Abstract
OBJECTIVE
To evaluate the effects of pentoxifylline on hydrochloric acid-induced lung lesions in rats subjected to mechanical ventilation.
METHODS
Twenty male, adult Wistar-EPM-1 rats were anesthetized and randomly grouped (n=5 animals per group) as follows: control-MV (mechanical ventilation, MV group); bilateral instillation of HCl (HCl group); bilateral instillation of HCl followed by pentoxifylline (50 mg/kg bw) infusion (HCl+PTX group) and pentoxifylline infusion followed by bilateral instillation of HCl (PTX+HCl group). At 20, 30, 90 and 180 min after treatments, the blood partial pressures of CO2 and O2 were measured. The animals were euthanized, and bronchoalveolar lavages were taken to determine the contents of total proteins, corticosterone and TNF-α. Samples of lung tissue were used for histomorphometric studies and determining the wet-to-dry (W/D) lung weight ratio.
RESULTS
In the MV group, rats had alveolar septal congestion, and, in the HCl group, a remarkable recruitment of neutrophils and macrophages into the alveoli was noticed; these events were reduced in the animals with PTX+HCl. The partial pressure of oxygen increased in PTX+HCl animals (121±5 mmHg) as compared with the HCl (62±6 mmHg) and HCl+PTX (67±3 mmHg) groups within 30 minutes. TNF-α levels in bronchoalveolar lavage were significantly higher in the HCl group (458±50 pg/mL), reduced in the HCl+PTX group (329±45 pg/mL) and lowest in the PTX+HCl group (229±41 pg/mL). The levels of corticosterone in bronchoalveolar lavage were significantly lower in the HCl (8±1.3 ng/mL) and HCl+PTX group (16±2 ng/mL) and were highest in the PTX+HCl (27±1.9 ng/mL).
CONCLUSION
Pretreatment with PTX improves oxygenation, reduces TNF-α concentration and increases the concentration of corticosterone in bronchoalveolar lavage upon lung lesion induced by HCl.
Keywords: Acid aspiration, Acute lung injury, Mechanical ventilation, Corticosterone, Cytokine
INTRODUCTION
Gastric acid aspiration is a common etiology of acute lung injury (ALI) and is initially characterized by a chemical “burn” of the pulmonary epithelium, with a subsequent influx of extracellular fluid into the alveolar space that leads to pulmonary edema. Acid aspiration-induced lung injury is an important cause of acute respiratory distress syndrome (ARDS), which has a high mortality rate.1,2
The effect of acid instillation into the lung has been extensively studied.4,5 Acid airway aspiration has been reported as the cause of 10% to 30% of all anesthetic-related deaths.6,7 Following aspiration, patients usually develop symptoms consistent with microvascular lung injury, such as pulmonary edema and decreased lung compliance, which ultimately lead to increased work for breathing, hypoxemia, damage to the alveolar-capillary membrane, increased alveolar protein content and induced pulmonary macrophage accumulation.8,9 Tissue cytokine levels may be directly associated with further accumulation and activation of pulmonary macrophages, thus increasing lung injury. Cytokines mediate the induction and amplification of the inflammatory response to various types of injury, including hemorrhagic and endotoxic shock.10
Critical illness, such as trauma, pneumonia, sepsis, and major surgery, is accompanied by activation of the hypothalamus-pituitary-adrenal (HPA) axis. Serum adrenocorticotropic hormone (ACTH) and glucocorticoid concentrations are increased, and activation of this axis appears to be an important component of the general adaptation to stress.11
Normal activation and functioning of the HPA axis have an important role in modulating the response to critical illness and systemic inflammation.11 A deficiency in the HPA axis could lead to unchecked or ongoing activation of inflammatory mediators, cardiovascular instability, loss of vascular integrity and possible persistent airspace inflammation and injury, which would lead to an exuberant post-injury fibroproliferative response in the lung.12 The factors determining glucocorticoid response at the receptor and molecular level are complex and probably explain the individual variation and tissue specificity in systemic response.13 Basal circulating concentrations of glucocorticoids are complex and have also been observed to be unchanged,14 increased15 or decreased16 in rodents. Corticosterone measurements were performed using the plasma, urine and excrement from these animals. Unfortunately, we did not find detail in the literature about the measurement in broncoalveolar lavage (BAL).
Pentoxifylline (PTX) is a nonselective phosphodiesterase inhibitor with important immuno-regulatory actions. Its anti-inflammatory properties have recently been investigated by the ARDS Clinical Trials Network in a large, prospective, placebo-controlled multicenter study17 in addition to other experimental studies.18
This study was designed to further analyze the effects of PTX treatment immediately before and after hydrochloric acid instillation (HCl). To accomplish this, we determined protein leakage, tumor necrosis factor-alpha (TNF-α) and corticosterone concentrations in BALs and lung wet-to-dry ratios.
