Abstract
Synopsis
Reports that elasmobranchs (sharks, skates, and rays) may have a low incidence of disease have stimulated interest in understanding the role of their immune system in this apparent resistance. Although research in this area may potentially translate into applications for human health, a basic understanding of the elasmobranch immune system components and how they function is essential. As in higher vertebrates, elasmobranch fishes possess thymus and spleen, but in the absence of bone marrow and lymph nodes, these fish have evolved unique lymphomyeloid tissues, namely epigonal and Leydig organs. As conditions for short-term culture of elasmobranch immune cells have become better understood, the opportunity to examine functional activity of cytokine-like factors derived from conditioned culture medium has resulted in the identification of growth inhibitory activity against a variety of tumor cell lines. Specifically, the medium enriched by short term culture of bonnethead shark (Sphyrna tiburo) epigonal cells (epigonal conditioned medium, ECM) has been shown to inhibit the growth of mammalian tumor cell lines, including fibrosarcoma (WEHI-164), melanoma (A375.S2), B-cell lymphoma (Daudi), T-cell leukemia (Jurkat), pancreatic cancer (PANC-1), ovarian cancer (NIH:OVCAR-3), and three breast carcinoma cell lines (MCF7, HCC38, Hs578T). Of the cell lines tested, WEHI-164, A375.S2, Daudi, and Jurkat cells were among the most sensitive to growth inhibitory activity of ECM whereas PANC-1 and NIH:OVCAR-3 cells were among the least sensitive. In addition, ECM demonstrated preferential growth inhibition of malignant cells in assays against two different malignant/non-malignant cell line pairs (HCC38/HCC38 BL and Hs 578T/Hs 578Bst). Separation of protein components of ECM using SDS-PAGE resulted in a very reproducible pattern of three major bands corresponding to molecular sizes of approximately 40–42 kD, 24 kD, and 17 kD. Activity is lost after heating at 75°C for 30 min, and can be diminished by treatment with proteinase K and protease. Activity is not affected by treating with trypsin, DNase I or RNase A.
Introduction
Studies in the field of ecological immunology, which focuses on examining immune function in the context of evolution and ecology, can have significant implications for public health. Much can be learned from immune defense mechanisms of non-traditional animal models. In fact, reliance on traditional animal models may actually lead to inaccurate assessments of risk in others species, including humans (Haley 2003; Levin and others 2005a,5b; Mori and others 2006).
Elasmobranch fish, which include the sharks, skates, and rays, have many advantages as animal models for biomedical research, as described by Luer (1989). Public health implications of elasmobranch-derived substances have increased in scope and number over the past two decades, with many researchers exploring components of the elasmobranch immune system for compounds which may have implications for public health. Through these efforts, several compounds from shark tissues have demonstrated cytotoxic activity against tumor cells (Snodgrass and others 1976; Pettit and Ode 1977), antiangiogenic activity (Lee and Langer 1983; Luer 1986; Moriarty and others 1995; Sadownik and others 1995; Sills and others 1998; Cho and Kim 2002; Li and others 2002), and antibiotic activity (Moore and others 1993).
Elasmobranchs fill a unique immunological niche in that they are phylogenetically the most primitive group of vertebrates to possess all the components necessary for an adaptive immune system (Litman and others 1999; Flajnik and Rumfelt 2000). Cartilaginous fishes are the most primitive vertebrates with immunoglobulin (Ig) (Marchalonis and Edelman 1965; Kokubu and others 1988a,1988b; Anderson and others 1999), T-cell receptors (TCR) (Rast and others 1997), the major histocompatibility complex (MHC) (Kasahara and others 1992; Bartl and Weissman 1994; Bartl and others 1997), RAG-mediated rearrangement, somatic hypermutation, and the presence of primary and secondary lymphoid tissues as components of the immune system (reviewed by Flajnik and Rumfelt 2000). Elasmobranchs lack both bone marrow and a true lymphatic system, but have evolved specific lymphomyeloid tissues that play significant roles in immune system development and function (reviewed in Luer and others 2004). Lymphomyeloid sites unique to this subclass of fish include the epigonal and Leydig organs, associated with the gonads and the esophagus, respectively. The epigonal and Leydig organs are thought to be vital in generating the immune repertoire in chondrichthyan fishes (Walsh and others 1993; Miracle and others 2001; Luer and others 2004) and express high levels of RAG and TdT, indicating that these tissues are primary lymphoid organs (that is, bone marrow equivalents) in cartilaginous fishes (Miracle and others 2001; Rumfelt and others 2002). Such unique lymphomyeloid sites in elasmobranchs may represent a site of novel immune regulators.
As in higher vertebrates, the regulation of elasmo-branch immune responses likely involves intercellular signaling molecules such as cytokines. To date, only a few cytokine genes have been identified in cartilaginous fish, and include IL-1β (Bird and others 2002) and IL-8 (Inoue and others 2003). Developments in the ability to culture lymphoid cells isolated from chondrichthyan fishes (Walsh and Luer 1998) led to a search for cytokines produced by shark immune cells in culture. In screening for cytokine-like bioactivity, short-term cultures of immune cells from several shark and skate species were examined for growth inhibitory activity against mammalian tumor cell lines. Media conditioned by cultures of epigonal tissue from bonnethead sharks (Sphyrna tiburo) contained the most reproducible and most potent growth inhibitory activity. This paper summarizes this bio-activity as it relates to relative potency and specificity against a variety of clinically relevant mammalian tumor cell lines.
Materials and methods
Culture of elasmobranch epigonal cells
Epigonal organs were collected from mature bonnet-head sharks (S. tiburo) under aseptic conditions and rinsed thoroughly with sterile elasmobranch-modified phosphate buffered saline (E-PBS; Walsh and Luer 2004). Gonadal tissue remaining with the epigonal organ was removed by careful dissection. To express the cells, epigonal tissue was finely minced and placed into 75 mm2 sterile tissue culture flasks (Falcon). Epigonal cells were resuspended in sterile elasmobranch-modifed RPMI (E-RPMI), prepared as described in Walsh and Luer (2004). DNase I (Sigma Chemical Co, St Louis, MO) was added to a final concentration of 15 U/ml, and epigonal cells were cultured at 25°C and 5% CO2 for 2–4 days. In some of the studies, bonnethead shark spleen cells were isolated and cultured under the same conditions.
