Abstract
The innate immune system is primarily responsible for prevention of infection of the skin by pathogens, but is also important in control of inflammation. The components of innate immunity are frequently misunderstood based on a historical bias for leukocyte-mediated immune defense. Many participating cell types are often overlooked, in particular epithelial cells that provide an early and critical step to innate immune defense. This review will discuss our epithelial barrier to infection with emphasis on how microbes subvert this system, and human diseases associated with these events.
Introduction
The continuing emergence of antibiotic resistance in human pathogenic microorganisms, and the widespread morbidity and mortality associated with infectious disease, highlight the importance of understanding the barriers to microbial invasion. Our planet is estimated to have in excess of 1 ×108 different microbial species that inhabit every conceivable environmental nitch [1]. Despite this extreme diversity, no more than 1,200 microbial species have ever been described as contributing to infections in humans. This low rate of virulence from a large and diverse microbiome demonstrates the near perfection of our immune barrier.
Innate immunity is often defined as a rapid, first line defense system providing protection against infection. This system functions without prior exposure to the microbe. However, innate defense is often misinterpreted as a process that exists independently of the “adaptive” immune protection system; a process dependant on antigen presentation and clonal leukocyte amplification. These systems are not distinct. Abundant evidence supports a close interplay between the early microbial defense process and the secondary response that occurs as an adaptation to microbial exposures. Each process influences the other. A modern definition of innate immunity recognizes its role as a director of adaptive immune responses and its responsiveness to an environment that is subsequently changed by the development of adaptive immunity. Therefore, the innate immune response should be thought of as consisting of five elements that include both physical and chemical constitutive protection and the response process once the basic barrier is breached (Table 1).
Table 1.
The Five Elements of Innate Immune Defense
| 1) Physical barrier to microbial entry and physical danger |
| 2) Constitutive chemical shield to inhibit microbial growth and invasion |
| 3) Recognition system to identify the entry of foreign microbes |
| 4) Inducible antimicrobial response triggered by the recognition system |
| 5) Cellular recruitment process to amplify and enhance defense |
It is implicit that the innate immune system must begin with epithelia since these cell layers are positioned at the interface between the host and external environment. Understanding innate immune defense from this perspective offers the opportunity to rethink strategies for improving microbial defense and anti-infective therapy.
Microbial recognition and response
Our understanding of the molecular elements of microbial recognition and responses has advanced rapidly. There is currently direct evidence for a wide variety of extracellular, cell membrane, endosomal and cytoplasmic molecules whose responsibility lies in recognition of molecules produced by microbes. This group of molecules is sometimes referred to as pathogen-associated molecular patterns or PAMPs. This term is somewhat of a misnomer as host recognition elements responsible for detection of PAMPs can also detect molecules produced by nonpathogenic microorganisms or released by the host itself [2]. Nevertheless, the concept of PAMPs has been essential in furthering our understanding of innate immune defense systems. The traditional understanding of microbial recognition is that binding of a PAMP to a cognate pattern recognition receptor (PRR) starts a downstream signaling cascade leading to activation of an antimicrobial response network involving inflammatory cytokines, interferons and direct antimicrobial elements [3] [4] . More recent progress in understanding these signaling networks has shown that cell-specific expression of distinct groups of recognition elements dictates the pattern of response. Furthermore, the interaction or “cross-talk” of these recognition systems can lead to suppression of inflammation instead of activation [5]. Currently, this field is of great therapeutic interest as pharmacologic manipulation of the microbial recognition system offers an opportunity to either augment or suppress the immune defense.
Unexpected associations have emerged between systems that can control microbial recognition. For example, several recent studies have demonstrated that Vitamin D influences the expression and function of microbial recognition elements such as Toll Like Receptor-2 (TLR2) [6,7]. Furthermore, the innate antimicrobial recognition system provides an excellent example of the interplay between primary innate antimicrobial responses and adaptive responses dependent on antigen presentation. For example, the capacity of antigen presenting cells to function and instruct T-cell development is strongly influenced by the TLRs [8]. A full discussion of the many diverse functions of PRRs is beyond the scope of this brief review. However, it is important to acknowledge that the innate recognition system for microbes or injury is the initial signal for triggering a broader antimicrobial response and instructs host regulatory pathways for either increasing or decreasing inflammation. This microbial recognition system acts both on a constitutive level and when there has been a failure in physical and chemical defense systems. In the latter case, the innate antimicrobial response system is activated.
Antimicrobial Peptides (AMPs)
There are several mechanisms for direct antimicrobial response that include production of reactive oxygen species, change in pH, production of lipids, and the release of a wide range of antimicrobial proteins. Peptides with the capacity to directly kill or inhibit the growth of microbes are collectively known as Antimicrobial Peptides (AMPs) [9]. Since the AMPs represent an ideal example of how an innate barrier system incorporates both direct antimicrobial actions and indirect effects to modify the physical barrier and control the inflammatory response, the remainder of this review will focus on these molecules.
