Abstract
Objectives
We assessed the association between depression and sudden cardiac death (SCD) and cardiac events among individuals without baseline coronary heart disease (CHD).
Background
Depression is a risk factor for cardiac events and mortality among those with CHD, possibly from arrhythmia.
Methods
We studied depressive symptoms, and a proxy variable for clinical depression consisting of severe symptoms and/or antidepressant medication use, and their relationship to cardiac events in the Nurses’ Health Study. Questionnaires in 1992, 1996, and 2000 assessed symptoms with the Mental Health Index (MHI-5), and antidepressant use was assessed in 1996 and 2000. Primary endpoints included SCD, fatal CHD, and non-fatal myocardial infarction (MI).
Results
Among 63,469 women without prior CHD/stroke in 1992, 7.9% had MHI-5 scores (<53) previously found to predict clinical depression. Depressive symptoms were associated with CHD events, and the relationship was strongest for fatal CHD, where the association remained significant even after controlling for CHD risk factors (HR=1.49; 95% CI 1.11–2.00 for MHI-5 score<53). In models from 1996 onward, our proxy variable for clinical depression was most associated with SCD in multivariable models (HR=2.33, 95% CI 1.47–3.70), and this risk was primarily due to a specific relationship between antidepressant use and SCD (HR=3.34, 95% CI 2.03–5.50).
Conclusions
In this cohort of women without baseline CHD, depressive symptoms were associated with fatal CHD, and a measure of clinical depression including antidepressant use was specifically associated with SCD. Although antidepressant use may be a marker of worse depression, its specific association with SCD merits further study.
CONDENSED ABSTRACT
We prospectively analyzed the association between depression and cardiac events in the Nurses’ Health Study. Symptoms of depression as measured by Mental Health Index (MHI-5) score were directly associated with risk of CHD events, and the relationship was strongest for fatal CHD. A proxy variable for clinical depression comprised of MHI-5 score<53 or antidepressant use was strongly associated with sudden cardiac death (SCD), primarily due to a specific relationship between antidepressant use and SCD. Although antidepressant use may be a marker of worse depression, its specific association with SCD merits further study.
Keywords: sudden cardiac death, coronary disease, epidemiology, women
INTRODUCTION
Depression has been identified as a possible risk factor for an adverse prognosis and reduced survival after myocardial infarction (MI),1 and several studies have suggested that this poor prognosis may be due to an arrhythmic mechanism.2–4 Abnormalities in heart rate variability, levels of inflammatory biomarkers, platelet activation, omega-3-fatty acid levels, and plasma norepinephrine have been identified as potential mediators of this adverse prognosis.5–10 Although depression appears to be a marker of increased risk post MI, it is currently unclear whether depression can be considered an independent risk factor11 or whether treatment lowers this risk. The largest randomized trial thus far, The ENRICHD trial, did not show improvement in death or recurrent MI among post-MI patients treated with a strategy of cognitive behavior therapy and selective serotonin reuptake inhibitors (SSRI).12
The relationship between depression and coronary heart disease (CHD) incidence is even less certain with fewer observational studies reporting positive associations1 and no randomized treatment trials. Also, there has been no large prospective assessment of depression and sudden cardiac death (SCD) risk among individuals without pre-existing CHD. The Nurses’ Health Study presented a unique opportunity to analyze the prospective relationship between depression and risk of SCD and other cardiac events, while controlling for updated traditional and nontraditional CHD risk factors, in a large cohort of women without baseline CHD.
METHODS
The Nurses’ Health Study Cohort
The NHS began in 1976 when 121,701 female registered nurses, ages 30 to 55, completed a questionnaire about their medical history, CHD risk factors, and lifestyle factors. The cohort has been followed every two years with mailed questionnaires that update exposure information and inquire about newly diagnosed medical illnesses. Deaths are reported by next-of-kin or postal authorities, or identified through the National Death Index. Family members are asked for permission to obtain further information from medical records and are interviewed about the circumstances surrounding the death if not adequately documented in the medical record. Subjects or family members provided written, informed consent, and the study was approved by the institutional review board of Partners HealthCare System, Boston, Massachusetts. For this analysis, we excluded participants with a prior history of CHD, stroke, or cancer at baseline.
Depression Measure
Self-reported symptoms of depression and use of antidepressant medication were used as measures of depression. Depressive symptoms were assessed in 1992, 1996, and 2000 with the Mental Health Index (MHI-5), a five-item subscale of the Short-Form 36 health status survey13–14 designed to capture psychological distress versus well-being.15–16 The MHI-5 asks respondents how much of the time over the past month (all, most, good bit, some, little, or none) they felt nervous, felt so down that nothing could cheer them up, felt calm and peaceful, felt down and blue, or felt happy. The scale is scored from 0 to 100, with lower scores indicating more depressive symptoms. The MHI-5 has been shown to have high sensitivity and specificity for major depression, with an area under the receiver-operating characteristic curve of 0.88 to 0.91 for the detection of mood disorders or major depression. In accordance with a prior study using this scale,17 we divided the participants into 4 categories of depressive symptoms according to their MHI-5 score (77–100, 76–85, 53–75, 0–52).
For our clinical depression analyses, we created a proxy measure for clinical depression consisting of a low MHI-5 score or reported regular antidepressant medication use. Participants were first asked to report regular antidepressant medication use in 1996, the baseline year for these analyses. This information was updated in 2000, when participants were asked to specifically report their regular use during the past two years of fluoxetine, sertraline, paroxetine, citalopram, or other antidepressants, of which the tricyclic antidepressants amitriptyline, imipramine, and nortriptyline were provided as examples. Because an MHI-5 score of 52 or lower has been found to be predictive of major depression,18 this cutoff was used to define the group with “clinically significant depressive symptoms” and was combined with antidepressant use for the clinical depression analyses. Our proxy variable was found to be highly correlated with a report of being previously diagnosed with clinical depression on the 2000 questionnaire (correlation coefficient 0.49, p<0.001).
Endpoint Definition
The study end points included incident cases of SCD, fatal CHD, and nonfatal MI that occurred after return of the 1992 questionnaire and before June 1, 2004. The specific details regarding the classification of SCD in this cohort are described in detail elsewhere.19 Briefly, a cardiac death was considered sudden if the death or cardiac arrest that precipitated death occurred within one hour of symptom onset as documented by medical records or next-of-kin reports. Unwitnessed deaths that could have occurred within one hour of symptom onset with autopsy findings consistent with SCD were considered probable SCDs and were also included in the analysis. Secondary analyses were performed using SCD or death during sleep in the absence of prior known symptoms.