Preparation of epigonal conditioned medium (ECM)
After 2–4 days of culture, cell-free-ECM was prepared by aspirating the culture supernatants, centrifuging the supernatants twice at 20,000 g for 25 min at 4°C, and dialyzing them against several changes of 50 mM ammonium bicarbonate at 4°C for 4–5 days using 6–8000 MWCO dialysis tubing (Spectrum). Dialyzed conditioned media preparations were lyophilized and stored at −80°C. Protein concentration of ECM was determined using the Bradford assay (BioRad) and approximate molecular weights of ECM protein components were determined using SDS–PAGE.
Tumor cell lines
Cell lines were obtained from the American Type Culture Collection (ATCC; Manasass, VA) and were maintained according to requirements specific for each cell line. The cell lines that were tested and the corresponding tissue origins for each cell line are listed in Table 1. The cell lines used included WEHI-164, A375.S2, Jurkat, PANC-1, NIH:OVCAR-3, MCF7, and Daudi, as well as two malignant/non-malignant cell line pairs, HCC38/HCC38 BL and Hs 578T/Hs 578Bst. These cell lines were selected in order to encompass a broad range of tumor types, including cell lines originating from a variety of tissues as well as from both hard and soft tumors. Additionally, malignant/non-malignant cell line pairs were chosen in order to assess the effects of ECM on non-malignant pheno-types. Cells were cultured in 96-well microtiter plates (Costar). Original seeding density varied with cell line type, and was dependent on individual cell line requirements.
Table 1.
Percent growth inhibition (%GI) observed in tumor and normal cell lines treated with 1.0 mg/ml ECM for 72 h
| Cell line | Origin | %GI ± SEM | N |
|---|---|---|---|
| WEHI 164 | Fibrosarcoma | 92.7 ± 1.9 | 9 |
| Daudi | Burkitt’s B cell lymphoma | 92.0 ± 3.0 | 3 |
| A375.S2 | Malignant melanoma | 65.8 ± 3.0 | 26 |
| Jurkat | Acute T-cell leukemia | 58.3 ± 7.7 | 4 |
| MCF7 | Breast adenocarcinoma | 52.2 ± 4.3 | 18 |
| PANC-1 | Pancreatic epitheloid carcinoma | 49.1 ± 6.1 | 7 |
| Hs 578T | Ductal carcinoma, breast | 27.2 ± 6.7 | 9 |
| HCC38 | Primary ductal carcinoma, breast | 26.1 ± 2.1 | 9 |
| Hs 578Bst | Breast, normal | 15.8 ± 5.0 | 7 |
| HCC38 BL | B lymphoblastoid, normal peripheral blood | 15.2 ± 3.6 | 10 |
| NIH:OVCAR-3 | Ovary, adenocarcinoma | 5.4 ± 1.7 | 11 |
N represents the number of epigonal cultures assayed for a particular cell line. All cell lines are human, with the exception of WEHI 164 (murine).
Growth inhibition assay
Growth inhibitory activity of ECM against cell lines was assessed using 1-(4,5-Dimethylthiazol-2-yl)-3,5-diphenylformazan (MTT) (Mosmann 1983). ECM samples were resuspended in the cell culture medium specific for each cell line at a concentration of 4 mg/ml and filter-sterilized through a 0.2 µm syringe filter. Cells were co-cultured with ECM in concentrations ranging from 0.25 to 2 mg/ml for 72 h at 37°C, 5% CO2 in a humidified atmosphere. After 72 h incubation, 100 µl supernatant was removed from each well. MTT, 25 µl of 5 mg/ml stock solution prepared in PBS, was added to each well and microtiter plates were returned to the incubator for 4 h. After 4 h, 100 µl of a solubilizing solution (0.1 N HCl, 10% SDS) was added to each well and the plates were incubated overnight at 37°C. After incubation overnight, MTT conversion was measured using a microplate reader (BioRad Model 550) with absorbance measured at 570 nm minus background absorbance at 630 nm. Percent growth inhibition was calculated using the following formula: %GI = (CTL Abs570–630 − TRT Abs570–630)/CTL Abs570–630 × 100.
Enzyme treatments
Experiments to assess the effects of nucleases and proteolytic enzymes on ECM activity evaluated growth inhibition of malignant melanoma cell line A375.S2, using ECM that had been treated with deoxyribonuclease I (DNase I, EC 3.1.21.1), ribonuclease A (RNase A, EC 3.1.27.5), protease (Type VIII-A, EC 3.4.21.14), proteinase K (EC 3.4.21.64), or trypsin (EC 3.4.21.4). All enzymes were purchased from Sigma Chemical Co, and with the exception of DNase I, all enzymes were immobilized on agarose beads. Prior to use, beads were washed twice with sterile water by centrifuging at 500 g for 10 min at 4°C, followed by two washes with the cell culture medium used for culturing cell line A375.S2 (Eagle’s Minimal Essential Medium, MEM). Protein concentrations of bovine serum albumin (Sigma) as a control for proteolytic digestion and ECM were adjusted to 6 mg/ml. An equal volume of ECM and immobilized enzyme on agarose beads in cell culture media was used for each digestion. Final concentrations of the enzyme treatments were 20 U/ml DNase I, 250 µg/ml RNase A, 1 U/ml protease, 5 U/ml proteinase K, and 10 U/ml trypsin. Enzyme treatments were performed overnight at 37°C with gentle rocking, with total protein remaining in enzyme-treated BSA controls and ECM determined using the Bradford assay. Complete digestion of BSA occurred with all proteolytic enzyme treatments. No digestion of BSA occurred with DNase or RNase.
Statistical analyses
Statistical analyses were performed using Sigma-Stat Version 2.03. For all data, means ± SEM are presented. Data that passed tests for both normality and equal variance were analyzed using either one-way analysis of variance (ANOVA) or Student’s t-test. Data that did not satisfy requirements for normality and equal variance were analyzed using either non-parametric rank sum test or one-way ANOVA on Ranks. P-values less than 0.05 were considered significant.
Results
Growth inhibitory activity of ECM was assessed against nine cell lines of various tumor origins and two non-malignant cell lines. Growth inhibition of seven tumor cell lines as a function of ECM protein concentration is shown in Figure 1. Three of the dose–response curves include data for growth inhibition resulting from co-culture with spleen conditioned media (SCM). Little or no inhibitory activity of tumor cell growth was apparent in SCM. As a result, SCM has become useful as negative control material for ECM activity.
Fig. 1.
