexane/EtOAc = 30/70, 74%). 1H NMR (CDCl3, 400 MHz) δ 7.3 (m,, 5H), 5.1 (m, 2H), 4.26 (s, 1H), 3.90 (d, J = 9.7 Hz, 2H), 3.4 (m, 2H), 1.8 – 2.2 (m, 6H); 13C NMR (CDCl3, 100 MHz) δ 172.4, 156.1, 136.6, 128.7 (2 carbons), 128.2, 128.0 (2 carbons), 79.9, 71.6, 60.8, 47.7, 31.3, 29.2, 28.9, 24.7; ESI/APCI Calcd for C16H19N2O3+ ([M+H]+) m/e 287.1390; found m/e 287.1401.
Compound 2
A solution of compound 1 (1.50 g, 0.89 mmol) and NaN3 (0.12 g, 1.78 mmol) in DMF was stirred at 80°C overnight. TLC showed the completion of the reaction (Rf = 0.50 eluted with EtOAc/MeOH = 97/3). The reaction was filtered through Celite and the residue was washed with EtOAc. After the removal of the solvent followed by purification with a gradient column chromatography (CH2Cl2:MeOH = 100:0 to 90:10), the product was obtained as a white solid (0.81 g, 63%). 1H NMR (CD3COCD3, 400 MHz) δ 7.2 - 7.4 (m, 30H), 6.56 (m, 2H), 6.36 (d, J = 9.6 Hz, 1H), 4.8 – 5.3 (m, 18H), 4.5 (m, 4H), 3.8 – 4.1 (m, 6H), 3.3 – 3.9 (m, 20H); 13C NMR (CD3COCD3, 100 MHz) δ 157.6 (2 carbons), 157.4, 156.8, 156.5 (2 carbons), 137.4 – 137.7 (6 carbons), 127.7 – 128.7 (30 carbons), 110.0, 100.3, 99.1, 86.6, 80.5, 79.4, 74.8, 74.4, 73.1, 72.6, 72.3, 71.7, 70.3, 67.9, 66.0 – 67.9 (8 carbons), 56.6, 53.2, 51.9, 51.3, 42.6, 41.3, 34.8; ESI/APCI Calcd for C71H81N9O24Na ([M+Na]+) m/e 1466.5292; found m/e 1466.5263.
Compound 3
To a solution of compound 3 (1.98 g, 1.37 mmol) in THF (20 mL) and several drops of water, PMe3 in THF (8.2 mL, 8.2 mmol) was added. The reaction was stirred at 50°C for an hour and TLC showed the completion of the reaction (Rf = 0.02 eluted with EtOAc/MeOH = 97/3). After the removal of the solvent followed by purification with a gradient column chromatography (CH2Cl2:MeOH = 100:0 to 70:30), the product was obtained as a white solid (1.80 g, 93%). 1H NMR (CD3OD, 400 MHz) δ 7.3 – 7.4 (m, 30H), 5.1 (m, 10H), 3..3 – 3.9 (m, 37H); 13C NMR (CD3OD, 100 MHz) δ 157.6 – 158.0 (6 carbons), 136.9 – 137.1 (6 carbons), 127.7 – 128.4 (30 carbons), 110.9, 98.8, 98.1, 86.6, 79.4, 78.7, 74.8, 74.0, 73.3, 71.9, 71.8, 71.1, 70.3, 67.8, 56.2, 52.9, 51.7, 50.8, 43.4, 42.0, 41.1, 39.0, 33.6, 30.4, 29.6, 28.9, 24.4, 23.7, 22.8; ESI/APCI Calcd for C71H84N7O24 ([M+H]+) m/e 1418.5568; found m/e 1418.5536.
Compound 4a
1H NMR (D2O, 400 MHz) δ 8.08 (s, 1H), 5.95 (d, J = 3.4 Hz, 1H), 5.34 (s, 1H), 4.3 (t, J = 5.0 Hz, 2H), 4.14 (s, 2H), 4.06 (t, J = 9.7 Hz, 1H), 3.8 – 4.0 (m, 6H), 3.8 (m, 2H), 3.6 – 3.8 (m, 3H), 3.4 – 3.5 (m, 4H), 3.2 – 3.4 (m, 11H), 2.3 (m, 1H), 2.0 (m, 1H), 1.82 (s, 3H); 13C NMR (D2O, 100 MHz) δ 174.8, 159.4, 142.8, 125.5, 110.1, 95.5, 95.0, 84.9, 79.9, 76.8, 75.3, 73.5, 73.1, 72.4, 70.6, 70.4, 69.9, 68.2, 67.8, 67.5, 53.5, 52.2, 51.0, 49.9, 48.7, 47.0, 41.9, 40.7, 40.2, 38.6, 28.3, 22.0; ESI/APCI Calcd for C32H58N11O15 ([M+H]+) m/e 836.4108; found m/e 836.4107.
Compound 4b
1H NMR (D2O, 400 MHz) δ 7.80 (s, 1H), 5.78 (d, J = 3.7 Hz, 1H), 5.30 (d, J = 3.8 Hz, 1H), 5.21 (m, 1H), 4.5 (m, 2H), 4.2 (m, 1H), 3.9 (m, 1H), 3.7 – 3.9 (m, 6H), 3.5 (m, 2H), 3.3 (m, 5H), 3.2 (m, 3H), 2.62 (t, J = 7.4 Hz, 2H), 2.22 (m, 1H), 1.97 (m, 1H), 1.6 (m, 2H), 1.1 – 1.2 (m, 8H), 0.75 (m, 3H); 13C NMR (D2O, 100 MHz) δ 149.4, 123.9, 109.9, 95.9, 95.4, 85.4, 79.9, 78.0, 77.2, 73.2, 73.0, 70.9, 69.3, 68.9, 67.8, 67.5, 53.8, 51.7, 51.0, 50.3, 48.8, 46.8, 40.7, 40.3, 30.8, 30.4, 28.8, 27.9, 24.5, 22.1,13.5; ESI/APCI Calcd for C31H60N9O12 ([M+H]+) m/e 750.4356; found m/e 750.4333.