Fatal CHD was defined as ICD-9 codes 410 to 412 if confirmed by hospital records or autopsy, or if CHD was the most likely cause and was listed as the cause of death on the death certificate, along with evidence of prior CHD. We designated as probable CHD those cases in which CHD was the underlying cause on the death certificate but for which no medical records concerning the death were available, and included these cases in the analysis.
All women who reported having a nonfatal MI were asked for permission to review their medical records. MIs were confirmed according to World Health Organization criteria by physicians blinded to exposure status. MIs that required hospital admission and for which confirmatory information was obtained by interview or letter, but for which no medical records were available, were designated as probable and included in the analysis.
Statistical Analyses
We computed age-adjusted means or proportions of cardiovascular risk factors across categories of MHI-5 score reported in 1992. Baseline measurements of cardiac risk factors were compared across MHI-5 category using Mantel-Haenszel chi-squared tests for categorical variables and linear regression for continuous variables.
For the depressive symptom analysis, women who responded to the MHI-5 items on any of the 1992, 1996, or 2000 questionnaires were included in the analysis from the time of their earliest questionnaire response. Participants with a prior history of CHD, stroke, or cancer in the baseline year were excluded from both analyses. For each woman we calculated person-months of follow-up from the date of return of the earliest questionnaire to date of first endpoint, death, or to June 1, 2004, whichever came first. We used Cox proportional hazards models in order to estimate age- and multivariable- adjusted hazard ratios. MHI-5 score was treated as a time-dependent variable utilizing the most recent exposure to predict outcome, and the last observation was carried forward for those with missing values at a particular time point. Tests for linear trend were performed by including MHI-5 score as a continuous variable in separate proportional hazards models.
Two multivariable models were performed; the first model (Multivariable model I) simultaneously adjusted for updated coronary risk factors except for reported non-fatal CHD during follow-up, hypertension, and diabetes, three possible biologic intermediates in the relationship between depression and heart disease outcome. The first model included variables for age, beginning year of follow-up, smoking status (never, past, current 1–14 cigarettes/day, 15–24, ≥25), body mass index (<25 kg/m2, 25–29.9, ≥30), alcohol intake (0, <5 g/day, 5–14, ≥15), menopausal status and postmenopausal hormone use, usual aspirin use (<1/week, 1–6, and 7+), multivitamin use, vitamin E supplement use, hypercholesterolemia, family history of MI (no, prior to 60, after age 60), history of stroke, n-3-fatty acid intake (quintiles), alpha linolenic acid intake (quintiles), and moderate/vigorous physical activity (0, 1–1.9 hours/week, 2–3.9, ≥4). The second multivariable model (Multivariable model II) included the variables in model I, with the addition of non-fatal CHD during follow-up, hypertension and diabetes.
For the clinical depression analysis, unadjusted Kaplan-Meier curves of time to cardiac event were estimated using 1996 baseline measurements of our proxy variable for clinical depression. Cox proportional hazards analyses included women who responded to the MHI-5 items and antidepressant medication questions on the 1996 or 2000 questionnaire, from the time of their earliest questionnaire response. Depression exposure was treated as a time-dependent variable, and Multivariable models I and II included the same updated time dependent covariates as described above. All reported P values are two-sided. Statistical analysis was performed using SAS statistical software (SAS Institute Inc, Cary, NC), Version 8.2.
Sensitivity Analyses
To evaluate the possibility of reverse causality associated with the development of non-fatal CHD, we performed analyses excluding individuals who reported being diagnosed with another non-fatal CHD (angina, CABG, or MI) endpoint prior to the study outcome in question. We also evaluated for an interaction between MHI-5 score and prior diagnosis of CHD in the entire population, through the inclusion of cross-product terms in our proportional hazards models. In addition, we performed sensitivity analyses of our results for non-fatal MI by excluding cases not confirmed by medical record review.
RESULTS
Depressive Symptoms
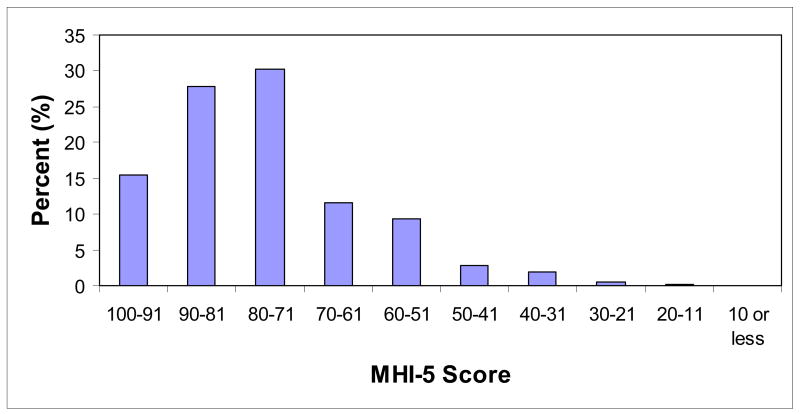
In 1992, 63,469 women of 102,482 (61.9%) total participants without prior coronary heart disease, stroke, or cancer responded to the items of the MHI-5. The median MHI-5 score was 80 (IQR 68–88), and 4,994 women (7.9% of the total) had MHI-5 score<53, which has been shown to be predictive of clinical depression (Figure 1).18 Compared with women with MHI-5 score 86–100, women with worse symptoms of depression, as represented by lower MHI-5 score, were younger and more likely to report a history of hypertension, diabetes, and high cholesterol (Table 1). Women with more depressive symptoms were also more likely to be smokers, obese, and less physically active. N-3-fatty acid intake was lower among participants with worse symptoms of depression, and regular aspirin and multivitamin use was higher.
Figure 1.
Distribution of MHI-5 score among 63,469 women without prior coronary heart disease, stroke, or cancer in 1992.
Table 1.