MATERIALS AND METHODS
Surgical Procedure
All procedures were approved by the Institutional Research Committee at Federal University of São Paulo, in accordance with the National Institutes of Health (NIH) Guidelines Regarding Animal Experimentation.
Twenty (n= 20) male Wistar rats weighing 268 to 312 g were fasted overnight with free access to water. Animals were group-housed in standard Plexiglas bins (2–3 rats per cage). Rats (n=5 in every group) were anesthetized intraperitoneally (i.p.) with sodium thiopental (40 mg/kg body weight, Thionembutal-ABBOTT, SP, Brazil). Muscle relaxation was maintained with 0.8 mg/kg pancuronium bromide (Pavulon-Organon, São Paulo, Brazil) i.p. Animals were placed in the supine position, and 1% lidocaine was injected subcutaneously at the tracheal region.
After anesthesia, a tracheotomy was performed, and a 14G cannula was inserted approximately 1 cm above the carina. A hind paw vein (used for drug infusions and compensation of fluid losses) and the left internal carotid artery (used to obtain arterial blood for gases determination) were exposed and cannulated using PE-50 tubing (Intramedic Polyethylene Tubing, Becton Dickinson, NJ, USA). Normothermia was maintained with an underlying electric hotplate (37 ± 0.6oC [mean±SD]).
Mechanical Ventilation
After reaching a surgical plane of anesthesia and muscle relaxation, the animals were ventilated using a rodent ventilator (683 model, Harvard Apparatus; Holliston, MA, USA) for 3 h using a tidal volume (VT) established at approximately 6 mL/kg, a positive end-expiratory pressure (PEEP) of 5 cmH2O, a fraction of inspired oxygen (FiO2) of 1.0, a respiratory rate (RR) of 30–50 bpm and an inspiration/expiration (I/E) ratio of 1:2. A venous catheter was placed in the jugular vein to allow the infusion of isotonic saline solution and sodium pentobarbital whenever needed.
Groupings:
Animals were divided into 4 different groups (n=5 in each group). The first group (Control-MV) received only mechanical ventilation; the second group (HCl) received hydrochloric acid instillation; the third group (HCl+PTX) received HCl instillation followed by PTX infusion; and the fourth group (PTX+HCl) received PTX infusion 10 min prior to HCl instillation.
Acid Instillation Lung Injury:
After 10 min connected in the ventilator for stabilization, the lung injury was performed using a 0.1 N HCl solution (2 mL/kg) instilled through a side port adaptor of the endotracheal tube during the inspiratory phase of respiration. Blood samples were collected 30 seconds before lung injury and the protocol was started.
Pentoxifylline administration:
HCl+PTX animals received 50 mg/kg PTX (Pentox, FARMASA, São Paulo, SP, Brazil) in a bolus after the lung injury induction. The animals in the PTX+HCl group received the PTX infusion 10 min prior to instillation of HCl. The PTX pre- or post-HCl instillation and HCl animals received PTX or normal saline through the tail vein18.
Measurements
Arterial blood gases measurement:
Arterial blood gases (arterial partial pressure of oxygen [PaO2] and carbon dioxide [PaCO2]) were determined in blood samples collected with sterile vented plastic syringes (PICO 70, Radiometer, Copenhagen, Denmark) and measured 20, 30, 90 and 180 min after initiation of MV using an automatic AVL-Compact3 device (Roche Diagnostic, Mannheim, Germany).
BAL:
At the end of 3 h, the animals were euthanized (1 mL/100 g bw T-61 Euthanasia Solution, Hoechst & Roussel). A sternotomy was performed, and the lungs and appending structures were removed and weighed. The left lung was tied, and the right lung was washed three times with 30 mL/kg cold sterile phosphate-buffered saline (PBS; pH 7.2–7.4). Lavage fluids were kept separate and centrifuged (at 400 x g for 10 min) at 4 ºC, and the supernatants were frozen immediately on dry ice and stored at −80 ºC.
Protein leakage:
A 1-mL aliquot of BAL was used to measure total protein using the Lowry method.18
W/D weight ratio of lungs:
After bronchoalveolar lavage, the right lung was placed in a drying oven for 72 h at 60 °C and then re-weighed. The W/D ratios were then calculated.
Corticosterone Detection:
Corticosterones were measured in duplicate using a radioimmunoassay (Coat-A-Count Rat Corticosterone, DPC Diagnostic, CA, USA) in the BAL, according to the manufacturer’s instructions, and the countings were performed using a Gamma Counter (Gamma Master 1277 model, LKB Wallac, Sweden). The assay sensitivity was 5.7 ng/mL; the inter-assay and intra-assay coefficients of variation were 4.8% to 5.8% and 4.0% to 4.3%, respectively.