Compound 4c
1H NMR (D2O, 400 MHz) δ 8.39 (s, 1H), 5.97 (d, J = 3.6 Hz, 1H), 5.40 (d, J = 2.9 Hz, 1H), 5.33 (s, 1H), 4.9 (dd, J = 14 Hz, J = 1.0 Hz, 1H), 4.81 (dd, J = 15 Hz, J = 7.4 Hz, 1H), 4.61 (t, J = 5.7 Hz, 1H), 4.5 (m, 4H), 4.5 (s, 2H), 4.35 (t, J = 5.3 Hz, 1H), 4.22 (t, J = 3.0 Hz, 1H), 4.12 (dd, J = 7.6 Hz, J = 2.9 Hz, 1H), 4.0 (m, 1H), 3.98 (t, J = 9.2 Hz, 2H), 3.8 – 3.9 (m, 3H), 3.7 (m, 2H), 3.6 (m, 1H), 3.4 – 3.5 (m, 6H), 3.2 – 3.3 (m, 3H), 2.3 (m, 1H), 1.7 (m, 1H); 13C NMR (D2O, 100 MHz) δ 137.2, 128.4, 110.4, 95.7, 95.4, 85.3, 79.8, 77.0, 76.9, 73.1, 73.0, 71.0, 70.6, 69.5, 69.0, 67.9, 67.6, 53.9, 52.9, 51.3, 51.1, 50.4, 49.0, 42.5 (2 carbons), 40.8, 40.5, 30.1; ESI/APCI Calcd for C28H55N10O12 ([M+H]+) m/e 723.3995; found m/e 723.4022.
Compound 4d
1H NMR (D2O, 400 MHz) δ 8.14 (s, 1H), 7.88 (t, J = 8.6 Hz, 2H), 7.73 (m, 3H), 7.68 (t, J = 8.2 Hz, 1H), 7.49 (t, J = 8.1 Hz, 1H), 7.4 (m, 3H), 5.94 (d, J = 3.9 Hz, 1H), 5.32 (d, J = 3.5 Hz, 1H), 5.21 (d, J = 1.5 Hz, 1H), 4.5 (m, 2H), 4.2 (m, 2H), 4.1 (m, 1H), 3.8 – 3.9 (m, 6H), 3.7 (m, 1H), 3.6 (m, 1H), 3.5 (m, 2H), 3.2 – 3.4 (m, 9H), 2.31 (dt, J = 8.4 Hz, J = 4.1 Hz, 1H), 1.73 (m, 1H); 13C NMR (D2O, 100 MHz) δ 169.7, 157.7, 147.5, 144.8, 142.2, 137.9, 131.3, 130.4, 129.2 (2 carbons), 128.3, 128.1, 127.9 (2 carbons), 125.0, 124.8, 123.0, 118.2, 110.0, 95.7, 95.2, 85.0, 79.9, 76.9, 76.2, 73.1, 72.6, 70.7, 70.3, 69.7, 68.4, 67.8, 67.5, 53.6, 52.2, 50.9, 50.0, 48.7, 40.7, 40.2, 35.1, 28.9; ESI/APCI Calcd for C42H59N11O13 ([M+H]+) m/e 948.4186; found m/e 948.4208.
Compound 4e
1H NMR (D2O, 400 MHz) δ 8.22 (s, 1H), 5.85 (d, J = 3.5 Hz, 1H), 5.30 (d, J = 2.9 Hz, 1H), 5.24 (d, J = 1.5 Hz, 1H), 4.8 (dd, J = 15Hz, J = 2.8 Hz, 2H), 4.52 (t, J = 5.9 Hz, 1H), 4.5 (m, 1H), 4.32 (s, 2H), 4.27 (t, J = 5.1 Hz, 1H), 4.14 (t, J = 3.0 Hz, 1H), 4.05 (dd, J = 4.6 Hz, J = 2.9 Hz, 1H), 3.96 (m, 1H), 3.85 (t, J = 9.3 Hz, 2H), 3.8 (m, 1H), 3.6 (m, 1H), 3.51 (s, 1H), 3.3 -3.4 (m, 5H), 3.2 (m, 4H), 2.66 (m, 3H), 2.20 (m, 1H), 1.57 (m, 1H); 13C NMR (D2O, 100 MHz) δ 138.5, 127.1, 110.3, 95.6, 95.4, 85.4, 79.7, 78.0, 76.8, 73.0, 72.9, 71.0, 70.5, 69.3, 69.2, 67.9, 67.6, 53.9, 52.7, 51.0, 50.4, 48.9, 42.7, 40.7, 40.4, 32.3, 31.2; ESI/APCI Calcd for C27H52N10O12Na ([M+Na]+) m/e 731.3658; found m/e 731.3693.
Compound 4f
1H NMR (D2O, 400 MHz) δ 8.18 (s, 1H), 5.90 (d, J = 3.2 Hz, 1H), 5.31 (s, 1H), 5.21 (s, 1H), 4.53 (m, 1H), 4.46 (d, J = 8.9 Hz, 2H), 4.27 (m, 1H), 4.1 (m, 2H), 3.8 – 3.9 (m, 5H), 3.1 – 3.6 (m, 14H), 2.20 (m, 2H), 1.9 (m, 3H), 1.6 (m, 3H); 13C NMR (D2O, 100 MHz) δ 144.4, 124.9, 110.4, 95.6, 95.3, 85.4, 82.0, 79.7, 77.0, 76.9, 75.2, 72.9, 71.0, 70.5, 69.3, 69.0, 67.9, 67.6, 53.8, 52.0, 51.0, 50.4, 48.9, 45.1, 40.7, 40.4, 30.5, 28.3, 21.7, 21.6; ESI/APCI Calcd for C30H57N10O12 ([M+H]+) m/e 749.4152; found m/e 749.4165.
Compound 4g
1H NMR (D2O, 400 MHz) δ 8.19 (s, 1H), 6.04 (d, J = 3.9 Hz, 1H), 5.36 (d, J = 3.1 Hz, 1H), 5.26 (d, J = 1.6 Hz, 1H), 4.53 (t, J = 5.2 Hz, 1H), 4.39 (m, 1H), 4.26 (m, 3H), 4.0 – 4.1 (m, 2H), 3.9 – 4.0 (m, 4H), 3.7 – 3.8 (m, 2H), 3.2 – 3.5 (m, 10H), 2.42 (m, 1H), 1.97 (m, 1H); 13C NMR (D2O, 100 MHz) δ 140.3, 126.1, 110.2, 95.5, 95.1, 85.0, 80.0, 76.8, 75.0, 73.0, 72.5, 70.6, 70.4, 70.0, 68.2, 67.8, 67.5, 53.4, 52.7, 51.0, 49.9, 48.6, 40.7, 40.3, 34.2, 28.1; ESI/APCI Calcd for C26H50N10O12 ([M+Na]+) m/e 717.3502; found m/e 717.3525.