Coronary heart disease risk factors* among 63,469 women without prior coronary heart disease, stroke, or cancer according to depression symptom category in 1992
| Depression symptom score | ||||
|---|---|---|---|---|
| 86–100 (Referent) | 76–85 | 53–75 | 0–52 (Depressive) | |
| No. of women (% total) | 18631 (29.4) | 22814 (36.0) | 17030(26.8) | 4994 (7.9) |
| Mean Age (SD) † | 59.6 (7.1) | 58.3 (7.2) | 57.6 (7.2) | 56.7 (7.4) |
| Smoking (%)† | ||||
| Past | 7453 (39.9) | 9521 (41.7) | 7199 (42.5) | 2068 (41.8) |
| Current 1–14 cigarettes/day | 952 (5.1) | 1305 (5.7) | 1120 (6.6) | 320 (6.4) |
| Current 15–24 cigarettes/day | 865 (4.8) | 1187 (5.2) | 1167 (6.8) | 384 (7.4) |
| Current 25 + cigarettes/day | 366 (2.0) | 478 (2.1) | 503 (2.9) | 241 (4.8) |
| Reported Diagnosis of: | ||||
| Diabetes (%)† | 809 (4.1) | 973 (4.3) | 865 (5.3) | 294 (6.3) |
| High Cholesterol (%)† | 7692 (39.7) | 9951 (43.7) | 7813 (46.9) | 2417 (50.4) |
| Hypertension (%)† | 5562 (28.3) | 7165 (31.5) | 5768 (34.9) | 1774 (38.0) |
| Body Mass Index ≥ 30 kg/m2 (%)† | 3001 (16.2) | 3773 (16.5) | 2968 (17.3) | 1038 (20.7) |
| Parental History of MI prior to age 60 (%)‡ | 2399 (13.1) | 3007 (13.2) | 2282 (13.3) | 686 (13.2) |
| Alcohol Intake (%)† | ||||
| 0.1–4.9 g/day | 5357 (29.0) | 6592 (28.9) | 4946 (28.9) | 1347 (26.6) |
| 5.0–14.9 g/day | 3018 (16.1) | 3793 (16.6) | 2705 (15.9) | 682 (13.6) |
| 15.0 + g/day | 1510 (7.9) | 1874 (8.2) | 1314 (7.8) | 386 (7.8) |
| Aspirin Use (7+/week, %)† | 2177 (11.5) | 2797 (12.3) | 2505 (14.8) | 859 (17.2) |
| Post Menopausal (%)† | 14945 (75.6) | 17177 (75.5) | 12347 (75.5) | 3467 (76.0) |
| N-3-fatty acid intake as % total | 0.145 (.152) | 0.143 (.143) | 0.139 (.133) | 0.136 (.146) |
| calories (SD)† | ||||
| Alpha linolenic acid intake as % total calories (SD) | 0.530 (.152) | 0.527 (.149) | 0.526 (.150) | 0.528 (.156) |
| Vitamin supplement use | ||||
| Vitamin E (%) | 3262 (17.2) | 3939 (17.3) | 2981 (17.8) | 868 (17.8) |
| Multivitamin (%)† | 7661 (40.7) | 9759 (42.8) | 7421 (43.8) | 2206 (44.7) |
| Moderate/vigorous physical activity† | ||||
| 0–1.9 hours/week (%) | 5637 (30.3) | 7493 (32.8) | 5840 (34.2) | 1699 (33.9) |
| 2–3.9 hours/week (%) | 3236 (17.6) | 4011 (17.6) | 2640 (15.4) | 671 (13.1) |
| ≥4 hours/week (%) | 4109 (22.1) | 4352 (19.1) | 2663 (15.6) | 612 (12.1) |
g=grams, kg/m2=kilograms per square meter, SD=standard deviation.
Age-adjusted.
P<0.01
Table 2 displays the association between depressive symptoms, as measured by the MHI-5 score, and risk of SCD, fatal CHD, and non-fatal MI. Hazard ratios are shown for each MHI-5 category, compared with the reference category (MHI-5 score 86–100). In age-adjusted proportional hazards analyses, a lower MHI-5 score (i.e., more severe depressive symptoms) was associated with an increased risk of all three CHD outcomes. However, these associations were attenuated in multivariable analyses that included CHD risk factors except for possible biological mediators (Multivariable model I), and relationships for SCD and non-fatal MI became non-significant in the fully adjusted model (Multivariable model II). The relationship between MHI-5 score and CHD mortality was attenuated but remained statistically significant (HR=1.49 for MHI-5 score<53; 95% CI 1.11–2.00; p trend=0.007) in the fully adjusted multivariable model. When deaths that occurred during sleep without prior symptoms were included in the SCD endpoint (number of endpoints increased from 138 to 176), the association between symptoms of depression and SCD also remained significant, but attenuated, in the full multivariable model (HR=1.51 for MHI-5 score<53; 95% CI 0.89–2.56; p trend=0.01).
Table 2.
Long-term relative risks* (95% confidence interval) of mortality/myocardial infarction among 81,875 women without prior coronary heart disease, stroke, or cancer at baseline followed from 1992 to 2004†, according to categories of depression symptom score
| Depression symptom score | |||||
|---|---|---|---|---|---|
| Outcome | 86–100 (Referent) | 76–85 | 53–75 | 0–52 (Depressive) | P for trend‡ (continuous) |
| Person-years | 311391 | 293969 | 201794 | 54932 | |
| Sudden Cardiac Death | |||||
| No. of cases | 46 | 37 | 43 | 12 | |
| Age-adjusted | 1.0 | 0.98 (0.64–1.51) | 1.72 (1.13–2.61) | 1.96 (1.03–3.71) | <0.001 |
| Multivariable I | 1.0 | 0.91 (0.59–1.41) | 1.36 (0.89–2.08) | 1.36 (0.71–2.61) | 0.050 |
| Multivariable II | 1.0 | 0.87 (0.56–1.34) | 1.27 (0.83–1.94) | 1.21 (0.63–2.33) | 0.13 |
| Fatal CHD | |||||
| No. of cases | 171 | 147 | 159 | 64 | |
| Age-adjusted | 1.0 | 1.01 (0.81–1.25) | 1.67 (1.35–2.08) | 2.81 (2.11–3.75) | <0.001 |
| Multivariable I | 1.0 | 0.93 (0.74–1.15) | 1.30 (1.04–1.62) | 1.79 (1.33–2.40) | <0.001 |
| Multivariable II | 1.0 | 0.86 (0.69–1.07) | 1.14 (0.91–1.42) | 1.49 (1.11–2.00) | 0.007 |
| Myocardial infarction | |||||
| No. of cases | 406 | 392 | 298 | 87 | |
| Age-adjusted | 1.0 | 1.10 (0.95–1.26) | 1.27 (1.09–1.47) | 1.49 (1.18–1.87) | <0.001 |
| Multivariable I | 1.0 | 1.05 (0.92–1.21) | 1.14 (0.98–1.33) | 1.24 (0.98–1.57) | 0.02 |
| Multivariable II | 1.0 | 1.03 (0.89–1.18) | 1.08 (0.93–1.25) | 1.13 (0.90–1.43) | 0.19 |
CHD=coronary heart disease
Hazard ratios from proportional hazards models with time-dependent variables.
