Abstract
Chronic ischemic left ventricular (LV) dysfunction is present in a number of clinical syndromes in whom myocardial revascularization results in an improvement of LV function, patients functional class and their survival. Early diagnosis of and treatment of viability is essential. Coronary arteriography is of limited value in diagnosis of viability. Non-invasive testing is essential for diagnosis which can be matched to the pathophysiologic changes that occur in hibernating myocardium. However, no single test has a perfect, or near perfect, sensitivity and specificity, and thus, a combination of tests are usually needed. Algorithms are developed to integrate these tests in clinical decision making.
Scientific discoveries tend to be made
not by those who seek to prove a hypothesis,
but by those who keep their eyes open……..
Isaac Newton
Albert Einstein 1
It was believed that left ventricular (LV) dysfunction (LVD) at rest was the result of ongoing ischemia or myocardial infarction. 33 years ago studies of patients undergoing coronary artery bypass graft surgery for angina led to the discovery of improvement or even normalization of the LVD with myocardial revascularization, that is, there was viable myocardium [Hibernating Myocardium (HM)] in areas of LV dysfunction.2,3 It took almost 12+ years for the concept of HM to be clinically acceptable. In the ensuing years certain clinical issues have been recognized:
HM has been documented in a number of clinical syndromes besides angina (stable/unstable). These include acute myocardial infarction, LV aneurysm, heart failure, aborted sudden death, anomalous left coronary artery from the pulmonary artery and valve disease with LV dysfunction.
Revascularization of HM results in an improvement of regional and global LV systolic function4, remodeling is reversed5,6, survival is increased7 and there is a decrease of the composite of myocardial infarction, heart failure and unstable angina.8 Importantly, revascularization of LVD in the absence of significant amounts of viable myocardium was not of demonstrable beneficial clinical effects.7
The lumen of the vessel distal to a severe coronary obstruction is related to disease of the vessel as well as to the amount of MBF. After revascularization, the lumen may increase depending on the increase of MBF. 2 The ability to judge the amount of MBF from a coronary arteriogram is limited. Moreover, when the reduction in MBF is large at rest even a “small” increase of MBF with revascularization may improve patient outcomes but to a greater degree (Table 1).
One vessel disease can also result in changes in the remote areas without associated coronary artery disease. The remote areas show: a) Reduced coronary vasodilator reserve and altered metabolism 9; b) Histological changes similar to those seen in areas of HM in an experimental study with left anterior descending artery (LAD) stenosis10 ; and c) Improvement/ normalization of LV function after revascularization of the areas of HM with left anterior descending coronary artery occlusion. 6
About two-thirds of patients with HF in the developed world have underlying coronary artery disease and these patients have a significantly worse prognosis than patients with nonischemic heart failure.11 In the Christmas trial, 29% of patients had HM and 19% had reversible perfusion defect;12 these patients had greater improvement of LV function.
Early revascularization of HM is associated with greater improvement of LV function 7 and also of patient survival.13
There are major limitations to diagnosing HM by invasive techniques.
TABLE 1.
SUGGESTED CHANGE IN PATIENT OUTCOMES WITH THE SAME INCREASE OF MBF IN TWO DIFFERENT CLINICAL SITUATIONS “A” AND “B”
| CHANGES AFTER REVASCULARIZATION |
|||||||
|---|---|---|---|---|---|---|---|
| Clinical Situation | MBF | Increase of MBF | CCVS Angina Class | NYHA Functional Class | |||
| Before | After | Before | After | Before | After | ||
| A | 100 | 130 | 30 | II | 0 to I | II | I |
| B | 30 | 60 | 30 | IV | I to II | IV | II |
MBF = Myocardial blood flow (ml/min/100g of muscle)
CCVS = Canadian Cardiovascular Society
NYHA = New York Heart Association
These findings have emphasized the need for and importance of non-invasive tests to diagnose and quantitate the viable myocardium in areas of LV dysfunction.
This review is limited to assessment of myocardial viability only in chronic LV dysfunction and will not attempt to differentiate between stunning and hibernation. It will focus on the important issues for the non-invasive cardiac imager, namely: 1) Pathophysiology of myocardial hibernation as it pertains to the imager and imaging targets; 2) Identification of viable myocardium and prediction of functional outcomes by various imaging modalities; 3) Integrated imaging schema which can be used in the clinical setting to identify viable myocardium; and 4) Issues involved in clinical decision making.
PATHOPHYSIOLOGY OF CHRONIC LV DYSFUNCTION
FLOW-FUNCTION ALTERATIONS
Experimental Studies
The fundamental derangement is reduced myocardial blood flow (MBF), specifically subendocardial MBF (SE-MBF). In normal animals, the subendocardium governs transmural contraction and the subendocardium receives more MBF per unit of muscle than the epicardium.14 In conscious dogs, there is a sensitive coupling between SE-MBF and function so that only a to 20% reduction in SE-MBF15 can cause severe regional dysfunction, and the relationship between systolic thickening and SE-MBF is more or less linear and dependent on the hemodynamic situation.16 There is a 2 to 1 relationship in the reduction of SE-MBF to reduction of transmural MBF (TM-MBF).16-19 For example, a 25% reduction in TM-MBF results in a 50% reduction in SE-MBF (Figure 1). A 50% reduction in TM-MBF in anesthetized dog20 and 75% reduction in SE-MBF in the conscious dog results in akinesis of LV wall whereas subepicardial MBF does not correlate to transmural LV wall function14. In animals with coronary arterial obstruction following relief of ischemia, the ischemia-induced vasodilatation is maldistributed in the myocardium supplied by the obstructed coronary artery possibly due to microcirculatory changes. As a result, the increased volume of MBF from the hyperemia is directed to a greater extent to the epicardium and the subendocardium remains ischemic at least for a period of time.21 Thus, relief of ischemia and even restoration of TM-MBF does not necessarily mean SE-MBF is normal or has been restored to pre-ischemic levels.
Figure 1. Relationship of subendocardial to transmural blood flow with reductions of transmural blood flow.
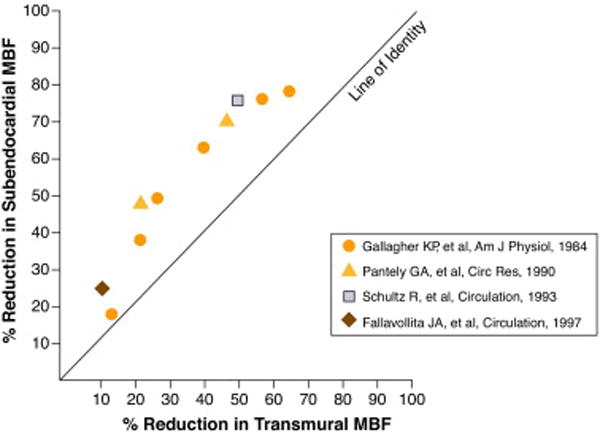
Reduction of subendocardial myocardial blood flow (SE-MBF) is shown on the vertical axis and transmural myocardial blood flow (TM-MBF) on the horizontal axis. For 25-50% reduction of TM – MBF, the SE-MBF is reduced by 50-75%. Data from experimental studies were adapted and/or calculated from references 16-19. Original figure.
Acute and short-term animal studies have shown that episode(s) of ischemia lead to the development of HM.22
Experimental studies of adaptation to chronic fixed coronary stenosis have shown: a) MBF may be normal or only mildly diminished in the early period but regional contraction may already be reduced (“stunning”) but over the subsequent time period reduction in MBF and function are matched (“hibernation”)14 ; and b) Progression to matched decreases in flow and function (HM) also occurs very early when a 15-minute partial coronary occlusion is followed by reperfusion through a critical stenosis (“hibernation”).10
Clinical Studies
The few studies that showed blood flow was “not reduced” measured only TM-MBF by PET; at that time it was not possible to measure SE-MBF in humans. The weight of evidence shows patients with HM have reduced TM-MBF at rest 14 which implies SE-MBF is significantly reduced to a much greater extent.
Until recently it was not possible to measure SE-MBF in humans with any degree of precision. Using cardiovascular magnetic resonance imaging (CMR), Selvanayagam and colleagues24 have documented in patients with HM, SE-MBF is reduced at rest when compared to areas without significant coronary stenosis in the same patients and following revascularization SE-MBF and LV function are normalized/improved (Figure 2). This led Klocke to conclude this study “…provides convincing evidence for the hibernation paradigm.” 25
Figure 2. Relationship of mean corrected subendocardial blood flow to transmural grade of myocardial hyperenhancement.
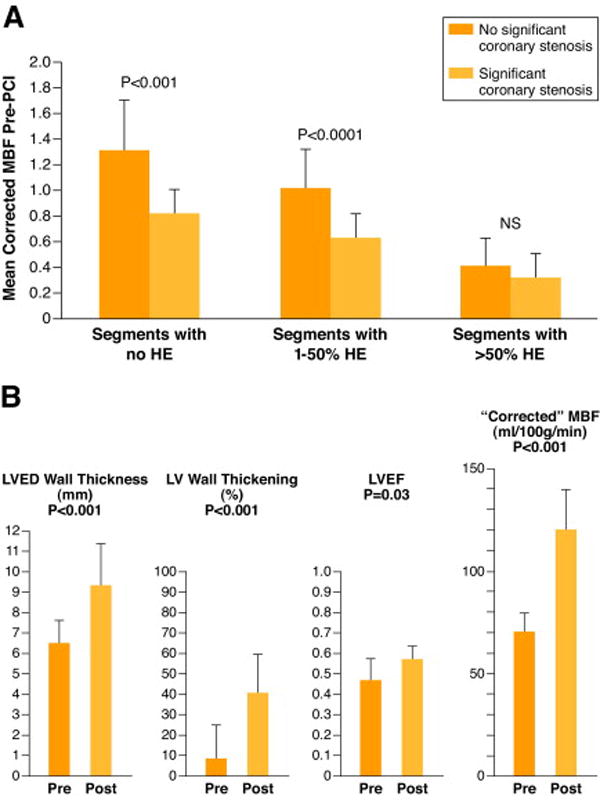
Relationship of mean corrected myocardial blood flow (MBF) that is SE-MBF, to transmural grade of myocardial hyperenhancement (HE) before percutaneous catheter intervention (PCI) is shown (A). Comparison is made between segments subtended by stenosed and nonstenosed coronary arteries. NS = nonsignificant (P > 0.05). (B), pre- and post PCI data are shown in pink and red respectively for LVED = left ventricular end-diastolic, LV=left ventricular, LVEF=left ventricular ejection fraction and corrected MBF=Subendocardial myocardialblood flow. Figure drawn from data of Selvanayagam JB et al (Reference 24). Figure permission requested from Circulation.
Coronary flow reserve (CFR) is reduced in people with hyperlipidemia, in those with coronary atherosclerosis, and in patients with obstructive coronary artery disease. Thus, reduced CFR is not just a phenomenon of HM or stunning.