Compound 4h
1H NMR (D2O, 400 MHz) δ 7.96 (s, 1H), 5.90 (d, J = 3.8 Hz, 1H), 5.31 (d, J = 3.3 Hz, 1H), 5.24 (d, J = 1.6 Hz, 1H), 4.48 (m, 2H), 4.39 (s, 2H), 4.26 (t, J = 4.6 Hz, 1H), 4.14 (t, J = 3.1 Hz, 1H), 3.8 – 3.9 (m, 5H), 3.74 (m, 1H), 3.6 (dd, J = 9.0 Hz, J = 7.0 Hz, 1H), 3.51 (m, 1H), 3.2 – 3.4 (m, 12H), 2.96 (td, J = 12.9 Hz, J = 3.0 Hz, 2H), 2.6 (m, 1H), 23 (dt, J = 8.6 Hz, J = 4.2 Hz, 1H), 2.0 (m, 2H), 1.7 – 1.8 (m, 3H); 13C NMR (D2O, 100 MHz) δ 176.5, 145.2, 124.6, 110.2, 95.6, 95.2, 85.2, 79.9, 76.9, 76.8, 73.0, 72.8, 70.8, 70.4, 69.5, 68.6, 67.8, 67.5, 53.7, 52.3, 51.0, 50.1, 48.7, 43.3 (2 carbons), 40.7, 40.3, 39.8, 34.6, 29.0, 25.2 (2 carbons); ESI/APCI Calcd for C32H59N11O13Na ([M+Na]+) m/e 828.4186; found m/e 828.4170.
Compound 4i
1H NMR (D2O, 400 MHz) δ 8.01 (s, 1H), 5.98 (d, J = 4.0 Hz, 1H), 5.34(d, J = 3.5 Hz, 1H), 5.24 (s, 1H), 4.4 – 4.5 (m, 4H), 4.31 (t, J = 2.9 Hz, 1H), 4.26 (t, J = 5.4 Hz, 1H), 4.14 (t, J = 3.0 Hz, 1H), 3.97 (t, J = 9.0 Hz, 1H), 3.95 (dd, J = 10.5 Hz, J = 9.0 Hz, 1H), 3.9 (m, 3H), 3.8 (s, 1H), 3.64 (t, J = 10 Hz, 1H), 3.2 – 3.5 (m, 13H), 2.4 (m, 2H), 2.0 (m, 3H), 1.84 (dd, J = 14.0 Hz, J = 12.0 Hz. 1H); 13C NMR (D2O, 100 MHz) δ 169.8, 144.6, 124.7, 110.1, 95.7, 95.1, 84.9, 80.0, 77.0, 75.1, 73.1, 72.5, 70.6, 70.4, 69.8, 68.2, 67.8, 67.5, 59.9, 53.4, 52.3, 50.9, 49.8, 48.6, 46.7, 40.7, 40.2, 34.8, 29.8, 28.1, 24.0; ESI/APCI Calcd for C31H58N11O13 ([M+H]+) m/e 792.4210; found m/e 792.4236.
Compound 5a
1H NMR (D2O, 400 MHz) δ5.80 (d, J = 3.8 Hz, 1H), 5.30 (d, J = 10.7 Hz, 1H), 5.19 (d, J = 1.3 Hz. 1H), 4.33 (t, J = 4.9 Hz, 1H), 4.22 (t, J = 5.0 Hz, 1H), 4.16 (t, J = 2.9 Hz, 1H), 3.8 – 3.9 (m, 4H), 3.7 (m, 1H), 3.65 (t, J = 9.6 Hz, 1H), 3.55 (dd, J = 12.8 Hz, J = 3.8 Hz, 1H), 3.5 (m, 1H), 3.2 – 3.4 (m, 10H), 2.61 (m, 1H), 2.17 (t, J = 7.2 Hz, 2H), 1.5 (m, 1H), 1.2 (m, 2H), 1.2 (m, 8 H), 0.74 (t, J = 6.5 Hz, 3H); 13C NMR (D2O, 100 MHz) 178.0, 109.3, 95.9, 95.2, 85.2, 80.8, 77.5, 76.5, 73.6, 72.7, 70.7, 70.3, 69.3, 68.5, 67.8, 67.5, 53.6, 51.0, 49.9, 48.8, 41.5, 40.7, 40.3, 36.1, 30.9, 27.8, 28.2, 25.6, 22.1, 13.5; ESI/APCI Calcd for C30H60N7O13 ([M+H]+) m/e 726.4244; found m/e 726.4226.
Compound 5b
1H NMR (D2O, 400 MHz) δ 5.72 (d, J = 3.6 Hz, 1H), 5.28 (d, J = 4.1 Hz, 1H), 5.18 (d, J = 1.4 Hz, 1H), 4.31 (t, J = 3.6 Hz, 1H), 4.22 (t, J = 4.0 Hz, 1H), 4.17 (t, J = 4.9 Hz, 2H), 4.12 (t, J = 3.0 Hz, 1H), 3.95 (m, 1H), 3.7 – 3.8 (m, 3H), 3.4 – 3.6 (m, 3H), 3.3 – 3.4 (m, 6H), 3.2 (m, 3H), 2.2 (m, 3H), 1.6 (m, 1H), 1.48 (m, 2H), 1.2 (m, 26H), 0.74 (m, 3H); 13C NMR (D2O, 100 MHz) δ 178.1, 109.4, 96.0, 95.5, 85.4, 80.6, 77.5, 73.6, 73.0, 70.9, 70.3, 69.4, 69.1, 67.8, 67.6, 53.9, 51.0, 50.2, 49.0, 41.2, 40.7, 40.3, 36.1, 31.4, 28.5 – 29.0 (12 carbons), 25.6, 22.2, 13.6; ESI/APCI Calcd for C39H78N7O13 ([M+H]+) m/e 852.5652; found m/e 852.5633.
Compound 5c
1H NMR (D2O, 400 MHz) δ 5.75 (d, J = 3.3 Hz, 1H), 5.31 (s, 1H), 5.17 (s, 1H), 4.35 (t, J = 4.6 Hz, 1H), 4.1 – 4.2 (m, 4H), 3.95 (m, 1H), 3.8 -3.9 (m, 3H), 3.62 (t, J = 8.8 Hz, 1H), 3.5 (m, 3H), 3.1 – 3.4 (m, 8H), 2.2 (m, 3H), 1.6 (m, 1H), 1.49 (m, 2H), 1.16 (m, 30H), 0.76 (m, 3H); 13C NMR (D2O, 100 MHz) δ 177.6, 109.4, 96.0, 95.6, 85.1, 80.3, 77.6, 73.4, 72.9, 71.1, 70.3, 69.4, 67.9, 67.7, 54.0, 51.1, 50.3, 49.0, 41.5, 40.7, 40.4, 39.0, 36.2, 31.7, 28.9 – 29.9 (14 carbons), 25.8, 22.5, 13.9; ESI/APCI Calcd for C41H82N7O13 ([M+H]+) m/e 880.5965; found m/e 880.5959.