AMPs are a primary system for protection against infection, exhibit broad-spectrum activity against bacteria, fungi and viruses, and are evolutionarily ancient. In fact, it is thought that all life forms produce AMPs, such that even simple single cell organisms can gain a protective advantage in their environment. In human tissues such as skin or gut, the expression of AMPs can occur as part of the constitutive innate immune barrier, or can be increased when triggered by PRRs in response to injury or infection [10] [11]. AMP gene families in humans include the defensins and cathelicidins, first discovered in neutrophils and epithelia for their antimicrobial properties [12], and many other peptides and proteins originally known for activity as chemokines, enzymes, enzyme inhibitors and neuropeptides. Thus, the broad definition of an AMP encompasses a large and diverse group of proteins.
Although the sequences of AMPs are variable, these peptides are often cationic and 20 to 60 amino acids in length. Although significant structural variation exists between classes, AMPs typically assemble into final structures that are amphipathic and thus have hydrophobic and hydrophilic surfaces. This property enables them to interact in both the aqueous environment and within lipid-rich target membranes. The molecular mechanisms responsible for microbial killing depend upon the charge and membrane-binding characteristics of the individual peptides, and a variety of models have been proposed to explain how specific AMPs disrupt membranes [13]. Depending on the AMP class, the peptide may assemble to form a true pore, penetrate and disrupt the membrane, or integrate and disorganize the membrane. In all cases, the toxicity of the peptide depends on both AMP and the specific composition of the target membrane. In this way, an AMP can demonstrate selectivity, disrupting target cells without necessarily harming the cell that produced it.
More recent studies of AMPs such as cathelicidins and β-defensins have shown that they not only kill microbes but also critically influence host cell functions. Therefore, the term AMP is somewhat incomplete, and many of the peptides in this group might be better called “alarmins” to recognize their capacity to alert host cells to the potential for infection or the presence of injury [14] . Several alternative models have emerged to explain how these peptides can elicit host cell responses (Figure 1). For example, human cathelicidin peptide LL-37 has been implicated as both a selective activator of the cell surface receptor FPRL1 [15], and as an indirect modifier of the EGR receptor [16]and of TLR-4 [17]. These interactions, combined with the direct antimicrobial action of an AMP, make LL-37 a powerful early regulator of the microbial response within the epithelium.
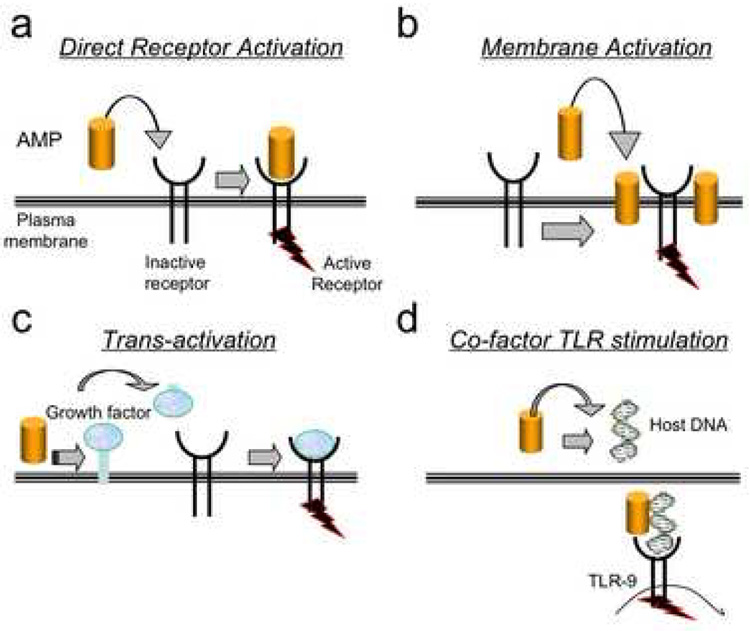
Figure 1. Molecular models for cell activation by antimicrobial peptides.
a. Direct receptor activation model predicts the host defense peptide interacts with the receptor and induces a change in conformation and subsequent downstream signaling. B. The trans-activation response reflects an indirect activation of the signaling receptor. This can occur by release of a membrane bound growth factor that subsequently binds to its specific receptor and activates it. C. Antimicrobial peptides integrate within plasma membranes. In this model the presence of the peptide in the membrane surrounding the receptor leads to a change in activity of this receptor. This can be an activation or inactivation event. D. Antimicrobial peptides can bind DNA. This model suggests the association of the antimicrobial peptide LL-37 with host DNA results in a complex that can activate TLR-9 to stimulate interferon release. All models may co-exist and reflect specific cell type responses. Cell activation by antimicrobial peptides normally leads to increased protection against infection and wound repair. However, in situations of abnormal expression these events can lead to inflammatory disease.