Women who responded to the MHI-5 items on any of the 1992, 1996, or 2000 questionnaires and were free of prior coronary heart disease, stroke, or cancer at the time of their earliest questionnaire response were included in the analysis from this timepoint on. Please see methods for description of variables for Multivariable models I and II.
P value reflects test for linear trend using MHI-5 score as continuous variable.
To evaluate the sensitivity of these results to possible reverse causality associated with the development of non-fatal CHD, we performed analyses excluding all women who reported being diagnosed with another non-fatal CHD event (angina, CABG, or MI) prior to each study outcome. The hazard ratio for each MHI-5 score category (data not shown), as well as the overall association between depression score and SCD (p for trend=0.079), fatal CHD (p for trend=0.008), and non-fatal MI (p for trend=0.28) were not materially different from our primary analyses. There was also no evidence for an interaction between MHI-5 score and CHD during follow-up for any of the CHD outcomes. In addition, for analyses of non-fatal MI, exclusion of cases not confirmed by medical record review (24.6 % of cases) did not materially change the results compared with our primary analyses.
Clinical Depression and Antidepressant Use
In 1996, 4,280 women (6.0 percent of the cohort in that year) had an MHI-5 score<53, and in 2000, 3,399 women (4.8%) had MHI-5 score<53. In 1996, 4,769 women (6.7%) and in 2000, 6,869 women (9.7%) reported use of medications that are commonly used as antidepressants. In 2000, among those reporting antidepressant use, 61 percent reported using an SSRI either sertraline, fluoxetine, paroxetine, or citalopram), and 39 percent reported “other antidepressant use”. MHI-5 score<53 and antidepressant use were both significantly correlated with a report of a diagnosis of clinical depression on the 2000 questionnaire (correlation coefficient 0.21 and 0.54 respectively).
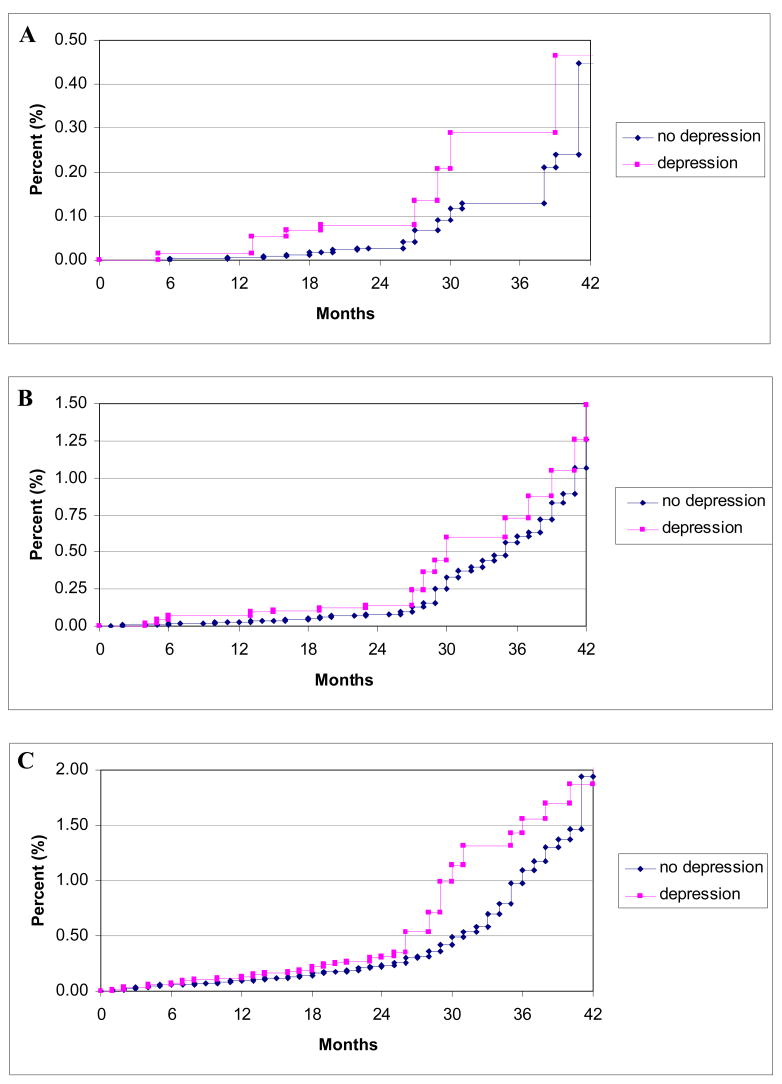
Utilizing this information on antidepressant use, we evaluated the association between a proxy variable for clinical depression, which consisted of either MHI-5 score<53 or use of antidepressant medication, and cardiac events. Unadjusted Kaplan-Meier curves using 1996 baseline data for our proxy measure are shown in Figure 2, and multivariable proportional hazards analyses using time-varying measurements are shown in Table 3. Similar to the results for MHI-5 score, the proxy variable for clinical depression was associated with increased risk of all three CHD outcomes in age-adjusted and multivariable models excluding biological intermediates. However, the elevation in risk was greatest for SCD, where a 2.33 fold association (95% CI 1.47–3.70, p<0.001) persisted in the full multivariable model (Multivariable Model II). In comparison, the associations for fatal CHD (HR 1.37, 95% CI 1.04–1.81; P=0.03) and non-fatal MI (HR 1.20, 95% CI 0.99–1.46; P=0.06) were markedly attenuated and only marginally significant after full multivariable adjustment. Similar to the results for depressive symptoms, these results for the proxy measure of clinical depression were not materially altered when women who developed an intervening non-fatal CHD event (angina, CABG, or MI) were excluded from the analysis (for SCD, HR 2.20, 95% CI 1.35–3.57, p=0.002; for fatal CHD, HR 1.31, 95% CI 0.96–1.78, p=0.09; for non-fatal MI, HR 1.25, 95% CI 1.03–1.53, p=0.027).
Figure 2.
Unadjusted Kaplan-Meier curves of time to first event for sudden cardiac death (A), fatal coronary heart disease (B), and non-fatal myocardial infarction (C), according to depression status as defined by MHI-5 score<53 or reported use of antidepressant medication at baseline starting in 1996. Curves are truncated at 42 months. P values for log rank test=0.02 for sudden cardiac death, 0.31 for fatal coronary heart disease, and 0.80 for non-fatal myocardial infarction.
Table 3.