Compound 5d
1H NMR (D2O, 400 MHz) δ 8.13 (t, J = 8.8 Hz, 2H), 7.98 (dd, J = 8.0 Hz, J = 2.0 Hz, 1H), 7.95 (s, 1H), 7.87 (t, J = 8.4 Hz,1H), 7.70 (t, J = 7.2 Hz, 1H), 7.6 (m, 3H), 7.4 (m, 1H), 5.71 (m, 1H), 5.36 (d, J = 3.7 Hz, 1H), 5.2 (m, 1H), 4.55 (t, J = 5.3 Hz, 1H), 4.3 (m, 3H), 4.2 (m, 1H), 4.1 (m, 1H), 4.0 (m, 2H), 3.7 – 3.9 (m, 3H), 3.6 (m, 3H), 3.5 (m, 2H), 3.3 – 3.4 (m, 2H), 3.1 - 3.3 (m, 2H), 3.0 - 3.1 (m, 2H), 2.8 (m, 1H), 2.22 (m, 1H), 1.6 (m, 1H); 13C NMR (D2O, 100 MHz) δ 170.6, 158.1, 147.0, 142.8, 137.0, 131.6, 130.6, 129.5 (2 carbons), 128.6, 128.4, 128.0 (2 carbons), 124.9, 123.0, 118.7, 109.8, 95.8, 95.5 (2 carbons), 85.3, 80.3, 77.8, 73.6, 73.2, 70.8, 70.4, 69.2, 67.6, 67.2, 66.0, 53.9, 51.1, 50.3, 49.0, 41.5, 40.4, 40.2, 32.0; ESI/APCI Calcd for C41H82N7O13 ([M+H]+) m/e 845.4040; found m/e 845.4021.
Compound 5e
1H NMR (D2O, 400 MHz) δ 5.80 (s, 1H), 5.31 (d, J = 3.3 Hz, 1H), 5.22 (s, 1H), 4.40 (t, J = 5.2 Hz, 1H), 4.2 – 4.3 (m, 6H), 4.13 (t, J = 3.1 Hz, 1H), 3.8 – 3.9 (m, 5H), 3.74 (s, 1H), 3.65 (t, J = 9.7 Hz, 1H), 3.5 (m, 4H), 3.2 – 3.4 (m, 4H), 3.07 (t, J = 7.2 Hz, 2H), 2.3 (m, 1H), 2.1 (m, 1H), 1.9 (m, 1H), 1.7 (m, 1H); 13C (D2O, 100 MHz) δ 176.1, 109.5, 95.8, 95.3, 85.0, 80.4, 77.4, 73.5, 72.7, 70.8, 70.3, 69.7, 69.5, 69.0, 67.9, 67.6, 53.8, 51.0, 50.2, 48.9, 41.3, 40.7, 40.3, 36.9, 31.2, 30.1, 30.0; ESI/APCI Calcd for C27H54N8O14Na ([M+Na]+) m/e 737.3652; found m/e 737.3682.
Compound 5f
1H NMR (D2O, 400 MHz) δ 5.88 (d, J = 3.9 Hz, 1H), 5.32 (d, J = 3.5 Hz, 1H), 5.21 (d, J = 1.5 Hz, 1H), 4.35 (t, J = 5.4 Hz, 1H), 4.26 (t, J = 5.3 Hz, 1H), 4.22 (d, J = 3.7 Hz, 1H), 4.1 (m, 2H), 4.03 (t, J = 9.0 Hz, 1H), 3.9 (m, 2H), 3.78 (s, 1H), 3.74 (m, 2H), 3.71 (t, J = 9.4 Hz, 1H), 3.6 (m, 1H), 3.2 – 3.5 (m, 11H), 2.35 (m, 1H), 1.80 (dd, J = 24.0 Hz, J = 14.6 Hz, 1H); 13C (D2O, 100 MHz) δ 170.0, 109.2, 95.9, 95.6, 84.8, 80.6, 78.3, 77.0, 73.3, 72.8, 70.8, ,70.3, 69.5, 69.1, 67.9, 67.6, 53.8, 51.0, 50.3, 49.0, 41.8, 40.7 (2 carbons), 40.3, 30.0; ESI/APCI Calcd for C25H51N8O13 ([M+H]+) m/e 671.3570; found m/e 671.3591.
Compound 5g
1H NMR (D2O, 400 MHz) δ 5.88 (d, J = 3.9 Hz, 1H), 5.34 (d, J = 3.8 Hz, 1H), 5.22 (s, 1H), 4.31 (t, J = 5.3 Hz, 1H), 4.26 (t, J = 2.0 Hz, 1H), 4.18 (t, J = 4.9 Hz, 2H), 4.13 (t, J = 3.0 Hz, 1H), 4.0 – 4.1 (m, 2H), 3.9 – 4.0 (m, 3H), 3.74 (dd, J = 3.1 Hz, J = 1.5 Hz, 1H), 7.72 (d, J = 9.7 Hz, 1H), 3.2 – 3.6 (m, 11H), 2.36 (dt, J = 8.5 Hz, J = 4.2 Hz, 1H), 1.81 (m, 1H), 1.45 (d, J = 7.1 Hz, 3H); 13C NMR (D2O, 100 MHz) δ 171.6, 108.8, 95.9, 95.2, 84.6, 80.9, 77.0, 73.4, 72.4, 70.6, 70.3, 69.8, 68.6, 67.8, 67.7, 53.5, 51.0, 50.0, 49.3, 48.8, 41.7, 40.8, 40.2, 27.0, 16.9; ESI/APCI Calcd for C25H53N8O13 ([M+H]+) m/e 685.3727; found m/e 685.3735.
Compound 5h
1H NMR (D2O, 400 MHz) δ 5.91 (d, J = 4.0 Hz, 1H), 5.35 (d, J = 3.9 Hz, 1H), 5.21 (d, J = 1.7 Hz, 1H), 4.30 (t, J = 9.2 Hz, 1H), 4.31 (d, J = 10.8 Hz, 1H), 4.3 (m, 1H), 4.1 – 4.2 (m, 2H), 3.9 – 4.0 (m, 3H), 3.7 (m, 2H), 3.3 – 3.6 (m, 15H), 2.4 (m, 2H), 2.0 (m, 3H), 1.86 (m, 1H); 13C NMR (D2O, 100 MHz) δ 170.3, 108.8, 95.9, 95.2, 84.5, 81.0, 77.1, 75.2, 73.4, 72.3, 70.5, 70.2, 70.0, 68.4, 67.8, 67.7, 60.1, 53.3, 50.9, 49.8, 48.7, 46.6, 41.8, 40.7, 40.2, 29.9, 28.0, 24.1; ESI/APCI Calcd for C28H54N8O13Na ([M+Na]+) m/e 733.3703; found m/e 733.3725.