Cytokine Assay:
TNF-α concentrations in BAL were determined using a rat-specific, commercially available enzyme immunoassay [ELISA] (R&D Systems, Minneapolis, USA).
Histology and morphometry:
Lungs were removed en bloc and inflated at a pressure of 20 cmH2O with 4% paraformaldehyde in PBS at room temperature to fix the tissue. Left lung fragments were washed in 50mM phosphate buffer, pH 7.4, dehydrated in graded concentrations of ethanol and then embedded in paraffin. From each lung, 4 μm sections were obtained and stained with hematoxylin and eosin (HE) to evaluate lung morphology and morphometry. The slides were systematically scanned in a microscope using a 400X magnification. Macrophage and neutrophil counts were performed in the alveolar lumen septae by image capture with a light microscope using 400X magnification (Axiolab Standart 2.0, Carl Zeiss) coupled to a video camera (AxionCam, Carl Zeiss).
STATISTICAL ANALYSIS
Data were expressed as means ± standard deviation (SD). Values were compared between groups using an ANOVA test for multiple comparisons, and repeated-measures ANOVA was used to compare measurements within groups along the different time periods. Statistical analyses were performed using a standard computer software package (GraphPad Prisma, GraphPad Software, San Diego, CA, USA). P-values less than 0.05 were considered statistically significant.
RESULTS
Blood Gases Analyses
In the HCl and HCl+PTX groups, the PaO2 values decreased after 30 min (62±6.1 mmHg and 67±3.4 mmHg, values were higher in the respectively, Table 1), and PaO2 PTX+HCl group [121±5.3 mmHg at 30 min; 287±7 mmHg at 90 min; and 377±12.4 at 180 min] compared to the HCl and HCL+PTX groups in the same time point (Table 1). PaCO2 values were higher in the HCl and HCl+PTX groups compared to the Control-MV and PTX+HCl groups (Table 1) after 30, 90 and 180 min. No significant differences were observed among groups at the 20 min time point.
Table 1.
Pentoxifylline (PTX) and hydrochloric acid (HCl) effects on the arterial blood levels of PaO2 and PaCO2 at 20, 30, 90 and 180 minute time points. Data are shown as mean±SD.
| PaO2 (mmHg) | PaCO2 (mmHg) | |||||||
|---|---|---|---|---|---|---|---|---|
| 20 min | 30 min | 90 min | 180 min | 20 min | 30 min | 90 min | 180 min | |
| C-MV | 124±3.6 | 288±11.1a | 356±7.2b | 397±8.3c | 38±3.1 | 36±1.5a | 36±1.3b | 37±2.3c |
| HCl | 127±1.8 | 62±6.1 | 177±6.6 | 127±4.7 | 36±3 | 54±2.5 | 55±2.6 | 61±4 |
| HCl+PTX | 118±6.7 | 67±3.4 | 114±7.5 | 254±14.6 | 39±2.4 | 54±1.5 | 49±0.8 | 53±2.9 |
| PTX+HCl | 121±7.1 | 121±5.3* | 287±7# | 377±12.4¥ | 36±0.8 | 50±2.0* | 45±3.8§ | 46±5.1¥ |
No differences were observed at the 20 minute time point in the PaO2 and PaCO2 levels among all groups.
p<0.05 compared with HCl and HCl+PTX groups in the PaO2 and PaCO2 measurements (30 min);
p<0.05 compared with HCl and HCl+PTX groups in the PaO2 measurements (90 min);
p<0.05 compared with HCl group in the PaCO2 measurement (90 min);
p<0.05 compared with HCl and HCl+PTX groups in the PaO2 and PaCO2 measurements (180 min);
p<0.05 compared with all other groups in the PaO2 and PaCO2 measurements (30 min);
p<0.05 compared with all other groups in the PaO2 and PaCO2 measurements (90 min);
p<0.05 compared with all other groups in the PaO2 and PaCO2 measurements (180 min).
W/D weight of lungs, total proteins, TNF-α and corticosterone in the BAL
W/D ratios were higher in the HCl group as compared to the HCl+PTX and PTX+HCl groups; and the W/Ds were lower in the PTX+HCl animals compared with the HCl+PTX group (Table 2).
Table 2.
