Abstract
The skin continuously encounters microbial pathogens. To defend against this, cells of the epidermis and dermis have evolved several innate strategies to prevent infection. Antimicrobial peptides are one of the primary mechanisms used by the skin in the early stages of immune defense. In general, antimicrobial peptides have broad antibacterial activity against gram-positive and negative bacteria and also show antifungal and antiviral activity. The antimicrobial activity of most peptides occurs as a result of unique structural characteristics that enable them to disrupt the microbial membrane while leaving human cell membranes intact. However, antimicrobial peptides also act on host cells to stimulate cytokine production, cell migration, proliferation, maturation, and extracellular matrix synthesis. The production by human skin of antimicrobial peptides such as defensins and cathelicidins occurs constitutively but also greatly increases after infection, inflammation or injury. Some skin diseases show altered expression of antimicrobial peptides, partially explaining the pathophysiology of these diseases. Thus, current research suggests that understanding how antimicrobial peptides modify susceptibility to microbes, influence skin inflammation, and modify wound healing, provides greater insight into the pathophysiology of skin disorders and offers new therapeutic opportunities.
Keywords: antimicrobial peptides, defensin, cathelicidin, dermcidin, innate immunity, skin diseases
The human epidermis forms a protective shield consisting of a dense mechanical barrier composed of the cornified envelope, keratin, and lipid layers to prevent invasion of microbes. However, resident cells of the epidermis not only form a passive mechanical barrier but also secret molecules to fight against exogenous pathogens. This initial defense response is defined as the “innate immune” response of the skin and has three major functions; 1) recognition of pathogens, 2) induction of molecules to activate host cells whose purpose is to eliminate pathogens, and 3) secretion of molecules to directly kill pathogens. This review will focus on one group of molecules that have important actions in both of the latter two functions of innate immunity, the “antimicrobial peptides”.
Antimicrobial peptides (AMPs)1 have been found in vertebrates and in vertebrates, and act in organisms with complex adaptive immune systems as well as those that lack an acquired immune system. Although the sequences of AMPs are variable, many peptides have broad-spectrum antimicrobial activity against bacteria, fungi, and viruses. These peptides are usually cationic and 20 to 60 amino acids in length. The cationic nature of these peptides allow them to bind to negatively charged bacterial membrane molecules such as lypopolysaccharude and lipoteichoic acid [1-4].
AMPs are usually organized structurally such that they have hydrophobic and hydrophilic sides. This enables them to interact in both the aqueous environment and the lipid-rich membrane. These peptides can act together with other proteins with antimicrobial activity, like lactoferrin, lysozyme, bactericidal/permeability-increasing protein, phospholipase A2 and antileukoproteas in neutrophils to increase the potency of the combinded mixture [5-7]. Extensive studies of these evolutionally conserved molecules have shown that they not only kill microbes but also have multiple functions against the host cells themselves. Therefore, the term AMP is in fact a bit of a misnomer, and many of the peptides in this group might be better called “alarmins” to recognize their capacity to alert host cells to the potential for infection or the presence of injury [8]. Continued progress in understanding new aspects of the behavior of these unique and ubiquitous small peptides is rapidly increasing our knowledge of the innate immune systems of many species, including humans. Human skin is a major source of AMPs. The AMPs produced in human skin include defensins, cathelicidins, dermcidin, and other short proteins first discovered for other biological activities such as neuropeptides and chemokines. Many other larger proteins with direct antimicrobial action also can be found in the skin such as lysozyme, elastase, complement, S100 proteins, and others. This paper summarizes AMPs identified in resident cells of the skin, and their functions in skin homeostasis and dynamics.
Defensins
The defensins are a group of small cationic peptides and are categorized in three subfamilies, α, β-, and circular θ-defensins. The consensus sequences of defensin mature peptides contain six conserved cysteines, which form three disulfides bridges and β-hairpin structures (figure 1) [9]. α- and β-defensins are distinguished by the position of three disulfides bridges; α—defensins have disulfides bridges between cysteines 1-6, 2—4, and 3-5, and β-defensins between cysteines 1-5, 2-4, and 3-6 [10, 11]. Six α- defensin peptides have been identified from 5 genes in human, HNP (human neutrophil peptide)-1 to -4, HD-5, and HD-6 [12]. HNP-1, -2, and 3 were first identified in neutrophils and are stored in azurophilic granules [13]. HNP-2 is a truncated form of HNP-1 or HNP-3 peptides and derived from HNP-1 or -3 genes [14]. α-defensins HNP-1, -2, and -3 are mainly produced by neutrophils and, to date, not isolated from human keratinocytes or cells derived from skin appendages. HD-5 and HD-6 are predominantly expressed in Paneth cells in small intestine [15]. Recent computational genomic approach revealed 5 additional α-defensin pseudogenes, DEFA7P ∼ DEFA11P and these genes products have not been identified so far [16]. Expression and function of four β-defensins (hBD-1 to 4) are well characterized, and keratinocytes express hBD-1 to 4. Computational genomic research predicts 28 new human β-defensins [17], and the expression of these genes in human skin cells is unknown. θ-defensins were primarily found in primates [18]. Although a θ-defensin pseudogene has been found in human bone marrow, the peptide has not been identified in humans to date [19].
Figure 1. Human defensin peptide sequences.
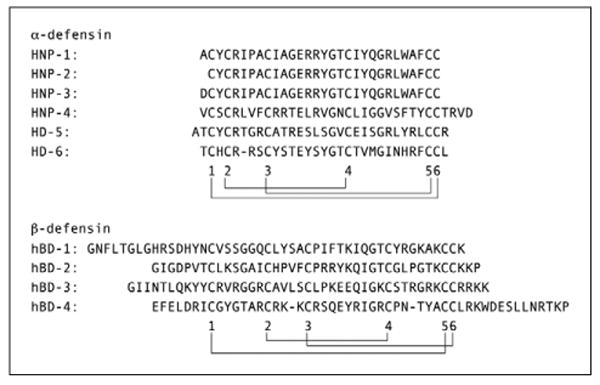
Alignment of the six human α-defensin peptides and four β-defensin peptides. The location of the six cysteins and the disulfide bond connectivity are indicated with digits and lines, respectively.
Regulation of defensin expression and activity
The genomic structure of defensins consists of two exons and one intron, and this translates to a proprotein consisting of an N-terminal signal peptide and C-terminal mature peptide [16, 20, 21]. HNP-1 and -3 genes (DEFA1 and DEFA3) and hBD-1 ∼ 4 genes are clustered in chromosome 8p23.1, which is known to be a frequent site of chromosomal rearrangements, and copy numbers of defensin genes vary with individuals [22, 23]. Genomic copy number of defensins correlate with levels of those messenger RNA transcript [22, 23]. hBD-1 constitutively expresses at low levels in human epidermis and sweat gland ducts [24-26], whereas hBD-2 to -3 are inducible in keratinocytes by bacterial infection, cytokines (IL-1α, IL-1β and TNF-α), and differentiation [27, 28]. hBD-2 ∼ 4 can also be induced by calcium and PMA (phorbol 12-myristate 13-acetate, and can be inhibited or suppressed by retinoic acid pretreatment, indicating retinoic acid is an important regulator of the innate immune system in epidermis [29]. Propionibacterium acnes and lipopolysaccharide induce hBD-2 in sebocytes [30]. Defensins are secreted as proproteins and post-translational processing cleaves out the C-terminal mature peptide from α–defensin proprotein [31]. Local proteases affect processing of defensins, and multiple forms of N-terminal truncated α- defensins have been identified in various tissue or cells [32-35]. Although defensin processing enzymes in human skin are unknown, the requirement for processing of the proprotein to activate AMP activity predicts that proteases in skin will affect the activity of defensins.