Evolution of Microbial Immunity to AMPs
For any bacterial species whose ecology includes colonization of humans, evolutionary selective pressure is exerted through perpetual exposure to our AMPs. The relative sensitivity or resistance of a given bacterial species to AMPs and other front line effectors of innate immunity essentially dictates its virulence potential, since the spectrum of human infectious disease can be viewed as those disorders arising from failures of innate immunity. While transient or fixed host immune susceptibility states (e.g. AIDS, chemotherapy, surgical wounds) contribute greatly to this dynamic, it is clear that enhanced resistance to AMP killing is a hallmark feature of several invasive human pathogens. For example, Salmonella spp. are characteristically resistant to cationic AMPs such as defensins and cathelicidins, and in turn frequently associated with systemic dissemination; conversely, strains of the closely genetically-related Escherichiae coli are generally sensitive to AMPs and are more likely associated with mucosal infections and toxin-mediated disease effects [18].
The importance of AMPs in mammalian innate defense to bacterial infection has been clearly established through experimental manipulation of mice. For example, the knockout mouse lacking cathelicidin is more susceptible to bacterial infection of the skin [19], conjuctivae [20], gastrointestinal tract [21], urinary tract [22], and bloodstream [23]. Conversely, enhanced resistance to bacterial infection is provided by augmenting cathelicidin levels by transgenics [24], viral gene therapy [25], or pharmacologic administration [26]. Consequently, loss of virulence in mouse infection models has allowed corroboration of candidate bacterial AMP resistance factors identified by altered susceptibility during in vitro testing. These studies have revealed a surprisingly diverse of strategies deployed by leading human bacterial pathogens to resist the action of AMPs.
One path to resistance shared by several human bacterial pathogens involves introducing chemical modifications to normally anionic constituents of their cell surfaces, thereby increasing net positive charge to repulse rather than attract cationic AMPs. Additional pathogenic species have evolved membrane pumps for active efflux of AMPs. Bacteria can also secrete factors that inactivate AMPs through direct binding or proteolytic degradation. Finally, certain pathogens take one step further and blunt innate defense by directly downregulating host cell expression of AMPs. The emergence of bacterial resistance is controlled by transcriptional regulatory networks induced upon sensing of the AMP by the pathogen [27,28]. Certain bacterial species express multiple AMP resistance mechanisms, which contribute synergistically to impair host innate immune clearance – this concept will be illustrated in the next two sections for Staphylococcus aureus (SA) and group A Streptococcus (GAS), invasive pathogens that represent the two leading agents of human skin and soft tissue infections. A schematic illustration of mechanisms deployed by these preeminent human pathogens to avoid innate immune defense is shown in Figure 2.
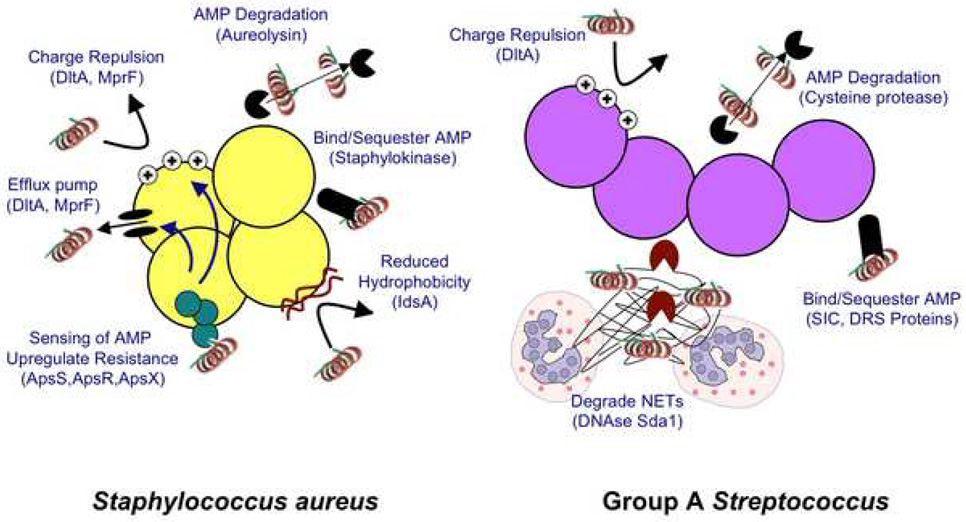
Figure 2. Mechanisms for microbial resistance to antimicrobial peptides.
Microbes have evolved a wide range of strategies to resist killing by antimicrobial peptides. Known resistance mechanisms for Staphylococcus aureus and Group A Streptococcus are illustrated here. Major systems include degradation of the peptide by proteases, inactivating the peptide by binding and sequestration, active transport of the peptide away from the cell, alteration of membrane sensitivity by decreasing the capacity of the peptide to bind bacterial membrane. These strategies can lead to increased disease as a consequence of enhanced virulence.
Infections due to microbial resistance to the innate immune response
Staphylococcus aureus
SA is a prominent cause of wound infections, cellulitis, abscesses, osteomyelitis, septic arthritis, endocarditis and septicemia, and exhibits significantly higher minimum inhibitory concentrations to human AMPs than observed in related organisms [29]. The best appreciated mechanisms of SA resistance to AMPs center on modifications of teichoic acid in its cell wall. Generally, bacterial teichoic acids are polyanionic because of abundant phosphate groups in their repeating structure, helping to attract host cationic AMPs. However, the gene products of the dltABCD operon incorporate D-alanine into the SA teichoic acid through an ester bond that instead leaves the positively changed amino group exposed [30]. SA with dlt operon mutations have increased cell-surface negative charge and are more sensitive to killing by human α-defensins and cathelicidin as well as variety of other cationic AMPs [31]. Similarly, positive charge modification of SA membrane phosphotidylglycerol with L-lysine through the action of the mprF gene is shown to enhance SA resistance to cationic AMPs [32].