Hazard ratios (95% confidence interval) for mortality/MI among 75,718 women without prior coronary heart disease, stroke, or cancer followed at baseline followed from 1996 to 2004, according to depression score less than 53 or use of antidepressant medication (in 1996 or 2000).*
| Outcome | Hazard Ratio (95% CI) | P value |
|---|---|---|
| Sudden Cardiac Death (n=99) | ||
| Age-adjusted | 2.91 (1.85–4.57) | <0.001 |
| Multivariable I | 2.49 (1.57–3.94) | <0.001 |
| Multivariable II | 2.33 (1.47–3.70) | <0.001 |
| Fatal CHD (n=342) | ||
| Age-adjusted | 1.87 (1.42–2.46) | <0.001 |
| Multivariable I | 1.54 (1.17–2.03) | 0.002 |
| Multivariable II | 1.37 (1.04–1.81) | 0.03 |
| Myocardial infarction (n=811) | ||
| Age-adjusted | 1.41 (1.17–1.71) | <0.001 |
| Multivariable I | 1.28 (1.05–1.56) | 0.01 |
| Multivariable II | 1.20 (0.99–1.46) | 0.06 |
CHD=coronary heart disease
Please see methods for description of variables for Multivariable models I and II.
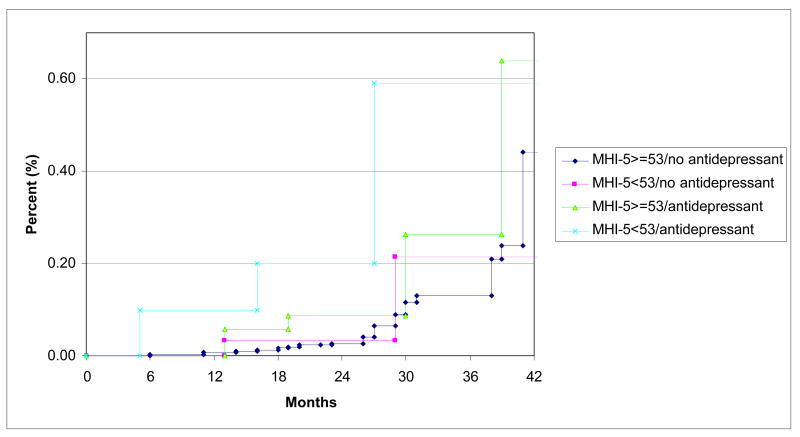
To further evaluate the elevated risk of SCD associated with the composite clinical depression measure, we assessed the individual components (MHI-5 score<53 and antidepressant use) simultaneously in proportional hazard models (Table 4). In these models, antidepressant medication use was associated with a markedly elevated risk of SCD in the fully adjusted models (HR 3.34, 95% CI 2.03–5.50), whereas MHI-5 score<53 no longer conferred an elevated risk (HR 1.04, 95% CI 0.51–2.12). By contrast, there was no relationship between antidepressant use and fatal CHD (HR 1.07, 95% CI 0.75–1.53) or non-fatal MI (HR 1.21, 95% CI 0.96–1.53) in these models. Unadjusted Kaplan-Meier curves of time to SCD stratified by antidepressant use and MHI-5 score<53 at baseline in 1996 are shown in Figure 3. In a secondary analysis of data from 2000 to 2004 with separate variables for SSRI use and “other antidepressant use” (31 total SCDs), we found similar hazard ratios for the two categories of medications in age-adjusted analyses (HR=5.07, 95% CI 1.73–14.8 for SSRI use, HR=3.19, 95% CI 0.92–11.00 for other antidepressant use).
Table 4.
Hazard ratios (95% confidence interval) for mortality/MI among 75,718 women without prior coronary heart disease, stroke, or cancer followed from 1996 to 2004, from multivariable models that included depression score less than 53 and use of antidepressant medication (in 1996 or 2000).*
| Depression Score < 53 | Antidepressant Medication Use | |||
|---|---|---|---|---|
| Outcome | Hazard Ratio (95% CI) | P value | Hazard Ratio (95% CI) | P value |
| Sudden Cardiac Death (n=99) | ||||
| Age-adjusted | 1.36 (0.67–2.77) | 0.40 | 3.59 (2.20–5.86) | <0.001 |
| Multivariable I | 1.09 (0.53–2.24) | 0.81 | 3.53 (2.15–5.81) | <0.001 |
| Multivariable II | 1.04 (0.51–2.12) | 0.92 | 3.34 (2.03–5.50) | <0.001 |
| Fatal CHD (n=342) | ||||
| Age-adjusted | 2.07 (1.43–2.99) | <0.001 | 1.30 (0.91–1.84) | 0.15 |
| Multivariable I | 1.58 (1.09–2.29) | 0.02 | 1.19 (0.83–1.70) | 0.34 |
| Multivariable II | 1.41 (0.97–2.04) | 0.07 | 1.07 (0.75–1.53) | 0.71 |
| Myocardial infarction (n=814) | ||||
| Age-adjusted | 1.14 (0.85–1.54) | 0.38 | 1.38 (1.10–1.74) | 0.006 |
| Multivariable I | 1.05 (0.78–1.41) | 0.77 | 1.27 (1.01–1.60) | 0.04 |
| Multivariable II | 0.98 (0.73–1.33) | 0.91 | 1.21 (0.96–1.53) | 0.10 |
CHD=coronary heart disease
Please see methods for description of variables for Multivariable models I and II.
Figure 3.
Unadjusted Kaplan-Meier curves of time to sudden cardiac death stratified by MHI-5 score<53 and reported use of antidepressant medication at baseline starting in 1996. P value for log rank test across strata=0.01.
DISCUSSION
In this prospective study among women without known cardiovascular disease at baseline, symptoms of depression were directly associated with risk of CHD events in age-adjusted and multivariable models excluding potential biologic intermediates. Symptoms were most strongly related to fatal CHD events, where the association remained significant even after controlling for all CHD risk factors. However, depressive symptoms were also associated with multiple risk factors for CHD, and the relationships between depressive symptoms and all three endpoints, including fatal CHD, were attenuated in multivariable analyses that adjusted for updated CHD risk factor status. These CHD risk factors may act as biologic intermediates in the relationship between depressive symptoms and cardiac events, given their strong association with depression in this and prior studies.20 When we examined a proxy variable for clinical depression that consisted of either MHI-5 score<53 or use of antidepressant medication, a strong association with risk of SCD emerged that was not attenuated in multivariable models. When examined separately, this increased risk appeared to result primarily from a specific elevation in the risk of SCD among women who reported antidepressant use. Neither depression score nor antidepressant use was significantly associated with non-fatal events in multivariable models.