Effects of pentoxifylline (PTX) treatment before or after hydrochloric acid (HCl) instillation on the levels of total protein (TP) in bronchoalveolar lavage and wet-to-dry ratio (W/D). Data are shown as mean±SD.
| Control-MV | HCl | HCl+ PTX | PTX + HCl | |
|---|---|---|---|---|
| TP (mg/mL) | 20.4±0.6 | 49.6±4.3* | 36±3.1 | 28±2.5# |
| W/D (g) | 9.4±1.3 | 14±1.4* | 11.2±1.8 | 8.6±0.5# |
p<0.05 compared with Control, HCl+PTX and PTX+HCl.
p<0.05 compared with HCl and HCl+PTX.
The total proteins in BAL decreased in the PTX+HCl group compared to HCl+PTX [28±2.5 mg/mL versus 36±3.1 mg/mL, respectively]. In the Control-MV group, the total protein values in BAL were 20.4±0.6 mg/mL (Table 2).
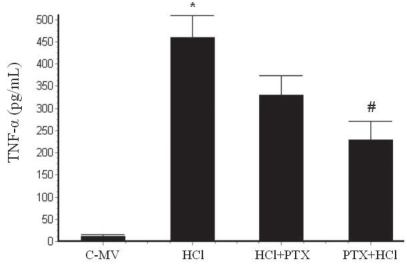
The TNF-α concentration in BAL was significantly higher from 458.4±50.5 pg/mL [HCl] to 329.4±44.9 pg/mL [HCl+PTX] and 229.4±41.1 pg/mL [PTX+HCl] (p<0.001) (Figure 1).
Figure 1.
Effect of pentoxifylline (PTX) on TNF-α levels in BAL from rats with mechanically ventilated controls (C-MV) and instilled with HCl. *p<0.05 versus the HCl+PTX and PTX+HCl groups, and #p<0.05 versus the HCl and the HCl+PTX groups. Values are presented as mean ± SD.
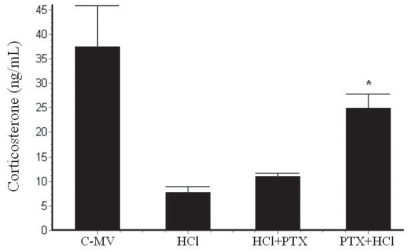
The corticosterone level in the BAL (Figure 2) was higher in the Control-MV group (41.4±5.2 ng/mL; p<0.001) compared to all other groups. The corticosterone concentrations were lower in the HCl group (7.6±1.3 ng/mL) and the HCl+PTX group (16±2.0 ng/mL) compared to the PTX+HCl group (27.4±1.9 ng/mL).
Figure 2.
Effects of HCl instillation and pentoxyfilline (PTX) pre- or post-treatments on corticosterone levels in BAL from rats under mechanical ventilation. *p<0.05 versus the HCl and the HCl+PTX groups. Values are presented as mean ± SD.
Histopatology and morphometric analyses
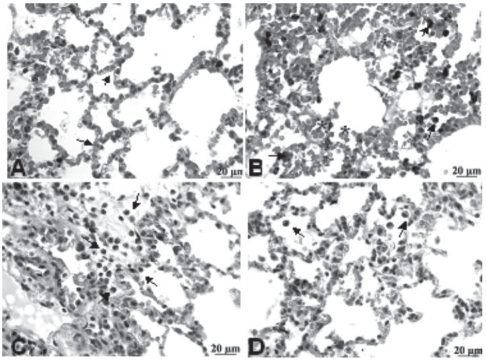
The light microscopic appearance of lung tissue of all four groups after 3 h of mechanical ventilation is shown in Figure 3. In the group Control-MV, pulmonary parenchyma showed typical cavities, septs with congested capillaries and few macrophages and neutrophils inside (Figure 3A). In the HCl group, alveolar hemorrhage, infiltration of macrophages with hemosiderin pigment and alveolar wall dilation were observed (Figure 3B). In the HCl+PTX group, areas of hemorrhage in the alveoli, macrophage influx and dilation of alveolar septae could be observed (Figure 3C). In the PTX+HCl group, a greatly decreased volume of macrophages and red blood cells infiltrates in lung tissue. (Figure 3D).
Figure 3.
Photomicrographs of HE-stained rat lungs using 400X magnification. (A) C-MV (control, mechanical ventilation) exhibits dilated and congested alveolar septa (arrows); (B) HCl group shows macrophages (arrow) and erythrocytes inside alveolus (asterisk); (C) HCl+PTX group exhibits some inflammatory infiltration, numerous macrophages, neutrophils (arrows) and red blood cells; (D) PTX+HCl group, showing mild alveolar edema and few macrophages in the alveolar lumen (arrow).