Certain SA strains harbor a multiresistance plasmid pSK1 that encodes the QacA efflux pump. SA positive for QuacA may exhibit higher levels of resistance to a cationic AMPs, as demonstrated experimentally for the platelet-derived AMP, tPMP [33]. The metalloprotease aureolysin is released by SA and can degrade human cathelicidin LL-37 in a dose and time-dependent manner [34]; strains producing lower levels of aureolysin were found to be more susceptible to cathelidicin killing. A proteolytic activty released by SA also inactivates lactoferricin B, a cationic AMP derived from the N-terminus of mammalian lactoferrin [35].
The SA exoprotein staphylokinase (SK) is well known for its ability to activate host plasminogen. It is now appreciated that SK is independently able to directly bind α-defensins produced by human neutrophils, inhibiting their bactericidal activity [36]. Testing of a panel of SA strains found that those producing SK were resistant to α-defensins, and that addition of purified SK to SK-negative SA cultures rescued them from α-defensin killing [36]. Interestingly, SA upregulates cathelicidin expression during infection, and the binding of cathelicidin to SK augments the ability of this virulence factor to activate plasminogen, promote fibrinolysis, and allow bacterial dissemination [37].
The SA surface anchored IsdA protein, first studied in the context of iron acquisition, is now also known to reduce the overall hydrophobicity of the bacterium, thereby blocking the action of AMPs including cathelicidins and defensins, as well as the antibacterial properties of fatty acids present in human serum [38]. IsdA is upregulated by SA in vivo and in response to encountering neutrophils and their release of effector molecules such as oxidants and AMPs through the respiratory burst and degranulation. Global regulation of AMP defense mechanisms in SA is provided by the three-component sensing system, ApsS, ApsR and ApsX [39]. Thus SA has evolved to avoid the metabolic expenditure associated with enhanced AMP defense until presented with the selective pressure in vivo.
Group A Streptococcus
GAS is also a leading bacterial pathogen of humans, producing a wide range of diseases from simple mucosal infections such as pharyngitis and impetigo to life-threatening invasive conditions such as necrotizing fasciitis and toxic-shock syndrome. The placement of AMP defense as a critical determining factor in the outcome of GAS disease has been well illustrated by genetic studies in the mouse model. Elimination of the gene Cnlp encoding the sole murine cathelicidin mCRAMP rendered the knockout mice mice highly susceptible to necrotizing skin infection produced by GAS [19]; conversely, a GAS mutant in transcriptional regulator crgR increased cathelicidin resistance and virulence of GAS in normal mice [40]. Consistent with a front line role of AMPs in GAS defense, keratinocyte-specific expression of porcine cathelicidin in transgenic mice restricted GAS disease progression in the skin infection model [24].
One specific mechanism contributing to GAS AMP resistance is shared with SA – GAS possesses a dltABCD operon that serves to incorporate positively charge residues into its cell wall lipoteichoic acid, leading to electrostatic repulsion of AMPs, thus promoting resistance to cathelicidins and to neutrophil killing [41]. GAS also produces a broad spectrum cysteine protease, SpeB, and the activity of SpeB GAS supernatants has been shown to degrade human cathelicidin LL-37 [42]. Through a complex interaction, secreted SpeB is trapped on the bacterial surface by host α2-macroglobulin that is bound by the cell-wall anchored GAS protein GRAB; the retained SpeB is capable of cleaving and inactivating LL-37 and protecting the bacteria against its antimicrobial action [43]. A surface-anchored protein known as LSA, representing the largest ORF in the GAS genome, also affords the pathogen a level protection from cathelicidin AMP action through an as yet undetermined mechanism [44].
M1 serotype strains of GAS, commonly associated with invasive infections including necrotizing fasciitis, release a small peptide known as SIC that binds and inhibits the activity of human cathelicidins, α- and β-defensins, and lysozyme [45]. Recently it has been shown distantly related small peptide known as DRS is produced by M12 GAS strains, another common serotype associated with invasive infections, and this peptide like SIC can function to inactive host b-defensins [46].
Finally, the recent discovery and appreciation of the function of neutrophil extracellular traps (NETs) in pathogen killing has opened up a new avenue for exploring cationic AMP function in innate defense. NETs consist of released chromatin and granule contents that together form a fibrous network that bind bacteria and allow killing through the action of proteases and AMPs [47]. GAS may escape from NETs by expression of the potent DNAse Sda1 which degrades the chromatin fibers, allowing the bacteria to avoid local entrapment [48]. The acquisition of the bacteriophage encoding Sda1 appears to be a sentinel event in the evolution of the globally disseminated M1 clone that is the leading cause of invasive GAS infections , as it offers selection pressure for a genetic and phenotypic shift leading to upregulation of numerous virulence phenotypes, including the AMP resistance peptide SIC [49].