Irrespective of rest flow, CFR is almost always reduced in viable but dysfunctional myocardium, albeit more severely in segments with low flow at rest. The severity of CFR reduction directly impacts on the ability of viable myocardium to improve its contraction upon inotropic stimulation, as this requires increases in MBF. Viable segments without contractile reserve (CR) usually have lower CFR than viable segments with CR. The response of viable segments to these stimuli varies greatly from segment to segment as well as with the intensity and the duration of stimulation. Many viable segments in HM exhibit a biphasic response when challenged by increasing levels of inotropic stimulation.26 At low levels of stimulation, and provided that sufficient CFR is present, systolic wall thickening usually increases and starts earlier in systole. At higher levels, when the increase in demand cannot be matched anymore by further increases in MBF, systolic function again deteriorates and can even become worse than at baseline. The observation of this biphasic pattern is important to rule out other causes of regional dysfunction such as the presence of a subendocardial scar or remodelled myocardium, these two conditions being characterized by a sustained CR at both low and high levels of inotropic stimulation. About 20-25% of viable segments do not improve functionally during inotropic stimulation. These segments usually have mild reduction of resting MBF, avidly take up glucose under fasting conditions and have an almost completely exhausted MBF reserve. This prevents them from increasing their oxygen consumption upon inotropic stimulation and hence to increase their contractile function. Apart from the exhaustion of MBF reserve, other factors, like the presence and severity of cardiomyocytes alterations or down regulation of ß-adrenoreceptors, may also contribute to the lack of CR in apparently viable segments.
STRUCTURAL ALTERATIONS IN LV DYSFUNCTION
Besides the changes in resting MBF and CFR, several structural alterations affect cardiomyocytes and extra cellular matrix in patients with chronic HM.22,27 These structural changes are mostly encountered in the areas of dysfunction, but can also be seen in remote normally contractile regions. Most of the available information on these structural changes has been gathered from studies in which human myocardial biopsy specimens that were harvested at the time of coronary bypass surgery and also from experimental studies of HM.
Microcirculation
Histological analysis of human dysfunctional myocardium demonstrated the microvasculature was better preserved in segments that improved in function after revascularization than in those that remained dysfunctional. This is particularly true for the capillaries whose density and cross-sectional area are usually within the normal range in viable segments.28 In contrast, there is greater heterogeneity among persistently dysfunctional segments despite revascularization, and approximately half of them show significant capillary rarefaction.
Myoctes
The primary alteration is the depletion of contractile elements in the cardiomyocytes. In some cells, this is limited to the vicinity of the nucleus, whereas in others it is very extended, leaving only few or no sarcomeres at the cell periphery. The space previously occupied by the myofilaments is usually filled with glycogen. The mitochondria are increased in number and display alterations in size and shape. Nuclei are usually tortuous, and show uniformly dispersed heterochromatin. Sarcoplasmic reticulum is virtually absent, as are T-tubules. At the molecular level, the expression of myosin, actin, titin and α-actinin is usually reduced. Cytoskeletal proteins such as desmin, tubulin and vinculin are disorganized. The expression of connexin-43, a major gap junction protein, and that of nuclear A-type lamins are also reduced.
Extracellular matrix
Shows increased amounts of type I collagen, type III collagen and fibronectin in chronic LVD. Within the widened interstitium, an increased number of vimentin-positive cells (endothelial cells and fibroblasts) and macrophages can usually be seen.
The severity of the structural changes that affect chronically dysfunctional myocardium are likely to impact on its ability to respond to an inotropic stimulus and on the speed at which it may or may not recover after revascularization. Segments with less fibrosis or with less severe cardiomyocyte alterations are more likely to improve in function following the administration of dobutamine or after revascularization. Current data suggests a spectrum of myocardial dysfunction may exist in patients with coronary artery disease.
PATHOPHYSIOLOGICAL TARGETS FOR THE IMAGER
Based on the understanding of the pathophysiology of myocardial viability, a number of these targets can be clinically imaged. The following section describes each of the imaging techniques that can be used to image the key pathphysiologic substrates of: a) Fibrosis and/or cellular viability; b) Flow-metabolism; c) Microcirulation; and d) Contractile reserve.
A. Assessment of fibrosis and/or cellular viability
The most important determinant of the return of resting contraction following revascularization is the severity of the underlying tissue fibrosis, whether interstitial or infarct-related. Imaging methods can directly assess the presence of tissue fibrosis, such as delayed enhancement by CMR, or its inverse, i.e. the mass of viable cardiomyocytes, such as thallium and metabolic imaging using positron emission tomography (PET), or consequences of fibrosis such as wall thickness/chamber size (Echocardiography and CMR). However, in the absence of flow or CR measurements, images obtained at rest while sensitive, cannot distinguish between viable and remodeled myocardium (especially in the remote myocardium) and therefore lack specificity.
ECHOCARDIOGRAPHY
Spatial extent of LV scar
LV volume measurement is a guide to the degree of irreversible LV damage. A very dilated LV (end-diastolic volume greater than twice the upper limit of normal) is unlikely to demonstrate significant global functional recovery (eg. improvement of LV ejection fraction >5%), as this degree of LV remodeling is usually caused by a large number of scarred segments. 2D echocardiography underestimates LV volumes, LV opacification improves the reliability of these measurements. 3 dimensional echocardiography permits more accurate volume measurements without needing to make geometric assumptions which is desirable if the LV has been involved in multiple previous myocardial infarctions.29
The involvement of ≥4 ventricular wall segments by scar (ie thinned segments) also identifies the LV that is unlikely to show global functional recovery after revascularization. Extensive infarction also reduces LV compliance, producing a restrictive filling pattern. A short mitral E wave deceleration time is associated with a small number of viable segments or a large number of scar segments, and deceleration time is directly related to the degree of LV ejection fraction change after revascularization, with >5% improvement being unusual with a deceleration time of <180 ms.30 Segments with significant transmural extent of infarction become retracted and fibrotic as the infarct heals. LVend-diastolic wall thickness of ≤0.5-0.6 cm is associated with akinesis or even dyskinesis, and these segments usually demonstrate no CR in response to dobutamine.30,31 A thinned segment is very unlikely to recover (≤ 5% probability to recover); thinning to ≤ 0.5-0.6 cm has a sensitivity of 94% for infarction but its specificity is much lower. A segment of > 0.5-0.6cm may recover (specificity 48%) and is more likely to do so if it augments in response to dobutamine; the combination of wall thickness with augmentation at dobutamine stress (see below) gives a sensitivity of 88% and specificity of 77% for prediction of functional recovery.30,31 Dobutamine stress and single photon emission computed tomography (SPECT) provide similar degree of additional information to wall thickness.31
Transmural extent of scar
Increasing transmural extent of scar is inversely related to the likelihood of functional recovery after revascularization and correlates with the degree of reduction of radial function. Wall motion scoring is too insensitive to distinguish minor gradations of function. The thickness of subendocardial scar may be identified using myocardial contrast echocardiography; the relative thickness of scar and perfused myocardium corresponds with transmural extent of scar by MRI. New echocardiographic indices of LV deformation are sensitive to the reductions in function caused by non-transmural scar. Tissue velocity-based longitudinal strain and strain-rate are reduced with subendocardial scar, although the relationship is non-linear because of the importance of the subendocardium to longitudinal function. Studies using speckle-based (“two-dimensional”) strain32 have shown radial or circumferential strain to be impaired in proportion to the transmural extent of scar.
Direct evidence of viability from resting images
Myocardial deformation is impaired in proportion to the extent of myocardial fibrosis, so the magnitude of strain is an index of the amount of chronically dysfunctional tissue. Unfortunately, this association is not sufficiently robust to be used in decision-making, probably because the parameters are load-dependent. Although tissue velocity is less useful than strain for the assessment of regional changes because it is less site-specific and influenced by tethering of adjacent segments, its high temporal resolution (especially using pulsed-wave Doppler) may allow the measurement of pre-ejection velocity, which may be a load-independent marker. Preserved pre-ejection velocity has been reported to be a reasonable resting marker of viability, and is a predictor of the likelihood of functional recovery and favorable outcome.33
MAGNETIC RESONANCE IMAGING (CMR)
Late gadolinium enhancement (LGE)
Gadolinium chelates are extracellular/interstitial contrast agents that enhance T1 relaxation in regions of infarction as a consequence of the increased volume of distribution of the chelates within collagenous scar and their delayed washout due to reduced capillary density.34 The end result is that regions of myocardial infarction (MI) appear bright on inversion recovery images acquired 10-20 minutes after gadolinium administration. The spatial extent of LGE closely mirrors the distribution of myocyte necrosis early after MI and that of collagenous scar seen at 8 weeks.35 Furthermore, studies have shown that in regions of the heart subjected to reversible injury, the retention of contrast does not occur.36 Regions of chronically dysfunctional myocardium often consist of an admixture of reversibly injured and irreversibly injured (infarcted) myocardium.37 The power of LGE resides in its ability to distinguish these two states within the same segment of myocardium (Figure 3).
Figure 3. Contrast-enhanced images obtained by MRI in chronic left ventricular dysfunction.
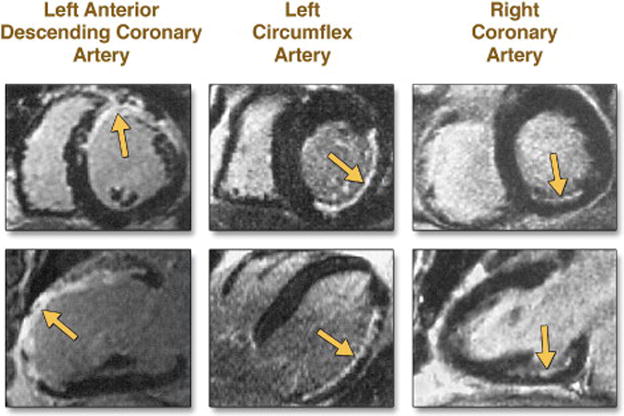
Late gadolinium-enhanced images are shown in a short-axis view (upper panels) and a long-axis view (lower panels) in three patients. Hyperenhancement is present (arrows) in various coronary-perfusion territories — the left anterior descending coronary artery, the left circumflex artery, and the right coronary artery — with a range of transmural involvement. From Kim et al (40), with permission
The ability of LGE to identify and characterize myocardial scar has been directly compared to both SPECT and PET. In a study of 91 patients with suspected or known coronary artery disease, SPECT failed to correctly identify nearly half of the subendocardial infarcts that were identified by LGE.38 Another study compared LGE to PET in 31 patients with ischemic HF (10). Infarct mass correlated well between the two modalities (r=0.81, p<0.0001), but LGE more frequently identified subendocardial scar than PET did, again reflecting its superior spatial resolution. In fact, when the transmural extent of scar is <37%, PET defines the segment as viable due to the presence of sufficient signal from remaining viable midwall and subepicardium.39 When scar is >37% transmural, PET defines the segment as nonviable.