Compound 5i
1H NMR (D2O, 400 MHz) δ 7.55 (d, J = 7.9 Hz, 1H), 7.44 (d, J = 8.3 Hz, 1H), 7.26 (s, 1H), 7.17 (t, J = 7.1 Hz, 1H), 7.10 (t, J = 7.8 Hz, 1H), 5.66 (d, J = 3.7 Hz, 1H), 5.19 (d, J = 3.4 Hz, 1H), 4.94 (s, 1H), 4.26 (t, J = 6.9 Hz, 2H), 4.1 (m, 2H), 3.6 – 4.0 (m, 8H), 3.1 – 3.5 (m, 12H), 2.22 (dt, J = 8.8 Hz, J = 4.6 Hz, 1H), 1.6 (m, 1H), 1.2 (m, 1H); 13C NMR (D2O, 100 MHz) δ 170.7, 136.3, 126.8, 125.3, 122.5, 119.8, 118.4, 112.4, 108.5, 106.7, 96.0, 95.9, 84.7, 80.6, 78.0, 76.7, 70.9, 71.3, 70.0, 69.4, 69.3, 67.8, 67.5, 53.9, 53.8, 51.2, 50.8, 50.3, 49.1, 41.2, 40.7, 40.3, 30.2, 27.1; ESI/APCI Calcd for C34H58N9O13 ([M+H]+) m/e 800.4149; found m/e 800.4139.
Compound 5j
1H NMR (D2O, 400 MHz) δ 5.95 (d, J = 3.9 Hz, 1H), 5.35 (d, J = 3.1 Hz, 1H), 4.38 (t, J = 5.4 Hz, 1H), 4.26 (t, J = 3.2 Hz, 2H), 4.1 – 4.2 (m, 4H), 3.9 – 4.0 (m, 5H), 3.6 – 3.8 (m, 3H), 3.2 – 3.5 (m, 11H), 2.42(dt, J = 8.5 Hz, J = 4.3 Hz, 1H), 1.86 (q, J = 12.6 Hz, 1.H); 13C NMR (D2O, 100 MHz) δ 168.8, 109.3, 95.6, 95.2, 84.6, 80.3, 76.7, 75.1, 73.3, 72.4, 70.6, 70.3, 69.9, 68.3, 67.8, 67.6, 60.5, 54.5, 53.3, 51.0, 49.9, 48.7, 41.8, 40.7, 40.2, 28.1; ESI/APCI Calcd for C26H53N8O14 ([M+H]+) m/e 701.3676; found m/e 701.3695.
Compound 5k
1H NMR (D2O, 400 MHz) δ 5.75 (d, J = 3.5 Hz, 1H), 5.32 (d, J = 4.1 Hz, 1H), 5.21 (d, J = 1.5 Hz, 1H), 4.30 (t, J = 5.3 Hz, 1H), 4.26 (t, J = 3.7 Hz, 1H), 4.1 – 4.2 (m, 3H), 3.9 – 4.0 (m, 2H), 3.8 – 3.9 (m, 3H), 3.7 (m, 1H), 3.6 – 3.7 (m, 2H), 3.2 – 3.5 (m, 11H), 2.9 (t, J = 7.7 Hz, 1H), 2.2 (m, 1H), 1.8 (m, 2H), 1.6 (m, 3H), 1.38 (m, 2H); 13C NMR (D2O, 100 MHz) δ 170.9, 109.0, 96.0, 95.9, 84.8, 80.9, 77.1, 73.2, 72.8, 70.8, 70.2, 69.5, 69.1, 67.8, 67.7, 53.9, 53.3, 51.0, 50.2, 49.0, 42.0, 40.8, 40.3, 39.2 (2 carbons), 30.8, 30.0, 26.6, 21.7; MALDI Calcd for C29H59N9O13Na ([M+Na]+) m/e 764.4125; found m/e 764.4163.
Compound 5l
This compound was prepared by subjecting compound 32 to hydrogenation and sequential purification. 1H NMR (D2O, 300 MHz) δ 5.85 (d, J = 4.2 Hz, 1H), 5.31 (d, J = 4.1 Hz, 1H), 5.20 (d, J = 1.4 Hz, 1H), 4.35 (t, J = 4.8 Hz, 1H), 4.1 – 4.2 (m, 4H), 3.8 – 4.0 (m, 4H), 3.7 (m, 2H), 3.2 – 3.6 (m, 11H), 2.4 – 2.6 (m, 5H), 1.90 (m, 1H); 13C NMR (D2O, 100 MHz) δ 177.4, 175.6, 84.8, 80.8, 77.2, 75.3, 75.1, 73.5, 72.2 (2 carbons), 70.4, 70.2, 69.8 (2 carbons), 68.1, 67.7, 67.4, 53.3, 50.8, 49.7, 48.6, 41.0, 40.6, 40.1, 30.4, 29.5, 28.0; MALDI Calcd for C21H57N7O15Na ([M+Na]+) m/e 736.3335; found m/e 736.3331.
Compound 5m
1H NMR (D2O, 300 MHz) δ 5.87 (d, J = 7.9 Hz, 1H), 5.34 (d, J = 3.8 Hz, 1H), 5.20 (s, 1H), 4.40 (t, J = 5.5 Hz, 1H), 3.8 – 4.3 (m, 10H), 3.7 (m, 2H), 3.2 – 3.7 (m, 10H), 2.41 (dt, J = 8.6 Hz, J = 4.1 Hz, 1H), 1.90 (m, 1H), 1.46 (d, J = 6.9 Hz, 3H); 13C NMR (D2O, 100 MHz) δ 171.5, 108.8, 95.7, 95.2, 84.5, 80.9, 76.8, 75.2, 73.4, 72.2, 70.5, 70.2, 69.8, 68.2, 67.7, 67.5, 53.3, 50.9, 49.7, 49.2, 48.6, 40.6, 40.2, 28.0, 16.9; MALDI Calcd for C26H52N8O13Na ([M+Na]+) m/e 707.3546; found m/e 707.3518.
Compound 5n
1H NMR (D2O, 300 MHz) δ 5.69 (d, J = 3.8 Hz, 1H), 5.28 (d, J = 5.0 Hz, 1H), 5.20 (s, 1H), 4.33 (t, J = 5.1 Hz, 1H), 4.1 – 4.2 (m, 5H), 3.6- 3.8 (m, 9H), 3.1 – 3.5 (m, 10H), 2.19 (dt, J = 11.0 Hz, J = 4.5 Hz, 1H), 1.55 (m, 1H); 13C NMR (D2O, 100 MHz) δ 170.2, 109.0, 95.9, 84.8, 80.4, 79.1, 77.0, 73.2, 72.9, 70.9, 70.2, 69.4, 69.2, 67.8, 67.6, 61.1, 54.9, 53.9, 52.0, 50.9, 50.3, 49.0, 41.5, 40.6, 40.3, 30.7; MALDI Calcd for C26H52N8O14Na ([M+Na]+) m/e 723.3495; found m/e 723.3494.