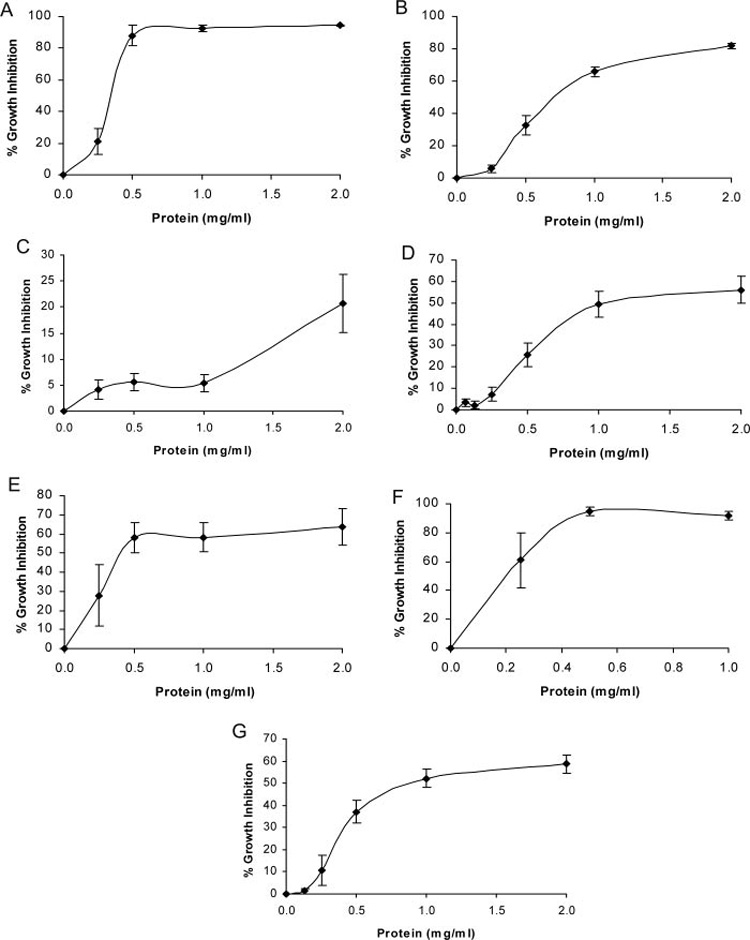
Growth inhibitory activity of epigonal conditioned medium against cell lines of various tumor origins. (A) WEHI 164 (fibrosarcoma), N = 9; (B) A375.S2 (malignant melanoma), N = 26; (C) NIH:OVCAR-3 (ovarian carcinoma), N = 11; (D) PANC-1 (pancreatic carcinoma), N = 7; (E) Jurkat (T-cell leukemia), N = 4; (F) Daudi (B-cell lymphoma), N = 3; (G) MCF7 (breast carcinoma), N = 18.
Each cell line tested responded differently to treatment with ECM. Treatment of a cell line derived from mouse fibrosarcoma, WEHI 164, with 0.5 mg/ml ECM (Figure 1A) resulted in a growth inhibition of ~85%. Increasing the ECM protein concentration to 1.0 and 2.0 mg/ml resulted in 92 and 94% growth inhibition, respectively. Bioactivity of ECM against malignant melanoma (A375.S2) cells (Figure 1B) increased more gradually, with 32% growth inhibition when cells were treated with 0.5 mg/ml and 65 and 82% growth inhibition in response to treatment with 1.0 and 2.0 mg/ml, respectively. A markedly different pattern of growth inhibition was observed when cells derived from an ovarian cancer (NIH:OVCAR-3) were treated with ECM (Figure 1C). Very little (<10%) growth inhibition was observed in response to treatment with ECM in the concentration range of 0.25–1.0 mg/ml protein and only 33% growth inhibition in response to treatment with ECM at 2.0 mg/ml protein concentration. Growth inhibition of a pancreatic cancer cell line, PANC-1, is shown in Figure 1D. When these cells were treated with ECM, ~27% cytotoxicity was observed in response to 0.5 mg/ml protein, 49% cytotoxicity was observed in response to 1.0 mg/ml protein, and 56% cytotoxicity was observed in response to 2.0 mg/ml protein. Inhibition of the growth of a T-lymphocyte leukemic cell line (Jurkat E-6; Figure 1E) resulted in a similar growth inhibition pattern compared to WEHI cells in which near maximal growth inhibition was reached in response to 0.5 mg/ml protein (58% growth inhibition).
Activity of ECM against two cell lines originating from soft tumors, a T-cell leukemia (Jurkat) and a Burkitt’s lymphoma (Daudi), was also examined. Treatment of Jurkat cells with 1.0 and 2.0 mg/ml protein resulted in 58 and 63% growth inhibition, respectively. With the Daudi cell line, almost 100% inhibition of cell growth was observed (Figure 1F) following treatment with ECM at 0.5 and 1.0 mg/ml protein.
Figure 1G shows the growth inhibition of a breast carcinoma cell line, MCF7, in response to treatment with ECM. Approximately 40% growth inhibition resulted from treatment with 0.5 mg/ml, 50% growth inhibition resulted from treatment with 1.0 mg/ml, and 60% growth inhibition resulted from treatment 2.0 mg/ml ECM.
To support the growth inhibition data obtained using the MTT assay, photomicrographs depicting growth properties and cell morphologies of two tumor cell lines, A375.S2 and MCF7, following culture in the presence and absence of ECM are shown in Figure 2. Untreated A375.S2 cells (Figure 2A) displayed growth characteristics typical of this cell line including confluence, adherence, and an elongated appearance. Following 48 h of incubation with ECM, A375.S2 cells had lost their characteristic growth properties and had become dramatically fewer in number, detached, and rounded up (Figure 2B). Untreated MCF7 cells (Figure 2C) displayed growth characteristics typical of this cell line including both suspended and adherent cells, primarily as three-dimensional clusters or aggregates that reached 70–80% confluence. Following co-culture with ECM for 48 h, MCF7 cells had lost their characteristic growth properties, aggregates were decreased in size and number, and cells appeared granular and somewhat crenated (Figure 2D). Moreover, growth inhibitory effects of ECM appeared to be permanent, since normal growth of cell lines could not be reestablished even after washing the inhibited cells with fresh culture medium.
Fig. 2.