The consensus sequences of defensin mature peptides contain six conserved cysteines, which form three disulfides bridges and β-hairpin structures. Formation of disulfides bridges in hBD-3 is affected by the oxidative conditions and can affect its function as a chemoattractive molecule [36]. Crystallographic studies showed HNP-3 forms a symmetric dimer [37], and hBD-2 can further form an octamer [38], whereas hBD-1 forms asymmetric dimers and does not form higher oligomers though the monomer structure is similar with hBD-2 [39]. Further studies are required to elucidate precise relations between structural changes and functions of defensins. Genome wide search has predicted more than 30 β-defensins [17]. These peptides have a conserved cysteine motif, but the amino acid contents are variable. Potentially, these polymorphisms of hBD peptides might have served to adapt activity to various microbes.
Functions of defensins
α- and β-defensins show a broad antibacterial activity against gram-positive and negative bacteria [12, 13], and have antifungal activity [40, 41]. Binding of the positively charged defensin with the negatively charged bacterial membrane precedes membrane permeabilization and is thought to be the mechanism of bacterial killing by defensins. Defensins also have antiviral properties against adenovirus [42], papilloma virus [43], human immunodeficiency virus (HIV) [44, 45], and herpes simplex virus (HSV) [46]. Variable mechanisms have been proposed for the action of defensins against viral infection. hBD-2 and hBD-3 modulate cell surface CXCR4 coreceptor expression on immunocompetent cells and suppressed HIV infection in vitro [47, 48]. HSV infects cells using HSV glycoprotein B as a ligand to host surface heparan sulfate [49]. HNPs 1, 2, 3, and HD 5 bind HSV glycoprotein B, and HNP-4 and HD 6 bind heparan sulfate to inhibit HSV infection in vivo and in vitro [46]. hBD-3 binds both of HSV glycoprotein B and heparan sulfate [46]. HNPs 1, 2, 3, and HD 5 block virion escape of papilloma pseudoviruses from endocytic vesicles that lead microbes to lysosomes in vitro [43]. Thus, defensins inhibit multi steps of microbe infectivity with variable mechanisms.
Although directly antimicrobial in vitro, the effect of defensins on mammalian cells is an important component of how these peptides affect immunity. α- and β–defensins modify cell migration and maturation. β-defensins are chemoattractive for immature-dendritic cells and memory T-cells through chemokine receptor CCR6 activation [50]. Mouse β-defensin-29 induces angiogenesis by recruiting bone marrow-derived dendritic cells and inducing endothelial-like differentiation thorough CCR6 activation in tumor vasculization [51]. In contrast, α-defensin HNPs inhibit endothelial cell migration by inhibition of binding of the endothelial cell α5β1 integrin to fibronectin [52]. Murine β-defensin 2 acts on TLR-4 and induces dendritic cell maturation [53].
Defensins also induce cytokines and other molecules secreted from host cells. α -defensin [HNPs] up-regulate the expression of TNF-α and IL-1β in monocytes activated with Staphylococcus aureus [54]. Defensins induce IL-8 and proinflammatory cytokines in lung epithelial cells [55, 56]. hBD-1 ∼ 4 induce IL-18 in human primary keratinocytes [57]. HNP-1 and -4 induce histamine release from mast cells [58], and hBD-2 ∼ -4 induces histamine and prostaglandin D2 release from mast cells [59, 60]. Coadministration of α-defensin (HNP-1, -2) and β-defensin (hBD-1, -2) augments antigenspecific serum IgG production in mice immunized intra-nasally with the antigen ovalbumin [61, 62]. In sum, current evidence of the multiple functions of defensins in addition to their antimicrobial properties suggest that defensins work in both innate and adaptive immunity.
Cathelicidin
Cathelicidin is named for the conserved prosequence domain of the precursor protein that resembles the cathelin protein, originally isolated as a cathepsin L inhibitor [63]. The structure of the cathelicidin proprotein consists of a N-terminal signal domain, a highly conserved prosequence domain (cathelin domain), and the C-terminal peptide domain (figure 2A) [64]. Cathelicidin is secreted as a proprotein that consists of the cathelin domain and C-terminal cationic domain, and this proprotein is inactive as an antimicrobial molecule [65]. Post-transcriptional processing cleaves out the C-terminal peptide from the prosequence and makes the active AMPs [66, 67]. C-terminal peptides of cathelicidins in different species include β-sheets and linear peptides rich in proline or tryptophan but most, including the human cathelicidin LL-37, is an amphipathic cationic peptide deduced to be α-helical in some buffer conditions [68]. Most cathelicidin peptides form an α-helical structure, which has both a hydrophobic and a hydrophilic side (figure 2D). This amphipathic structure and cationic charge enables cathelicidin peptides to interact in the aqueous environment, the lipid-rich membrane, and bind negatively charged bacterial membranes.
Figure 2. Human cathelicidin hCAP18 proprotein structure and sequence.
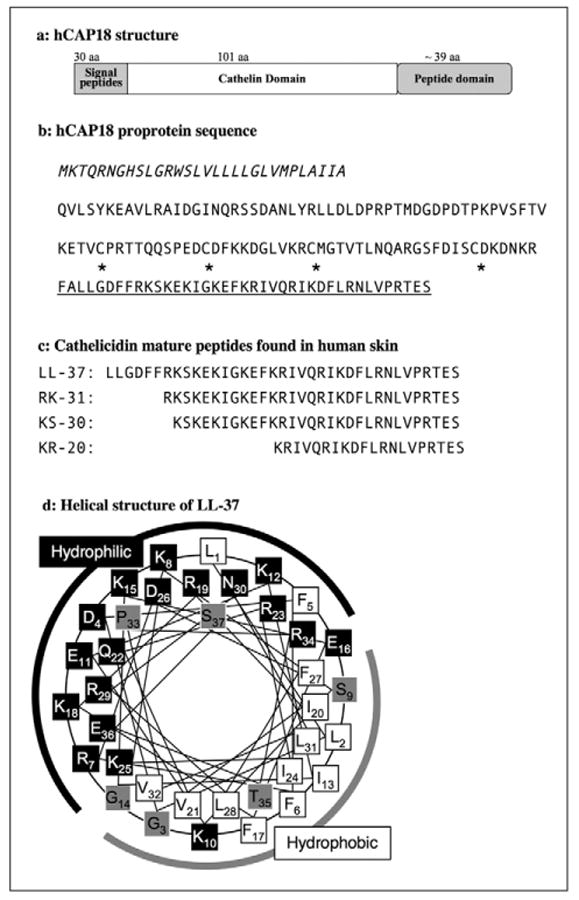
A) Schematic representation of the hCAP18 proprotein structure. B) Italic characters indicate signal peptide, and mature peptide domain of LL-37 is underlined. Asterisks indicate four cysteins, which are conserved in the cathelicidin proprotein between species and form disulfide bonds in the cathelin domain. Postsecretory processing generates cathelicidin mature peptides although the sizes and sequences of mature peptides are varied and depend on tissues or cell-specific proteases. C) Four representative human skin cathelicidin peptides LL-37, RK-31, KS-30, and KR-20 are listed. d: α-helical wheel of LL-37. Black boxes indicate hydrophilic amino acids, white boxes indicate hydrophobic amino acids, and gray boxes are amphiphilic amino acids [165]. Numeric numbers indicate the order in the peptide. A black line indicates the hydrophilic side and a gray line indicates the hydrophobic side of the peptides.
