Abstract
PURPOSE
To ascertain perioperative morbimortality and identify prognostic factors for mortality among patients ≥55 years who undergo non-cardiac surgery.
METHODS
A retrospective cohort of 403 patients relating to perioperative morbidity-mortality. Data were collected from a standardized protocol on gender, age, comorbidities, medications used, smoking, alcohol abuse, chronic use of benzodiazepine, nutritional status, presence of anemia, activities of daily living, American Society of Anesthesiology classification, Detsky’s modified cardiac risk index - American College of Physicians, renal function evaluation, pulmonary risk according to the Torrington scale, risk of thromboembolic events, presence of malignant disease and complementary examinations.
RESULTS
The mean age of the subjects was 70.8 ± 8.1 years. The “very old” (≥80 years) represented 14%. The mortality rate was 8.2%, and the complication rate was 15.8%. Multiple logistic regression showed that a history of coronary heart disease (OR: 3.75; p=0.02) and/or valvular heart disease (OR: 31.79; p=0.006) were predictors of mortality. The American Society of Anesthesiology classification was shown to be the best scale to mark risk (OR: 3.01; p=0.016). Nutritional status was a protective factor, in which serum albumin increases of 1 mg/dl decreased risk by 63%.
DISCUSSION
The results indicate that serum albumin, coronary heart disease, valvular heart disease and the American Society of Anesthesiology classification could be prognostic predictors for aged patients in a perioperative setting. In this sample, provided that pulmonary, cardiac and thromboembolic risks were properly controlled, they did not constitute risk factors for mortality. Furthermore, continuous effort to learn more about the preoperative assessment of elderly patients could yield intervention possibilities and minimize morbimortality.
Keywords: Surgery, Preoperative, Mortality, Risk, Evaluation
INTRODUCTION
In past several decades, it was commonly recommended that surgery for the elderly be avoided because of their frailty and increased risk when undergoing a major operative procedure. However, recent advances in anesthetic practice and surgical techniques, including minimally invasive surgical approaches, have enabled sicker and older patients to be eligible for procedures that were more risky in the past.1
The perioperative care of elderly people is influenced by the particular alterations to aging, such as the presence of comorbidities, the use of multiple medications and the severity of the disease requiring intervention. Atypical presentations of diseases, diminished heart and lung reserves and alterations in the pharmacodynamics and pharmacokinetics of drugs are also observed.2,3
It must be kept in mind that much of the current management during the perioperative period for elderly patients is based on data from the non-elderly adult population. There is a need to define specific parameters for the elderly, and particularly for the very old.4
The concern now is that, because an increasing number of sicker and older patients are having surgery, perioperative morbidity and mortality rates may be increasing. As a result, there is a renewed interest in identifying factors associated with adverse postoperative outcomes to develop strategies to improve the perioperative care of and outcomes for geriatric surgical patients. Under these conditions, perioperative evaluation gives rise to the need for an comprehensive approach, with the objectives of minimizing risk as well as maintaining or recovering previous functional abilities.2
Design
This retrospective cohort study investigated perioperative morbidity-mortality among elderly inpatients of Hospital das Clínicas between February and November 2004. The study was conducted by administering a structured preoperative evaluation and postoperative follow-up until the outcome of death or discharge.
SAMPLE AND METHODS
Preoperative evaluations were performed on patients of both sexes aged 55 years and over. The exclusion criterion was failure to proceed with surgery.
After ethical approval, a single researcher reviewed the medical records made available by the Medical Archives Service and information present in the preoperative assessment file, with the objective of retrieving the clinical data.
The following data were collected from a standardized protocol: sex, age, comorbidities, medications used, smoking, alcohol abuse, chronic use of benzodiazepine, nutritional status, presence of anemia, activities of daily living (ADL), American Society of Anesthesiology (ASA) classification, Detsky’s modified cardiac risk index – American College of Physicians (ACP), pulmonary risk according to the Torrington scale, risk of thromboembolic events, presence of malignant disease and complementary examinations (electrocardiogram, chest radiograph, hemogram, urea, creatinine, albumin, electrolytes and glycemia).
The primary outcome was the occurrence of in-hospital death.
After clinical evaluation and risk estimative, the CARES group (Clinical Assessment and Research in Elderly Surgical patients) applies guidelines focused on minimize risk including to star the beta blockade treatment to maintaining the heart rate below 70 beats per minute for high and intermediate cardiac risk, as suggest by the international recommendations.8–14 Stress imaging testing like sestamibi SPECT scan was indicated in selected cases.