The utility of LGE for identifying the likelihood of functional recovery after revascularization was demonstrated in a study of 50 patients imaged before and after revascularization.40 Eighty percent of patients had some region of LGE with a mean signal intensity that was 530% of that seen in normal segments. Functional recovery inversely correlated with the transmural extent of scar. In segments with no LGE, 78% demonstrated recovery of function. Only 1 of 58 segments with >75% transmural extent, however, showed any improvement following revascularization, thus demonstrating the powerful negative predictive value of this finding. Several subsequent studies have further supported the LGE imaging approach for predicting functional recovery. 41,42 The standard CMR pulse sequence used for LGE imaging is an inversion recovery (IR) gradient echo technique.43 which has been carefully validated in a canine model. This pulse sequence requires selection of an inversion time that optimally nulls normal myocardium. A recently developed phase-sensitive inversion recovery (PSIR) technique is less sensitive to the choice of an incorrect inversion time and may therefore be more efficient.44 Additional novel approaches include a subtractive inversion recovery technique using both a long and short inversion time,45 that improves infarct-blood pool contrast by 247 ± 136% compared to magnitude inversion recovery while maintaining signal difference-to-noise ratio for infarct-myocardium. However, subtraction techniques are prone to misregistration. Another approach to improve the differentiation of the blood pool and infarcted subendocardium is a multi-contrast method using a combination of T1 and T2-weighted imaging.46 For patients who cannot hold their breath, a single shot sub second technique was recently validated with slightly lower sensitivity and overall accuracy but still quite useful in the acutely ill patient nonetheless.47
End-diastolic wall thickness and residual rim of viability
Studies with both echocardiography and cine CMR have established end-diastolic wall thickness as an important parameter in the assessment of myocardial viability, although its accuracy, using fluorodeoxyglucose (FDG) PET as the reference standard, is less than that of CR in response to dobutamine.48 In one study, segments that failed to improve following revascularization had a significantly lower end-diastolic wall thickness (6mm vs. 9.8mm, p <0.001) than those that showed functional improvement.49 More important than end-diastolic wall thickness alone may be the residual thickness of the unenhanced rim of viable myocardium beyond the area of LGE in the subendocardium. One study compared en-diastolic wall thickness and viable rim with FDG PET in a group of 22 patients with ischemic cardiomyopathy.39 Receiver-operator characteristic analysis demonstrated greater area under the curve for viable rim compared to end-diastolic wall thickness (0.95 vs. 0.86, respectively). Optimal cutoff values for viability were 5.4 mm for end-diastolic wall thickness and 3.0 mm for the viable rim.
RADIONUCLIDE IMAGING: SPECT
Tracers used with SPECT or PET to image MBF are commonly referred to as “perfusion tracers”, their uptake and retention mechanisms require viable myocellular membranes. Thus, uptake and visualization of myocardial regions with perfusion tracers requires the presence of working, viable myocytes. This information is the converse of direct and indirect fibrosis imaging described above by echocardiography and CMR. Thallium-201 redistribution, which reflects myocardial potassium space with SPECT, and rest sestamibi imaging (with or without nitrates), which reflects mitochondrial membrane integrity are the two most common “perfusion” tracers used to image cellular viability.
The evaluation of myocardial ischemia and viability by thallium scintigraphy has occupied a rather unique place in the management of patients with known or suspected coronary artery disease since late 1970’s.50,51 Like potassium, thallium is transported across the sarcolemma membrane via the Na-K ATPase system. The initial extraction and distribution of thallium in the myocardium is primarily a function of blood flow (either during stress or at rest) and is unaffected by hypoxia, chronic hypoperfusion (hibernation), or postischemic dysfunction (stunning), unless myocardial infarction is present. The later distribution of thallium (3-4 hr or 24 hr after stress or rest injection), termed redistribution phase, is flow-independent and is a function of regional blood volume and myocardial potassium space reflecting cellular viability.51,52 Thus, thallium defects on early rest images that “fill-in” on delayed, redistribution phase (termed reversible defect) represents a scintigraphic pattern of HM. In contrast, because thallium is not actively taken up in regions of scarred myocardium, defects on rest images that persist on redistribution images (termed irreversible or fixed defect) represent scarred myocardium.
There are a number of thallium protocols that are used clinically for the detection of myocardial viability. Among the range of choices, 2 protocols are optimized for viability detection: 1) rest-redistribution and 2) stress-4-hour redistribution-reinjection imaging (Figure 4). The former assesses myocardial viability alone53,54 while the latter assesses myocardial ischemia and viability.55,56 A pooled analysis of rest-redistribution and stress-redistribution-reinjection thallium studies reported a relatively high sensitivity (80%-90% range) and modest specificity (54%-80% range) for the prediction of recovery of regional function after revascularization.57,58 However, these conclusions must be viewed in the context of the limitations of pooled data analysis. When taking into consideration regions with reversible defects (ischemia) and success of revascularization (reexamining regional perfusion or vessel patency after revascularization) stress-redistribution-reinjection thallium imaging yields excellent positive and negative predictive accuracy (both in the 80%-90% range) for recovery of function after revascularization.55,56
Figure 4. Thallium-201 imaging for myocardial viability.
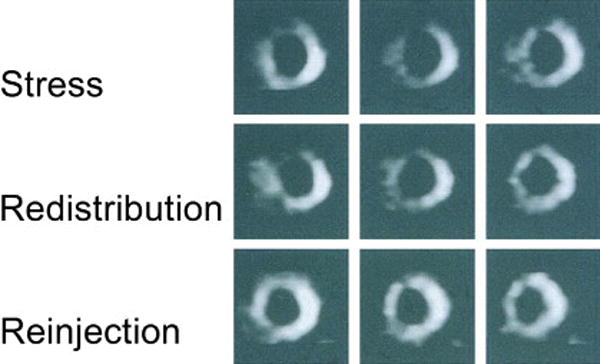
Short-axis 201Thallium tomograms during stress, redistribution, and reinjection imaging in a patient with coronary artery disease. There are extensive 201Thallium abnormalities in the anterior and septal regions during stress that persist on redistribution images but improve markedly on reinjection images. From Dilsizian et al (55), with permission.
In the case of technetium-99m based perfusion tracers, both sestamibi and tetrofosmin are lipophilic cationic complexes that are retained within the mitochondria due to a large negative transmembrane potential. Because accumulation and retention of sestamibi and tetrofosmin are related to energy-dependent processes which maintain mitochondrial membrane polarization, they may also serve as markers of cellular viability. The administration of nitrates to improve resting MBF prior to injection of sestamibi or tetrofosmin appears to improve slightly the ability of these tracers to detect cellular viability.59
With regard to prediction of improvement in LV function after revascularization, both thallium and technetium-99m based techniques have been shown to be reasonably accurate. Reported sensitivities for recovery of ventricular function are in the 75-85% range, with positive- and negative-predictive values of about 70% and 90%, respectively.57,58,60 With additional enhancements to imaging protocols, such as nitrate-enhanced rest perfusion imaging and gated SPECT for assessment of function, the accuracy of these techniques is even higher.59,61
B. Assessment of Flow-metabolism relationship
Viable myocardial segments display a variety of perfusion patterns.
RADIONUCLIDE IMAGING: PET
Decreased regional myocardial tracer uptake at rest could reflect either lack of cell membrane integrity in an area of scarred myocardium or reduced MBF secondary to HM (dysfunctional but viable). Therefore, in patients with chronic ischemic LV dysfunction, myocardial “perfusion” tracers alone may not reliably differentiate hibernating from scarred myocardium. In that setting, flow-independent probes that assess intact cellular metabolic processes may be used as an adjunct to resting myocardial blood flow.
18F-2-fluoro-2-deoxyglucose (FDG)
Despite the excellent flow kinetics and biological properties of thallium, attenuation of photons is a potential limitation for thallium in patients with large body habitus. PET systems have generally better sensitivity and spatial resolution than SPECT systems, and provide more accurate attenuation correction. Thus, metabolic imaging with FDG PET may provide incremental information to thallium regarding myocardial viability, especially in patients with severely impaired LVD.62 High-energy phosphates, such as ATP, are generated in the myocardium by oxidative phosphorylation and glycolysis. In the normal myocardium, utilization of free fatty acids is the preferred metabolic pathway for ATP production. In the setting of myocardial ischemia, where local oxygen supply is reduced, the myocardium shifts ATP production from fatty acid metabolism (which occurs in the mitochondria and is oxygen-dependent) to glucose utilization (which occurs in the cytoplasm and is oxygen-independent).
The principle of using a metabolic tracer for assessing myocardial viability is based on the concept that viable tissue is metabolically active, while scarred tissue is metabolically inactive. The glucose analogue FDG may be preserved or increased in hypoperfused but viable myocardium, termed metabolism/perfusion mismatch (Figure 5) 62a. Conversely, FDG uptake will be decreased or absent in hypoperfused and scarred myocardium, termed metabolism/perfusion match. The metabolism/perfusion mismatch pattern represents a scintigraphic marker of HM. PET imaging in dysfunctional myocardium had positive and negative predictive values of 85% and 92% respectively for recovery of function after revascularization.63-69 A subsequent European multicenter trial in 178 patients confirmed the high sensitivity of PET mismatch pattern for predicting functional recovery after revascularization70.
Figure 5. PET imaging and perfusion-metabolism mismatch in hibernation.
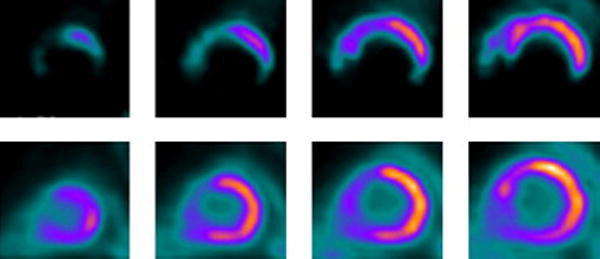
PET scan showing perfusion (top) metabolism (bottom) mismatch in hibernating heart as an example of preserved cardiometabolic reserve. Rubidium-82 positron emission tomograms in short–axis view (top row) show markedly decreased perfusion defects in the apical, inferior, inferolateral and septal regions of the left ventricle at rest, which extends from distal to basal slices. [18F] 2-deoxy, 2-fluoroglucose (FDG) images acquired under glucose loaded condition (lower row) show perfusion-metabolism mismatch pattern (the scintigraphic hallmark of hibernation) in all abnormally perfused myocardial regions at rest, with exception of the anteroseptal region, which demonstrates matched perfusion-metabolism pattern (compatible with scarred myocardium).
The prognostic significance of perfusion-metabolism mismatch pattern has been demonstrated in several nonrandomized, retrospective studies with PET. Patients with perfusion-metabolism mismatch pattern (HM) who were treated with revascularization had lower ischemic events and deaths when compared to those treated medically.7,71,72 Moreover, the extent of the PET mismatch pattern correlated with improvement in LV function, clinical course and magnitude of heart failure symptoms after revascularization.73 Patients with matched defects (concordant reduction in regional perfusion and metabolism), indicating scarred myocardium displayed no such difference in outcomes or clinical benefit from revascularization.7
The principal limitation for the widespread application of PET imaging for the assessment of myocardial metabolism is its availability. Currently in the United States, there are approximately 1,600 PET cameras versus 12,000 SPECT cameras. Thus, PET is not readily available for most practicing cardiologists. Because SPECT cameras are more prevalent than PET cameras in the community and cardiology offices, investigators have recently focused their attention on developing gamma-emitting metabolic radiotracers, such as β-Methyl-p-[123I]-iodophenyl-pentadecanoic acid (BMIPP), in order to make the assessment of myocardial metabolism readily available to patients presenting with symptoms of heart failure or LVD.74
β-Methyl-p-[123I]-iodophenyl-pentadecanoic acid (BMIPP)
BMIPP is a fatty acid analogue that provides insight into myocardial metabolism using SPECT cameras. In a recent study, delayed recovery of myocardial metabolism after ischemic injury, termed ischemic memory, was shown with BMIPP.+ Fatty acid metabolism remained suppressed for a prolonged time (up to 30 hours following an ischemic episode) even after perfusion had returned to normal. 74,75
Uptake of BMIPP from the plasma into myocardial cells occurs via CD36 transporter protein present on the sarcolemmal membrane. Retention of BMIPP in the myocardium most likely reflects activation of fatty acids by coenzyme A, and indirectly, of cellular ATP production resulting from fatty acid metabolism. Thus, in the setting of myocardial ischemia, reduction in ATP production secondary to diminished fatty acid metabolism is mirrored by decreased myocardial BMIPP uptake. Although BMIPP is approved for clinical use in Japan, it has not yet received approval by the Food and Drug Administration in the United States. In the clinical setting, the finding of persistent and prolonged disturbances in BMIPP uptake, long after resolution of ischemic symptoms, may provide a scintigraphic imprint as to the underlying cause of chronic left ventricular dysfunction.