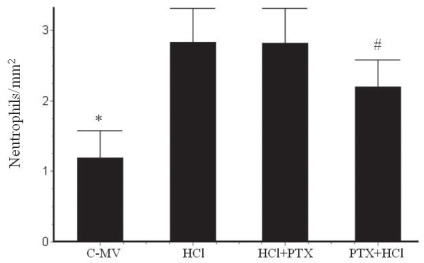
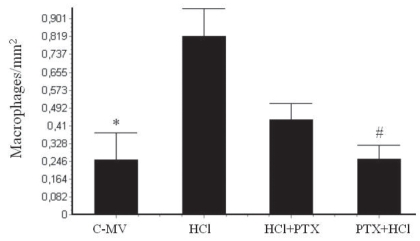
In the morphometric analysis, there was an increase of neutrophils (Figure 4) in the HCl (2.83 ± 0.5/mm2) and HCl+PTX (2.81 ± 0.5/mm2) groups compared with the control (1.13 ± 0.3/mm2). A significant difference in this regard (p<0.05) was observed between the HCl+PTX (2.81± 0.5/mm2) and PTX+HCl (2.2 ± 0.4/mm2) groups. The macrophage counts (Figure 5) were high in the HCl and HCl+PTX groups (0.82 ± 0.13/mm2 and 0.43 ± 0.08*/mm2, respectively; *p<0.05). The macrophage count was lower in the control and PTX+HCl groups (0.25 ± 0.12/mm2 and 0.26 ± 0.06/mm2, respectively).
Figure 4.
Effects of PTX on morphometric counts of neutrophils in the lung tissue in the groups C-MV, HCl, HCl+PTX and PTX+HCl. *p<0.05 versus all the other groups, and #p<0.05 versus the HCl and the HCl+PTX groups. Values are presented as mean ± SD.
Figure 5.
Effects of PTX on morphometric counts of macrophages per alveolar area in the lung tissue in the groups C-MV, HCl, HCl+PTX and PTX+HCl. *p<0.05 versus the HCl and HCl+PTX groups, and #p<0.05 versus the HCl and the HCl+PTX groups. Values are presented as mean ± SD.
DISCUSSION
The present study demonstrated that pretreatment with after lung instillation PTX promoted the recovery of PaO2 of HCl and reduced both cytokine levels and the total protein content in BAL. The histological study among the experimental groups, particularly those with PTX pretreatment, indicated a significant reduction of macrophage and neutrophil infiltrate. On the other hand, PTX administration after HCl instillation was not able to reduce, nor did it prevent, the disruption of the alveolar surface. The drug was chosen following previous experimental data obtained in our laboratory.18,36
We speculate that the findings from the current study may be of significance regarding a number of important related areas. First, pulmonary dysfunction worsens with mechanical ventilation. Second, pretreatment with PTX reduced the pulmonary dysfunction and inhibited TNF-alpha and PMN cells influx into the lung. Third, corticosterone may play a role in stabilizing the local inflammatory process.
In animals that received HCl instillation followed by mechanical ventilation (MV), there was a reduction of PaO2; in those post-infused with PTX, the oxygenation improved, and an even better oxygenation level was observed in the group pretreated with PTX. In rabbits, Nishina et al. (1998) demonstrated that pretreatment with lidocaine promoted the recovery of oxygenation after HCl-induced lung lesion model.19
In our model, we demonstrated that PTX reduces the lung lesions caused by HCl instillation, reducing both the total protein content in BAL and edema as assessed by the W/D ratio. Inflammatory mediators traffic neutrophils into the lungs, which causes a protein-rich pulmonary edema, one of the typical features of the pulmonary insult by acid aspiration, essentially because of the endothelial lesion.20
Morphometric analysis demonstrated an increase of both macrophages and neutrophils in the lungs previously instilled with HCl. However, an important reduction of both macrophages and neutrophils was observed in lungs of animals pretreated with PTX. Studies by Schreiber and colleagues (2006) demonstrated that the lung tissue of non-mechanically ventilated animals subjected to a unilateral acid instillation produced a strong inflammatory response.21
Several animal models of lung lesions are well-described in the literature as completely satisfactory and relevant to human disease states. The animal model used in the present study was chosen in view of the fact that more then 30% of human cases of acute pulmonary failure/ARDS are associated with gastric contents aspiration.22–24
Pretreatment with PTX also reduced the levels of TNF-α, and some studies have shown that PTX reduces the haemodynamic equilibrium disruption observed in cases such as hypovolemic shock, endotoxaemic shock, gastric aspiration and TNF-induced pulmonary lesions.25
During severe disease, neurohumoral factors, cytokines and endothelins, among others, activate the HPA axis, thereby resulting in the production and increase of plasma levels of cortisol.26 In rodents, even though the increase of plasma corticosteroids is a normal event in response to stress and lesion, there is some debate on what could be a ‘normal’ response to stress and how such a response would affect the normal or the deleterious defense mechanisms of the body.27 In humans, the rise of circulating cortisol is well-described in both ARDS and the normal stress response.27 Besides, low levels of cortisol are associated with the worsening of the organic conditions in patients with sepsis.28 Sebaldt et al.(1990) demonstrated that high daily oral doses of glycocorticoids (60 mg prednisone/day) given in the morning for 7 days inhibit the synthesis and the appearance of leukotrienes in the BAL of healthy volunteers,29 but the subjects in their study were not under MV. There are diverse, and sometimes opposing, effects of the glucocorticoids, because they are dependent on the cell type upon which these drugs act.30
Data reported by Bernard et al. (1987) suggest that high-dose methylprednisolone does not affect the outcome in patients with established ARDS due to sepsis, aspiration or a mixed cause.31
The neuroendocrine responses mediated by the HPA axis are associated with various feedback mechanisms. It is conceivable that the reactions to stress have different degrees of specificity, and that they may or may not develop with proper homeostasis maintenance. This can be observed through the corticosterone concentrations in the BAL observed in the present study.