Regulation of cathelicidin activity
Cathelicidin expression and function is regulated by two major steps; transcription to mRNA and post-translational processing to active peptides. In the human genome, the cathelicidin exons 1-4 are found on chromosome 3p21. These are transcribed as a single gene, CAMP (cathelicidin antimicrobial peptide), which translates to a 18 kDa proprotein referred to as “hCAP18” (human cationic antimicrobial protein 18 kDa). The other nomenclature commonly used to describe the protein is “hCAP18/LL-37” because LL-37 was the first isolated mature peptide dominantly expressed in neutrophils [67, 69]. In human keratinocytes, cathelicidin is inducible with skin inflammation from basal expression levels that are low and barely detectible [70]. 1,25-dehydroxy vitamin D3 is a potent inducer of cathelicidin mRNA transcription and the presence of vitamin D3 seems to be essential to cathelicidin induction in skin infection and wounding [71-73]. hCAP18 is stored in lamellar bodies in keratinocytes and secreted in the granular to spinous layer of the epidermis [74]. After secretion, local proteases cleave the c-terminal peptides to form active AMPs. As the proteolytic activity of various cells and tissues differs, hCAP18 can be processed to multiple mature peptides in addition to the form LL-37 found in neutrophils. Nomenclature for these alternatively processed cathelicidin peptides follows the format set for LL-37 which defines the first two N-terminal amino acids and the length of peptide. For example, LL-37 consists of 37 amino acids starting with two leucines, cleaved in neutrophils from hCAP18 by proteinase 3 [67]. On the skin surface, SCTE (stratum corneum tryptic enzyme, kallikrein 5/hK5) first processes hCAP18 to LL-37 and a combination of SCTE and SCCE (stratum corneum chymotryptic protease, kallikrein 7/hK7) further process to smaller peptides known as RK-31 and KS-30 (figure 2C) [66, 75]. These peptides (RK-31 and KS-30) in human skin have increased antimicrobial activity and show different ability to induce cytokines than LL-37, suggesting postsecretory enzymatic processing is a key step to dictate the activity and function of cathelicidin peptides [75, 76].
Functions of cathelicidin
Human cathelicidin peptides have broad antimicrobial activity against gram positive and negative bacteria [77-79], vaccinia virus [80], and fungi [81, 82]. Cathelicidin peptides are cationic and, like defensins, thought to directly bind to the anionic cell wall and membrane of the microbe, increasing permeability of the microbes' cell wall [83, 84]. Human and Rabbit cathelicidin CAP18 has also been identified as a lipopolysaccharide (LPS)-binding protein, and pretreatment with cathelicidin peptides suppress LPS-dependent TNF-α expression from CD14(+) cells and protected mice from LPS lethality [4, 85, 86]. Furthermore, LL-37 can directly inhibit the function of TLR4 on dendritic cells, preventing their maturation as well as their response to LPS or other TLR4 ligands such as low molecular weight Hyaluronan [87, 88]. These observations, which unlike any other AMP have also been validated in vivo by mouse genetic models, suggests cathelicidin can both kill microbes and modulate the endotoxin response of the host [77, 87].
In addition to actions against microorganisms, LL-37 induces cellular signaling and activates keratinocytes and leukocytes. LL-37 is chemoattractive to neutrophils, monocytes, and T cells by activating the G-protein-coupled receptor FPRL1 (formyl peptide receptor-like 1) [89]. LL-37 also signals throught FPRL1 to induce angiogenesis [90]. Transactivation of the epidermal growth factor receptor (EGFR) by LL-37 was observed in human epidermal keratinocytes, and this induces keratinocyte migration [91]. Cathelicidin also induces proinflammatory cytokine secretion. LL-37 stimulates IL-8 secretion from human epidermal keratinocytes and airway epithelial cells via direct or indirect activation of epidermal growth factor receptor [76, 92]. LL-37 induces IL-18 via p38 and ERK1/2 MAP kinase pathway in keratinocytes [57]. Another G-protein-coupled receptor P2X7 can also be activated by LL-37, inducing IL-1β processing and release from LPS-primed monocytes [93]. These reports show the many functions of cathelicidin peptides to activate the innate immune system of host cells in response to microbes. An explanation for these diverse effects, acting through multiple specific cell surface receptors such as TLR4, EGFR, FPRL1 and P2X7, is that association of the LL- 37 peptide with the membrane surrounding the receptor, rather than specific binding to the receptor itself, which leads the structures changes on cell membrane and the observed cell activation events [87].
Dermcidin
Dermcidin (DCD) was identified in the eccrine gland [94]. Dermcidin gene and the mature peptide (principally DCD-1L, 47 aa) have been identified in humans, but not isolated from other species to date. In contrast to the defensins and cathelicidins, Dermcidin is constitutively secreted in human sweat and not inducible by skin injury or inflammation [95]. Dermcidin is also secreted as proprotein. Postsecretory processing by cathepsin D cleaves the peptide from the C-terminus of the proprotein, and dermcidin peptides are distributed to the skin surface with sweat [96]. YP-30 peptide, which consist of 30 aa and is derived from N-terminal side of dermcidin proprotein, works as a survival factor in developing neural cells and peripheral blood mononuclear cells in thymus [97], indicating dermcidin proprotein generates at least two functionally different peptides (figure 3A).
Figure 3. Human Dermcidin amino acid sequence.
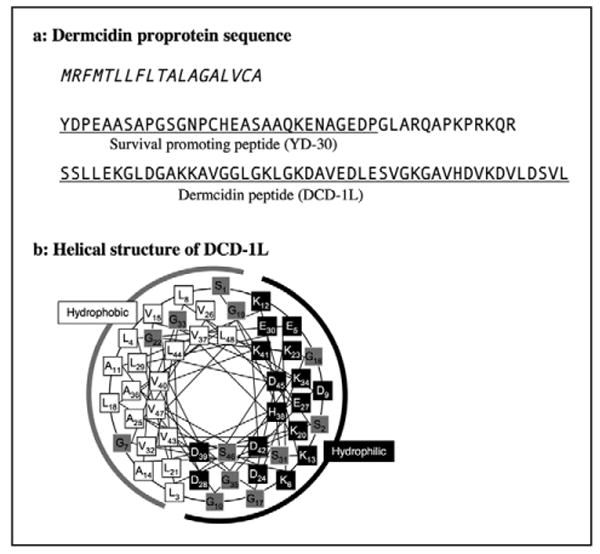
A) Italic characters indicate the signal peptide, and functional peptide domains are underlined. Dermcidin proprotein generates at least two different peptides; surviving promoting peptide (YD-30) and dermcidin antimicrobial peptide (DCD-1L). B) -helical wheel of DCD-1L. Black boxes indicate hydrophilic amino acids, white boxes indicate hydrophobic amino acids, and gray boxes are amphiphilic amino acids [165]. Numeric numbers indicate the order in the peptide. A black line indicates the hydrophilic side and a gray line indicates the hydrophobic side of the peptides.