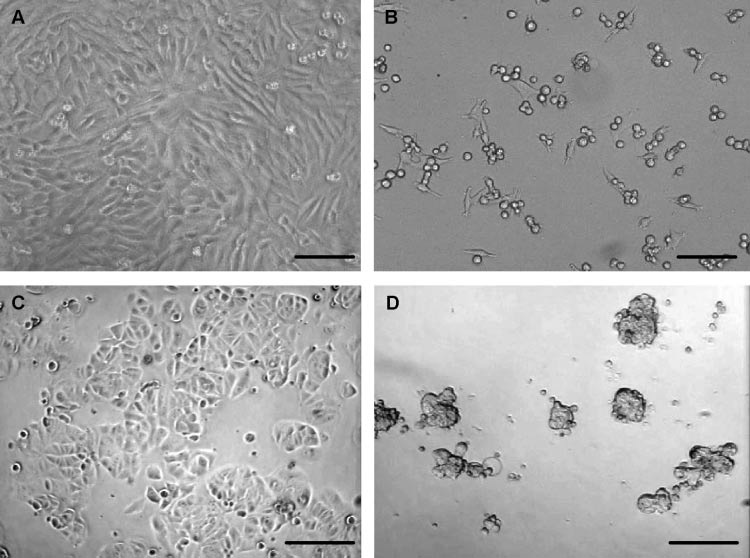
Photomicrographs showing cellular morphologies and growth properties of two tumor cell lines after 48 h of culture in the presence and absence of ECM. (A) A375.S2 (malignant melanoma), untreated; (B) A375.S2, co-cultured with ECM; (C) MCF7 (breast carcinoma), untreated; (D) MCF7, co-cultured with ECM. A and B, bar = 50 µm; C and D, bar = 100 µm.
As a way to assess relative potency and specificity of activity, growth inhibition of 11 tumor cell lines by ECM at a protein concentration of 1.0 mg/ml is shown in Table 1. At this protein concentration, growth inhibitory activity ranged from ~5% in the least sensitive cell line (NIH:OVCAR-3) to >90% in the most sensitive cell lines (WEHI 164 and Daudi). The fibrosarcoma (WEHI 164) cell line was consistently the most sensitive (93% inhibition), followed by the B cell lymphoma (Daudi; 92%), malignant melanoma (A375.S2; 66%), T cell leukemia (Jurkat; 58%), and pancreatic carcinoma (PANC-1; 49%). Of the three breast carcinoma cell lines, MCF-7 was more sensitive (52%) than either Hs 578T (27%) or HCC38 (26%). The ovarian carcinoma (NIH:OVCAR-3) cell line (5%) and the two non-malignant cell lines, normal lymphoblastoid (HCC38 BL; 15%) and normal breast cells (Hs 578Bst; 16%) were the least sensitive.
In an effort to compare relative susceptibility of tumor cell lines versus normal cell lines, two different malignant/non-malignant cell line pairs were co-cultured with ECM. Results from these growth inhibitory studies are shown in Figure 3 and Table 1. Inhibition of HCC38 (malignant breast) by ECM was significantly greater (P < 0.05) than inhibition of HCC38 BL (normal lymphoblastoid) at 0.5, 1.0, and 2.0 mg/ml protein, while inhibition of Hs 578T (malignant breast, ductal) was significantly greater (P < 0.05) than inhibition of Hs 578Bst (normal breast) only at 2.0 mg/ml protein. Growth inhibition by bonnethead shark SCM was minimal against either of the cell line pairs (data not shown).
Fig. 3.
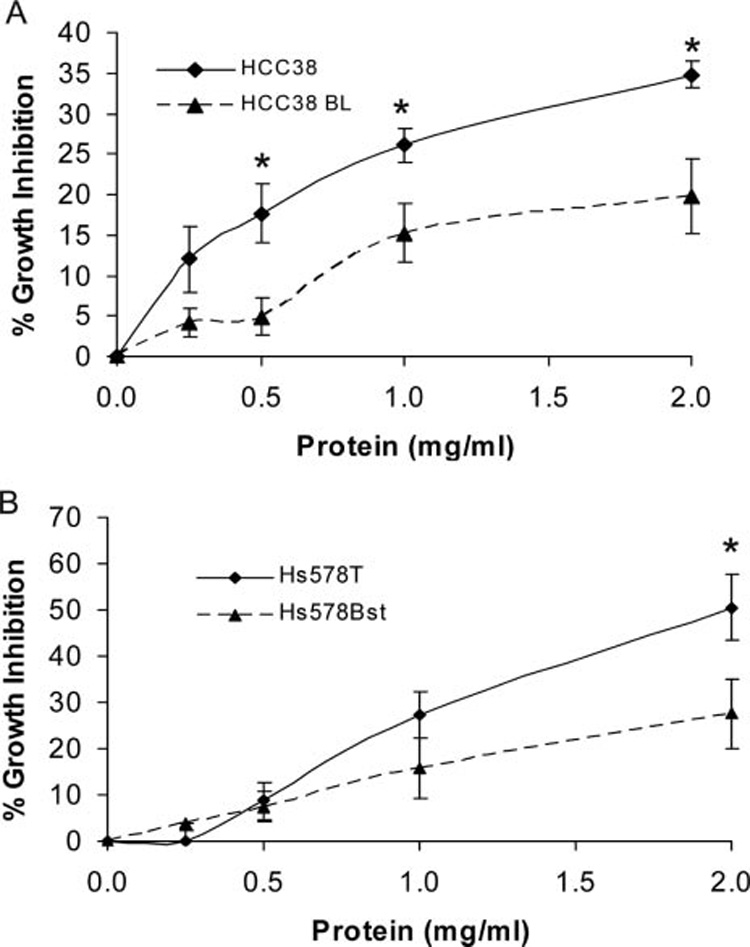
Treatment of malignant/non-malignant cell line pairs with epigonal conditioned medium, ECM. (A) HCC38 (malignant breast), N = 9 and HCC38 BL (normal lymphoblastoid), N = 10. Inhibition of HCC38 by ECM is significantly greater (P < 0.05) than inhibition of HCC38 BL at 0.5, 1.0, and 2.0 mg/ml protein. (B) Hs 578T (malignant breast, ductal), N = 9 and Hs 578Bst (normal breast), N = 7. Inhibition of Hs 578T by ECM is significantly greater (P < 0.05) than inhibition of Hs 578Bst at 2.0 mg/ml protein.
Separation of protein components of ECM using SDS–PAGE resulted in a very reproducible pattern of three major bands corresponding to molecular sizes of ~40–42, 24, and 17 KD (Figure 4). Protein profiles of conditioned media from spleen and peripheral blood leukocytes (PBL) had very little similarity to the protein profile of ECM. In addition, as shown in Figure 1, supernatants of conditioned media from short-term culture of S. tiburo spleen cells did not demonstrate appreciable inhibition of tumor cell growth.