The chronic renal failure stage ≥ 3 should carefully balancing fluids, avoid nephrotoxic drugs and use renal clearance to corrected antibiotic. These patients were seeing besides a nephrologist. The high respiratory risk was target to physiotherapy. We oriented non pharmacological preventive measures from thromboembolism for all patients and heparin therapy for high and intermediate risk. We accompanying all patients until discharge and complications were registered.
The variables relating to death (p< 0.10) in the univariate analysis were grouped for applying logistic regression, and each of the groups was reduced by means of a stepwise selection process. Considering the variables thus selected, the backward stepwise method was applied to obtain the principal predictors of mortality. The Hosmer-Lemeshow test was used for adjustment of the end model.
For the statistical analysis, the MINITAB 14 program was used for descriptive analysis and comparison between patients with opposite outcomes. For continuous variables, Student’s t-test or the non-parametric Kruskal-Wallis test were used. Qualitative variables were compared using the Pearson Chi-Square or Fisher’s exact tests. Two-sided p-values < 0.05 were considered significant.
RESULTS
Descriptive and univariate analysis
Among 508 patients enrolled, 105 (20.7%) fulfilled the exclusion criteria. Thus, 403 patients were studied.
The mean age was 70.8 ± 8.1 years, with a range from 55 to 92 years. The very elderly (≥ 80 years) represented 14% (Table 1). Elective surgery performs 88.9%, 67.2% cases were in the digestive system with malignant disease in 58% (Table 2).
Table 1.
Clinical characteristics of cohort patients, according to the outcomes.
| SG (n=370) | DG (n=33) | p-value | |
|---|---|---|---|
| Age (years)a | 70.7 ± 8.1 | 72.3 ± 7.3 | 0.249 |
| Male sex - n (%) | 176 (48) | 23 (70) | 0.017 |
| ADL dependence - n (%) | 05 (02) | 01 (11) | 0.177 |
| Malignancy - n (%) | 206 (56) | 28 (85) | <0.001 |
SG – survivor group; DG – death group; ADL – activities of daily living;
M ± SD-mean ± standard deviation.
Table 2.
Surgical types evaluated
| Type of Surgery | n | % |
|---|---|---|
| Digestive system elective | 271 | 67.2 |
| Emergency surgery | 44 | 10.9 |
| Gynecological | 38 | 9.4 |
| Urological | 11 | 2.7 |
| Vascular | 10 | 2.5 |
| Ear, nose and throat | 10 | 2.5 |
| Head and neck | 07 | 1.7 |
| Plastic surgery | 07 | 1.7 |
| Ophthalmological | 05 | 1.2 |
Women predominated (51%), but there were more deaths among men (p= 0.017; OR: 2.26; 95% CI: 1.08 – 4.72). Regardless of age, the number of comorbidities, the presence of malignant disease as a surgical diagnosis and ASA and Detsky surgical risk classifications presented similar distributions between the sexes.
The comorbidities are listed in table 3. The number of diseases (3.9 ± 1.7) or the number of medications used (2.6 ± 1.8) per patient did not correlate with death. When specific diseases were evaluated, we was found that dyslipidemia, chronic kidney disease, valve disease (aortic or mitral), anemia, and history of coronary artery disease contributed towards increased surgical risk. The use of benzodiazepine longer than three months (4.7%), alcoholism (4.7%) and current smoking (11.9%) or stop smoking longer than three months (28.3%) did not increase the mortality related to the operation.
Table 3.
Cohort patients: comorbidities, according to the outcomes
| Comorbities | SG n(%) | DG n(%) | p-value |
|---|---|---|---|
| Hypertension | 213 (58) | 24 (73) | 0.09 |
| Anemia | 100 (27) | 17 (52) | 0.004 |
| Diabetes mellitus | 76 (21) | 7 (21) | 0.93 |
| Dyslipidemia | 39 (11) | 8 (24) | 0.04 |
| Coronary disease | 21 (06) | 8 (24) | <0.001 |
| Cardiac failure | 25 (07) | 3 (09) | 0.47 |
| Hypothyroidism | 27 (07) | 0 (00) | 0.15 |
| COPDa | 24 (06) | 1 (03) | 0.70 |
| Stroke | 19 (05) | 3 (09) | 0.40 |
| Atrial fibrillation | 17 (05) | 4 (12) | 0.08 |
| Chronic kidney disease stage ≥3 | 11 (03) | 7 (21) | <0.001 |
| Depression | 13 (04) | 2 (06) | 0.35 |
| Body mass index <16 | 6 (02) | 5 (16) | <0.001 |
| Dementia | 6 (02) | 2 (06) | 0.13 |
| Heart valve disease | 1 (0.3) | 3 (09) | <0.001 |
SG - survivor group; DG - death group;
chronic obstructive pulmonary disease.