Compound 5o
1H NMR (D2O, 400 MHz) δ 5.86 (d, J = 3.9 Hz, 1H), 5.33 (d, J = 3.6 Hz, 1H), 5.22 (s, 1H), 4.3 – 4.4 (m, 2H), 4.3 (m, 1H), 4.1 – 4.2 (m, 4H), 3.9 – 4.0 (m, 3H), 3.2 – 4.0 (m, 14H), 2.4 (M, 2H), 2.0 (m, 4H), 1.9 (m, 1H); 13C NMR (D2O, 100 MHz) δ 170.4, 109.1, 95.8, 95.3, 84.7, 80.9, 77.0, 75.3, 73.5, 72.4, 70.7, 70.3, 70.0, 68.3, 67.8, 67.6, 60.0, 53.4, 51.0, 49.8, 48.7, 46.8, 41.8, 40.7, 40.4, 30.2, 28.1, 24.1; ESI/APCI Calcd for C28H55N8O13 ([M+H]+) m/e 711.3883; found m/e 711.3873.
Compound 5p
1H NMR (D2O, 400 MHz) δ 5.90 (d, J = 4.0 Hz, 1H), 5.34 (d, J = 3.4 Hz, 1H), 5.21 (d, J = 1.5 Hz, 1H), 4.39 (t, J = 6.5 Hz, 1H), 4.26 (m, 1H), 4.1 – 4.3 (m, 4H), 3.9 – 4.0 (m, 4H), 3.7 (m, 2H), 3.2 – 3.6 (m, 11H), 2.91 (t, J = 7.6 Hz, 2H), 2.41 (dt, J = 8.4 Hz, J = 4.0 Hz, 1H), 1.8 – 2.0 (m, 3H), 1.6 – 1.7 (m, 2H), 1.4 (m, 2H); 13C NMR (D2O, 100 MHz) δ 170.6, 109.1, 95.6, 95.3, 84.6, 80.8, 76.6, 75.2, 73.3, 72.4, 70.7, 70.4, 69.9, 68.4, 67.8, 67.6, 53.4, 53.3, 51.0, 49.9, 48.7, 41.7, 40.8, 40.3, 39.2, 30.7, 28.1, 26.6, 21.7; ESI/APCI Calcd for C29H59N9O13Na ([M+Na]+) m/e 764.4125; found m/e 764.4116.
Compound 33
To a solution of compound 3 (0.20 g, 0.14 mmol) in anhydrous DMF (5 mL), succinic anhydride (0.02 g, 0.20 mmol) was added. After being stirred overnight at room temperature overnight, the reaction mixture was concentrated. After removal of the solvent followed by a fast gradient column chromatography (eluted from CH2Cl2/MeOH = 100/0 to 80/20), the product, 32 was obtained as a solid and subjected to the next step without further purification. The acid crude product was re-dissolve in DMF (8 mL) and added with compound 3 (0.20 g, 0.14 mmol), Et3N (0.04 mL, 0.28 mmol), HOBt (0.030 g, 0.21 mmol) and EDC (0.040 g, 0.21 mmol). After being stirred overnight at room temperature overnight, the reaction mixture was concentrated and diluted with EtOAc. The organic solution was washed with saturated NaHCO3(aq), water, brine and dried over anhydrous Na2SO4. After removal of the solvent followed by a fast gradient column chromatography (eluted from CH2Cl2/MeOH = 100/0 to 80/20), the product was obtained as a solid subjected to hydrogenation without further purification. 1H NMR (D2O, 400 MHz) δ 5.94 (d, J = 3.8 Hz, 2H), 5.37 (d, J = 3.5 Hz, 2H), 5.22 (s, 2H), 4.40 (t, J = 5.2 Hz, 2H), 4.2 – 4.3 ( m, 8H), 3.9 (m, 6H), 3.8 (m, 6H), 3.2 – 3.6 (m, 22H), 2.5 (s, 4H), 2.43 (dt, J = 8.8 Hz, J = 4.5 Hz, 2H), 1.87 (m, 2H); 13C NMR (D2O, 100 MHz) δ 175.5 (2 carbons), 109.4 (2 carbons), 95.7 (2 carbons), 95.0 (2 carbons), 84.7 (2 carbons), 80.5 (2 carbons), 77.3 (2 carbons), 75.2 (2 carbons), 73.4 (2 carbons), 72.4 (2 carbons), 70.6 (2 carbons), 70.4 (2 carbons), 69.9 (2 carbons), 68.3 (2 carbons), 67.8 (2 carbons), 67.5 (2 carbons), 53.5 (2 carbons), 51.0 (2 carbons), 49.9 (2 carbons), 48.8 (2 carbons), 41.8 (2 carbons), 40.8 (2 carbons), 40.3 (2 carbons), 31.1 (2 carbons), 28.1 (2 carbons); ESI/APCI Calcd for C50H97N14O26 ([M+H]+) m/e 1309.6693; found m/e 1309.6684.
Compound 5q
1H NMR (D2O, 400 MHz) δ 5.95 (d, J = 3.9 Hz, 1H), 5.33 (d, J = 3.2 Hz, 1H), 5.20 (s, 1H), 4.42 (t, J = 5.2 Hz, 1H), 4.28 (m, 2H), 4.14 (m, 3H), 3.8 – 4.0 (m, 7H), 3.7 (m, 2H), 3.3 – 3.5 (m, 11H), 2.42 (dt, J = 12.4 Hz, J = 4.0 Hz, 1H), 1.91 (dd, J = 12.2 Hz, J = 3.6 Hz, 1H); 13C NMR (D2O, 100 MHz) δ 172.0, 168.2, 109.7, 95.6, 94.8, 84.8, 80.2, 77.5, 75.1, 73.4, 72.4, 70.6, 70.4, 69.8, 68.3, 67.8, 67.4, 53.5, 51.0, 49.9, 48.8, 42.9, 41.9, 40.8, 40.7, 40.2, 28.2; ESI/APCI Calcd for C27H54N9O14 ([M+H]+) m/e 728.3785; found m/e 728.3780.