Fig. 4.
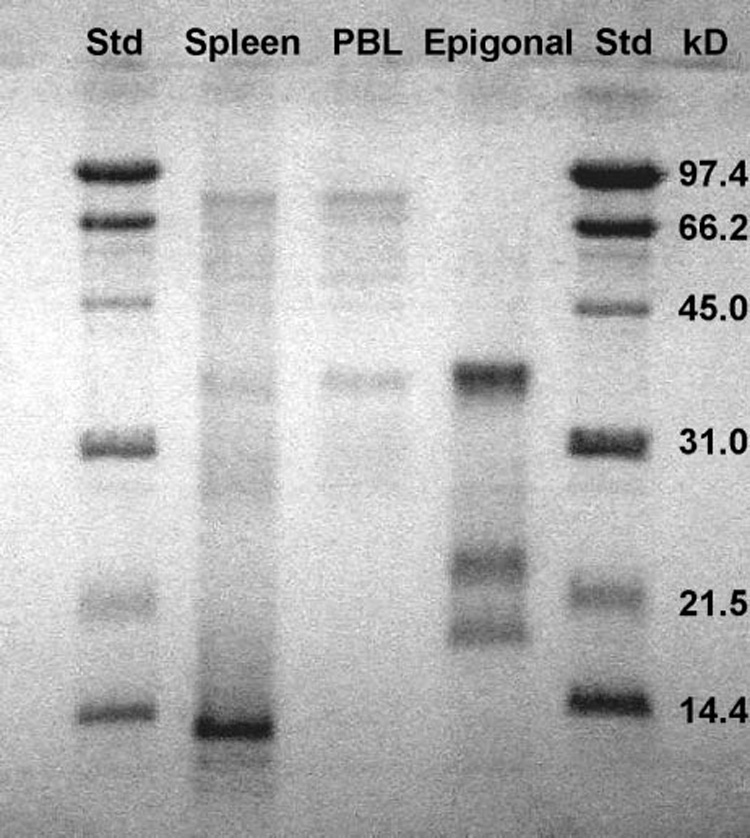
SDS polyacrylamide gel electrophoretic separation of conditioned medium (CM) proteins from short-term cultures of S. tiburo spleen cells, epigonal cells, and peripheral blood leukocytes (PBL). Lanes 1 and 5: MW standards; Lane 2: spleen CM; Lane 3: PBL CM; Lane 4: epigonal CM.
Effects of temperature and enzyme treatment on the bioactivity of ECM are shown in Figure 5–Figure 7. Although inhibition of tumor cells was not diminished after heating ECM at 56°C for 30 min, it was almost completely destroyed after heating at 75 or 100°C for 30 min (Figure 5). Bioactivity of ECM could also be reduced by selected proteolytic enzymes (Figure 6). Treatment of ECM with proteinase K resulted in a greater than 30% reduction in bioactivity, while treatment with non-specific protease resulted in a more than 60% reduction in inhibition of growth. Bioactivity of ECM was not sensitive to all proteolytic enzyme treatments, however, since growth inhibition of tumor cells was not affected by treatment with trypsin. Nucleic acids do not appear to be responsible for bioactivity, since treatment of epigonal culture supernatants with either DNase or RNase did not affect tumor cell growth inhibitory activity (Figure 7).
Fig. 5.
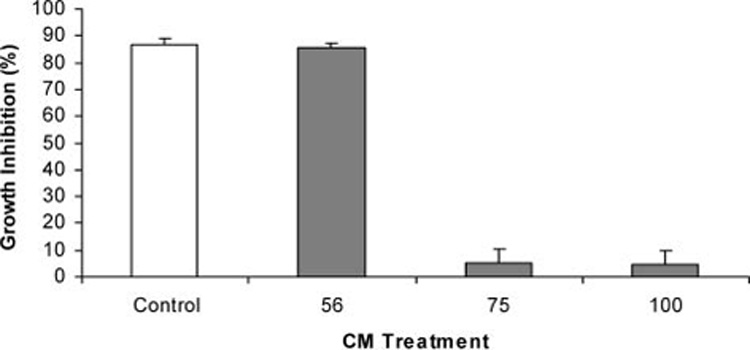
Effect of heat on growth inhibitory activity of ECM against A375.S2 (malignant melanoma) cell line. Growth inhibitory activity was measured using MTT after 72 h of co-culture with ECM (0.75 mg/ml protein) that had been heated for 30 min at 56°C, 75°C, or 100°C.
Fig. 7.
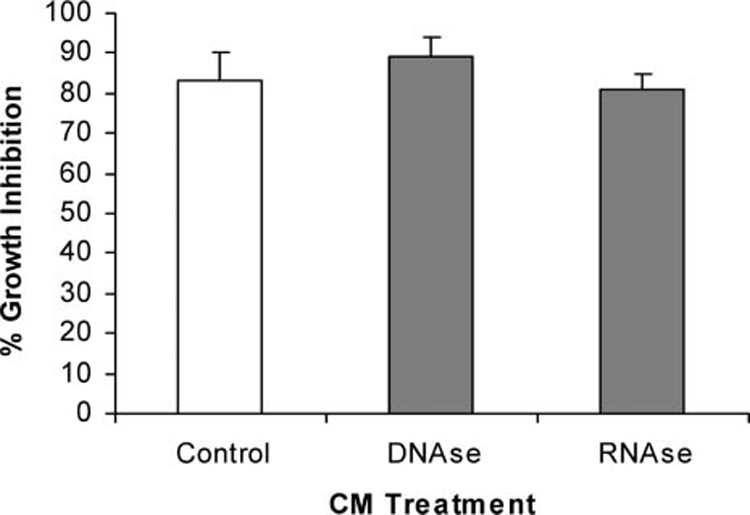
Effect of nucleases on growth inhibitory activity of ECM against A375.S2 (malignant melanoma) cell line. Growth inhibitory activity was measured using MTT after 72 h of co-culture with ECM (0.75 mg/ml protein) that had been incubated for 24 h with deoxyribonuclease (DNase I) or ribonuclease (RNase A).
Fig. 6.