The nutritional profile (Table 4) was seen to be a good prognostic factor, given that the mean hemoglobin, albumin and body mass index were lower among the patients who died, and these patients presented means that were below the reference values normally used.
Table 4.
Evolution related to nutritional assessment in the cohort
| Reference values | SG (n=370) M ± SD a | DG (n=33) M ± SD a | p-value | |
|---|---|---|---|---|
| Body mass index – kg/m2 | 20–25 | 24.6±5.3 | 21.3±5.0 | 0.011 |
| Hemoglobin – g/dL | 12–18 | 12.5±2.2 | 11.2±2.1 | 0.002 |
| Albumin – g/dL | 3.5–5.0 | 3.7±0.7 | 2.9±0.9 | <0.001 |
| Lymphocytes – 103 cell/mm3 | 1.0–3.4 | 1.7±0.7 | 1.4±0.7 | 0.084 |
SG - survivor group; DG - death group; M ± SD
-mean ± standard deviation.
General surgical risk, as classified by the ASA (Table 5), was shown to be progressively greater in accordance with the ASA grading, and it was correlated with mortality.
Table 5.
Evolution related to ASA classification
| ASA | SG n(%) | DG n(%) |
|---|---|---|
| I | 56 (15) | 00 (00) |
| II | 292 (79) | 27 (82) |
| III | 22 (06) | 05 (15) |
| IV | 00 (00) | 01 (03) |
| Chi-squared for trend p= 0.001 | ||
SG – survivor group; DG – death group; ASA – American Society of Anesthesiology.
For Detsky’s modified cardiac risk index, 208 (52%) patients were classified as low risk, 193 (48%) as intermediate risk and 2 (0.5%) as high risk. The mortality rate was 7.2% for the low-risk category, 8.8% for those at intermediate risk and 50.0% for those at high risk, which did not represent any significant difference.
The pulmonary and renal risks were low for most of the patients, however the risk of thromboembolism was almost high (73%). The risk of thromboembolic events and the high renal risk correlated with death, in despite of what was found for pulmonary risk (Table 6).
Table 6.
Evolution related to the renal, thromboembolism and pulmonary risk
| Risk | Low n(%) | Moderate n(%) | High n(%) |
|---|---|---|---|
| Renal risk; p=0.02 | |||
| SG (n=370) | 304 (82) | 39 (11) | 27 (07) |
| DG (n=033) | 23 (70) | 03 (09) | 07 (21) |
| Thromboembolism risk; p<0.001 | |||
| SG (n=370) | 03 (01) | 105 (28) | 262 (71) |
| DG (n=033) | 00 (00) | 02 (06) | 31 (94) |
| Pulmonary risk; p=0.20 | |||
| SG (n=370) | 298 (80) | 69 (19) | 03 (01) |
| DG (n=033) | 23 (70) | 10 (30) | 00 (00) |
SG – survivor group, DG – death group.
This sample presented a mortality rate of 8.2% and a non-fatal complications rate of 15.8% (Table 6). In particular, cardiac (6%), renal (5%), pulmonary (7%), operative (wound dehiscence, hemorrhage, fistula, etc; 8%) and infectious (14%) complications contributed to greater mortality (Table 7).
Table 7.
Complications of cohort patients, according to the outcomes
| Complication | SG n(%) | DG n(%) | p-value |
|---|---|---|---|
| Infectious | 31 (8) | 25 (76) | <0.001 |
| Surgical | 21 (6) | 11 (33) | <0.001 |
| Pulmonary | 16 (4) | 13 (39) | <0.001 |
| Cardiac | 16 (4) | 9 (27) | <0.001 |
| Renal | 5 (1) | 15 (45) | <0.001 |
| Electrolytic disorders | 12 (3) | 2 (6) | 0.320 |
| Delirium | 10 (3) | 3 (9) | 0.081 |
| Venous thromboembolic | 2 (0.5) | 1 (3) | 0.226 |
| Anesthetic | 2 (0.5) | 0 (0) | 1.000 |
SG – survivor group; DG – death group.