C. Assessment of Microcirculation
Contrast Echocardiography
The coronary microcirculation is preserved in myocardium with chronic ischemic LV dysfunction. The micro bubbles in echocardiographic contrast agents are intravascular tracers and the intensity of their reflected signal can be used to demonstrate the presence and relative size of the microcirculation, while their rate of accumulation is related to coronary flow. Myocardial contrast enhancement of dysfunctional segments at rest is therefore a potential marker for viable myocardium. The value of contrast echocardiography for this purpose has been reported in over ten studies, with sensitivities ranging from 62-92%, and specificity from 67-87%. Nonetheless, this technique remains technically challenging and may be difficult to use in all myocardial segments due to issues of shadowing and attenuation.
D. Assessment of Contractile Reserve (CR)
Resting scar imaging and perfusion imaging does not permit distinction between viable and remodeled myocardium. Assessment of MBF or CR is therefore mandatory combined with perfusion, metabolic or scar imaging.
Dobutamine Stress Echocardiagraphy
Viable myocardium has been shown to augment function in response to inotropes (eg. dobutamine, amrinone), coronary vasodilators (eg. dipyridamole), arterial vasodilators (eg. levosimandan) and low level exercise. However, the most widely used stressor to augment function is low-dose dobutamine (LDDE), with an average sensitivity of 75-80% and a specificity of 80-85% for the prediction of functional recovery both early after infarction as well as in the setting of chronic LV dysfunction. Augmentation of function in response to low-dose dobutamine is a more reliable predictor of recovery than improvement at peak dose (eg. 40 mcg/kg/min). Nonetheless, the peak-dose response is important, because the most reliable predictor of functional recovery is the biphasic response (ie. augmentation at low-dose with deterioration at peak-dose) indicating that the tissue is not only viable but also supplied by a stenosed infarct-related artery (Figure 6A-6C). The extent of viability is an important determinant of the likelihood of recovery of overall LV function (eg. characterized by an LVEF improvement of >5%). The presence of ≥4 segments with a biphasic response has a specificity and sensitivity of 80-90% for prediction of global functional recovery. Patients demonstrating a significant LVEF improvement with LDDE are also likely to show global functional recovery and reverse remodelling.76
Figure 6. Resting and dobutamine stress echo for myocardial thinning, contractile reserve and myocardial ischemia.
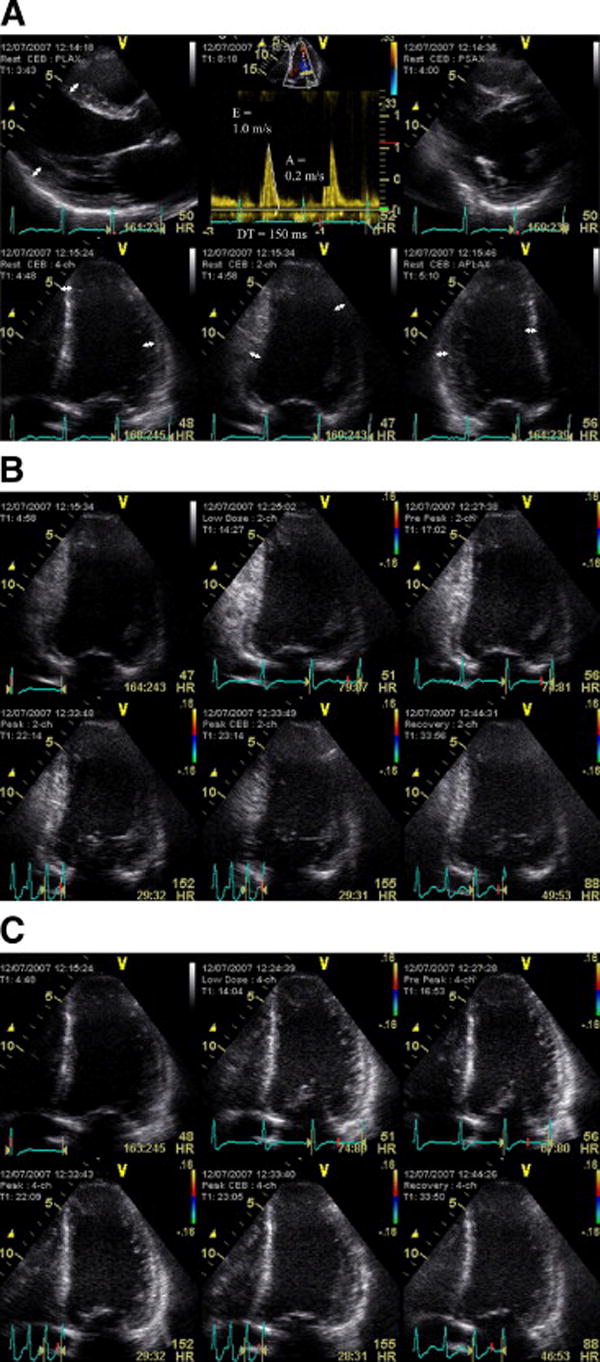
Integration of resting data and new technologies to predict functional recovery in a patient with severe dysfunction (LVEF 25%). The resting study (panel A) shows no areas of thinning (1 cm markers in all walls) despite severe LV dysfunction and borderline restrictive filling (deceleration time 150 ms, E/A >2). Dobutamine stress shows basal inferior ischemia and mid inferior biphasic response in panel B. Peak-dose (40 mcg/kg/min) dobutamine response showed hypokinetic septal and lateral walls had deteriorated at peak dose, consistent with extensive ischemia (panel C).
Echocardiography is an ideal initial screening test in these patients, because of its wide availability, low cost, and similar levels of accuracy to other more expensive modalities.58 Like any non-invasive test, there may be false-negative and false-positive responses with LDDE. The reliability of LDDE is influenced by a number of other factors apart from the extent of viable tissue, and false negative responses may be due to reduced substrate supply (compromised if the tissue is ischemic), loss of the myocardial contractile apparatus and excessive fibrosis (which may splint the segment and prevent it from shortening). If flow is severely compromised, the tissue may become ischemic before exhibiting an augmentation response. For this reason, LDDE should be performed with multiple low doses (eg. 5 and 10 mcg/kg/min - some authors have used a very low dose of 2.5 mcg/kg/min and doses higher than 10 mcg/kg/min), continually assessing the response, as augmentation may be very transient and ischemia is increasingly likely as the heart-rate increases. The chronotropic response to dobutamine usually starts at doses >10 mcg/kg/min, although the widespread use of long-acting beta blockers for HF has moved the “low-dose” threshold towards 20 mcg/kg/min. Another cause of false-negative responses is damage to the contractile apparatus within viable myocytes, caused by recurrent episodes of ischemia and stunning. Failure to respond to dobutamine may not indicate lack of viability, which may still be identified using PET; in these situations, prolonged follow-up may be needed to demonstrate functional recovery after revascularization. Non-transmural scar influences the likelihood of recovery of resting function, and may cause false positive responses. Subendocardial scar is a major contributor to resting dysfunction, so segments with non-transmural scar may respond to dobutamine because of augmentation of the subepicardium, but not recover resting function after revascularization. There are risks in the performance of peak dose dobutamine stress in individuals with severe LVD. The underlying reserve of the LV may be limited and hypotension, heart failure and serious arrhythmias may ensue if severe ischemia develops. These tests should be performed by experienced personnel, and the test may need to be stopped at the development of ischemia, and nitrates and oxygen may need to be administered. Nonetheless, there is no good evidence to indicate that there is a substantial increase of risk of dobutamine stress in the situation, compared to the usual risk of a significant adverse event in 3:1000 studies.
A problem relates to the challenges of interpreting LDDE. As with all stress echocardiographic techniques, wall motion scoring is limited by subjectivity and technical challenges. The measurement of myocardial deformation may prove to be a more reliable means of quantitation of this response. Strain-rate at LDDE correlates with the presence of myocardial viability evidenced by FDG imaging, with an optimal cut-point for this purpose being a SR increment of >0.23/second.77 A strain-rate increment of >0.25/second also predicts functional recovery after revascularization (sensitivity 80%, specificity 75%).78
Magnetic resonance imaging
The demonstration of CR is an established predictor of myocardial viability58 As with echocardiography, CMR assessment of CR is performed using the infusion of low doses of dobutamine (5-10 μg/kg/min). One study compared low dose dobutamine CMR to FDG-PET in 35 patients with chronic MI and segmental LVD.48 Using both EDWT and improvement in systolic thickening as markers of viability, CMR had a sensitivity, specificity, and diagnostic accuracy of 88%, 87%, and 92% respectively. Using improvement in LV function post-revascularization as the reference standard in the same patient group,49 dobutamine CMR had a sensitivity and specificity of 89% and 92%.
The utility of incorporating myocardial tagging for the assessment of intramyocardial functional reserve has also been demonstrated. In acute MI, the quantitative response to dobutamine is predictive of functional recovery, although the response in the subendocardium is quite complex.79 Studies in chronic ischemic heart disease before and after multi-vessel revascularization using tagged dobutamine CMR demonstrate that half of dysfunctional segments recover resting function, and, of the remainder, half demonstrate rest dysfunction but CR.80 Half of these latter segments in turn recover rest function when examined 3 years after revascularization.80
Some controversy exists as to which test performs best for predicting functional recovery among CMR-based techniques. LGE clearly identifies scar, but the relationship between scar and functional recovery in infarcts with 1-75% transmural infarction is complex. The performance of dobutamine CMR has been compared to LGE in this scenario.81 Although no difference was seen in the identification of viability in segments without LGE or those with scarring ≥75%, dobutamine CMR was superior in predicting recovery in zones in which LGE demonstrated between 1% and 75% transmural scar. 82 However, the performance of LGE varied greatly depending on the cutoff values used to define viability.82 Complementary use of dobutamine CMR and LGE may prove to be the optimal strategy for predicting post-revascularization functional recovery, as shown in a study of 15 patients studied before and 20 weeks after coronary artery bypass surgery.83 In segments with 1-50% infarct transmurality, as shown by LGE, dobutamine response was predictive of functional recovery, whereas the predictive value of LGE alone was intermediate. Wall thickening with dobutamine CMR before revascularization correlated with wall thickening at rest at follow-up.
This combined approach to defining viability by CMR may be warranted in certain clinical scenarios. While it may not be practical to perform low dose dobutamine infusion on all patients evaluated for viability, decision-making in those with multiple segments having 1-50% transmurality may benefit. In patients with no late enhancement or >50% late enhancement, there is little to gain by adding dobutamine, and LGE remains the preferred technique because of its ease of use.
E. Prognostic Implications of Myocardial Viability Testing
Beyond the assessment of mere presence or absence of myocardial viability and predictive values of the various tests for recovery of regional or global LV function, it is perhaps equally important (if not more important) to demonstrate a survival advantage offered by revascularization for patients with ischemic LV dysfunction with viable myocardium. In a pooled analysis consisting of 3088 patients in 24 studies, long-term survival after revascularization or medical therapy was determined independent of the type of viability testing, which included SPECT radionuclide imaging, PET or dobutamine echocardiography (7). In patients exhibiting predominantly viable myocardium, follow up on medical therapy was associated with very high risk, a 16% annual mortality. On the other hand, in similar patients, revascularization was associated with an 80% reduction in annual mortality (16% vs. 3.2%, p< 0.0001), compared with medical therapy (Figure 7). Importantly, patients with the most severe LV dysfunction derived the greatest benefit from revascularization (Table 1). With worsening LV EF, the survival benefit associated with revascularization of patients with viable myocardium increased proportionately (Figure 8) (7).