Cathelicidin and defensins are cationic peptides, which bind to and permeabilize bacterial membranes. In contrast, DCD-1L is an anionic peptide, and the molecular mechanism to kill bacteria appears different from cationic AMPs. Steffen et al. tested several truncated dermcidin peptides and showed that net charge of peptides did not affect bactericidal activity [98]. Dermcidin peptides also form an α-helical structure like the cathelicidin peptide and binds to bacterial membranes, but do not permeabilize bacterial membranes (figure 3B) [98]. Lai et al. demonstrated recombinant dermcidin peptides could have flexible structure with α-helical and β-sheet structures depending on buffer condition [99]. The structure flexibility of dermcidin peptides might determine the antimicrobial activity, and dermcidin likely kills microbes by a completely different mechanism from cathelicidin and defensins. Lai et al. tested antimicrobial activity of recombinant DCD–1L with the condition of 10 mM sodium phosphate buffer (pH 6.5) with 100 mM sodium chloride and successfully showed the potent antimicrobial activity against S. aureus, E. coli, and C. albicans [99], however, synthetic dermcidin peptides have little antimicrobial activity when tested in systems alongside cathelicidins with the condition of 10% tryptic soy broth in 10 mM phosphate buffer (pH 7.2) [75]. Thus, Dermcidin peptides appear to be expressed specifically and exclusively in human skin eccrine glands, but the precise functions and molecular mechanisms of dermcidin to kill bacteria remain to be elucidated.
Other antimicrobial proteins/peptides
Defensins, cathelicidins and dermcidin are all true peptides that were first discovered for their action to kill microbes, thus they have been grouped into the functionally defined family called AMPs. However, exploration of the microbial defense mechanisms have revealed antimicrobial activity for many other proteins and peptides that were known first for other functions. These include proteinase inhibitors, chemokines, and neural protein/peptides. Human skin expresses many of these molecules (table 1)
Table 1.
Antimicrobial molecuels identified in human skin
| Skin components/cells | Antimicrobial molecules |
|---|---|
| Epidermis, hair follicle/keratinocyte | hBD-1 to -4 cathelicidin (LL-37, RK-31, KS-30, KR-20) RNase 7 S100A7/psoriasin lactoferrin/lactoferricin SLPI/ALP elafin/ESI/SKALP α-MSH (meranocyte stimulating hormone) [162, 163] |
| Sweat gland/eccrine cells | cathelicidin (RK-31, KS-30, KR-20) dermcidin hBD-1, 2 [25, 164] |
| Sebaceous gland/sebocyte | hBD-1, 2 [156] |
| Neutrophils (infiltrated) | α-defensins (HNP 1-4) cathelicidin (LL-37) lactoferrin/lactoferricin elastase |
| Mast cell | cathelicidin elastase |
RNase 7 was identified in fractions of human skin extracts, which had antimicrobial activity, and inducible by IL-1 β and IFN-γ in cultured keratinocytes [100]. RNase 7 exhibits broad-spectrum antimicrobial activity against Gram-positive bacteria (S. aureus, Propionibacterium acnes), Gram-negative bacteria (Pseudomonas aeruginosa, E. coli) and the yeast Candida albicans. RNase 7 has homology to RNase A superfamily and has RNase activity. Other members of RNase A family, such as RNase 2/EDN (eosinophil-derived neurotoxin), RNase 3/ECP (eosinophil-cationic protein), RNase 5/angiogenin (mouse Angiogenin-4), also have antimicrobial activity and would be secreted from infiltrated cells in skin [101-104].
Several groups independently discovered S100A7/psoriasin. It was identified as a low molecular weight protein that is highly expressed in psoriatic skin [105]. S100A7/psoriasin is inducible in keratinocytes by differentiation [106, 107]. Psoriasin was also identified as a retinoic acid-inducible skin-specific gene (RIS-1) in human skin [108], and reported as calcium- and zinc- binding protein [106]. Recently, Glaser et al. reported S100A7/psoriasin has antimicrobial activity and treatment of human skin by antibodies against S100A7/psoriasin inhibited skin antibacterial activity against E. coli [109], suggesting S100A7/psoriasin is one of the innate skin barrier on human skin surface.
Lactoferrin is a member of the transferrin family and involved in iron metabolisim [110, 111]. Lactoferrin and lactoferricin, peptides derived from the amino terminus of Lactoferrin, have been shown to have antimicrobial activity against bacteria and viruses [112, 113], and appear to be two of the molecules in the innate immune system of neonates. Lactoferrin is detectible in human skin epidermis and can induce Langerhans cell migration [114], suggesting lactoferrin and derivative peptides may play a part in both the innate and adaptive immune system of human skin.
Human epidermis and epidermal keratinocytes express serine protease inhibitors, and these protease inhibitors influence epidermal differentiation. Some of these serine proteases inhibitors also have antimicrobial activity. The secretory leukocyte protease inhibitor (SLPI)/antiluekoprotease (ALP) is a cationic protein consisting of 107 amino acids [115, 116]. SLPI/ALP inhibits leukocyte-derived proteinases, also has anti- HIV-1 activity [117], antibacterial function against E. coli and S. aureus [118], and antifungal properties against Aspergillus fumigatus and Candida albicans [119]. SLPI/ALP is constitutively expressed in the human epidermis and increased expression was observed in injured and psoriatic epidermis [120]. The neutrophil elastase inhibitor, elafin/elastase-specific inhibitor (ESI)/skin-derived antileukoprotease (SKALP), was identified in bronchial mucus, and inhibits human neutrophil elastase and proteinase-3 [121]. Elafin/SKALP was independently identified as a human skin elastase inhibitor [122] and secreted from cultured human keratinocytes [123]. Mature elafin/SKALP protein is 57 aa, and is generated by posttranscriptional processing from the carboxyl-terminus of a 95 aa proprotein [123]. Elafin/SKALP has antimicrobial properties against P. auerginosa and S. aureus [124], and is increased in injured human skin and lesional epidermis of psoriasis patients [125, 126]. In vitro studies show proinflamatory cytokines TNF-α and IL-1β increased elafin/SKALP secretion from human keratinocytes [127, 128]. Inducible and constitutively expressed SLPI/ALP and elafin/SKALP might work as antimicrobial molecules and proteinase inhibitors, which suppress microbial infectivity and modulate inflammatory reactions by proteinases secreted from infiltrating cells.