Diseases due to inherent dysfunction of innate immunity
There is increasing evidence that a large number of human diseases are associated with defects in the innate immune defense system. These diseases may arise from abnormalities of excess or deficit, resulting in unchecked inflammation in auto-immune disorders or blunted immunity and predisposition to infectious diseases. Many of these diseases are a consequence of mutations in PRRs or their signaling elements [50]. In addition, abnormalities in expression or processing of AMPs are beginning to be associated with a range of human skin diseases. Here, like the situation with PRRs, abnormalities in AMPs can lead to either increased inflammation or increased infection.
Atopic dermatitis and infections due to a failure of host innate defense
Problems in AMP expression can lead to disease characterized by an increased susceptibility to infection. An excellent example of this is the disease atopic dermatitis (AD). Innate immunity plays an important role in AD. AD patients are particularly susceptible to recurrent skin infections, especially with SA [51]. Altered skin barrier function may partially explain SA colonization in AD, and a high percentage of these patients have mutations in filagrin [52], an important structural protein. However, considering that skin barrier defects also exist in psoriasis patients, who are by comparison more resistant to skin infection, a different explanation for microbial susceptibility of the AD patients was necessary. The explanation came with the discovery that AD skin has very low expression of multiple AMPs including cathelicidins and β-defensins [53]. This suppression of normal AMP expression is partially explained by an inhibitory effect of Th2 cytokines such as IL-4 and IL-13 that suppress β-defensin expression[54].
AMPs may also offer new insight into understanding viral skin infections. Correlations between the cutaneous proliferation of vaccinia virus with the lower expression of cathelicidin has been seen in mice [55] and this observation also supports the susceptibility of AD patients to eczema vaccinatum. This serious disorder underlies the contraindication for use of vaccinia in AD patients as immunization against smallpox. Induction of epidermal AMPs has also been shown during the development of verruca vulgaris and condyloma accuminatum [56], and these AMPs can act against HPV infection [57].
An association has emerged recently between the action of Vitamin D and resistance to infection. The expression of several important recognition and response elements are induced by the active form of Vitamin D; 1,25 OHD3. PRRs such as TLR2 and CD14, together with the AMP cathelicidin, are all increased by 1,25D3 . Upon injury of normal skin the enzyme responsible for 1-hydroxylation of 25D3 is induced and this induction leads to a local increase in 1,25 D3 [6]. The consequences of this system in human disease is still unfolding, but intriguing correlations between vitamin D nutritional status and inflammatory diseases and cancer are unfolding [58]. An association has been reported between tuberculosis and relative vitamin D deficiency, perhaps explained by the capacity of vitamin D to increase AMPs, which may lead to novel prevention strategies for this important infection [7].
Rosacea, Psoriasis and AMPs in inflammation
Recent evidence suggests that an excess in AMPs can exacerbate inflammatory responses. The skin disease rosacea is characterized by excessive inflammation, and blood vessel dilatation and proliferation in the face. Patients with rosacea were found to have an increase in the production of the cathelicidin precursor protein hCAP18. By itself this is not detrimental to the host, since hCAP18 is biologically inactive. Unfortunately, individuals with rosacea also have increased activity of the serine proteases (Kallkreins 5 and 7) responsible for processing hCAP18 [59]. This combination results in a shift in the composition of AMPs normally found on the surface of the skin and abnormal accumulation of LL-37. Support for an etiologic role this pathway in disease pathogenesis come from observations that administration of LL-37 to mice can directly stimulate an epidermal inflammatory and angiogenic response characteristic of the disease in humans [60].
The cathelicidin LL-37 is also elevated in several other human inflammatory disorders including psoriasis, lupus erythematosus, contact dermatitis [61], and erythema toxicum neonatorum [62]. In psoriasis, it has been recently proposed that the presence of LL-37 augments type-1 interferon release from plasmacytoid dendritic cells [63]. This response may occur though a mechanism such as illustrated in Figure 1 where the AMP combines with host DNA to trigger TLR9 activation.
Therapeutic implications and conclusions
The recent appreciation of innate immune barriers to infection offers new directions for treatment of infectious and inflammatory diseases. Several attempts have been made for development of AMPs as therapeutics. Although effective, the economical hurdle of cost of production of a peptide as an antibiotic has hindered progress. One solution to this problem is the recent development of alternative molecules that mimic AMP function but are more stable and less expensive to produce than peptides. Another approach is the development of compounds that induce an increase in AMP production. Vitamin D and its analogs are one example of this, and their application to human skin or cells in culture increases their capacity to kill pathogens such as S. aureus and Group A Streptococcus [6]. Another novel therapeutic approach involves targeting the transcriptional regulator hypoxia-inducible factor-1α (HIF-1α). HIF-1α is another factor that supports the production of cathelicidin AMPs in neutrophils and keratinocytes. Genetic and/or pharmacologic augmentation of HIF-1α upregulates cathelicidin transcript and protein production, enhancing the bactericidal capacity of host cells, and helping to restrict the progression of SA infection in a skin abscess [64]. Conversely, diseases that occur as a consequence of excessive innate immune response may be treated by inhibitors of these events. In the case of rosacea, this process is already in practice as a common therapy for this disease, administration of tetracycline based antibiotics, also reduces protease activity in the skin and tempers the pathogenic process.