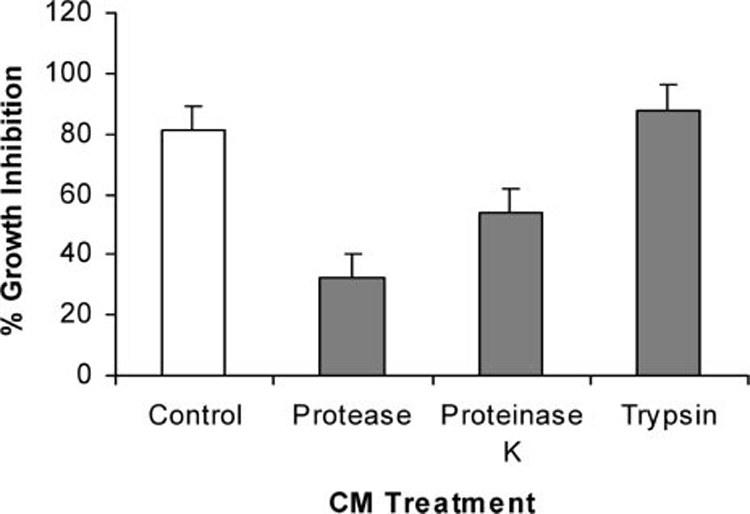
Effect of proteolytic enzymes on growth inhibitory activity of ECM against A375.S2 (malignant melanoma) cell line. Growth inhibitory activity was measured using MTT after 72 h of co-culture with ECM (0.75 mg/ml protein) that had been incubated for 24 h with non-specific protease, proteinase K, or trypsin.
Discussion
Elasmobranchs represent a novel and untapped source for compounds with the potential to benefit public health. Exploration of immune function in an animal group whose immune system appears to be effective in its own environment certainly may serve as a reasonable source of material for such applications. The studies described here indicate that short-term cultures of bonnethead shark epigonal cells produce a conditioned medium with a broad spectrum of growth inhibitory activity against a variety of mammalian tumor cell lines. Moreover, ECM appears to demonstrate preferential inhibition of malignant cells compared to non-malignant cells.
While growth inhibition was demonstrated for all nine cell lines, some cell lines were more sensitive than others, allowing for a more meaningful assessment of relative potency and specific activity (Figure 1 and Table 1). A comparison of the mean percent growth inhibition at the same protein concentration, 1.0 mg/ml, showed that the fibrosarcoma cell line was consistently the most sensitive, followed by the B cell lymphoma, malignant melanoma, T-cell leukemia, and pancreatic carcinoma. One of the three breast carcinoma cell lines, MCF7, was more sensitive than the other two, HCC38 and Hs 578T. The MCF7 cell line is estrogen receptor positive whereas HCC38 and Hs 578T are both estrogen receptor negative, which may lead to mechanistic information regarding the activity of ECM. The ovarian carcinoma cell line and two non-malignant cell lines, normal lymphoblastoid and normal breast cells, were the least sensitive. That differential effects are observed on cell lines of various tumor origins suggests that the inhibitory factor in ECM operates through a specific mechanism rather than through a generalized cytotoxic effect against tumor cell lines. Although the data are not reported here, preliminary experiments indicate that ECM induces apoptosis through the TRAIL (tumor necrosis factor-related apoptosis-inducing ligand) pathway. Studies to understand specific mechanisms of action of ECM are underway and will be described in subsequent published reports.
Two of the breast carcinoma cell lines, HCC38 and Hs 578T, were part of malignant/non-malignant cell line pairs. The HCC38 breast carcinoma cell line was paired with a normal lymphoblastoid (HCC38 BL) cell line (Gazdar and others 1998), while the Hs 578T breast carcinoma cell line was paired with normal breast cells (Hs 578Bst; Hackett and others 1977). In both cell line pairs, the normal cells originated from the same patient as the malignant cells. Results of co-culturing the malignant/non-malignant cell line pairs with the epigonal conditioned medium demonstrated that at most of the concentrations of proteins tested, growth inhibition of malignant cells was significantly greater (P < 0.05) than inhibition of non-malignant cells (Figure 3). In many cases, cytotoxic activity against malignant cells was at least twice the cytotoxic activity against non-malignant cells. Therefore, while growth inhibition of normal cells was observed, cytotoxic effects against malignant cells are considerably more pronounced.
The data reported here from enzyme treatment experiments are consistent with growth inhibitory properties of ECM being associated with protein. Under the denaturing conditions of SDS–PAGE, three protein bands corresponding to approximate molecular weights of 40–42, 24, and 17 kD are reproducibly visible. Under non-denaturing conditions, however, the inhibitory activity of ECM is retained following ultrafiltration with a 100 kD membrane (data not shown), suggesting that the bands on SDS may represent subunits of a protein with a larger molecular weight. Efforts are underway to purify and characterize protein and/or peptides responsible for growth inhibitory properties of ECM on the tumor cell lines. The observation that growth inhibitory activity is unaffected by treatment with trypsin is encouraging and suggests that peptides generated by tryptic digestion may retain bioactivity.
Public health implications of elasmobranch-derived substances have increased in recent years, with a number of compounds reaching clinical trials. One such example is squalamine, an aminosterol derived from shark liver first reported for its significant antibiotic activity (Moore and others 1993). Further study of squalamine revealed antiangiogenic activity as well (Moriarty and others 1995; Sadownik and others 1995; Sills and others 1998) which has been tested in Phase I and Phase II clinical trials (Bhargava and others 2001; Hao and others 2003; Herbst and others 2003). A compound isolated from shark cartilage, Neovastat (Æ-941), has been tested in Phase III clinical trials (Falardeau and others 2001) and has also shown promise as a potential therapeutic for airway asthmatic inflammation (Lee and others 2005).
The unique evolutionary niche that elasmobranchs fill make these fish extremely valuable for understanding the evolution of the immune system. The continued study of this group will provide valuable insight into the origins of specific immunity and how the adaptive immune system has evolved that allows higher vertebrates, including humans, to survive constant invasion by a variety of infectious microorganisms and toxins. As a result, the elasmo-branch immune system, with its unique lymphomyeloid tissues, may serve as an innovative source for novel compounds with potential benefits for human health.
Conclusions
Elasmobranch fish represent a unique animal model potentially harboring novel products with relevance to public health. Ongoing studies to identify immune regulatory factors in the serum-free media conditioned by short-term cultures of epigonal cells have resulted in the isolation of bioactive compounds with potent growth inhibitory activity against several mammalian tumor cell lines and may represent novel cytokines with potential applications for human health.