Antimicrobial protein/peptides in skin diseases
Altered expression of antimicrobial peptide/proteins has been recognized in some skin diseases and appears to play a role in determining susceptibility of patients with skin disorders to pathogens.
Psoriasis
Lesional skin of psoriasis patients increases expression of several antimicrobial molecules. Cathelicidin and hBD-2 mRNA and protein expression were observed to be increased in lesional skin of psoriasis patients [129, 130]. In fact, hBD-3, like hBD-2, was first identified in psoriatic scales [28]. HNP-1, -2, and 3 are detectible in psoriasis scale [131], though HNP-1-3 are exclusively produced by neutrophils and expression in human keratinocytes is not clear to date. S100A7 expression is elevated in psoriasis as named “psoriasin” [105]. SKALP/elafin and SLPI/ALP are upregulated in psoriatic epidermis [120, 125]. Increased antimicrobial molecule expression might explain the decrease susceptibility of psoriatic patients to infection [132].
Atopic dermatitis
In contrast to psoriatic patients, lesional skin of atopic dermatitis (AD) patients shows less expression of AMPs than would be predicted based on the inflammation and skin damage at this site [130]. Decreased hBD-2 and hBD-3 expression in atopic dermatitis was also observed, and Th-2 cytokines IL-4 and IL-13 suppress hBD-2 and hBD-3 mRNA induction by TNF-α in keratinocytes [130, 133]. Howell et al. reported elevated IL-10 gene expression in AD skin lesions, and treatment of AD skin explants with anti-IL-10 augmented the expression of both hBD-2 and LL-37 [134]. Reduced amounts of dermcidin expression in sweat from AD patients were also reported [135], as were multiple other AMPs as detected by gene microarray [136]. Decreased expression of these AMPs can explain the increased susceptibility of AD patients to skin infection. Association of single nucleotide polymorphisms (SNPs) in hBD-1 with atopic dermatitis have also been identified, though the biological role of these SNPs are not determined [137, 138].
Rosacea
Lesional skin of the rosacea has abnormally high cathelicidin expression [139]. Mass spectrometry analysis of these cathelicidin peptides revealed that rosacea patients process cathelicidin abnormally into forms of peptide that are not found in normal skin. The post-translational processing abnormality of these cathelicidin peptides was due to an increase in stratum corneum tryptic enzyme (SCTE/ kallikrein 5) [66, 139]. The significance of these observations was linked to the pathophysiology of this disorder since the peptides found in rosacea, but not those in normal skin, induced cytokine secretion from human keratinocytes and caused skin inflammation and telangiectacia in mice [139]. Therefore, this convergence of elevated antimicrobial peptide production and excessive enzymatic processing provides an explanation for the constellation of signs and symptoms in patients with this common skin disease. These observations are the first report to show that an abnormality of antimicrobial peptides can exacerbate inflammatory skin diseases.
Severe congenital neutropenia/Kostmann syndrome
Severe congenital neutropenia (SCN; or Kostmann syndrome, morbus Kostman) shows another example of AMP disfunction leading to human disease associated with increased infections. SCN is an autosomal recessive disorder characterized by a maturation arrest of myelopoiesis at the level of promyelocytes [140]. Although the treatment of SCN patients with recombinant human granulocyte colony stimulating factor (G-CSF) leads to a significant increase in circulating neutrophils followed by a clinical improvement, patients still have recurrent infections and periodontal disease. Examination of neutrophils from Kostmann patients revealed a lack of cathelicidin/LL-37 expression and reduced concentrations of α-defensins HNP1-3, although the oxidative burst function of these neutrophils was normal [141]. This indicates proper expression of AMPs is necessary for the antibiotic function of neutrophils.
Skin injury
AMPs were first discovered in skin by the analysis of skin wounds [1]. Cathelicidin is inducible in human epidermal keratinocytes at the wound site [142]. Growth factors that are induced during injury such as TGF-β and INF-γ induce the 1-hydroxylation of 25OH-Vitamin D, thus activating it, and enabling the greatly enhanced expression of several genes involved in the innate immune response including cathelicidin and TLR2 [143]. In addition, other genes such as insulin-like growth factor-1 and transforming growth factor-α can also induce a small increase in cathelicidin, hBD-3, and SLPI/ALP in cultured keratinocytes [144]. Infiltrated neutrophils, which constitutively express cathelicidin and α-defensins, are also a major source of AMPs in skin injury. Cole et al. reported an inhibition of neutrophil elastase prevents cathelicidin activation to mature AMPs and increases growth of bacteria in a porcine skin wound chamber assay [145]. The expression of hBD-2 is greatly decreased in the full-thickness burn wounds and burn fluid [146, 147]. Subcutaneous injection of Adenovirus vectors expressing human cathelicidin (hCAP18) reduced pseudomonas applied on rat burn wounds [148], and topical application of pig cathelicidin Protegrin-1 in rat burn wounds significantly improved bacterial clearance [149]. These reports suggest that AMP expression in wounds affects skin susceptibility to infection. AMPs not only kill bacteria but also promote wound healing itself. β-defensins hBD-2, -3, and -4 increase human keratinocyte migration and proliferation along with intracellular calcium mobilization and EGFR phosphorylation [150]. In contrast to increased cathelicidin expression in the acute wounds, Heilborn et al. demonstrated the absence of cathelicidin expression at the chronic wound stage. They further have shown that neutralizing antibody against LL-37 inhibited re-epithelization of wounded organcultured human skin [151]. α-defensin HNP-1 increased the expression of pro-alpha1 collagen and decreased the expression of matrix metalloproteinase-1 in cultured human fibroblasts, suggesting that HNP-1 promotes extracellular matrix deposition and controls its degradation [152]. Pig cathelicidin peptide PR-39 induces syndecan-1 and -4 in wounds, and these extracellular matrix molecules promote wound healing [1]. Application of LL-37 also promotes angiogenesis through activating FPRL1 (formyl peptide receptor-like 1) on endothelial cells, and mice lacking cathelicidin have decreased vascularization during wound repair [90]. These observations suggest that the induction and activation of AMPs after injury has a dual function, both preventing infection and assisting wound healing.
Viral infection
The cathelicidin antimicrobial peptide LL-37 is induced within the epidermis during the development of condyloma acuminatum and verruca vulgaris [153]. Although the significance of the increased AMP expression in skin viral infection is unclear, some reports suggest a correlation between decreased antimicrobial molecules and viral infections in the skin and keratinocytes. Human and mouse cathelicidin reduces vaccinia virus plaque formation in vitro and mice lacking cathelicidin showed more vaccinia pox formation than in control mice [80, 154]. Skin from AD patients with eczema herpeticum showed reduced expression of cathelicidin, and cathelicidin deficient mice showed higher levels of HSV-2 replication [154]. Decreased expression of defensins in the skin of AD patients also may explain their susceptibility to HSV infections [46]. These reports suggest patients with AD, which have increased viral infections as manifested by the characteristic clinical symptoms of eczema herpeticum and eczema vaccinatum, may be susceptible due to a deficiency of AMPs in the skin.