Overall, much has been learned in the past few years regarding the innate barrier to microbial disease. Unexpected associations have emerged between processes long thought to be independent. The immune defense strategy is seen today as an integrated system that first depends on an efficient barrier to infection and a rapid response when this barrier is broken. Leukocyte recruitment, once considered the mainstay of the immune response, is an important but secondary event in the struggle against infection. This new insight into the function of our immune barrier offers promising new therapeutic alternatives.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shouche YS, Patole MS. Microbial diversity: No limits? Current Science. 2005;88(9):1370–1371. [Google Scholar]
- 2.Taylor KR, et al. Recognition of hyaluronan released in sterile injury involves a unique receptor complex dependent on TLR4, CD44 and MD-2. J Biol Chem. 2007 doi: 10.1074/jbc.M606352200. [DOI] [PubMed] [Google Scholar]
- 3.Takeuchi O, Akira S. Signaling pathways activated by microorganisms. Curr Opin Cell Biol. 2007;19(2):185–191. doi: 10.1016/j.ceb.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Meylan E, et al. Intracellular pattern recognition receptors in the host response. Nature. 2006;442(7098):39–44. doi: 10.1038/nature04946. [DOI] [PubMed] [Google Scholar]
- 5.Hu X, et al. Crosstalk among Jak-STAT, Toll-like receptor, and ITAM-dependent pathways in macrophage activation. J Leukoc Biol. 2007;82(2):237–243. doi: 10.1189/jlb.1206763. [DOI] [PubMed] [Google Scholar]
- 6.Schauber J, et al. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J Clin Invest. 2007;117(3):803–811. doi: 10.1172/JCI30142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu PT, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 8.Weck MM, et al. TLR ligands differentially affect uptake and presentation of cellular antigens. Blood. 2007;109(9):3890–3894. doi: 10.1182/blood-2006-04-015719. [DOI] [PubMed] [Google Scholar]
- 9.Radek K, Gallo R. Antimicrobial peptides: natural effectors of the innate immune system. Semin Immunopathol. 2007;29(1):27–43. doi: 10.1007/s00281-007-0064-5. [DOI] [PubMed] [Google Scholar]
- 10.Dorschner RA, et al. Cutaneous injury induces the release of cathelicidin antimicrobial peptides active against group A Streptococcus. J Invest Dermatol. 2001;117(1):91–97. doi: 10.1046/j.1523-1747.2001.01340.x. [DOI] [PubMed] [Google Scholar]
- 11.Schauber J, et al. Control of the innate epithelial antimicrobial response is cell-type specific and dependent on relevant microenvironmental stimuli. Immunology. 2006;118(4):509–519. doi: 10.1111/j.1365-2567.2006.02399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Selsted ME, et al. Purification and antibacterial activity of antimicrobial peptides of rabbit granulocytes. Infec. Immun. 1984;45:150–154. doi: 10.1128/iai.45.1.150-154.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henzler Wildman KA, et al. Mechanism of lipid bilayer disruption by the human antimicrobial peptide, LL-37. Biochemistry. 2003;42(21):6545–6558. doi: 10.1021/bi0273563. [DOI] [PubMed] [Google Scholar]
- 14.Oppenheim JJ, Yang D. Alarmins: chemotactic activators of immune responses. Curr Opin Immunol. 2005;17(4):359–365. doi: 10.1016/j.coi.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 15.De Y, et al. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000;192(7):1069–1074. doi: 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tjabringa GS, et al. The antimicrobial peptide LL-37 activates innate immunity at the airway epithelial surface by transactivation of the epidermal growth factor receptor. J Immunol. 2003;171(12):6690–6696. doi: 10.4049/jimmunol.171.12.6690. [DOI] [PubMed] [Google Scholar]
- 17.Di Nardo A, et al. Cathelicidin antimicrobial peptides block dendritic cell TLR4 activation and allergic contact sensitization. J Immunol. 2007;178(3):1829–1834. doi: 10.4049/jimmunol.178.3.1829. [DOI] [PubMed] [Google Scholar]
- 18.Eckmann L. Defence molecules in intestinal innate immunity against bacterial infections. Curr Opin Gastroenterol. 2005;21(2):147–151. doi: 10.1097/01.mog.0000153311.97832.8c. [DOI] [PubMed] [Google Scholar]
- 19.Nizet V, et al. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414(6862):454–457. doi: 10.1038/35106587. [DOI] [PubMed] [Google Scholar]
- 20.Huang LC, et al. Cathelicidin-deficient (Cnlp −/−) mice show increased susceptibility to Pseudomonas aeruginosa keratitis. Invest Ophthalmol Vis Sci. 2007;48(10):4498–4508. doi: 10.1167/iovs.07-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iimura M, et al. Cathelicidin mediates innate intestinal defense against colonization with epithelial adherent bacterial pathogens. J Immunol. 2005;174(8):4901–4907. doi: 10.4049/jimmunol.174.8.4901. [DOI] [PubMed] [Google Scholar]