In healthy volunteers, Hubbard et al. (1994) found endogenous BAL levels of cortisone and cortisol of 380 and 153 pg/mL, respectively.32 Later, Baker et al. (2000) determined the concentration of cortisol in BALs of 11 patients with acute tuberculosis and 13 healthy volunteers and found that the diseased patients had significantly elevated cortisol levels in BAL.33
In addition to HCl-induced stress, which causes per se tissue lesions, MV also caused pulmonary lesions and vasoconstriction, though to a lower degree. Accordingly, various studies have demonstrated an increase of inflammatory mediators produced by the lung after MV.18, 34, 35
In the present study, the observed reduction of corticosterone levels in BALs of the group subjected to HCl instillation plus MV can be viewed as an indication that the direct chemical stress coupled with the forced ventilation reduced the passage of corticosterone into the alveolar spaces. Such a reduction could be related to the increase of the alveolar pressure caused by MV, which, in turn, would alter the alveolar vascular resistance. This would then be followed by a narrowing of the blood vessels around alveoli, an alteration of the alveolar-arterial gradient and, hence, the pulmonary distensibility. Though this study could not unequivocally prove that mechanical ventilation completely blocked the corticosteroid influx into the alveolar space, several other studies have indicated this possibility.29,31,32 The higher concentration of corticosterone in the Control-MV group is certainly not a normal value, and other evaluations in a mechanical ventilation-free group are in order. The measurement of corticosterone in blood samples of the same animal in every time point of the experiment was not possible due to the high volume necessary for the assay (0.5 mL per time point), leading to the risk of hypovolemic shock.
CONCLUSION
In conclusion, the data presented suggest that pentoxifylline modulates the production/release of a proinflammatory cytokine (TNF-α) and inflammatory cells, thereby improving oxygenation during the course of an experimental model of HCl-induced lung inflammation.
ACKNOWLEDGMENTS
We are especially grateful to Wagner Rogério Souza de Oliveira of the Division of Experimental Surgery (UNIFESP) and Ricardo Santos Simões of the Division of Histology and Structural Biology (UNIFESP). This work was supported, in part, by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP; grant 03/14098-0).
REFERENCES
- 1.Pepe PE, Potkin RT, Reus DH, Hudson LD, Carrico CJ. Clinical predictors of the adult respiratory distress syndrome. Am J Surg. 1982;144:124–30. doi: 10.1016/0002-9610(82)90612-2. [DOI] [PubMed] [Google Scholar]
- 2.Nader ND, Davidson BA, Tait AR, Holm BA, Knight PR. Serine antiproteinase administration preserves innate superoxide dismutase levels after acid aspiration and hyperoxia but does not decrease lung injury. Anesth Analg. 2005;101:213–9. doi: 10.1213/01.ANE.0000152188.65226.FE. [DOI] [PubMed] [Google Scholar]
- 3.Knight PR, Druskovich G, Tait AR, Johnson KJ. The role of neutrophils, oxidants and proteases in the pathogenesis of acid pulmonary injury. Aneshesiology. 1992;77:772–8. doi: 10.1097/00000542-199210000-00023. [DOI] [PubMed] [Google Scholar]
- 4.Modelska K, Pittet JF, Folkesson HG, Courtney Broaddus V, Matthay MA. Acid-induced lung injury: Protective effect of anti-interleukin-8 pretreatment on alveolar epithelial barrier function in rabbits. Am J Respir Crit Care Med. 1999;160:1450–6. doi: 10.1164/ajrccm.160.5.9901096. [DOI] [PubMed] [Google Scholar]