Other skin diseases with altered AMP expression
The expression of AMPs are typically increased in association with an inflammatory response, therefore it is not particularly surprising that an increase in AMPs have been reported in several inflammatory skin diseases. Higher amounts of cathelicidin is seen in systemic lupus erythematosus and nickel allergy [70]. LL-37 is increased in the neutrophils, eosinophils, and dendritic cells in the skin of erythema toxicum neonatorum patients [155]. Lesions of acne vulgaris have a marked increase of hBD-2 expression [156], potentially triggered by Propionibacterium acnes infection [157]. hBD-2 is also upregulated in superficial folliculitis [158]. hBD-1 ∼ -3 mRNA levels were higher in lesional skin of localized screloderma than unaffected skin and skin from healthy volunteers, and decreased hBD-1 and hBD-2 expression followed ultraviolet A1 phototherapy that resulted in clinical improvement of localized screloderma [159]. High expression of HNP-3 has been seen in cutaneous T-cell lymphoma patients [160]. Basal cell carcinoma (BCC) tumor showed increased hBD-2 and decreased hBD-1 mRNA expression compared to normal skin [161]. The precise functions of AMPs in these skin diseases remain to be elucidated.
Conclusion
Current accumulated knowledge has revealed that these small peptides, so-called AMPs, have various functions against microbes and host cells. Our primary understanding of these molecules as antimicrobials define them as a part of innate immunity, however, this might be an oversimplification of the function of the AMPs. AMPs affect the host by an incompletely understood mechanism that results in a change in cytokines, cell migration, cell proliferation, cell maturation, and extracellular matrix synthesis. It is reasonable to assume that AMPs act in part as an “alarmin” to participate in danger signals, and in part as a simple antibiotic shield. As AMPs are inducible, not only by microbial infection, but also by tissue injury, they represent a primary defense system of the skin. Also, as some of AMPs function to alter dendritic cell function, the AMPs act as a bridge between innate and acquired immunity, serving at the interface between evolutionarily ancient and modern immune programs. Clinically, the physiological significance of altered expression of many of the AMPs remains to be elucidated. Human skin constitutively expresses low amounts of β-defensins and cathelicidins, and many AMPs increase following infection, inflammation, injury, and epidermal differentiation. As cathelicidin expression in mouse skin modifies susceptibility to microbes, exploring of the function and dynamics of other AMPs in human skin will likely explain other important aspects of the pathology infectious and other skin diseases. The successful therapeutic application of AMPs will be the next challenge, as lack of or decreased AMP expression in atopic dermatitis, chronic wounds, and skin burns appears at least partially responsible for the susceptibility of these disorders to infection.
Acknowledgments
This work was supported by NIH R01-AI052453, R01-AR45676, The National Rosacea Society, and a VA Merit Award to RLG, the Association for Preventive Medicine of Japan to K.Y.
Footnotes
The abbreviations used are: AMP, antimicrobial peptide; HNP, human neutrophils peptide; HD, human defensin; hBD, human beta defensin; CAMP, cathelicidin antimicrobial peptide; mCRAMP, mouse cathelin-related antimicrobial peptide; hCAP18, human cationic antimicrobial protein 18 kDa; SCTE, stratum corneum tryptic enzyme (kallikrein 5/KLK5, hK5); SCCE, stratum corneum chymotryptic protease (kallikrein 7/KLK7, hK7); SLPI, secretory leukocyte protease inhibitor; ALP, antiluekoprotease; ESI, elastase-specific inhibitor, SKALP, skin-derived antileukoprotease; HIV, human immunodeficiency virus; HSV, herpes simplex virus; LPS, lypopolysaccharide; FPRL1, formyl peptide receptor-like 1; EGF, epidermal growth factor; AD, atopic dermatitis; SCN, severe congenital neutropenia;
References
- 1.Gallo RL, Ono M, Povsic T, Page C, Eriksson E, Klagsbrun M, Bernfield M. Syndecans, cell surface heparan sulfate proteoglycans, are induced by a prolinerich antimicrobial peptide from wounds. Proc Natl Acad Sci USA. 1994;91:11035–9. doi: 10.1073/pnas.91.23.11035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peschel A, Otto M, Jack RW, Kalbacher H, Jung G, Gotz F. Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J Biol Chem. 1999;274:8405–10. doi: 10.1074/jbc.274.13.8405. [DOI] [PubMed] [Google Scholar]
- 3.Kristian SA, Datta V, Weidenmaier C, Kansal R, Fedtke I, Peschel A, Gallo RL, Nizet V. D-alanylation of teichoic acids promotes group a streptococcus antimicrobial peptide resistance, neutrophil survival, and epithelial cell invasion. J Bacteriol. 2005;187:6719–25. doi: 10.1128/JB.187.19.6719-6725.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larrick JW, Hirata M, Balint RF, Lee J, Zhong J, Wright SC. Human CAP18: a novel antimicrobial lipopolysaccharide-binding protein. Infect Immun. 1995;63:1291–7. doi: 10.1128/iai.63.4.1291-1297.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ganz T, Weiss J. Antimicrobial peptides of phagocytes and epithelia. Semin Hematol. 1997;34:343–54. [PubMed] [Google Scholar]
- 6.Bals R, Wang X, Zasloff M, Wilson JM. The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc Natl Acad Sci USA. 1998;95:9541–6. doi: 10.1073/pnas.95.16.9541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sorensen O, Arnljots K, Cowland JB, Bainton DF, Borregaard N. The human antibacterial cathelicidin, hCAP-18, is synthesized in myelocytes and metamyelocytes and localized to specific granules in neutrophils. Blood. 1997;90:2796–803. [PubMed] [Google Scholar]
- 8.Oppenheim JJ, Yang D. Alarmins: chemotactic activators of immune responses. Curr Opin Immunol. 2005;17:359–65. doi: 10.1016/j.coi.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Lehrer RI, Lichtenstein AK, Ganz T. Defensins: Antimicrobial and Cytotoxic Peptides of Mammalian Cells. Annu Rev Immunol. 1993;11:105–28. doi: 10.1146/annurev.iy.11.040193.000541. [DOI] [PubMed] [Google Scholar]