- 22.Chromek M, et al. The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nat Med. 2006;12(6):636–641. doi: 10.1038/nm1407. [DOI] [PubMed] [Google Scholar]
- 23.Bergman P, et al. Induction of the Antimicrobial Peptide CRAMP in the Blood Brain Barrier and Meninges after Meningococcal Infection. Infect Immun. 2006 doi: 10.1128/IAI.01043-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee PH, et al. From The Cover: Expression of an additional cathelicidin antimicrobial peptide protects against bacterial skin infection. Proc Natl Acad Sci U S A. 2005;102(10):3750–3755. doi: 10.1073/pnas.0500268102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bals R, et al. Transfer of a cathelicidin peptide antibiotic gene restores bacterial killing in a cystic fibrosis xenograft model. J Clin Invest. 1999;103(8):1113–1117. doi: 10.1172/JCI6570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giacometti A, et al. The antimicrobial peptide BMAP-28 reduces lethality in mouse models of staphylococcal sepsis. Crit Care Med. 2004;32(12):2485–2490. doi: 10.1097/01.ccm.0000148221.09704.22. [DOI] [PubMed] [Google Scholar]
- 27.McPhee JB, et al. Cationic antimicrobial peptides activate a two-component regulatory system, PmrA-PmrB, that regulates resistance to polymyxin B and cationic antimicrobial peptides in Pseudomonas aeruginosa. Mol Microbiol. 2003;50(1):205–217. doi: 10.1046/j.1365-2958.2003.03673.x. [DOI] [PubMed] [Google Scholar]
- 28.Bader MW, et al. Recognition of antimicrobial peptides by a bacterial sensor kinase. Cell. 2005;122(3):461–472. doi: 10.1016/j.cell.2005.05.030. [DOI] [PubMed] [Google Scholar]
- 29.Peschel A, et al. Staphylococcus aureus resistance to human defensins and evasion of neutrophil killing via the novel virulence factor MprF is based on modification of membrane lipids with l-lysine. J Exp Med. 2001;193(9):1067–1076. doi: 10.1084/jem.193.9.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peschel A, et al. Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J Biol Chem. 1999;274(13):8405–8410. doi: 10.1074/jbc.274.13.8405. [DOI] [PubMed] [Google Scholar]
- 31.Kristian SA, et al. MprF-mediated lysinylation of phospholipids in Staphylococcus aureus leads to protection against oxygen-independent neutrophil killing. Infect Immun. 2003;71(1):546–549. doi: 10.1128/IAI.71.1.546-549.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kristian SA, et al. Alanylation of teichoic acids protects Staphylococcus aureus against Toll-like receptor 2-dependent host defense in a mouse tissue cage infection model. J Infect Dis. 2003;188(3):414–423. doi: 10.1086/376533. [DOI] [PubMed] [Google Scholar]
- 33.Kupferwasser LI, et al. Plasmid-mediated resistance to thrombin-induced platelet microbicidal protein in staphylococci: role of the qacA locus. Antimicrob Agents Chemother. 1999;43(10):2395–2399. doi: 10.1128/aac.43.10.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sieprawska-Lupa M, et al. Degradation of human antimicrobial peptide LL-37 by Staphylococcus aureus-derived proteinases. Antimicrob Agents Chemother. 2004;48(12):4673–4679. doi: 10.1128/AAC.48.12.4673-4679.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ulvatne H, et al. Lactoferricin B inhibits bacterial macromolecular synthesis in Escherichia coli and Bacillus subtilis. FEMS Microbiol Lett. 2004;237(2):377–384. doi: 10.1016/j.femsle.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 36.Jin T, et al. Staphylococcus aureus resists human defensins by production of staphylokinase, a novel bacterial evasion mechanism. J Immunol. 2004;172(2):1169–1176. doi: 10.4049/jimmunol.172.2.1169. [DOI] [PubMed] [Google Scholar]
- 37.Braff MH, et al. Staphylococcus aureus exploits cathelicidin antimicrobial peptides produced during early pneumonia to promote staphylokinase-dependent fibrinolysis. J Infect Dis. 2007;195(9):1365–1372. doi: 10.1086/513277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clarke SR, et al. The Staphylococcus aureus surface protein IsdA mediates resistance to innate defenses of human skin. Cell Host Microbe. 2007;1(3):199–212. doi: 10.1016/j.chom.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 39.Li M, et al. Gram-positive three-component antimicrobial peptide-sensing system. Proc Natl Acad Sci U S A. 2007;104(22):9469–9474. doi: 10.1073/pnas.0702159104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nizet V. Antimicrobial peptide resistance mechanisms of human bacterial pathogens. Curr Issues Mol Biol. 2006;8(1):11–26. [PubMed] [Google Scholar]