Multivariate analysis
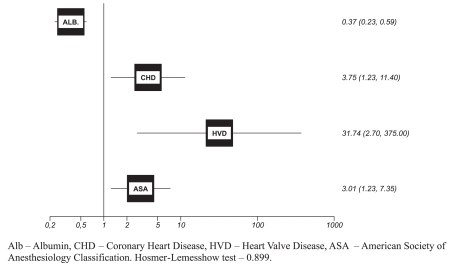
Multivariate analysis via multiple logistic regression (Figure 1) indicated the variables that were prognostic for mortality. The presence of coronary disease (odds ratio: 3.75; p=0.02) almost quadrupled the risk of death, while heart valve disease (odds ratio, 31.79; p=0.006) increased the risk 31-fold.
Figure 1.
Odds of death related to the prognostic factors on perioperative period
Nutritional status was shown to be a protective marker: a serum albumin increase of 1 mg/dl decreased the risk of mortality by 63%.
ASA classification was shown to be better for estimating prognoses that the other risk scales used. On this scale, a one-unit increase tripled the chance of death.
The Hosmer-Lemeshow test (0.899) showed a good fit, 99.3% sensitivity, 25.0% specificity and 91.3% accuracy in retesting this model on the same sample.
DISCUSSION
The limitations of this study are related to its retrospective design, which favors under-diagnosing of complications. For instance, the low incidence of delirium (3%) found in this population can be cited. It is known that hypoactive delirium is a condition that is frequently under-diagnosed by health teams and its impact on mortality often ignored.5,6 Nevertheless, the deaths inside the hospital (primary outcome) were faithfully recorded by the institution. Standardization of the preoperative assessment file and the risk scales used gave uniformity to the data and made it easier to collect.
We worked with the bias that the majority of our patients underwent digestive system surgery. Incorporation of other surgical groups, such as those who underwent orthopedic surgery, urological surgery or neurosurgery, might have furnished different perspectives from that observed. Our population presented a high mean age and a high rate of comorbidities but a good functional capacity. Only 2% of our patients were dependent for Activities of Daily Living. Some comorbidities are related to worse perioperative prognosis as coronary artery disease and nowadays, there are many purposes to minimize this risk and the elderly is a population that potentially has beneficial effects of such interventions.8–14
Among the factors included in the multivariate analysis, the presence of mitral or aortic valve disease can be highlighted. The presence of symptomatic valve disease increases the surgical risk and involves a complex treatment that postpones possible non-cardiac surgery. Goldman’s scale contraindicates surgery in patients with severe aortic valve disease, and the management of such patients currently raises interesting controversies.15–20
A poor nutritional profile is a known risk factor for infectious complications and wound dehiscence, thereby contributing towards greater perioperative mortality. Nutritional support benefits patients with severe malnutrition in perioperative period.21–26 In the present study, there was greater correlation between mortality and the body mass index, albumin and hemoglobin. Albumin was highlighted in the logistic regression, in that an increase of 1 g/dl decreased the chance of death by 63%.
Regarding to the scales used for preoperatively evaluating the risks, there was a clear correlation between the ASA classification and mortality. It scores age and degree of clinical impairment by chronic diseases, where brief interventions generally produce disappointing results. A veterans’ study7 also saw the ASA classification as a precise predictor of mortality.21–26
The univariate association between thromboembolic risk and death reflects the particular criteria that scores high-risk (malignant disease, immobilization, cardiac insufficiency, prolonged surgery, etc.). The majority (73%) of this study population was at high risk, but because of the wide use of adequate prophylaxis, only three occurrences of this complication were confirmed (0.7%). In other words, the real occurrences of venous thromboembolism were unrelated to greater mortality. In the literature, it is predicted that deep vein thrombosis will occur at a rate of 10 to 20%, and symptomatic pulmonary thromboembolism will occur at a rate of 5 to 10% in the absence of prophylaxis.27
The modified Detsky index showed similar mortality between low and intermediate risk. This was probably due to the advent of the use of beta-blockers, which may significantly diminish the risk of perioperative cardiac events in patients at intermediate risk. Our group systematically indicates the use of beta-blockers for patients at intermediate and high cardiac risk and suggests that this medication should be maintained for patients who are already using it at home as suggest by the literature.8–14
Pulmonary risk based upon the Torrington scale was not a predictor of mortality. This is in line with the literature and the guidelines of the American College of Physicians (2006), in which pulmonary risk does not contraindicate surgery. We recommend clinical control and respiratory physiotherapy as indicated for patients at risk.4,27,28
We conclude that, provided pulmonary and cardiac conditions and the risk of thromboembolism are properly controlled, they do not constitute risk factors for mortality. Therefore, surgical procedures on elderly people with such conditions should not be contraindicated. Instead, risk should be managed with a preventive attitude. The impact that these clinical interventions produce on mortality predictors reinforces the need for a comprehensive approach towards the elderly.