Figure 7. Prognostic implications of myocardial viability testing.
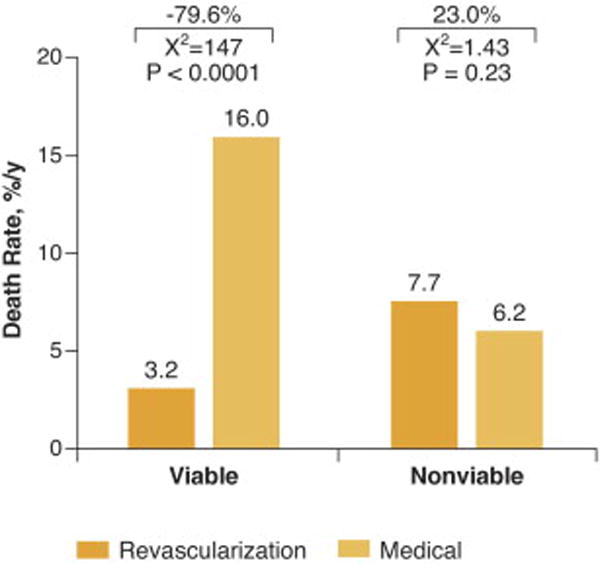
The data are derived from meta-analysis of 3088 patients with coronary artery disease and left ventricular dysfunction who underwent viability testing. Death rates for patients with and without myocardial viability treated by revascularization or medical therapy are shown. In patients with viable myocardium, there is 79.6% reduction in mortality among those who were treated with revascularization (p<0.0001). In contrast, among patients without evidence of viable myocardium, there was no significant difference in mortality with revascularization versus medical therapy. Adapted from Allman et al. (7).
Figure 8. Myocardial viability, LV function, and reduction in mortality after revascularization.
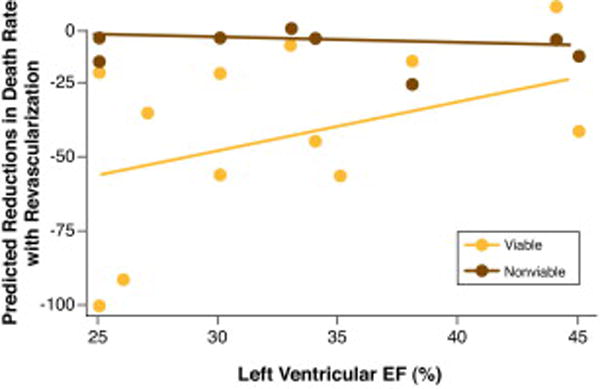
The relationship of reductions in death rate to resting left ventricular ejection fraction in patients who had revascularization. In patients with non-viable myocardium, there is no reduction of death with revascularization. In patients with viable myocardium, the lower the ejection fraction the greater is the reduction of deaths after revascularization. From Allman et al (7).
INTEGRATED IMAGING IN CLINICAL PRACTICE
None of the currently available imaging tools can address all the different aspects of the complex pathophysiology of myocardial viability and hibernation. Accordingly, they should often be used in combination in order to achieve the highest possible level of diagnostic accuracy. The clinician and non-invasive imager should note the following in the evaluation of reversible ischemic dysfunction:
The sensitivity, specificity, positive predictive and negative predictive values of the various tests for recovery of regional LV function and for recovery of global LV function.84 (Figures 9A-9D, 10)
These studies included various methodologies, patient populations and end-points, nd thus, should be interpreted with appropriate caution. Data obtained by direct comparison of dobutamine echocardiography and nuclear studies in the same patients in the same centers are shown in (Figures 11A, 11B).57
SPECT imaging with thallium-201 or technetium-99m perfusion tracers is more sensitive for detecting viable myocardium than dobutamine echocardiography. LDDE is more specific for predicting recovery of function after revascularization. Thus, markers of CR predict functional recovery with greater specificity than do markers of preserved cell membrane function.85 The recommended SPECT imaging is stress-redistribution-reinjection. It provides information about viability and ischemia. Those in whom stress cannot be performed, rest-redistribution can be used.
The retrospective nature of all available studies represents a weakness in the collective evidence base. These are the only data that are available and one should exercise some care in their application to patient management. However, it should be recognized that prospective, properly designed and executed studies will likely not be available for a considerable period of time. Moreover, the review that is cited 84 from which Figures 9A-9D and 10 are shown was comprehensive and included all available studies with adequate data and follow-up. This allows for a reasonable, and probably a very good, plan of decision making for patient management at the present time.
There is no perfect test (95 – 100 % sensitive and specific), and therefore there may be a need for combination of tests.
The judgment to use test(s) at any one medical center is dependent on the availability of the test(s) and the skill and experience in performing the tests and interpreting its results.
Mild-moderate improvement/severe defect on reinjection, ≤50% of thallium uptake on SPECT imaging and > 50% LGE on CMR indicates a small probability of LV function improvement after revascularization (Figure 12) 86
The patients should first have an echocardiogram/Doppler (or another test) to diagnose LV dysfunction.
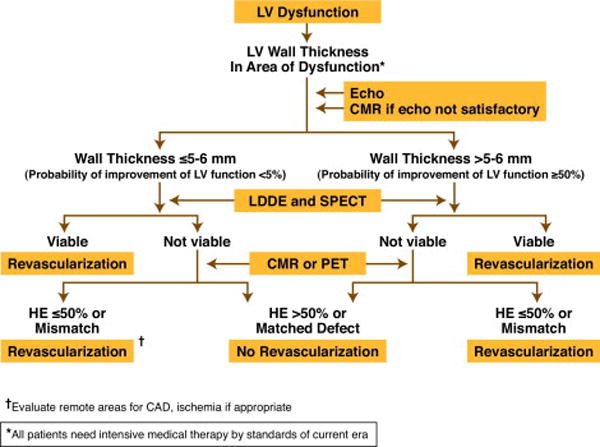
A suggested format for diagnosis of viable myocardium and need for myocardial revascularization is shown in Algorithm 1. The format of the proposed studies is the one which uses tests that are available at larger medical centers and there is good volume of data documenting the value of the tests. The abbreviations in the Algorithms are clarified in the Appendix.
A simplified format that proposes studies which are most widely available to most practicing clinical cardiologists is shown in Algorithm 2.
Criteria suggesting LV function is not likely to improve with revascularization are listed in Table 3.
Figure 9. Diagnostic accuracy of various techniques for functional recovery.
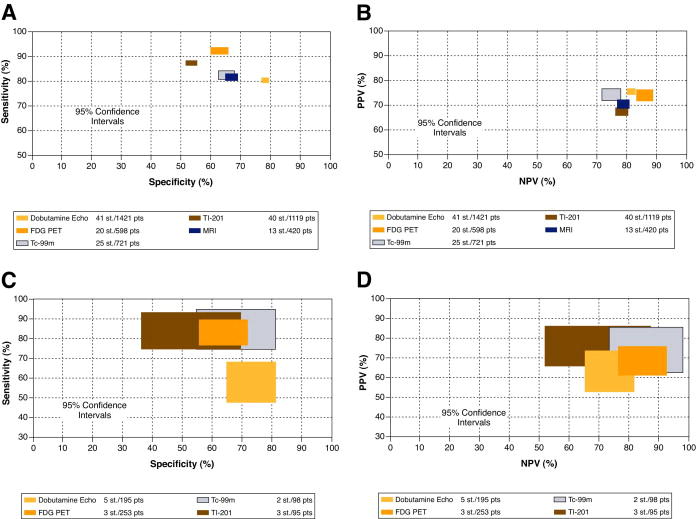
(A) Comparison of sensitivities and specificities (A) and predictive values (B) with 95% confidence intervals for the recovery of regional wall function. (C), Comparison of sensitivities and specificities (C) and predictive values (D) of the various techniques with 95% confidence intervals for prediction of the recovery of global left ventricular (LV) function. LV = left ventricle, Dobutamine echo = dobutamine echocardiography; FDG PET = F18 – fluorodexoxyglucose, positron emission tomography; Tc − 99m = Technetium – 99m-labeled agents; T1 − 201 = thallium 201; MRI = magnetic resonance imaging. PPV = positive predictive value, NPV = negative predictive value; St = Number of studies, pts = Number of patients. From Schinkel AFL et al. (Reference 84).
Figure 10. Diagnostic accuracy of magnetic resonance imaging for post-revascularization improvement in regional function.
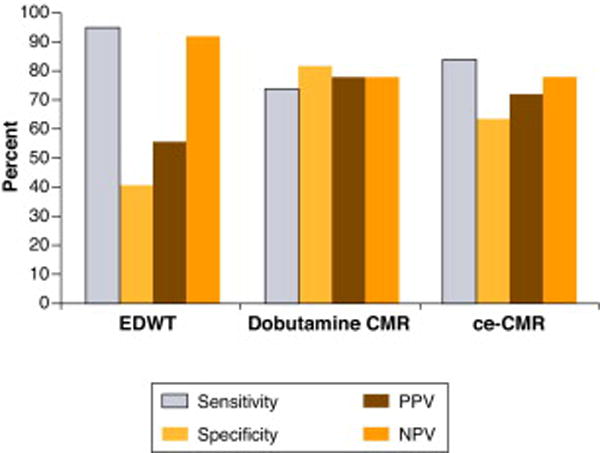
Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of data from various techniques with cardiovascular magnetic resonance (CMR). EDWT = end – diastolic wall thickness, Ce – CMR = contrast enhanced cardiovascular magnetic resonance. Figure developed from data of Schinkel AFL et al (Reference 84).
Figure 11 A + B. Comparison of diagnostic accuracy of dobutamine echocardiography and nuclear imaging.
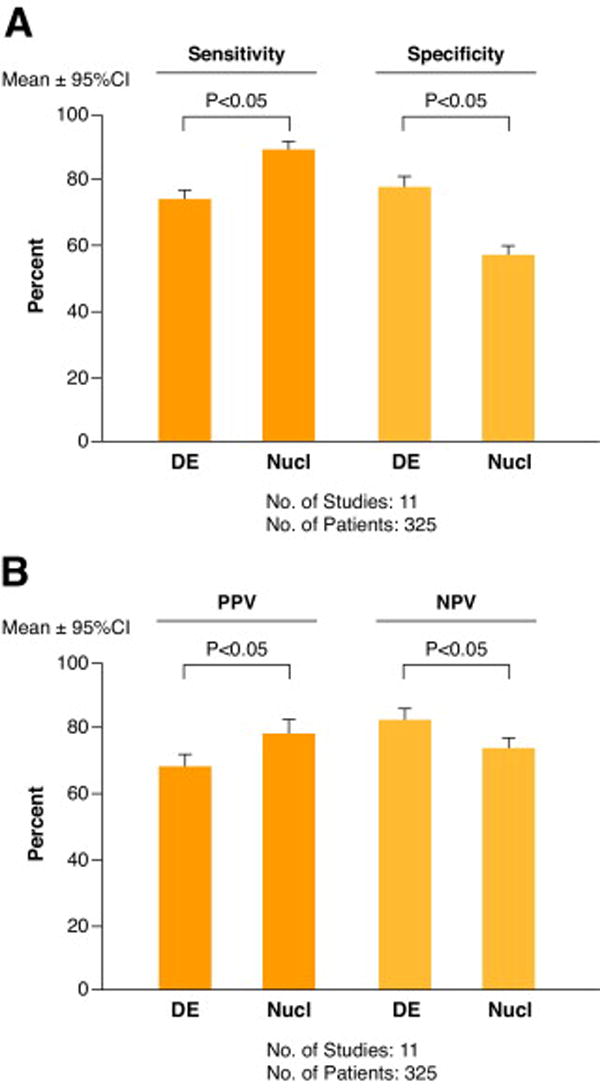
Sensitivity and specificity (A) :and positive (PPV) and negatiave (NPV) predictive value for predicting improvement in left ventricular function obtained by a direct comparison of dobutamine echocardiography (DE) and nuclear imaging (Nucl) of 325 patients in 11 studies. In each study the same patients underwent both tests at the same medical center. Figure developed from data of Bax JJ et al (reference 57).