- 41.Kristian SA, et al. D-alanylation of teichoic acids promotes group a streptococcus antimicrobial peptide resistance, neutrophil survival, and epithelial cell invasion. J Bacteriol. 2005;187(19):6719–6725. doi: 10.1128/JB.187.19.6719-6725.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmidtchen A, et al. Proteinases of common pathogenic bacteria degrade and inactivate the antibacterial peptide LL-37. Mol Microbiol. 2002;46(1):157–168. doi: 10.1046/j.1365-2958.2002.03146.x. [DOI] [PubMed] [Google Scholar]
- 43.Nyberg P, et al. alpha2-Macroglobulin-proteinase complexes protect Streptococcus pyogenes from killing by the antimicrobial peptide LL-37. J Biol Chem. 2004;279(51):52820–52823. doi: 10.1074/jbc.C400485200. [DOI] [PubMed] [Google Scholar]
- 44.Kwinn LA, et al. Genetic characterization and virulence role of the RALP3/LSA locus upstream of the streptolysin s operon in invasive M1T1 Group A Streptococcus. J Bacteriol. 2007;189(4):1322–1329. doi: 10.1128/JB.01256-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fernie-King BA, et al. The interaction of streptococcal inhibitor of complement (SIC) and its proteolytic fragments with the human beta defensins. Immunology. 2004;111(4):444–452. doi: 10.1111/j.0019-2805.2004.01837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fernie-King BA, et al. Streptococcal DRS (distantly related to SIC) and SIC inhibit antimicrobial peptides, components of mucosal innate immunity: a comparison of their activities. Microbes Infect. 2007;9(3):300–307. doi: 10.1016/j.micinf.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 47.Brinkmann V, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 48.Buchanan JT, et al. DNase expression allows the pathogen group A Streptococcus to escape killing in neutrophil extracellular traps. Curr Biol. 2006;16(4):396–400. doi: 10.1016/j.cub.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 49.Walker MJ, et al. DNase Sda1 provides selection pressure for a switch to invasive group A streptococcal infection. Nat Med. 2007;13(8):981–985. doi: 10.1038/nm1612. [DOI] [PubMed] [Google Scholar]
- 50.Marodi L, Notarangelo LD. Immunological and genetic bases of new primary immunodeficiencies. Nat Rev Immunol. 2007;7(11):851–861. doi: 10.1038/nri2195. [DOI] [PubMed] [Google Scholar]
- 51.Leung DY. Infection in atopic dermatitis. Curr Opin Pediatr. 2003;15(4):399–404. doi: 10.1097/00008480-200308000-00008. [DOI] [PubMed] [Google Scholar]
- 52.Palmer CN, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006;38(4):441–446. doi: 10.1038/ng1767. [DOI] [PubMed] [Google Scholar]
- 53.Ong PY, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347(15):1151–1160. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- 54.Howell MD, et al. Mechanism of HBD-3 deficiency in atopic dermatitis. Clin Immunol. 2006;121(3):332–338. doi: 10.1016/j.clim.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 55.Howell MD, et al. Selective killing of vaccinia virus by LL-37: implications for eczema vaccinatum. J Immunol. 2004;172(3):1763–1767. doi: 10.4049/jimmunol.172.3.1763. [DOI] [PubMed] [Google Scholar]
- 56.Conner K, et al. The antimicrobial peptide LL-37 is expressed by keratinocytes in condyloma acuminatum and verruca vulgaris. J Am Acad Dermatol. 2002;47(3):347–350. doi: 10.1067/mjd.2002.122190. [DOI] [PubMed] [Google Scholar]
- 57.Buck CB, et al. Human alpha-defensins block papillomavirus infection. Proc Natl Acad Sci U S A. 2006;103(5):1516–1521. doi: 10.1073/pnas.0508033103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ingraham BA, et al. Molecular basis of the potential of vitamin D to prevent cancer. Curr Med Res Opin. 2008;24(1):139–149. doi: 10.1185/030079908x253519. [DOI] [PubMed] [Google Scholar]
- 59.Yamasaki K, et al. Kallikrein-mediated proteolysis regulates the antimicrobial effects of cathelicidins in skin. Faseb J. 2006;20(12):2068–2080. doi: 10.1096/fj.06-6075com. [DOI] [PubMed] [Google Scholar]
- 60.Yamasaki K, et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med. 2007;13(8):975–980. doi: 10.1038/nm1616. [DOI] [PubMed] [Google Scholar]
- 61.Frohm M, et al. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J Biol Chem. 1997;272(24):15258–15263. doi: 10.1074/jbc.272.24.15258. [DOI] [PubMed] [Google Scholar]
- 62.Marchini G, et al. The newborn infant is protected by an innate antimicrobial barrier: peptide antibiotics are present in the skin and vernix caseosa. Br J Dermatol. 2002;147(6):1127–1134. doi: 10.1046/j.1365-2133.2002.05014.x. [DOI] [PubMed] [Google Scholar]
- 63.Lande R, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449(7162):564–569. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- 64.Zinkernagel AS, et al. Pharmacologic augmentation of hypoxia-inducible factor-1alpha with mimosine boosts the bactericidal capacity of phagocytes. J Infect Dis. 2008;197(2):214–217. doi: 10.1086/524843. [DOI] [PubMed] [Google Scholar]