- 10.Schneider JJ, Unholzer A, Schaller M, Schaefer-Korting M, Korting HC. Human defensins. J Mol Med. 2005;83:587. doi: 10.1007/s00109-005-0657-1. [DOI] [PubMed] [Google Scholar]
- 11.White SH, Wimley WC, Selsted ME. Structure, function, and membrane integration of defensins. Curr Opin Struct Biol. 1995;5:521. doi: 10.1016/0959-440x(95)80038-7. [DOI] [PubMed] [Google Scholar]
- 12.Ericksen B, Wu Z, Lu W, Lehrer RI. Antibacterial activity and specificity of the six human {alpha}-defensins. Antimicrob Agents Chemother. 2005;49:269–75. doi: 10.1128/AAC.49.1.269-275.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ganz T, Selsted ME, Szklarek D, Harwig SS, Daher K, Bainton DF, Lehrer RI. Defensins. Natural peptide antibiotics of human neutrophils. J Clin Invest. 1985;76:1427–35. doi: 10.1172/JCI112120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valore EV, Ganz T. Posttranslational processing of defensins in immature human myeloid cells. Blood. 1992;79:1538–44. [PubMed] [Google Scholar]
- 15.Mallow EB, Harris A, Salzman N, Russell JP, DeBerardinis RJ, Ruchelli E, Bevins CL. Human Enteric Defensins. J Biol Chem. 1996;271:4038–45. doi: 10.1074/jbc.271.8.4038. [DOI] [PubMed] [Google Scholar]
- 16.Patil A, Hughes AL, Zhang G. Rapid evolution and diversification of mammalian {alpha}-defensins as revealed by comparative analysis of rodent and primate genes. Physiol Genomics. 2004;20:1–11. doi: 10.1152/physiolgenomics.00150.2004. [DOI] [PubMed] [Google Scholar]
- 17.Schutte BC, Mitros JP, Bartlett JA, Walters JD, Jia HP, Welsh MJ, Casavant TL, McCray PB., Jr Discovery of five conserved beta-defensin gene clusters using a computational search strategy. Proc Natl Acad Sci USA. 2002;99:2129–33. doi: 10.1073/pnas.042692699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang YQ, Yuan J, Osapay G, Osapay K, Tran D, Miller CJ, Ouellette AJ, Selsted ME. A cyclic antimicrobial peptide produced in primate leukocytes by the ligation of two truncated alpha-defensins. Science. 1999;286:498–502. doi: 10.1126/science.286.5439.498. [DOI] [PubMed] [Google Scholar]
- 19.Cole AM, Hong T, Boo LM, Nguyen T, Zhao C, Bristol G, Zack JA, Waring AJ, Yang OO, Lehrer RI. Retrocyclin: a primate peptide that protects cells from infection by T- and M-tropic strains of HIV-1. Proc Natl Acad Sci USA. 2002;99:1813–8. doi: 10.1073/pnas.052706399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu L, Wang L, Jia HP, Zhao C, Heng HH, Schutte BC, McCray PB, Jr, Ganz T. Structure and mapping of the human beta-defensin HBD-2 gene and its expression at sites of inflammation. Gene. 1998;222:237–44. doi: 10.1016/s0378-1119(98)00480-6. [DOI] [PubMed] [Google Scholar]
- 21.Liu L, Zhao C, Heng HH, Ganz T. The human beta-defensin-1 and alphadefensins are encoded by adjacent genes: two peptide families with differing disulfide topology share a common ancestry. Genomics. 1997;43:316–20. doi: 10.1006/geno.1997.4801. [DOI] [PubMed] [Google Scholar]
- 22.Hollox EJ, Armour JA, Barber JC. Extensive normal copy number variation of a beta-defensin antimicrobial-gene cluster. Am J Hum Genet. 2003;73:591–600. doi: 10.1086/378157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aldred PM, Hollox EJ, Armour JA. Copy number polymorphism and expression level variation of the human alpha-defensin genes DEFA1 and DEFA3. Hum Mol Genet. 2005;14:2045–52. doi: 10.1093/hmg/ddi209. [DOI] [PubMed] [Google Scholar]
- 24.Fulton C, Anderson GM, Zasloff M, Bull R, Quinn AG. Expression of natural peptide antibiotics in human skin. Lancet. 1997;350:1750–1. doi: 10.1016/S0140-6736(05)63574-X. [DOI] [PubMed] [Google Scholar]
- 25.Ali RS, Falconer A, Ikram M, Bissett CE, Cerio R, Quinn AG. Expression of the peptide antibiotics human beta defensin-1 and human beta defensin-2 in normal human skin. J Invest Dermatol. 2001;117:106–11. doi: 10.1046/j.0022-202x.2001.01401.x. [DOI] [PubMed] [Google Scholar]
- 26.Zhao C, Wang I, Lehrer RI. Widespread expression of beta-defensin hBD-1 in human secretory glands and epithelial cells. FEBS Lett. 1996;396:319–22. doi: 10.1016/0014-5793(96)01123-4. [DOI] [PubMed] [Google Scholar]
- 27.Liu AY, Destoumieux D, Wong AV, Park CH, Valore EV, Liu L, Ganz T. Human beta-defensin-2 production in keratinocytes is regulated by interleukin-1, bacteria, and the state of differentiation. J Invest Dermatol. 2002;118:275–81. doi: 10.1046/j.0022-202x.2001.01651.x. [DOI] [PubMed] [Google Scholar]
- 28.Harder J, Bartels J, Christophers E, Schroder JM. Isolation and characterization of human beta -defensin-3, a novel human inducible peptide antibiotic. J Biol Chem. 2001;276:5707–13. doi: 10.1074/jbc.M008557200. [DOI] [PubMed] [Google Scholar]
- 29.Harder J, Meyer-Hoffert U, Wehkamp K, Schwichtenberg L, Schroder JM. Differential gene induction of human beta-defensins (hBD-1, -2, -3, and -4) in keratinocytes is inhibited by retinoic acid. J Invest Dermatol. 2004;123:522–9. doi: 10.1111/j.0022-202X.2004.23234.x. [DOI] [PubMed] [Google Scholar]
- 30.Nagy I, Pivarcsi A, Kis K, Koreck A, Bodai L, McDowell A, Seltmann H, Patrick S, Zouboulis CC, Kemeny L. Propionibacterium acnes and lipopolysaccharide induce the expression of antimicrobial peptides and proinflammatory cytokines/chemokines in human sebocytes. Microbes Infect. 2006;8:2195–205. doi: 10.1016/j.micinf.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 31.Ganz T, Liu L, Valore EV, Oren A. Posttranslational processing and targeting of transgenic human defensin in murine granulocyte, macrophage, fibroblast, and pituitary adenoma cell lines. Blood. 1993;82:641–50. [PubMed] [Google Scholar]
- 32.Bensch KW, Raida M, Magert HJ, Schulz-Knappe P, Forssmann WG. hBD-1: a novel beta-defensin from human plasma. FEBS Lett. 1995;368:331–5. doi: 10.1016/0014-5793(95)00687-5. [DOI] [PubMed] [Google Scholar]