Prior studies have examined the association between depression and incident cardiac events, with varying results. Two meta-analyses of observational studies both estimated that depression conferred a 1.6 fold increased risk of CHD;21–22 and similar to the relationship observed here for SCD, clinically relevant depression appeared to be a stronger predictor than depressive symptoms.22 With respect to prior large scale data among women, investigators from the Women’s Health Initiative (WHI) also found that depressive symptoms were associated with a significantly higher risk of fatal cardiovascular events (adjusted risk ratio 1.5, 95% CI 1.10–2.03) among 73,098 women without a history of cardiovascular disease over four years of follow-up.23 As observed in our study, relationships appeared stronger for fatal versus nonfatal events; however, separate risks were not reported for antidepressant use and SCD was not specifically examined.
Part of the association with fatal events observed in this and previous studies could be explained if part of the elevated mortality risk associated with depression were due to an increased risk of fatal ventricular arrhythmias. The strong association between our proxy measure of clinical depression and SCD supports this possibility. A large case control study also found a significant association between a diagnosis of clinical depression and out-of-hospital cardiac arrest independent of established CHD risk factors.2 Depressive symptoms have also been specifically associated with ventricular arrhythmias among individuals with implantable defibrillators.3 Possible mechanisms for the elevated risk of ventricular arrhythmias and SCD associated with depression include greater sympathetic nervous system activation,9 higher resting heart rates, increases in QT dispersion,24 and reduced heart rate variability8 among individuals with depression. Another possibility is that treatments for depression may elevate risk of ventricular arrhythmias. When examined separately, we found an elevated risk of SCD associated with antidepressant use, and not with more severe depressive symptoms according to MHI-5 score<53. It is also noteworthy that as opposed to the hazard ratio corresponding to MHI-5 score, the hazard ratio for SCD corresponding to antidepressant use was minimally attenuated despite adjustment for multiple coronary risk factors (HR in fully adjusted model 3.34, 95% CI 2.03–5.50).
In addition to our own study, prior observational studies have noted a higher mortality risk associated with antidepressant use in patients with heart failure25 and in patients undergoing coronary artery bypass graft surgery.26 Fluoxetine, which has been shown to cause direct and indirect reductions in the hERG potassium current in a human embryonic kidney cell model,27 has been reported to result in QT prolongation and torsades de pointes.28–29 Tricyclic antidepressants are also known to cause QT prolongation and ventricular arrhythmias in overdose, and in doses greater than 100 mg have been associated with SCD risk in a retrospective cohort study.30 In this same study, standard doses of SSRIs and TCAs were not associated with increased risk; however, the study was unable to control for other confounding factors.
Although proarrhythmic effects from antidepressant medications may have resulted in a higher risk of SCD in our study, it must be emphasized that observational studies cannot prove causality, and confounding by indication can never be completely ruled out. Since antidepressant use was more significantly correlated with a diagnosis of clinical depression than the MHI-5 score, it is entirely possible that antidepressant use identified participants with more severe depression that was not fully captured by the MHI-5 questionnaire. In addition, these risks need to be put in perspective and balanced against known benefits of these medications on depression. SCD among healthy women is an uncommon event, and although elevated, the absolute risk among women who reported antidepressant use in this study was still very low (46 SCDs per 100,000 person-years).
Although the individual risk is low, the possible public health implications of an arrhythmic risk from these commonly prescribed medications at a societal level remain large. This potential risk coupled with recent data suggesting that the clinical benefit of newer antidepressant agents may be less than previously thought31 highlights the need for large-scale randomized trials of antidepressant medications that are adequately designed and powered to detect important adverse cardiovascular outcomes such as proarrhythmia as well as efficacy. Although the Sertraline Antidepressant Heart Attack Randomized Trial (SADHART) trial was specifically designed to examine cardiovascular safety in patients with acute MI or unstable angina, the primary study endpoints were intermediate endpoints such as left ventricular ejection fraction and significant QTc prolongation.32 With only 369 patients enrolled, the trial had minimal power to examine hard endpoints such as SCD or fatal CHD. The largest randomized trial thus far, the ENRICHD trial, enrolled 2481 post-MI patients and did not find an increase or decrease in the risk of death or recurrent MI associated with cognitive behavioral therapy supplemented with SSRI, but raised the possibility of an interaction by sex, with higher risks of cardiovascular events among women treated with the intervention.12
Strengths of our study include the relatively large sample size, the long follow up period, rigorously documented CHD endpoints, and the updated measures of depression and cardiac risk factors over the course of follow-up. There are also additional limitations of the present study that warrant consideration. First, reverse causality may account for at least part of the association observed between depression and cardiovascular disease in this and other observational studies. Individuals who are diagnosed with CHD or with conditions that predispose them to coronary disease such as diabetes or hypertension may develop depression, and therefore, would also be at higher risk for fatal cardiac events. Our study excluded women with known CHD at baseline, and sensitivity analyses that excluded participants who reported CHD in follow-up did not significantly change our results, arguing that reverse causality by symptomatic CHD is less likely to be a significant source of bias. Although we attempted to adjust for as many potential confounding factors as possible in our multivariable analyses, there may still be residual confounding from CHD risk factors and prevalent CHD. Also, for the non-fatal MI analysis, women who are depressed my be more likely to self-report MI, and this could inflate the detection of MI, particularly with respect to the probable cases not confirmed by medical records. However, analyses excluding these probable cases revealed similar results.
Second, we did not have a measure of adherence to medications in our data. Because depression has previously been associated with reduced adherence to medications,33 adherence may be a confounder in our results. Also, we did not collect information on antidepressant dose, and therefore, it is not possible to analyze our data for a dose-response relationship between antidepressants and SCD. In addition, our analysis does not include other comorbid psychologic factors, such as anxiety, which may be collinear with depression and which has been correlated with arrhythmia.35 Another limitation is the relatively small number SCD events in our analyses (138 SCDs for analyses starting in 1992, 99 SCDs for analyses starting in 1996), compared with the fatal CHD and non-fatal MI endpoints. Finally, the Nurses’ Health study represents a relatively healthy group of mostly Caucasian females, and findings from this analysis may not be generalizable to other populations. However, the estimated 7.9% prevalence of depression as measured by an MHI-5 score <53 is comparable to the prevalence of major depression (4.8%) estimated among white women aged 45–54 years using a structured interview in the National Comorbidity Survey.36
In summary, in this prospective cohort of women without baseline cardiovascular disease, we found that symptoms of depression are associated with higher risks of cardiac events, and at least part of this association appears to be explained by differences in coronary risk factors, which may act as causal intermediates in the risk conferred by depression. When we utilized a proxy for clinical depression, which included antidepressant medication use, we observed a stronger association for SCD suggesting a possible pro-arrhythmic mechanism. Although antidepressant medication use may be a marker of worse depression, its specific association with elevated risk of SCD merits further study.