Figure 12. Comparison of diagnostic accuracy of nuclear and magnetic resonance imaging for functional recovery after revascularization.
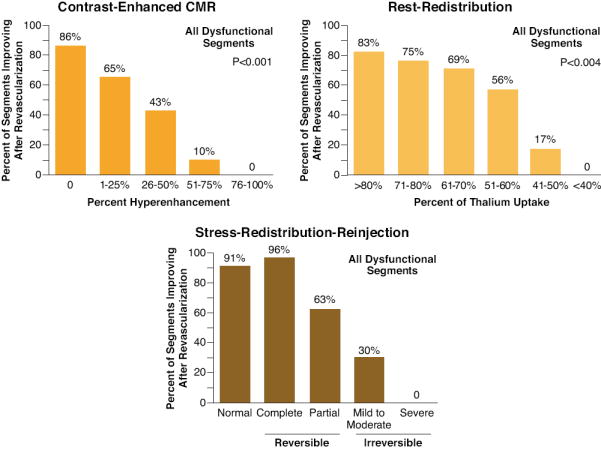
Relationships between recovery of function after revascularization with contrast-enhanced CMR, and 2 thallium protocols optimized for viability detection: 1) rest-redistribution and 2) stress-redistribution-reinjection imaging are shown. Irrespective of the imaging modality applied, the data suggest that recovery of function after revascularization is a continuum and is coupled to the ratio of viable to scarred myocardium within dysfunctional myocardial segments. The extent of infarct size on CMR or percent thallium defect on SPECT correlated with decreasing likelihood of functional recovery after revascularization. From Dilsizian V (reference 86).
Algorithm 1.
Algorithm 2.
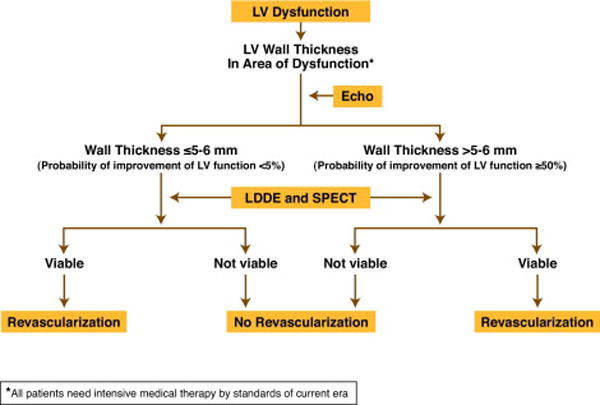
TABLE 3.
GLOBAL LV DYSFUNCTION AND MULTIVESSAL DISEASE
CRITERIA INDICATING LV FUNCTION LESS LIKELY TO IMPROVE WITH REVASCULARIZATION.
|
COMBINATION OF CRITERIA INDICATING LV FUNCTION NOT LIKELY TO IMPROVE WITH REVASCULARIZATION
|
Abbreviations: LV = Left ventricle; WT = Wall thickness; EF = Ejection Fraction; EDV/EDVI = End-diastolic volume/index; ESV/ESVI = End systolic volume/index; LDDE = Low dose dobutamine echocardiography; SPECT = Single photon emission computed tomography; CMR = Cardiovascular magnetic resonance; LGE = Late gadolinium enhancement; PET = Positron emission tomography; HM = Hibernating Myocardium
CLINICAL DECISION MAKING
There are several steps in the management of these patients.
There is a need for almost immediate diagnosis of LVD in the clinical syndromes described earlier in this symposium. Usually, this is best done by echocardiography/Doppler studies.
Appropriate test(s) for viability (± ischemia) should be performed very early in the management of these patients. Test(s) for viability and coronary arteriography are essential prior to a consideration for revascularization.
The entire clinical picture, including results and proper interpretation of the results of diagnostic tests, should be incorporated into clinical-decision making.
TABLE 2.
PATHOPHYSIOLOGY OF CORNARY BLOOD FLOW
|
It is also reduced in normal people and patients with significant coronary atherosclerosis.
SE-MBF = Subendocardial myocardial blood flow
TM-MBF = Transmural myocardial blood flow
Acknowledgments
Dr. Kramer was supported in part by RO1 HL075792 from National Institutes of Health, Bethesda, MD;
Dr. Marwick was supported by project grant 210217 from the National Health and Medical Research Council, Canberra, Australia (THM).
ABBREVIATIONS/ACRONYMS
- CFR
Coronary flow reserve
- CMR
Cardiovascular magnetic resonance
- CR
Contractile reserve
- HM
Hibernating myocardium
- LGE
Late gadolinium enhancement
- LVD
Left ventricular dysfunction
- MBF
Myocardial blood flow
- SE-MBF
Subencocardial MBF
- TM-MBF
Transmural MBF
Appendix 1
Abbreviations used in Algorithms
- LV
Left ventricle
- LVEF
LV ejection fraction
- CMR
Cardiovascular Magnetic Resonance
- LDDE
Low dose dobutamine echocardiography
- SPECT
Single-photon emission computed tomography
- PET
F 18-flouorodeoxyglucose and postitron emission tomography
- Revasc
Myocardial revascularization by PCI/C
- PCI
Percutaneous catheter intervention
- HM
Hibernating myocardium
- CABG
Coronary artery bypass graft surgery
- Revasc Special
Large LV area or critical LV area
- VD
Vessel disease
- EDVI
End-diastolic volume index
- ESVI
End-systolic volume index
- EDDI
End-diastolic dimension index
Footnotes
DISCLOSURE Dr. Rahimtoola has received Honoraria for educational lectures from American College of Cardiology Foundation; American College of Physicians; University of California Los Angeles; University of California Irvine; Cornell University; Creighton University; Thomas Jefferson University; Cedars-Sinai Medical Center; Harvard Medical School; University of Wisconsin; University of Hawaii; Cardiologists Association of Hong Kong, China; ATS; St. Jude Medical; Carbomedics; Merck; Pfizer; Edwards Life Sciences.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Los Angeles Times. 1997 Aug 17;:M4. [PubMed] [Google Scholar]
- 2.Rahimtoola SH. Coronary Bypass Surgery for Chronic Angina -1981. A perspective. Circulation. 1982;65:225–241. doi: 10.1161/01.cir.65.2.225. [DOI] [PubMed] [Google Scholar]
- 3.Rahimtoola S. The hibernating myocardium. Am Heart J. 1989;117:211–221. doi: 10.1016/0002-8703(89)90685-6. [DOI] [PubMed] [Google Scholar]
- 4.Ferrari R. Myocardial hibernation. An adaptive phenomenon? In: Yellon DM, Rahimtoola SH, Opic LH, editors. New Ischemic Syndromes. Authors Publishing House; New York: 1997. pp. 204–214. [Google Scholar]
- 5.Carluccio E, Biagioli P, Alunn G, et al. Patients with hibernating myocardium show altered left ventricular volumes and shape, which revert after revascularization: evidence that dyssynergy might directly induce cardiac remodeling. J Am Coll Cardiol. 2006;47:969–77. doi: 10.1016/j.jacc.2005.09.064. [DOI] [PubMed] [Google Scholar]
- 6.Rahimtoola SH, La Canna G, Ferrari R. Hibernating myocardium: Another piece of the puzzle falls into place. J Am Coll Cardiol. 2006;47:978–80. doi: 10.1016/j.jacc.2005.11.038. [DOI] [PubMed] [Google Scholar]
- 7.Allman KC, Shaw LJ, Hachamovitch R, Udelson JE. Myocardial viability testing and impact of revascularization on prognosis in patients with coronary artery disease and left ventricular dysfunction. J Am Coll Cardiol. 2002;39:1151–8. doi: 10.1016/s0735-1097(02)01726-6. [DOI] [PubMed] [Google Scholar]
- 8.Allman KC, Shaw LJ, Hachamovitch R, Udelson JE. Prognostic valve of myocardial viability testing: a meta-analysis. Circulation. 2000;102(SupplII):576. [Google Scholar]
- 9.Uren NG, Marraccini P, Gistri R, de Silva R, Camichi PG. Altered coronary vasodilator reserve and metabolism in myocardium subtended by normal Arteries in patients with coronary artery disease. J Am Coll Cardiol. 1993;22:650–8. doi: 10.1016/0735-1097(93)90172-w. [DOI] [PubMed] [Google Scholar]
- 10.Thomas SA, Fallavollita JA, Suzuki G, Borgers M, Canty JM., Jr Dissociation of regional adaptations to ischemia and global myolysis in an accelerated swine model of chronic hibernating myocardium. Circ Res. 2002;94:970–977. doi: 10.1161/01.res.0000040396.79379.77. [DOI] [PubMed] [Google Scholar]
- 11.Gheorghiade M, Sopko G, De Luca, et al. Navigating the crossroads of coronary artery disease and heart failure. Circulation. 2006;114:1202–13. doi: 10.1161/CIRCULATIONAHA.106.623199. [DOI] [PubMed] [Google Scholar]
- 12.Cleland JGF, Pennell DJ, Ray SG, et al. Myocardial viability as a determinat of the ejection fraction response to carvedilol in patients with heart failulre (Christmas trial): Randomized controlled trial. Lancet. 2003;362:14–21. doi: 10.1016/s0140-6736(03)13801-9. [DOI] [PubMed] [Google Scholar]
- 13.Tarakjik G, Brunken R, McCarthy PM, et al. Myocardial viability testing and the effect of early intervention in patients with advanced left ventricular dysfunction. Circulation. 2006;113:230–237. doi: 10.1161/CIRCULATIONAHA.105.541664. [DOI] [PubMed] [Google Scholar]
- 14.Heusch G, Schulz R, Rahimtoola SH. Myocardial hibernation: a delicate balance. Am J Physiol Heart Circ Physiol. 2005;228:H984–H999. doi: 10.1152/ajpheart.01109.2004. [DOI] [PubMed] [Google Scholar]
- 15.Vatner SF. Correlation between acute reductions in myocardial blood flow and function in conscious dogs. Circ Res. 1980;47:201–7. doi: 10.1161/01.res.47.2.201. [DOI] [PubMed] [Google Scholar]
- 16.Gallager KP, Matsuzaki M, Koziol JA, Kemper WS, Ross J., Jr Regional myocardial perfusion and wall thickening during ischemia in conscious dogs. Am J Physiol Heart Circ Physiol. 1984;247:H727–38. doi: 10.1152/ajpheart.1984.247.5.H727. [DOI] [PubMed] [Google Scholar]
- 17.Pantely GA, Malone SA, Rhen WS, et al. Regeneration of myocardial phosphocreative in pigs despite continued moderate ischemia. Circ Res. 1990;67:1481–93. doi: 10.1161/01.res.67.6.1481. [DOI] [PubMed] [Google Scholar]
- 18.Schulz R, Rose J, Martin D, Brodole OE, Heusch G. Development of short-term myocardial hibernation: its limitation by the severity of ischemia and inotropic stimulation. Circulation. 1993;88:684–95. doi: 10.1161/01.cir.88.2.684. [DOI] [PubMed] [Google Scholar]
- 19.Fallavollita JA, Perry BJ, Canty JM. 18-F-2 deoxyglucose deposition and regional flow in pigs with chronically dysfunctional myocardium. Evidence for transmural variations in chronic hibernating myocardium. Circulation. 1997;95:1900–9. doi: 10.1161/01.cir.95.7.1900. [DOI] [PubMed] [Google Scholar]
- 20.Edward NC, Sinusas AJ, Bergin JD, Watson DD, Ruiz M, Beller GA. Influence of subendocardial ischemia on transmural myocardial function. Am J Physol Heart Circ Physiol. 1992:H568–H576. doi: 10.1152/ajpheart.1992.262.2.H568. [DOI] [PubMed] [Google Scholar]
- 21.Bache RJ, Cobb FR, Greenfield JC., Jr Myocardial blood flow distribution during ischemia-induced coronary vasodilatation in the un-anesthetized dog. J Chin Invest. 1974;54:1462–72. doi: 10.1172/JCI107894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heusch G. Hibernating myocardium. Physiol Rev. 1998;78:1055–1085. doi: 10.1152/physrev.1998.78.4.1055. [DOI] [PubMed] [Google Scholar]