Procedure for MIC determination
A solution of selected bacteria was inoculated in the Trypticase Soy broth at 35°C for 1 - 2hrs. After which, the bacteria concentration was found, and diluted with broth, if necessary, to an absorption value of 0.08 to 0.1 at 625 nm. The adjusted inoculated medium (100 μL) was diluted with 10 mL broth, and then applied to a 96-well microtilter plate (50 μL). A series of solutions (50 μL each in 2-fold dilution) of the tested compounds was added to the testing wells. The 96-well plate was incubated at 35°C for 12 - 18 hrs. The minimum inhibitory concentration (MIC) is defined as the minimum concentration of compound needed to inhibit the growth of bacteria. The MIC results are repeated at least three times.
Supplementary Material
Acknowledgement
We acknowledge National Institutes of Health (AI053138) for financial support. We thank Prof. Mobashery of Notre Dame University for providing the pTZ19U-3 and pSF815 plasmids.
a Abbreviations
- SAR
Structural activity relationship
- AME
aminoglycoside-modifying enzyme
- MRSA
Methicillin-resistant Staphylococcus aureus
- VRE
vancomycin-resistant enterococci
- AHB
(S)-4-amino-2-hydroxybutanoyl
- APH
aminoglycoside phosphotransferases
- ANT
aminoglycoside nucleotidyltransferases
- Cbz or Z
carbobenzyloxy
- AAC
aminoglycoside acetyltransferase
- MIC
minimum inhibitory concentration
- rRNA
ribosomal RNA
- EDC
1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride
- HOBt
1-Hydroxybenzotriazole
Footnotes
Supporting Information Available: A table listing the appropriate analytical data including spectra of 1H and 13C NMR for the synthesized compounds, and 1H, 13C NMR, HPLC and HRMS of the assayed compounds. This material is available free of charge via the internet at http://pubs.acs.org.
References
- 1.Umezawa S, Tsuchiya T. In: Aminoglycoside Antibiotics. Umezawa H, Hooper IR, editors. Springer-Verlag; New York: 1982. pp. 37–110. [Google Scholar]
- 2.Haddad J, Kotra LP, Mobashery S. In: Glycochemistry Principles, Synthesis, and Applications. Wang PG, Bertozzi CR, editors. Marcel Dekker, Inc.; New York/Basel: 2001. pp. 353–424. [Google Scholar]
- 3.Wang J, Chang C-WT. In: Aminoglycoside Antibiotics. Arya DP, editor. John Wiley & Sons, Inc.; 2007. pp. 141–180. [Google Scholar]
- 4.Wang J, Li J, Chen H-N, Chang H, Tanifum CT, Liu H-H, Czyryca PG, Chang C-WT. Glycodiversification for optimization of the kanamycin class aminoglycosides. J. Med. Chem. 2005;48:6271–6285. doi: 10.1021/jm050368c. [DOI] [PubMed] [Google Scholar]
- 5.Li J, Chiang F-I, Chen H-N, Chang C-WT. Investigation of the regioselectivity for Staudinger reaction and its application for the synthesis of aminoglycosides with N-1 modification. J. Org. Chem. 2007;72:4055–4066. doi: 10.1021/jo062588j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asensio JL, Hidalgo A, Bastida A, Torrado M, Corzana F, Chiara JL, Garcia-Junceda E, Canada J, Jimenez-Barbero J. A simple structural-based approach to prevent aminoglycoside inactivation by bacterial defense proteins. conformational restriction provides effective protection against neomycin-B nucleotidylation by ANT4. J. Am. Chem. Soc. 2005;127:8278–8279. doi: 10.1021/ja051722z. [DOI] [PubMed] [Google Scholar]
- 7.Blount KF, Zhao F, Hermann T, Tor Y. Conformational constraint as a means for understanding RNA-aminoglycoside specificity. J. Am. Chem. Soc. 2005;127:9818–9829. doi: 10.1021/ja050918w. [DOI] [PubMed] [Google Scholar]
- 8.Kling D, Hesek D, Shi Q, Mobashery S. Design and synthesis of a structurally constrained aminoglycoside. J. Org. Chem. 2007;72:5450–5453. doi: 10.1021/jo0707636. [DOI] [PubMed] [Google Scholar]
- 9.Luedtke NW, Liu Q, Tor Y. RNA-ligand interactions: affinity and specificity of aminoglycoside dimers and acridine conjugates to the HIV-1 Rev response element. Biochemistry. 2003;42:11391–11403. doi: 10.1021/bi034766y. [DOI] [PubMed] [Google Scholar]
- 10.Boer J, Blount KF, Luedtke NW, Elson-Schwab L, Tor Y. RNA-Selective Modification by a Platinum(II) Complex Conjugated to Amino- and Guanidinoglycosides. Angew. Chem. Int. Ed. 2005;44:927–932. doi: 10.1002/anie.200461182. [DOI] [PubMed] [Google Scholar]
- 11.Quader S, Boyd SE, Jenkins ID, Houston TA. Multisite modification of neomycin B: combined Mitsunobu and click chemistry approach. J. Org. Chem. 2007;72:1962–1979. doi: 10.1021/jo0620967. [DOI] [PubMed] [Google Scholar]
- 12.Fujisawa K, Hashiya T, Kawaguchi H. Aminoglycoside antibiotics. VII. Acute toxicity. of aminoglycoside antibiotics. J. Antibiot. 1974;27:677–681. doi: 10.7164/antibiotics.27.677. [DOI] [PubMed] [Google Scholar]
- 13.Hainrichson M, Pokrovskaya V, Shallom-Shezifi D, Fridman M, Belakhov V, Shachar D, Yaronb S, Baasova T. Branched aminoglycosides: Biochemical studies and antibacterial activity of neomycin B derivatives. Bioorg. Med. Chem. 2005;13:5797–5807. doi: 10.1016/j.bmc.2005.05.058. [DOI] [PubMed] [Google Scholar]
- 14.Fridman M, Belakhov V, Yaron S, Baasov T. A new class of branched aminoglycosides: pseudo-pentasaccharide derivatives of neomycin B. Org. Lett. 2003;5:3575–3578. doi: 10.1021/ol035213i. [DOI] [PubMed] [Google Scholar]
- 15.Huisgen R. In: 1,3-Dipolar Cycloaddition Chemistry. Padwa A, editor. Wiley; New York: 1984. pp. 1–176. [Google Scholar]
- 16.Mingeot-Leclercq M-P, Glupczynski Y, Tulkens PM. Aminoglycosides: activity and resistance. Antimicrob. Agents Chemother. 1997;43:727–737. doi: 10.1128/aac.43.4.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kotra LP, Haddad J, Mobashery S. Aminoglycosides: perspective on mechanisms of action and resistance and strategies to counter resistance. Antimicrob. Agents Chemother. 2000;44:3249–3256. doi: 10.1128/aac.44.12.3249-3256.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wright GD. Aminoglycoside-modifying enzymes. Curr. Opin. Microbiol. 1999;2:499–503. doi: 10.1016/s1369-5274(99)00007-7. [DOI] [PubMed] [Google Scholar]