- 5.Doerschuk CM, Winn RK, Coxson HO, Harlan JM. CD18-dependent and –independent mechanisms of neutrophil emigration in the pulmonary and systemic microcirculation of rabbits. J Immunol. 1990;144:2327–33. [PubMed] [Google Scholar]
- 6.Bodlander FM. Deaths associated with anaesthesia. Br J Anaesth. 1975;47:36–40. doi: 10.1093/bja/47.1.36. [DOI] [PubMed] [Google Scholar]
- 7.Marx GF, Mateo CV, Orkin LR. Computer analysis of postanaesthetic deaths. Anaesthesiology. 1973;39:54–8. doi: 10.1097/00000542-197307000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Nagase T, Uozumi N, Ishii S, Kume K, Izumi T, Ouchi Y, et al. Acute lung injury by sepsis and acid aspiration: a key role for cytosolic phospholipase A2. Nature Immunol. 2000;1:42–6. doi: 10.1038/76897. [DOI] [PubMed] [Google Scholar]
- 9.Wang W, Tam WF, Hughes CCW, Rath S, Sen R. c-Rel is a target of pentoxifylline-mediated inhibition of T lymphocyte activation. Immunity. 1997;6:165–74. doi: 10.1016/s1074-7613(00)80423-9. [DOI] [PubMed] [Google Scholar]
- 10.Meng ZH, Dyer K, Billiar TR, Tweardy DJ. Essential role for IL-6 in postresuscitation inflammation in hemorrhagic shock. Am J Physiol Cell Physiol. 2001;280:C343–51. doi: 10.1152/ajpcell.2001.280.2.C343. [DOI] [PubMed] [Google Scholar]
- 11.Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med. 1995;332:1351–62. doi: 10.1056/NEJM199505183322008. [DOI] [PubMed] [Google Scholar]
- 12.MacLaren R, Jung R. Stress-dose corticosteroid therapy for sepsis and acute lung injury or acute respiratory distress syndrome in critically ill patients. Pharmacotherapy. 2002;22:1140–56. doi: 10.1592/phco.22.13.1140.33519. [DOI] [PubMed] [Google Scholar]
- 13.Lipworth BJ, Seckl JR. Measures for detecting systemic bioactivity with inhaled and intranasal corticosteroids. Thorax. 1997;52:476–82. doi: 10.1136/thx.52.5.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scapagnini U. Psychoneuroendocrinoimmunology: the basis for a novel therapeutic approach in aging. Psychoneuroendocrinoimmunology. 1992;17:411–20. doi: 10.1016/0306-4530(92)90046-a. [DOI] [PubMed] [Google Scholar]
- 15.Sapolsky RM, Krey LC, McEwen BS. The adrenocortical stress-response in the aged male rat: impairment of recovery from stress. Exp Gerontol. 1983;18:55–64. doi: 10.1016/0531-5565(83)90051-7. [DOI] [PubMed] [Google Scholar]
- 16.Ottenweller JE, Tapp WN, Pitman DL, Natelson BH. Interactions among the effects of aging, chronic disease, and stress on adrenocortical function in Syrian hamsters. Endocrinology. 1990;126:102–9. doi: 10.1210/endo-126-1-102. [DOI] [PubMed] [Google Scholar]
- 17.The ARDS Clinical Trials Network. Randomized, placebo-controlled trial of lisofylline for early treatment of acute lung injury and acute respiratory distress syndrome. Crit Care Med. 2002;30:1–6. doi: 10.1097/00003246-200201000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Oliveira-Junior IS, Pinheiro BV, Silva IDCG, Salomao R, Zollner RL, Beppu OS. Pentoxifylline decreases tumor necrosis factor and interleukin-1 during high tidal volume. Braz J Med Biol Res. 2003;36:1349–57. doi: 10.1590/s0100-879x2003001000011. [DOI] [PubMed] [Google Scholar]
- 19.Nishina K, Mikawa K, Takao Y, Shiga M, Maekawa N, Obara H. Intravenous lidocaine attenuates acute lung injury induced by hydrochloric acid aspiration in rabbits. Anesthesiology. 1998;88:1300–9. doi: 10.1097/00000542-199805000-00022. [DOI] [PubMed] [Google Scholar]
- 20.Goldman G, Welbourn R, Klausner JM, Kobzik L, Valeri CR, Shepro D, et al. Neutrophil accumulations due to pulmonary thromboxane synthesis mediate acid aspiration injury. J Appl Physiol. 1991;70:1511–7. doi: 10.1152/jappl.1991.70.4.1511. [DOI] [PubMed] [Google Scholar]