- 23.Fallavollita JA, Canty JM., Jr Differential 18F-2-deoxyglucose uptake inviable dysfunctional myocardium with normal resting perfusion. Circulation. 1999;99:2798–2805. doi: 10.1161/01.cir.99.21.2798. [DOI] [PubMed] [Google Scholar]
- 24.Selvanayagam JB, Jerosch-Herold M, Porto I, et al. Resting myocardial blood flow is impaired in hibernating myocardium: A magnetic resonance study of quantitative perfusion assessment. Circulation. 2005;112:3289–96. doi: 10.1161/CIRCULATIONAHA.105.549170. [DOI] [PubMed] [Google Scholar]
- 25.Klocke FJ. Resting blood flow in hypocontractile myocardium: Resolving the controversy. Circulation. 2005;112:3222–3224. doi: 10.1161/CIRCULATIONAHA.105.583344. [DOI] [PubMed] [Google Scholar]
- 26.Afridi I, Kleiman NS, Raizner AE, Zoghbi WA. Dobutamine echocardiography in myocardial hibernation. Optimal dose and accuracy in predicting recovery of ventricular function after coronary angioplasty. Circulation. 1995;91:663–70. doi: 10.1161/01.cir.91.3.663. [DOI] [PubMed] [Google Scholar]
- 27.Borgers M, Thoné F, Wouters L, Ausma J, Shivalkar B, Flameng W. Structural correlates of regional myocardial dysfunction in patients with critical coronary artery stenosis: Chronic hibernation? Cardiovasc Pathol. 1993;2:237–245. [Google Scholar]
- 28.Shimoni S, Frangogiannis NG, Aggeli CJ, et al. Microvascular structural correlates of myocardial contrast echocardiography in patients with coronary artery disease and left ventricular dysfunction: implications for the assessment of myocardial hibernation. Circulation. 2002;106:950–956. doi: 10.1161/01.cir.0000026395.19594.43. [DOI] [PubMed] [Google Scholar]
- 29.Lang RM, Mor-Avi V, Sugeng L, Nieman PS, Sahn DJ. Three-dimensional echocardiography: the benefits of the additional dimension. J Am Coll Cardiol. 2006;48:2053–69. doi: 10.1016/j.jacc.2006.07.047. [DOI] [PubMed] [Google Scholar]
- 30.Cwajg JM, Cwajg E, Nagueh SF, et al. End-diastolic wall thickness as a predictor of recovery of function in myocardial hibernation: relation to rest-redistribution T1-201 tomography and dobutamine stress echocardiography. J Am Coll Cardiol. 2000;35:1152–61. doi: 10.1016/s0735-1097(00)00525-8. [DOI] [PubMed] [Google Scholar]
- 31.La Canna G, Rahimtoola SH, Visioli O, et al. Sensitivity, specificity and predictive accuracies of non-invasive tests, singly and in combination, for diagnosis of hibernating myocardium. Eur Heart J. 2000;21:1358–67. doi: 10.1053/euhj.1999.2038. [DOI] [PubMed] [Google Scholar]
- 32.Chan J, Hanekom L, Wong C, Leano R, Cho GY, Marwick TH. Differentiation of subendocardial and transmural infarction using two-dimensional strain rate imaging to assess short-axis and long-axis myocardial function. J Am Coll Cardiol. 2006;48:2026–33. doi: 10.1016/j.jacc.2006.07.050. [DOI] [PubMed] [Google Scholar]
- 33.Penicka M, Tousek P, De Bruyne B, et al. Myocardial positive pre-ejection velocity accurately detects presence of viable myocardium, predicts recovery of left ventricular function and bears a prognostic value after surgical revascularization. Eur Heart J. 2007;28:1366–73. doi: 10.1093/eurheartj/ehl456. [DOI] [PubMed] [Google Scholar]
- 34.Wu KC, Lima JA. Noninvasive imaging of myocardial viability: current techniques and future developments. Circ Res. 2003;93:1146–58. doi: 10.1161/01.RES.0000103863.40055.E8. [DOI] [PubMed] [Google Scholar]
- 35.Kim RJ, Fieno DS, Parrish TB, et al. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age and contractile function. Circulation. 1999;100:1992–2002. doi: 10.1161/01.cir.100.19.1992. [DOI] [PubMed] [Google Scholar]
- 36.Rehwald WG, Fieno DS, Chen EL, Kim RJ, Judd RM. Myocardial magnetic resonance imaging contrast agent concentrations after reversible and irreversible ischemic injury. Circulation. 2002;105:224–9. doi: 10.1161/hc0202.102016. [DOI] [PubMed] [Google Scholar]
- 37.Mankad S, Khalil R, Kramer CM. MRI for the diagnosis of myocardial ischemia and viability. Curr Opin Cardiol. 2003;18:351–6. doi: 10.1097/00001573-200309000-00005. [DOI] [PubMed] [Google Scholar]
- 38.Wagner A, Mahrholdt H, Holly TA, et al. Contrast-enhanced MRI and routine single photon emission computed tomography (SPECT) perfusion imaging for detection of subendocardial myocardial infarcts: an imaging study. Lancet. 2003;361:374–9. doi: 10.1016/S0140-6736(03)12389-6. [DOI] [PubMed] [Google Scholar]
- 39.Kuhl HP, der Weerdt AV, Beek AM, Visser CA, Hanrath P, van Rossum AC. Relation of end-diastolic wall thickness and the residual rim of viable myocardium by magnetic resonance imaging to myocardial viability assessed by fluorine-18 deoxyglucose positron emission tomography. Am J Cardiol. 2006;97:452–7. doi: 10.1016/j.amjcard.2005.09.074. [DOI] [PubMed] [Google Scholar]
- 40.Kim RJ, Wu E, Rafael A, Chen E-L, Parker M, Judd RM. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343:1445–53. doi: 10.1056/NEJM200011163432003. [DOI] [PubMed] [Google Scholar]
- 41.Knuesel PR, Nanz D, Wyss C, et al. Characterization of dysfunctional myocardium by positron emission tomography and magnetic resonance: relation to functional outcome after revascularization. Circulation. 2003;108:1095–100. doi: 10.1161/01.CIR.0000085993.93936.BA. [DOI] [PubMed] [Google Scholar]
- 42.Schvartzman PR, Srichai MB, Grimm RA, et al. Nonstress delayed-enhancement magnetic resonance imaging of the myocardium predicts improvement of function after revascularization for chronic ischemic heart disease with left ventricular dysfunction. Am Heart J. 2003;146:535–41. doi: 10.1016/S0002-8703(03)00318-1. [DOI] [PubMed] [Google Scholar]
- 43.Simonetti OP, Kim RJ, Fieno DS, et al. An improved MR imaging technique for the visualization of myocardial infarction. Radiology. 2001;218:215–23. doi: 10.1148/radiology.218.1.r01ja50215. [DOI] [PubMed] [Google Scholar]
- 44.Kellman P, Arai AE, McVeigh ER, Aletras AH. Phase-sensitive inversion recovery for detecting myocardial infarction using gadolinium-delayed hyperenhancement. Magn Reson Med. 2002:372–83. doi: 10.1002/mrm.10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Foo TK, Wolff SD, Gupta SN, Kraitchman DL. Enhanced viability imaging: Improved contrast in myocardial delayed enhancement using dual inversion time subtraction. Magn Reson Med. 2005;53:1484–9. doi: 10.1002/mrm.20515. [DOI] [PubMed] [Google Scholar]
- 46.Kellman P, Chung Y-C, Simonetti O, McVeigh ER, Arai AE. Multicontrast delayed enhancement provides improved contrast between myocardial infarction and blood pool. J Magn Reson Imaging. 2005;22:605–13. doi: 10.1002/jmri.20426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sievers B, Elliott MD, Hurwitz LM, et al. Rapid Detection of Myocardial Infarction by Subsecond, Free-Breathing Delayed Contrast-Enhancement Cardiovascular Magnetic Resonance. Circulation. 2007;115:236–44. doi: 10.1161/CIRCULATIONAHA.106.635409. [DOI] [PubMed] [Google Scholar]
- 48.Baer FM, Both E, Schneider CA, Theissen P, Schicha H, Sechtem U. Comparison of low-dose dobutamine-gradient echo magnetic resonance imaging and positron emission tomography 18F-fluorodeoxyglucose in patients with chronic coronary artery disease. Circulation. 1995;91:1006–15. doi: 10.1161/01.cir.91.4.1006. [DOI] [PubMed] [Google Scholar]
- 49.Baer FM, Theissen P, Schneider CA, et al. Dobutamine magnetic resonance imaging predicts contractile recovery of chronically dysfunctional myocardium after successful revascularization. J Am Coll Cardiol. 1998;31:1040–8. doi: 10.1016/s0735-1097(98)00032-1. [DOI] [PubMed] [Google Scholar]
- 50.Zaret BL, Strauss HW, Martin ND, Wells HP, Flamm MD. Noninvasive regional myocardial perfusion with radioactive potassium: Study of patients at rest with exercise and during angina pectoris. N Engl J Med. 1973;288:809–812. doi: 10.1056/NEJM197304192881602. [DOI] [PubMed] [Google Scholar]
- 51.Pohost GM, Zir LM, Moore RH, McKusick KA, Guiney TE, Beller GA. Differentiation of transiently ischemic from infarcted myocardium by serial imaging after a single dose of thallium-201. Circulation. 1977;55:294–302. doi: 10.1161/01.cir.55.2.294. [DOI] [PubMed] [Google Scholar]
- 52.Kiat H, Berman DS, Maddahi J, et al. Late reversibility of tomographic myocardial thallium-201 defects: An accurate marker of myocardial viability. J Am Coll Cardiol. 1988;12:1456–63. doi: 10.1016/s0735-1097(88)80009-3. [DOI] [PubMed] [Google Scholar]
- 53.Gewirtz H, Beller GA, Strauss HW, et al. Transient defects of resting thallium scans in patients with coronary artery disease. Circulation. 1979;59:707–713. doi: 10.1161/01.cir.59.4.707. [DOI] [PubMed] [Google Scholar]
- 54.Ragosta M, Beller GA, Watson DD, Kaul S, Gimple LW. Quantitative planar rest-redistribution 201Tl imaging in detection of myocardial viability and prediction of improvement in left ventricular function after coronary artery bypass surgery in patients with severely depressed left ventricular function. Circulation. 1993;87:1630–1641. doi: 10.1161/01.cir.87.5.1630. [DOI] [PubMed] [Google Scholar]
- 55.Dilsizian V, Rocco TP, Freedman NM, Leon MB, Bonow RO. Enhanced detection of ischemic but viable myocardium by the reinjection of thallium after stress-redistribution imaging. N Engl J Med. 1990;323:141–146. doi: 10.1056/NEJM199007193230301. [DOI] [PubMed] [Google Scholar]