- 19.Vakulenko SB, Mobashery S. Versatility of aminoglycosides and prospects for their future. Clin. Microbiol. Rev. 2003;16:430–450. doi: 10.1128/CMR.16.3.430-450.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ida T, Okamoto R, Shimauchi C, Okubo T, Kuga A, Inoue M. Identification of aminoglycoside-modifying enzymes by susceptibility testing: epidemiology of methicillin-resistant Staphylococcus aureus in Japan. J. Clin. Microbiol. 2001;39:3115–3121. doi: 10.1128/JCM.39.9.3115-3121.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmitz FJ, Fluit AC, Gondolf M, Beyrau R, Lindenlauf E, Verhoef J, Heinz HP, Jones ME. The prevalence of aminoglycoside resistance and corresponding resistance genes in clinical isolates of staphylococci from 19 European hospitals. J. Antimicrob. Chemother. 1999;43:253–259. [PubMed] [Google Scholar]
- 22.Udo EE, Dashti AA. Detection of genes encoding aminoglycoside-modifying enzymes in staphylococci by polymerase chain reaction and dot blot hybridization. Int. J. Antimicrob. Agents. 2000;13:273–279. doi: 10.1016/s0924-8579(99)00124-7. [DOI] [PubMed] [Google Scholar]
- 23.Werner G, Hildebrandt B, Witte W. Aminoglycoside-streptothricin resistance gene cluster aadE-sat4-aphA-3 disseminated among multiresistant isolates of Enterococcus faecium. Antimicrob. Agents Chemother. 2001;45:3267–3269. doi: 10.1128/AAC.45.11.3267-3269.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKay GA, Thompson PR, Wright GD. Broad spectrum aminoglycoside phosphotransferase Type III from Enterococcus: overexpression, purification, and substrate specificity. Biochemistry. 1994:6936–6944. doi: 10.1021/bi00188a024. [DOI] [PubMed] [Google Scholar]
- 25.McKay GA, Wright GD. Kinetic mechanism of aminoglycoside phosphotransferase type IIIa. J. Biol. Chem. 1995:24686–24692. doi: 10.1074/jbc.270.42.24686. [DOI] [PubMed] [Google Scholar]
- 26.Russell RJM, Murray JB, Lentzen G, Haddad J, Mobashery S. The complex of a designer antibiotic with a model aminoacyl site of the 30S ribosomal subunit revealed by X-ray crystallography. J. Am. Chem. Soc. 2003;125:3410–3411. doi: 10.1021/ja029736h. [DOI] [PubMed] [Google Scholar]
- 27.Structures of 5″-substituted neomycins were docked to APH(3′)-IIIa and 16S ribosomal RNA. Models of the molecules of derivatized neomycins were prepared using HyperChem 7.5. Atomic charges were calculated using the AM1 semi-empirical method in vacuo. The conformations were based on the X-ray structures of complexes of neomycin with respective molecules. Orientations of the 5″ chains were adjusted manually and the structures were subject to geometry optimization (ligand molecules only). Then, the structures of the complexes were refined, by using AutoDock 4 as local minimum optimizer (Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, Belew RK, Olson AJ. J. Comput. Chem. 1998;19:1639–1662.). Visualizations were prepared using AutoDock Tools (Sanner MF. J. Mol. Graphics Mod. 1999;17:57–61.).
- 28.Francois B, Russell RJ, Murray JB, Aboul-ela F, Masquida B, Vicens Q, Westhof E. Crystal structures of complexes between aminoglycosides and decoding A site oligonucleotides: role of the number of rings and positive charges in the specific binding leading to miscoding. Nucleic Acids Res. 2005;33:5677–5690. doi: 10.1093/nar/gki862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fong DH, Berghuis AM. Substrate promiscuity of an aminoglycoside antibiotic resistance enzyme via target mimicry. EMBO J. 2002;21:2323–2331. doi: 10.1093/emboj/21.10.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rasheed JK, Anderson GJ, Yigit H, Queenan AM, Domenech-Sanchez A, Swenson JM, Biddle JW, Ferraro MJ, Jacoby GA, Tenover FC. Characterization of the extended-spectrum β-lactamase reference strain, Klebsiella pneumoniae K6 (ATCC 700603), which produces the novel enzyme SHV-18. Antimicrob. Agents Chemother. 2000;44:2382–2388. doi: 10.1128/aac.44.9.2382-2388.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hachiler H, Santanam P, Kayser FH. Sequence and characterization of a novel chromosomal aminoglycoside phosphotransferase gene, aph (3′)-IIb, in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 1996;40:1254–1256. doi: 10.1128/aac.40.5.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fridkin SK, Lawton R, Edwards JR, Tenover FC, McGowan JE, Jr., Gaynes RP. Monitoring antimicrobial use and resistance: comparison with a national benchmark on reducing vancomycin use and vancomycin-resistant enterococci. Emerg. Infect. Dis. 2002;8:702–707. doi: 10.3201/eid0807.010465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swenson JM, Clark NC, Sahm DF, Ferraro MJ, Doern G, Hindler J, Jorgensen JH, Pfaller MA, Reller LB, Weinstein MP, Zabransky RJ, Tenover FC. Molecular characterization and multilaboratory evaluation of Enterococcus faecalis ATCC 51299 for quality control of screening tests for vancomycin and high-level aminoglycoside resistance in Enterococci. J. Clin. Microbiol. 1995;33:3019–3021. doi: 10.1128/jcm.33.11.3019-3021.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fosso M, Zhang J, Wang J, Chang C-WT. Unpublished results.
- 35.Zhang J, Chen H-N, Chiang F-I, Takemoto JY, Bensaci M, Chang C-WT. Sonication-assisted library synthesis of oxazolidinone-carbohydrate conjugates. J. Comb. Chem. 2007;9:17–19. doi: 10.1021/cc060146f. [DOI] [PubMed] [Google Scholar]
- 36.Sajiki H, Hattori K, Hirota K. The formation of a novel Pd/C-ethylenediamine complex catalyst: chemoselective hydrogenation without deprotection of the O-benzyl and N-cbz groups. J. Org. Chem. 1998;63:7990–7992. [Google Scholar]
- 37.Li J, Chen H-N, Chang H, Wang J, Chang C-WT. Tuning the regioselectivity of Staudinger reaction for the facile synthesis of kanamycin and neomycin class antibiotics with N-1 modification. Org. Lett. 2005;7:3061–3064. doi: 10.1021/ol051045d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.