The results indicate that serum albumin, coronary heart disease, valvular heart disease and ASA classification could be prognostic predictors for aged patients in a perioperative setting. Furthermore, continuous efforts to learn more about the preoperative assessment of elderly patients could yield intervention possibilities and minimize morbimortality. More consistent data will come from prospective studies that are now in progress at our center.
REFERENCES
- 1.McGoldrick KE. The Graying of America: Anesthetic Implications for Geriatric Outpatients. ASA Refresher Courses in Anesthesiology. 2002;33:165–74. [Google Scholar]
- 2.Leung JM, Liu LL. Current Controversies in the Perioperative Management of Geriatric Patients. ASA Refresher Courses in Anesthesiology. 2001;29:175–87. [Google Scholar]
- 3.Muravchick S. Physiological Changes of Aging. ASA Refresher Courses in Anesthesiology. 2003;31:139–49. [Google Scholar]
- 4.Sitta MC, Machado AN, Lapa MS. Avaliação perioperatória. In: Jacob Filho W, Amaral JRG, editors. Avaliação Global do Idoso – Manual da Liga do Gamia. 1. São Paulo: Editora Atheneu; 2005. pp. 193–214. [Google Scholar]
- 5.Dibert C. Delirium and the older adult after surgery. Perspectives. 2004;28:10–6. [PubMed] [Google Scholar]
- 6.O’Brien D. Acute postoperative delirium: definitions, incidence, recognition, and interventions. J Perianesth Nurs. 2002;17:384–92. doi: 10.1053/jpan.2002.36783. [DOI] [PubMed] [Google Scholar]
- 7.Hamel MB, Henderson WG, Khuri SF, Daley J. Surgical outcomes for patients aged 80 and older: morbidity and mortality from major noncardiac surgery. J Am Geriatr Soc. 2005;53:424–9. doi: 10.1111/j.1532-5415.2005.53159.x. [DOI] [PubMed] [Google Scholar]
- 8.Mangano DT, Layug EL, Wallace A, Tateo I. Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery. Multicenter Study of Perioperative Ischemia Research Group. N Engl J Med. 1996;335:1713–20. doi: 10.1056/NEJM199612053352301. [DOI] [PubMed] [Google Scholar]
- 9.Yang H, Raymer K, Butler R, Parlow J, Roberts R. The effects of perioperative beta-blockade: results of the Metoprolol after Vascular Surgery (MaVS) study, a randomized controlled trial. Am Heart J. 2006;152:983–90. doi: 10.1016/j.ahj.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 10.Ferguson TB, Jr, Coombs LP, Peterson ED. Preoperative beta-blocker use and mortality and morbidity following CABG surgery in North America. Jama. 2002;287:2221–7. doi: 10.1001/jama.287.17.2221. [DOI] [PubMed] [Google Scholar]
- 11.McGory ML, Maggard MA, Ko CY. A meta-analysis of perioperative beta blockade: what is the actual risk reduction? Surgery. 2005;138:171–9. doi: 10.1016/j.surg.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 12.Auerbach AD, Goldman L. beta-Blockers and reduction of cardiac events in noncardiac surgery: clinical applications. Jama. 2002;287:1445–7. doi: 10.1001/jama.287.11.1445. [DOI] [PubMed] [Google Scholar]
- 13.Auerbach AD, Goldman L. beta-Blockers and reduction of cardiac events in noncardiac surgery: scientific review. Jama. 2002;287:1435–44. doi: 10.1001/jama.287.11.1435. [DOI] [PubMed] [Google Scholar]
- 14.Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med. 2005;353:349–61. doi: 10.1056/NEJMoa041895. [DOI] [PubMed] [Google Scholar]