Acknowledgments
Supported by Grants CA-87969, HL-34594, and HL-03783 from the National Institutes of Health.
We are indebted to the participants in the Nurses’ Health Study for their outstanding commitment and cooperation., and to Julie Pester, Lisa Dunn, and Barbara Egan for their expert assistance.
ABBREVIATIONS LIST
- CHD
coronary heart disease
- SCD
sudden cardiac death
- MHI
Mental Health Index
- MI
myocardial infarction
- SSRI
selective serotonin reuptake inhibitor
- CABG
coronary artery bypass graft
- HR
hazard ratio
Footnotes
Disclosures
The authors have no disclosures to report.
References
- 1.Frasure-Smith N, Lespérance F. Recent evidence linking coronary heart disease and depression. Can J Psychiatry. 2006;51(12):730–737. doi: 10.1177/070674370605101202. [DOI] [PubMed] [Google Scholar]
- 2.Empana JP, Jouven X, Lemaitre RN, Sotoodehnia N, Rea T, Raghunathan TE, Simon G, Siscovick DS. Clinical depression and risk of out-of-hospital cardiac arrest. Arch Intern Med. 2006;166:195–200. doi: 10.1001/archinte.166.2.195. [DOI] [PubMed] [Google Scholar]
- 3.Whang W, Albert CM, Sears SF, Jr, Lampert R, Conti JB, Wang PJ, Singh JP, Ruskin JN, Muller JE, Mittleman MA. Depression as a predictor for appropriate shocks among patients with implantable cardioverter-defibrillators: results from the Triggers of Ventricular Arrhythmias (TOVA) study. J Am Coll Cardiol. 2005;45:1090–1095. doi: 10.1016/j.jacc.2004.12.053. [DOI] [PubMed] [Google Scholar]
- 4.Carney RM, Blumenthal JA, Stein PK, Watkins L, Catellier D, Berkman LF, Czajkowski SM, O’Connor C, Stone PH, Freedland KE. Depression, heart rate variability, and acute myocardial infarction. Circulation. 2001;104:2024–2028. doi: 10.1161/hc4201.097834. [DOI] [PubMed] [Google Scholar]
- 5.Bush DE, Ziegelstein RC, Patel UV, Thombs BD, Ford DE, Fauerbach JA, McCann UD, Stewart KJ, Tsilidis KK, Patel AL, Feuerstein CJ, Bass EB. Post-myocardial Infarction Depression. Evidence Report/Technology Assessment No. 123. AHRQ Publication Number 05-E018-1. Rockville, MD: Agency for Healthcare Research and Quality; 2005. [PMC free article] [PubMed] [Google Scholar]
- 6.Vaccarino V, Johnson BD, Sheps DS, Reis SE, Kelsey SF, Bittner V, Rutledge T, Shaw LJ, Sopko G, Bairey Merz CN National Heart, Lung, and Blood Institute. Depression, inflammation, and incident cardiovascular disease in women with suspected coronary ischemia: the National Heart, Lung, and Blood Institute-sponsored WISE study. J Am Coll Cardiol. 2007;50:2044–2050. doi: 10.1016/j.jacc.2007.07.069. [DOI] [PubMed] [Google Scholar]
- 7.Carney RM, Rich MW, Tevelde A, Saini J, Clark K, Jaffe AS. Major depressive disorder in coronary artery disease. Am J Cardiol. 1987;60:1273–1275. doi: 10.1016/0002-9149(87)90607-2. [DOI] [PubMed] [Google Scholar]
- 8.Carney RM, Blumenthal JA, Stein PK, Watkins L, Catellier D, Berkman LF, Czajkowski SM, O’Connor C, Stone PH, Freedland KE. Depression, heart rate variability, and acute myocardial infarction. Circulation. 2001;104:2024–2028. doi: 10.1161/hc4201.097834. [DOI] [PubMed] [Google Scholar]
- 9.Carney RM, Freedland KE, Veith RC. Depression, the autonomic nervous system, and coronary heart disease. Psychosom Med. 2005;67(Suppl 1):S29–S33. doi: 10.1097/01.psy.0000162254.61556.d5. [DOI] [PubMed] [Google Scholar]
- 10.Gold PW, Wong ML, Goldstein DS, Gold HK, Ronsaville DS, Esler M, Alesci S, Masood A, Licinio J, Geracioti TD, Jr, Perini G, DeBellis MD, Holmes C, Vgontzas AN, Charney DS, Chrousos GP, McCann SM, Kling MA. Cardiac implications of increased arterial entry and reversible 24-h central and peripheral norepinephrine levels in melancholia. Proc Natl Acad Sci USA. 2005;102:8303–8308. doi: 10.1073/pnas.0503069102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nicholson A, Kuper H, Hemingway H. Depression as an aetiologic and prognostic factor in coronary heart disease: a meta-analysis of 6362 events among 146 538 participants in 54 observational studies. Eur Heart J. 2006;27:2763–2774. doi: 10.1093/eurheartj/ehl338. [DOI] [PubMed] [Google Scholar]
- 12.Berkman LF, Blumenthal J, Burg M, Carney RM, Catellier D, Cowan MJ, Czajkowski SM, DeBusk R, Hosking J, Jaffe A, Kaufmann PG, Mitchell P, Norman J, Powell LH, Raczynski JM, Schneiderman N Enhancing Recovery in Coronary Heart Disease Patients Investigators (ENRICHD) Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Randomized Trial. JAMA. 2003;289:3106–3116. doi: 10.1001/jama.289.23.3106. [DOI] [PubMed] [Google Scholar]
- 13.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 14.Ware JE, Snow KK, Kosinski M. SF-36 health survey: manual & interpretation guide. Lincoln, RI: QualityMetric Incorporated; 2000. [Google Scholar]
- 15.McHorney CA, Ware JE, Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36). II Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Ware J, Snow K, Kosinski M, et al. SF-36 Health Survey: Manual and interpretation guide. Boston, MA: The Health Institute, New England Medical Center; 1993. [Google Scholar]
- 17.Kroenke CH, Bennett GG, Fuchs C, Giovannucci E, Kawachi I, Schernhammer E, Holmes MD, Kubzansky LD. Depressive symptoms and prospective incidence of colorectal cancer in women. Am J Epidemiol. 2005;162:839–848. doi: 10.1093/aje/kwi302. [DOI] [PubMed] [Google Scholar]