- 21.Schreiber T, Hueter L, Gaser E, Schmidt B, Schwarzkopf K, Rek H, et al. PEEP has beneficial effects on inflammation in the injured and no deleterious effects on the noninjured lung after unilateral lung acid instillation. Intensive Care Med. 2006;35:740–9. doi: 10.1007/s00134-006-0117-6. [DOI] [PubMed] [Google Scholar]
- 22.Matthay M, Rosen GD. Acid aspiration induced lung injury. New insights and therapeutic options. Am J Respir Crit Care Med. 1996;154:277–8. doi: 10.1164/ajrccm.154.2.8756794. [DOI] [PubMed] [Google Scholar]
- 23.Knight PR, Kurek C, Davidson BA, Nader ND, Patel A, Sokolowski J, et al. Acid aspiration increases sensitivity to increased ambient oxygen concentration. Am J Respir Cell Mol Biol. 2000;278:L1240–7. doi: 10.1152/ajplung.2000.278.6.L1240. [DOI] [PubMed] [Google Scholar]
- 24.Balibrea JL, Arias-Díaz J. Acute respiratory distress syndrome in the septic surgical patient. World J Surg. 2003;27:1275–84. doi: 10.1007/s00268-003-7258-3. [DOI] [PubMed] [Google Scholar]
- 25.Kudoh I, Miyazaki H, Ohara M, Fukushima J, Tazawa T, Yamada H. Activation of alveolar macrophages in acid-injured lung in rats: different effects of pentoxifylline on tumor necrosis factor-alpha and nitric oxide production. Crit Care Med. 2001;29:1621–5. doi: 10.1097/00003246-200108000-00020. [DOI] [PubMed] [Google Scholar]
- 26.Thompson BT. Glucocorticoids and acute lung injury. Crit Care Med. 2003;31:S253–7. doi: 10.1097/01.CCM.0000057900.19201.55. [DOI] [PubMed] [Google Scholar]
- 27.Munford RS, Pugin J. Normal responses to injury prevent systemic inflammation and can be immunosuppressive. Am J Respir Crit Care Med. 2001;163:316–21. doi: 10.1164/ajrccm.163.2.2007102. [DOI] [PubMed] [Google Scholar]
- 28.Bollaert PE, Charpentier C, Levy B, Debouverie M, Audibert G, Larcan A. Reversal of late septic shock with supraphysiologic doses of hydrocortisone. Crit Care Med. 1998;26:645–50. doi: 10.1097/00003246-199804000-00010. [DOI] [PubMed] [Google Scholar]
- 29.Sebaldt R, Sheller J, Oates J, Roberts LJ, 2nd, FitzGerald G. Inhibition of eicosanoid biosynthesis by glucocorticoids in humans. Proc Natl Acad Sci USA. 1990;87:6974–8. doi: 10.1073/pnas.87.18.6974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gilbert KA, Petrovic-Dovat L, Rannels E. Hormonal control of compensatory lung growth. In: McDonald JA, editor. Lung Growth and Development. New York: Dekker; 1997. pp. 627–60. [Google Scholar]
- 31.Bernard GR, Luce JM, Sprung CL, Rinaldo JE, Tate RM, Sibbald WJ, et al. High-dose corticosteroids in patients with the adult respiratory distress syndrome. N Engl J Med. 1987;317:1565–70. doi: 10.1056/NEJM198712173172504. [DOI] [PubMed] [Google Scholar]
- 32.Hubbard WC, Bickel C, Scheimer RP. Simultaneous quantitation of endogenous levels of cortisone and cortisol in human nasal and bronchoalveolar lavage fluids and plasma via gas chromatography-negative ion chemical ionization mass spectrometry. Anal Biochem. 1994;221:109–17. doi: 10.1006/abio.1994.1386. [DOI] [PubMed] [Google Scholar]
- 33.Baker RW, Walker BR, Shaw RJ, Honour JW, Jessop DS, Lightman SL, et al. Increased cortisol: cortisone ratio in acute pulmonary tuberculosis. Am J Respir Crit Care Med. 2000;162:1641–7. doi: 10.1164/ajrccm.162.5.9912119. [DOI] [PubMed] [Google Scholar]
- 34.Ricard JD, Dreyfuss D, Saumon G. Ventilator induced lung injury. Eur Respir J. 2003;22:2s–9s. doi: 10.1183/09031936.03.00420103. [DOI] [PubMed] [Google Scholar]
- 35.Oliveira-Junior IS, Brunialti MK, Koh IH, Junqueira VB, Salomao R. Effect of pentoxifylline on lung inflammation and gas exchange in a sepsis-induced acute lung injury model. Braz J Med Biol Res. 2006;39:1455–63. doi: 10.1590/s0100-879x2006001100009. [DOI] [PubMed] [Google Scholar]