- 56.Kitsiou AN, Srinivasan G, Quyyumi AA, Summers RM, Bacharach SL, Dilsizian V. Stress-induced reversible and mild-to-moderate irreversible thallium defects: Are they equally accurate for predicting recovery of regional left ventricular function after revascularization? Circulation. 1998;98:501–508. doi: 10.1161/01.cir.98.6.501. [DOI] [PubMed] [Google Scholar]
- 57.Bax JJ, Poldermans D, Elhendy A, Boersma E, Rahimtoola SH. Sensitivity, specificity, and predictive accuracies of various noninvasive techniques for detecting hibernating myocardium. Curr Prob Cardiol. 2001;26:141–86. doi: 10.1067/mcd.2001.109973. [DOI] [PubMed] [Google Scholar]
- 58.Bax JJ, Wijns W, Cornel JH, Visser FC, Boersma E, Fioretti PM. Accuracy of currently available techniques for prediction of functional recovery after revascularization in patients with left ventricular dysfunction due to chronic coronary artery disease: comparison of pooled data. J Am Coll Cardiol. 1997;30:1451–1460. doi: 10.1016/s0735-1097(97)00352-5. [DOI] [PubMed] [Google Scholar]
- 59.Bisi G, Sciagra R, Santoro GM, et al. Technetium-99m-sestamibi imaging with nitrate infusion to detect viable hibernating myocardium and predict postrevascularization recovery. J Nucl Med. 1995;36:1994–2000. [PubMed] [Google Scholar]
- 60.Udelson JE, Coleman PS, Metherall JA, et al. Predicting recovery of severe regional ventricular dysfunction: comparison of resting scintigraphy with 201Tl and 99mTc-sestamibi. Circulation. 1994;89:2552–2561. doi: 10.1161/01.cir.89.6.2552. [DOI] [PubMed] [Google Scholar]
- 61.Levine MG, McGill CC, Ahlberg AW, et al. Functional assessment with electrocardiographic gated single-photon emission computed tomography improves the ability of technetium-99m sestamibi myocardial perfusion imaging to predict myocardial viability in patients undergoing revascularization. Am J Cardiol. 1999;83:1–5. doi: 10.1016/s0002-9149(98)00772-3. [DOI] [PubMed] [Google Scholar]
- 62.Srinivasan G, Kitsiou AN, Bacharach SL, Bartlett ML, Miller-Davis C, Dilsizian V. 18F-fluorodeoxyglucose Single Photon Emission Computed Tomography: Can it replace PET and thallium SPECT for the assessment of myocardial viability? Circulation. 1998;97:843–50. doi: 10.1161/01.cir.97.9.843. [DOI] [PubMed] [Google Scholar]
- 63.Tillisch JH, Brunken R, Marshall R, et al. Reversibility of cardiac wall-motion abnormalities predicted by positron tomography. N Engl J Med. 1986;314:884–888. doi: 10.1056/NEJM198604033141405. [DOI] [PubMed] [Google Scholar]
- 64.Tamaki N, Yonekura Y, Yamashita K, et al. Positron emission tomography using fluorine-18 deoxyglucose in evaluation of coronary artery bypass grafting. Am J Cardiol. 1989;64:860–865. doi: 10.1016/0002-9149(89)90832-1. [DOI] [PubMed] [Google Scholar]
- 65.Luciganani G, Paolini G, Landoni C, et al. Presurgical identification of hibernating myocardium by combined use of technetium-99m hexakis 2-methoxyisobutylisonitrile single photon emission tomography and fluorine-18-fluoro-2-deoxy-D-glucose positron emission tomography in patients with coronary artery disease. Eur J Nucl Med. 1992;19:874–881. doi: 10.1007/BF00168164. [DOI] [PubMed] [Google Scholar]
- 66.Nienaber CA, Brunken RC, Sherman CT, et al. Metabolic and functional recovery of ischemic human myocardium after coronary angioplasty. J Am Coll Cardiol. 1991;18:966–978. doi: 10.1016/0735-1097(91)90755-x. [DOI] [PubMed] [Google Scholar]
- 67.Marwick TH, MacIntyre WJ, LaFont A, Nemec JJ, Salcedo EE. Metabolic responses of hibernating and infarcted myocardium to revascularization: a follow-up study of regional perfusion, function, and metabolism. Circulation. 1992;85:1347–1353. doi: 10.1161/01.cir.85.4.1347. [DOI] [PubMed] [Google Scholar]
- 68.vom Dahl J, Eitzman DT, Al-Aouar ZR, et al. Relation of regional function, perfusion, and metabolism in patients with advanced coronary artery disease undergoing surgical revascularization. Circulation. 1994;90:2356–2366. doi: 10.1161/01.cir.90.5.2356. [DOI] [PubMed] [Google Scholar]
- 69.Flameng WJ, Shivalkar B, Spiessens B, et al. PET scan predicts functional recovery of left ventricular function after coronary artery bypass operation. Ann Thorac Surg. 1997;64:1694–701. doi: 10.1016/s0003-4975(97)00919-3. [DOI] [PubMed] [Google Scholar]
- 70.Gerber BL, Ordoubadi FF, Wijns W, et al. Positron emission tomography using (18)F-fluoro-deoxyglucose and euglycaemic hyperinsulinaemic glucose clamp: optimal criteria for the prediction of recovery of post-ischaemic left ventricular dysfunction. Results from the European Community Concerted Action Multicenter study on use of (18)F-fluoro-deoxyglucose Positron Emission Tomography for the Detection of Myocardial Viability. Eur Heart J. 2001;22(18):1691–701. doi: 10.1053/euhj.2000.2585. [DOI] [PubMed] [Google Scholar]
- 71.Eitzman D, Al-Aouar Z, Kanter H, et al. Clinical outcome of patients with advanced coronary artery disease after viability studies with positron emission tomography. J Am Coll Cardiol. 1992;20:559–565. doi: 10.1016/0735-1097(92)90008-b. [DOI] [PubMed] [Google Scholar]
- 72.Di Carli MF, Davidson M, Little R, et al. Value of metabolic imaging with positron emission tomography for evaluating prognosis in patients with coronary artery disease and left ventricular dysfunction. Am J Cardiol. 1994;73:527–533. doi: 10.1016/0002-9149(94)90327-1. [DOI] [PubMed] [Google Scholar]
- 73.Di Carli MF, Asgarzadie F, Schelbert HR, et al. Quantitative relation between myocardial viability and improvement in heart failure symptoms after revascularization in patients with ischemic cardiomyopathy. Circulation. 1995;92:3436–3444. doi: 10.1161/01.cir.92.12.3436. [DOI] [PubMed] [Google Scholar]
- 74.Kawai Y, Tsukamoto E, Nozaki Y, et al. Significance of reduced uptake of iodinated fatty acidanalogues for the evaluation of patients with acute chest pain. J Am Coll Cardiol. 2001;38:1888. doi: 10.1016/s0735-1097(01)01634-5. [DOI] [PubMed] [Google Scholar]
- 75.Dilsizian V, Bateman TM, Bergmann SR, et al. Metabolic imaging with β-methyl-ρ-[123I]-iodophenyl-pentadecanoic acid (BMIPP) identifies ischemic memory following demand ischemia. Circulation. 2005;112(14):2169–2174. doi: 10.1161/CIRCULATIONAHA.104.530428. [DOI] [PubMed] [Google Scholar]
- 76.Pasquet A, Lauer MS, Williams MJ, Secknus M-A, Lytle B, Marwick TH. Prediction of global left ventricular function after bypass surgery in patients with severe left ventricular dysfunction. Impact of pre-operative myocardial function, perfusion, and metabolism. Eur Heart J. 2000;21:125–36. doi: 10.1053/euhj.1999.1663. [DOI] [PubMed] [Google Scholar]
- 77.Hoffmann R, Altiok E, Nowak B, et al. Strain rate measurement by doppler echocardiography allows improved assessment of myocardial viability inpatients with depressed left ventricular function. J Am Coll Cardiol. 2002;39:443–9. doi: 10.1016/s0735-1097(01)01763-6. [DOI] [PubMed] [Google Scholar]
- 78.Hanekom L, Jenkins C, Jeffries L, et al. Incremental value of strain rate analysis as an adjunct to wall-motion scoring for assessment of myocardial viability by dobutamine echocardiography: a follow-up study after revascularization. Circulation. 2005;112:3892–900. doi: 10.1161/CIRCULATIONAHA.104.489310. [DOI] [PubMed] [Google Scholar]
- 79.Geskin G, Kramer CM, Rogers WJ, et al. Quantitative assessment of myocardial viability after infarction by dobutamine magnetic resonance tagging. Circulation. 1998;98:217–23. doi: 10.1161/01.cir.98.3.217. [DOI] [PubMed] [Google Scholar]
- 80.Samady H, Choi CJ, Ragosta M, Powers ER, Beller GA, Kramer CM. Electromechanical mapping identifies improvement in function and retention of contractile reserve after revascularization in ischemic cardiomyopathy. Circulation. 2004;110:2410–6. doi: 10.1161/01.CIR.0000145119.94542.AE. [DOI] [PubMed] [Google Scholar]
- 81.Wellnhofer E, Olariu A, Klein C, et al. Magnetic resonance low-dose dobutamine test is superior to scar quantification for the prediction of functional recovery. Circulation. 2004;109:2172–4. doi: 10.1161/01.CIR.0000128862.34201.74. [DOI] [PubMed] [Google Scholar]
- 82.Kim RJ, Manning WJ. Viability assessment by delayed enhancement cardiovascular magnetic resonance: will low-dose dobutamine dull the shine? Circulation. 2004;109:2476–9. doi: 10.1161/01.CIR.0000130730.63776.69. [DOI] [PubMed] [Google Scholar]
- 83.Bove CM, DiMaria JM, Voros S, Conaway MR, Kramer CM. Dobutamine Response and Myocardial Infarct Transmurality: Functional Improvement after Coronary Artery Bypass Grafting--Initial Experience. Radiology. 2006;240:835–41. doi: 10.1148/radiol.2403051150. [DOI] [PubMed] [Google Scholar]
- 84.Schinkel AFL, Bax JJ, Poldermane D, Elhendy A, Ferrari R, Rahimtoola SH. Hibernating myocardium: Diagnosis and patient outcomes. Curr Prob Cardiol. 2007;32:361–410. doi: 10.1016/j.cpcardiol.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 85.Bonow RO. Comments. Curr Probl Cardiol. 2007;32:381. [Google Scholar]
- 86.Dilsizian V. Cardiac magnetic resonance versus SPECT: Are all non-infarct myocardial regions created equal? J Nucl Card. 2007;14:9–14. doi: 10.1016/j.nuclcard.2006.12.143. [DOI] [PubMed] [Google Scholar]