Acknowledgments
This study was supported in part by NIH Award No. 1R21CA100523-01A2, the Elsa U. Pardee Foundation, the Ann and Alfred Goldstein Foundation, the Vernal W. and Florence H. Bates Foundation, and the Polly Loomis Endowment. The authors gratefully acknowledge the use of facilities at Mote Marine Laboratory (Sarasota, FL). The authors would also like to thank Dr. Jim Gelsleichter and John Tyminski for help with obtaining bonnethead shark specimens. U.S. Patent Number 6,908.627.
Footnotes
From the symposium “Ecological Immunology: Recent Advances and Applications for Conservation and Public Health” presented at the annual meeting of the Society for Integrative and Comparative Biology, January 4–8, 2006, at Orlando, Florida.
References
- Anderson MK, Strong SJ, Litman RT, Luer CA, Amemiya CT, Rast JP, Litman GW. A long form of the skate IgX gene exhibits a striking resemblance to the new shark IgW and IgNARC genes. Immunogenetics. 1999;49:49–67. doi: 10.1007/s002510050463. [DOI] [PubMed] [Google Scholar]
- Bartl S, Baish MA, Flajnik MF, Ohta Y. Identification of class I genes in cartilaginous fish, the most ancient group of vertebrates displaying an adaptive immune response. J Immunol. 1997;159:6097–7104. [PubMed] [Google Scholar]
- Bartl S, Weissman IL. Isolation and characterization of major histocompatibility complex class IIB genes from the nurse shark. Proc Natl Acad Sci USA. 1994;91:262–266. doi: 10.1073/pnas.91.1.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhargava P, Marshall JL, Dahut W, Rizvi N, Trocky N, Williams JI, Hait H, Song S, Holroyd KJ, Hawkins MJ. A Phase I and pharmacokinetic study of Squalamine, a novel antiangiogenic agent, in patients with advanced cancers. Clin Cancer Res. 2001;7:3912–3919. [PubMed] [Google Scholar]
- Bird S, Wang T, Zou J, Cunningham C, Secombes CJ. The first cytokine sequence within cartilaginous fish: IL-1β in the small spotted catshark (Scyliorhinus canicula) J Immunol. 2002;168:3329–3340. doi: 10.4049/jimmunol.168.7.3329. [DOI] [PubMed] [Google Scholar]
- Cho J, Kim Y. Sharks: a potential source of antiangiogenic factors and tumor treatments. Mar Biotechnol. 2002;4:521–525. doi: 10.1007/s10126-002-0064-3. [DOI] [PubMed] [Google Scholar]
- Falardeau P, Champagne P, Poyet P, Hariton C, Dupont E. Æ-941 (Neovastat), a naturally occurring multifunctional anti-angiogenic product in phase clinical trials. Semin Oncol. 2001;28:620–625. doi: 10.1016/s0093-7754(01)90035-1. [DOI] [PubMed] [Google Scholar]
- Flajnik MF, Rumfelt LL. The immune system of cartilaginous fish. Curr Top Microbiol Immunol. 2000;248:249–270. doi: 10.1007/978-3-642-59674-2_11. [DOI] [PubMed] [Google Scholar]
- Gazdar AF, Kurvari V, Virmani A, Gollahon L, Sakaguchi M, Westerfield M, Kodagoda D, Stasny V, Cunningham HT, Wistuba II, et al. Characterization of paired tumor and non-tumor cell lines established from patients with breast cancer. Int J Cancer. 1998;78:766–774. doi: 10.1002/(sici)1097-0215(19981209)78:6<766::aid-ijc15>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Hackett AJ, Smith HS, Springer EL, Owens RB, Nelson-Rees WA, Riggs JL, Gardner MB. Two syngeneic cell lines from human breast tissue: the aneuploid mammary epithelial (Hs578T) and the diploid myoepithelial (Hs578Bst) cell lines. J Natl Cancer Inst. 1977;58:1795–1806. doi: 10.1093/jnci/58.6.1795. [DOI] [PubMed] [Google Scholar]
- Haley PJ. Species differences in the structure and function of the immune system. Toxicology. 2003;188:49–71. doi: 10.1016/s0300-483x(03)00043-x. [DOI] [PubMed] [Google Scholar]
- Hao D, Hammond LA, Eckhardt SG, Patnaik A, Takimoto CH, Schwartz GH, Goetz AD, Tolcher AW, McCreery HA, Mamun K, et al. A Phase I and pharmacokinetic study of Squalamine, an aminosterol angiogenesis inhibitor. Clin Cancer Res. 2003;9:2465–2471. [PubMed] [Google Scholar]
- Herbst RS, Hammond LA, Carbone DP, Tran HT, Holroyd KJ, Desai A, Williams JI, Bekele BN, Hait H, Allgood V, et al. A Phase I/IIA trials of continuous five-day infusion of squalamine lactate (MSI-1256F) plus carboplatin and paclitaxel in patients with advanced non-small cell lung cancer. Clin Cancer Res. 2003;9:4108–4115. [PubMed] [Google Scholar]
- Inoue Y, Haruta C, Usui K, Moritomo T, Nakanishi T. Molecular cloning and sequencing of the banded dogfish (Triakis scyllia) interleukin-8 cDNA. Fish Shellfish Immunol. 2003;14:275–281. doi: 10.1006/fsim.2002.0432. [DOI] [PubMed] [Google Scholar]
- Kasahara M, Vazquez M, Sato K, McKinney EC, Flajnik MF. Evolution of the major histocompatibility complex: isolation of class II A cDNA clones from cartilaginous fish. Proc Natl Acad Sci USA. 1992;89:6688–6892. doi: 10.1073/pnas.89.15.6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokubu F, Litman R, Shamblott MJ, Hinds K, Litman GW. Diverse organization of immunoglobulin VH gene loci in a primitive vertebrate. EMBO J. 1988a;7:3413–3422. doi: 10.1002/j.1460-2075.1988.tb03215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokubu F, Hinds K, Litman R, Shamblott MJ, Litman GW. Complete structure and organization of immunoglobulin heavy chain constant region genes in a phylogenetically primitive vertebrate. EMBO J. 1988b;7:1979–1988. doi: 10.1002/j.1460-2075.1988.tb03036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A, Langer R. Shark cartilage contains inhibitors of tumor angiogenesis. Science. 1983;221:1185. doi: 10.1126/science.6193581. [DOI] [PubMed] [Google Scholar]