- 18.Berwick DM, Murphy JM, Goldman PA, et al. Performance of a five-item mental health screening test. Med Care. 1991;29:169–176. doi: 10.1097/00005650-199102000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Albert CM, Chae CU, Grodstein F, Rose LM, Rexrode KM, Ruskin JN, Stampfer MJ, Manson JE. Prospective study of sudden cardiac death among women in the United States. Circulation. 2003;107:2096–2101. doi: 10.1161/01.CIR.0000065223.21530.11. [DOI] [PubMed] [Google Scholar]
- 20.Skala JA, Freedland KE, Carney RM. Coronary heart disease and depression: a review of recent mechanistic research. Can J Psychiatry. 2006;51:738–745. doi: 10.1177/070674370605101203. [DOI] [PubMed] [Google Scholar]
- 21.Wulsin LR, Singal BM. Do depressive symptoms increase the risk for the onset of coronary disease? A systematic quantitative review. Psychosom Med. 2003;65:201–210. doi: 10.1097/01.psy.0000058371.50240.e3. [DOI] [PubMed] [Google Scholar]
- 22.Rugulies R. Depression as a predictor for coronary heart disease a review and meta-analysis. Am J Prev Med. 2002;23:51–61. doi: 10.1016/s0749-3797(02)00439-7. [DOI] [PubMed] [Google Scholar]
- 23.Wassertheil-Smoller S, Shumaker S, Ockene J, Talavera GA, Greenland P, Cochrane B, Robbins J, Aragaki A, Dunbar-Jacob J. Depression and cardiovascular sequelae in postmenopausal women. The Women’s Health Initiative (WHI) Arch Intern Med. 2004;164:289–298. doi: 10.1001/archinte.164.3.289. [DOI] [PubMed] [Google Scholar]
- 24.Carney RM, Freedland KE, Stein PK, Watkins LL, Catellier D, Jaffe AS, Yeragani VK. Effects of depression on QT interval variability after myocardial infarction. Psychosom Med. 2003;65:177–180. doi: 10.1097/01.psy.0000033129.21715.4b. [DOI] [PubMed] [Google Scholar]
- 25.Sherwood A, Blumenthal JA, Trivedi R, Johnson KS, O’Connor CM, Adams KF, Jr, Dupree CS, Waugh RA, Bensimhon DR, Gaulden L, Christenson RH, Koch GG, Hinderliter AL. Relationship of depression to death or hospitalization in patients with heart failure. Arch Intern Med. 2007;167:367–373. doi: 10.1001/archinte.167.4.367. [DOI] [PubMed] [Google Scholar]
- 26.Xiong GL, Jiang W, Clare R, Shaw LK, Smith PK, Mahaffey KW, O’Connor CM, Krishnan KR, Newby LK. Prognosis of patients taking selective serotonin reuptake inhibitors before coronary artery bypass grafting. Am J Cardiol. 2006;98:42–47. doi: 10.1016/j.amjcard.2006.01.051. [DOI] [PubMed] [Google Scholar]
- 27.Rajamani S, Eckhardt LL, Valdivia CR, Klemens CA, Gillman BM, Anderson CL, Holzem KM, Delisle BP, Anson BD, Makielski JC, January CT. Drug-induced long QT syndrome: hERG K+ channel block and disruption of protein trafficking by fluoxetine and norfluoxetine. Br J Pharmacol. 2006;149:481–489. doi: 10.1038/sj.bjp.0706892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Appleby M, Mbewu A, Clarke B. Fluoxetine and ventricular torsade – is there a link? Int J Cardiol. 1995;49:178–180. doi: 10.1016/0167-5273(94)02270-s. [DOI] [PubMed] [Google Scholar]
- 29.Wilting I, Smals OM, Holwerda NJ, Meyboom RH, de Bruin ML, Egberts TC. QTc prolongation and torsades de pointes in an elderly woman taking fluoxetine. Am J Psychiatry. 2006;163:325. doi: 10.1176/appi.ajp.163.2.325. [DOI] [PubMed] [Google Scholar]
- 30.Ray WA, Meredith S, Thapa PB, Hall K, Murray KT. Cyclic antidepressants and the risk of sudden cardiac death. Clin Pharmacol Ther. 2004;75:234–241. doi: 10.1016/j.clpt.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 31.Turner EH, Matthews AM, Linardatos E, Tell RA, Rosenthal R. Selective publication of antidepressant trials and its influence on apparent efficacy. N Engl J Med. 2008;358:252–260. doi: 10.1056/NEJMsa065779. [DOI] [PubMed] [Google Scholar]
- 32.Glassman AH, O’Connor CM, Califf RM, Swedberg K, Schwartz P, Bigger JT, Jr, Krishnan KR, van Zyl LT, Swenson JR, Finkel MS, Landau C, Shapiro PA, Pepine CJ, Mardekian J, Harrison WM, Barton D, Mclvor M. Sertraline Antidepressant Heart Attack Randomized Trial (SADHEART) Group. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA. 2002;288:701–109. doi: 10.1001/jama.288.6.701. [DOI] [PubMed] [Google Scholar]
- 33.Gehi A, Haas D, Pipkin S, Whooley MA. Depression and medication adherence in outpatients with coronary heart disease: findings from the Heart and Soul Study. Arch Intern Med. 2005;165:2508–2513. doi: 10.1001/archinte.165.21.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tew R, Guthrie EA, Creed FH, Cotter L, Kisely S, Tomenson B. A long-term follow-up study of patients with ischaemic heart disease versus patients with nonspecific chest pain. J Psychosom Res. 1995;39:977–985. doi: 10.1016/0022-3999(95)00065-8. [DOI] [PubMed] [Google Scholar]
- 35.Burg MM, Lampert R, Joska T, Batsford W, Jain D. Psychological traits and emotion-triggering of ICD shock-terminated arrhythmias. Psychosom Med. 2004;66:898–902. doi: 10.1097/01.psy.0000145822.15967.15. [DOI] [PubMed] [Google Scholar]
- 36.Blazer DG, Kessler RC, McGonagle KA, Swartz MS. The prevalence and distribution of major depression in a national community sample: the National Comorbidity Survey. Am J Psychiatry. 1994;151:979–986. doi: 10.1176/ajp.151.7.979. [DOI] [PubMed] [Google Scholar]