- 15.Zahid M, Sonel AF, Saba S, Good CB. Perioperative risk of noncardiac surgery associated with aortic stenosis. Am J Cardiol. 2005;96:436–8. doi: 10.1016/j.amjcard.2005.03.095. [DOI] [PubMed] [Google Scholar]
- 16.Kertai MD, Bountioukos M, Boersma E, et al. Aortic stenosis: an underestimated risk factor for perioperative complications in patients undergoing noncardiac surgery. Am J Med. 2004;116:8–13. doi: 10.1016/j.amjmed.2003.07.012. [DOI] [PubMed] [Google Scholar]
- 17.Christ M, Sharkova Y, Geldner G, Maisch B. Preoperative and perioperative care for patients with suspected or established aortic stenosis facing noncardiac surgery. Chest. 2005;128:2944–53. doi: 10.1378/chest.128.4.2944. [DOI] [PubMed] [Google Scholar]
- 18.Rimmerman CM. Optimizing the preoperative evaluation of patients with aortic stenosis or congestive heart failure prior to noncardiac surgery. Cleve Clin J Med. 2006;73 (Suppl 1):S111–5. doi: 10.3949/ccjm.73.suppl_1.s111. [DOI] [PubMed] [Google Scholar]
- 19.Goldman L. Aortic stenosis in noncardiac surgery: underappreciated in more ways than one? Am J Med. 2004;116:60–2. doi: 10.1016/j.amjmed.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Bach DS, Eagle KA. Perioperative assessment and management of patients with valvular heart disease undergoing noncardiac surgery. Minerva Cardioangiol. 2004;52:255–61. [PubMed] [Google Scholar]
- 21.Martindale RG, Maerz LL. Management of perioperative nutrition support. Curr Opin Crit Care. 2006;12:290–4. doi: 10.1097/01.ccx.0000235204.54579.14. [DOI] [PubMed] [Google Scholar]
- 22.Weimann A, Braga M, Harsanyi L, et al. ESPEN Guidelines on Enteral Nutrition: Surgery including organ transplantation. Clin Nutr. 2006;25:224–44. doi: 10.1016/j.clnu.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 23.Heys SD, Schofield AC, Wahle KW, Garcia-Caballero M. Nutrition and the surgical patient: triumphs and challenges. Surgeon. 2005;3:139–44. doi: 10.1016/s1479-666x(05)80033-2. [DOI] [PubMed] [Google Scholar]
- 24.Salvino RM, Dechicco RS, Seidner DL. Perioperative nutrition support: who and how. Cleve Clin J Med. 2004;71:345–51. doi: 10.3949/ccjm.71.4.345. [DOI] [PubMed] [Google Scholar]
- 25.Waitzberg DL, Saito H, Plank LD, et al. Postsurgical infections are reduced with specialized nutrition support. World J Surg. 2006;30:1592–604. doi: 10.1007/s00268-005-0657-x. [DOI] [PubMed] [Google Scholar]
- 26.Goonetilleke KS, Siriwardena AK. Systematic review of peri-operative nutritional supplementation in patients undergoing pancreaticoduodenectomy. Jop. 2006;7:5–13. [PubMed] [Google Scholar]
- 27.Caprini JA, Arcelus JI, Reyna JJ. Effective risk stratification of surgical and nonsurgical patients for venous thromboembolic disease. Seminars in hematology. 2001;38(2 Suppl 5):12–9. doi: 10.1016/s0037-1963(01)90094-0. [DOI] [PubMed] [Google Scholar]
- 28.Lawrence VA, Cornell JE, Smetana GW. Strategies to reduce postoperative pulmonary complications after noncardiothoracic surgery: systematic review for the American College of Physicians. Annals of internal medicine. 2006;144:596–608. doi: 10.7326/0003-4819-144-8-200604180-00011. [DOI] [PubMed] [Google Scholar]
- 29.Smetana GW, Lawrence VA, Cornell JE. Preoperative pulmonary risk stratification for noncardiothoracic surgery: systematic review for the American College of Physicians. Annals of internal medicine. 2006;144:581–95. doi: 10.7326/0003-4819-144-8-200604180-00009. [DOI] [PubMed] [Google Scholar]