- Lee S-Y, Paik S-Y, Chung S-Y. Neovastat (Æ-941) inhibits the airway inflammation and hyperresponsiveness in a murine model of asthma. J Microbiol. 2005;43:11–16. [PubMed] [Google Scholar]
- Levin M, Morsey B, Mori C, Nambiar PR, De Guise S. PCBs and TCDD, alone and in mixtures, modulate marine mammal but not B6C3F1 mouse leukocyte phagocytosis. J Toxicol Environ Health A. 2005a;68(3):635–656. doi: 10.1080/15287390590921766. [DOI] [PubMed] [Google Scholar]
- Levin M, Morsey B, Mori C, Nambiar PR, De Guise S. Non-coplanar PCB-mediated modulation of human leukocyte phagocytosis: a new mechanism for immunotoxicity. J Toxicol Environ Health A. 2005b;68(22):1977–1993. doi: 10.1080/15287390500227126. [DOI] [PubMed] [Google Scholar]
- Li D, Williams JI, Pietras RJ. Squalamine and cisplatin block angiogenesis and growth of human ovarian cancer cells with or without HER-2 gene overexpression. Oncogene. 2002;21:2805–2814. doi: 10.1038/sj.onc.1205410. [DOI] [PubMed] [Google Scholar]
- Litman GW, Anderson MK, Rast JP. Evolution of antigen binding receptors. Ann Rev Immunol. 1999;17:109–147. doi: 10.1146/annurev.immunol.17.1.109. [DOI] [PubMed] [Google Scholar]
- Luer CA. Inhibitors of angiogenesis from shark cartilage. Fed Proc Fed Am Soc Exp Biol. 1986;45:949. [Google Scholar]
- Luer CA. Elasmobranchs (sharks, skates, and rays) as animal models for biomedical research. In: Woodhead A, editor. Nonmammalian models for biomedical research. Boca Raton: CRC Press; 1989. pp. 121–147. [Google Scholar]
- Luer CA, Walsh CJ, Bodine AB. The immune system of sharks, skates, and rays. In: Carrier JC, Musick JA, Heithaus MR, editors. Biology of sharks and their relatives. Boca Raton: CRC Press; 2004. pp. 369–395. [Google Scholar]
- Marchalonis J, Edelman GM. Phylogenetic origins of antibody structure. I. Multichain structure of immunoglobulins in the smooth dogfish (Mustelus canis) J Exp Med. 1965;122:601–618. doi: 10.1084/jem.122.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miracle AL, Anderson MK, Litman RT, Walsh CJ, Luer CA, Rothenberg EV, Litman GW. Complex expression patterns of lymphocyte-specific genes during the development of cartilaginous fish implicate unique lymphoid tissues in generating an immune repertoire. Int Immunol. 2001;13:567–580. doi: 10.1093/intimm/13.4.567. [DOI] [PubMed] [Google Scholar]
- Moore KS, Wehrli S, Roder H, Rogers M, Forrest JN, McCrimmon D, Zasloff M. Squalamine: an aminosterol antibiotic from the shark. Proc Natl Acad Sci USA. 1993;90:1354–1358. doi: 10.1073/pnas.90.4.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori C, Morsey B, Levin M, Nambiar PR, De Guise S. Immunomodulatory effects of in vitro exposure to organochlorines on T-cell proliferation in marine mammals and mice. J Toxicol Environ Health A. 2006;69:283–302. doi: 10.1080/15287390500227472. [DOI] [PubMed] [Google Scholar]
- Moriarty RM, Enache LA, Kinney WA, Allen CS, Canary JW, Tuladhar SM, Liang G. Stereoselective synthesis of squalamine dessulfate. Tetrahedron Lett. 1995;36:5139–5142. [Google Scholar]
- Mosmann TJ. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Pettit GR, Ode RH. Antineoplastic agents L: isolation and characterization of sphyrnastatins 1 and 2 from the hammerhead shark Sphyrna lewini. J Pharm Sci. 1977;66:757. doi: 10.1002/jps.2600660546. [DOI] [PubMed] [Google Scholar]
- Rast JP, Anderson MK, Strong SJ, Luer C, Litman RT, Litman GW. α, β, γ, and δ T cell antigen receptor genes arose early in vertebrate phylogeny. Immunity. 1997;6:1–11. doi: 10.1016/s1074-7613(00)80237-x. [DOI] [PubMed] [Google Scholar]
- Rumfelt LL, McKinney EC, Taylor E, Flajnik MF. The development of primary and secondary lymphoid tissues in the nurse shark Ginglymostoma cirratum: B-cell zones precede dendritic cell immigration and T-cell zone formation during ontogeny of the spleen. Scan J Immunol. 2002;56:130–148. doi: 10.1046/j.1365-3083.2002.01116.x. [DOI] [PubMed] [Google Scholar]
- Sadownik A, Deng G, Janout V, Regen SL, Bernard EM, Kikuchi K, Armstrong D. Rapid construction of a squalamine mimic. J Am chem. Soc. 1995;117:6138–6139. [Google Scholar]
- Sills AK, Williams JI, Tyler BM, Epstein DS, Sipos EP, Davis JD, McLane MP, Pitchford S, Cheshire K, Gannon FH, et al. Squalamine inhibits angiogenesis and solid tumor growth in vivo and perturbs embryonic vasculature. Cancer Res. 1998;58:2784–2792. [PubMed] [Google Scholar]
- Snodgrass MJ, Burke JD, Meetz GD. Inhibitory effect of shark serum on the Lewis lung carcinoma. J Natl Cancer Inst. 1976;56:981. doi: 10.1093/jnci/56.5.981. [DOI] [PubMed] [Google Scholar]
- Walsh CJ, Luer CA. Comparative phagocytic and pinocytic activities of leucocytes from peripheral blood and lymphomyeloid tissues of the nurse shark (Ginglymostoma cirratum Bonaterre) and the clearnose skate (Raja eglanteria Bosc) Fish Shellfish Immunol. 1998;8:197–215. [Google Scholar]
- Walsh CJ, Luer CA, Bodine AB. Functional studies on elasmobranch epigonal cells in short-term culture. Florida Sci 56. 1993 Suppl 1:30. [Google Scholar]
- Walsh CJ, Luer CA. Elasmobranch hematology: identification of cell types and practical applications. In: Smith M, Warmolts D, Thoney D, Hueter R, editors. Elasmobranch Husbandry Manual: Proceedings of the First International Elasmobranch Husbandry Symposium 2001. Columbus: Ohio Biological Survey; 2004. pp. 309–326. [Google Scholar]