- 33.Valore EV, Park CH, Quayle AJ, Wiles KR, McCray PB, Jr, Ganz T. Human beta-defensin-1: an antimicrobial peptide of urogenital tissues. J Clin Invest. 1998;101:1633–42. doi: 10.1172/JCI1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bals R, Wang X, Wu Z, Freeman T, Bafna V, Zasloff M, Wilson JM. Human beta-defensin 2 is a salt-sensitive peptide antibiotic expressed in human lung. J Clin Invest. 1998;102:874–80. doi: 10.1172/JCI2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diamond DL, Kimball JR, Krisanaprakornkit S, Ganz T, Dale BA. Detection of beta-defensins secreted by human oral epithelial cells. J Immunol Methods. 2001;256:65–76. doi: 10.1016/s0022-1759(01)00442-2. [DOI] [PubMed] [Google Scholar]
- 36.Wu Z, Hoover DM, Yang D, Boulegue C, Santamaria F, Oppenheim JJ, Lubkowski J, Lu W. Engineering disulfide bridges to dissect antimicrobial and chemotactic activities of human beta-defensin 3. Proc Natl Acad Sci USA. 2003;100:8880–5. doi: 10.1073/pnas.1533186100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hill CP, Yee J, Selsted ME, Eisenberg D. Crystal structure of defensin HNP-3, an amphiphilic dimer: mechanisms of membrane permeabilization. Science. 1991;251:1481–5. doi: 10.1126/science.2006422. [DOI] [PubMed] [Google Scholar]
- 38.Hoover DM, Rajashankar KR, Blumenthal R, Puri A, Oppenheim JJ, Chertov O, Lubkowski J. The structure of human beta-defensin-2 shows evidence of higher order oligomerization. J Biol Chem. 2000;275:32911–8. doi: 10.1074/jbc.M006098200. [DOI] [PubMed] [Google Scholar]
- 39.Hoover DM, Chertov O, Lubkowski J. The structure of human beta-defensin-1: new insights into structural properties of beta-defensins. J Biol Chem. 2001;276:39021–6. doi: 10.1074/jbc.M103830200. [DOI] [PubMed] [Google Scholar]
- 40.Wilde CG, Griffith JE, Marra MN, Snable JL, Scott RW. Purification and characterization of human neutrophil peptide 4, a novel member of the defensin family. J Biol Chem. 1989;264:11200–3. [PubMed] [Google Scholar]
- 41.Hoover DM, Wu Z, Tucker K, Lu W, Lubkowski J. Antimicrobial Characterization of Human {beta}-Defensin 3 Derivatives. Antimicrob Agents Chemother. 2003;47:2804–9. doi: 10.1128/AAC.47.9.2804-2809.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bastian A, Schafer H. Human alpha-defensin 1 (HNP-1) inhibits adenoviral infection in vitro. Regul Pept. 2001;101:157–61. doi: 10.1016/s0167-0115(01)00282-8. [DOI] [PubMed] [Google Scholar]
- 43.Buck CB, Day PM, Thompson CD, Lubkowski J, Lu W, Lowy DR, Schiller JT. Human alpha-defensins block papillomavirus infection. Proc Natl Acad Sci USA. 2006;103:1516–21. doi: 10.1073/pnas.0508033103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun L, Finnegan CM, Kish-Catalone T, Blumenthal R, Garzino-Demo P, La Terra Maggiore GM, Berrone S, Kleinman C, Wu Z, Abdelwahab S, Lu W, Garzino-Demo A. Human beta-defensins suppress human immunodeficiency virus infection: potential role in mucosal protection. J Virol. 2005;79:14318–29. doi: 10.1128/JVI.79.22.14318-14329.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang TL, Vargas J, Jr, DelPortillo A, Klotman ME. Dual role of alpha-defensin- 1 in anti-HIV-1 innate immunity. J Clin Invest. 2005;115:765–73. doi: 10.1172/JCI200521948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hazrati E, Galen B, Lu W, Wang W, Ouyang Y, Keller MJ, Lehrer RI, Herold BC. Human alpha- and beta-defensins block multiple steps in herpes simplex virus infection. J Immunol. 2006;177:8658–66. doi: 10.4049/jimmunol.177.12.8658. [DOI] [PubMed] [Google Scholar]
- 47.Quinones-Mateu ME, Lederman MM, Feng Z, Chakraborty B, Weber J, Rangel HR, Marotta ML, Mirza M, Jiang B, Kiser P, Medvik K, Sieg SF, Weinberg A. Human epithelial beta-defensins 2 and 3 inhibit HIV-1 replication. AIDS. 2003;17:F39–F48. doi: 10.1097/00002030-200311070-00001. [DOI] [PubMed] [Google Scholar]
- 48.Feng Z, Dubyak GR, Lederman MM, Weinberg A. Cutting edge: human beta defensin 3--a novel antagonist of the HIV-1 coreceptor CXCR4. J Immunol. 2006;177:782–6. doi: 10.4049/jimmunol.177.2.782. [DOI] [PubMed] [Google Scholar]
- 49.WuDunn D, Spear PG. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J Virol. 1989;63:52–8. doi: 10.1128/jvi.63.1.52-58.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang D, Chertov O, Bykovskaia SN, Chen Q, Buffo MJ, Shogan J, Anderson M, Schroder JM, Wang JM, Howard OM, Oppenheim JJ. Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286:525–8. doi: 10.1126/science.286.5439.525. [DOI] [PubMed] [Google Scholar]
- 51.Conejo-Garcia JR, Benencia F, Courreges MC, Kang E, Mohamed-Hadley A, Buckanovich RJ, Holtz DO, Jenkins A, Na H, Zhang L, Wagner DS, Katsaros D, Caroll R, Coukos G. Tumor-infiltrating dendritic cell precursors recruited by a beta-defensin contribute to vasculogenesis under the influence of Vegf-A. Nat Med. 2004;10:950–8. doi: 10.1038/nm1097. [DOI] [PubMed] [Google Scholar]
- 52.Chavakis T, Cines DB, Rhee JS, Liang OD, Schubert U, Hammes HP, Higazi AA, Nawroth PP, Preissner KT, Bdeir K. Regulation of neovascularization by human neutrophil peptides (alpha-defensins): a link between inflammation and angiogenesis. FASEB J. 2004;18:1306–8. doi: 10.1096/fj.03-1009fje. [DOI] [PubMed] [Google Scholar]
- 53.Biragyn A, Ruffini PA, Leifer CA, Klyushnenkova E, Shakhov A, Chertov O, Shirakawa AK, Farber JM, Segal DM, Oppenheim JJ, Kwak LW. Toll-like receptor 4-dependent activation of dendritic cells by beta-defensin 2. Science. 2002;298:1025–9. doi: 10.1126/science.1075565. [DOI] [PubMed] [Google Scholar]
- 54.Chaly YV, Paleolog EM, Kolesnikova TS, Tikhonov II, Petratchenko EV, Voitenok NN. Neutrophil alpha-defensin human neutrophil peptide modulates cytokine production in human monocytes and adhesion molecule expression in endothelial cells. Eur Cytokine Netw. 2000;11:257–66. [PubMed] [Google Scholar]
- 55.van Wetering S, Mannesse-Lazeroms SP, van Sterkenburg MA, Hiemstra PS. Neutrophil defensins stimulate the release of cytokines by airway epithelial cells: modulation by dexamethasone. Inflamm Res. 2002;51:8–15. doi: 10.1007/pl00000282. [DOI] [PubMed] [Google Scholar]
- 56.Van Wetering S, Mannesse-Lazeroms SP, Van Sterkenburg MA, Daha MR, Dijkman JH, Hiemstra PS. Effect of defensins on interleukin-8 synthesis in airway epithelial cells. Am J Physiol. 1997;272:L888–L896. doi: 10.1152/ajplung.1997.272.5.L888. [DOI] [PubMed] [Google Scholar]
- 57.Niyonsaba F, Ushio H, Nagaoka I, Okumura K, Ogawa H. The human betadefensins (-1, -2, -3, -4) and cathelicidin LL-37 induce IL-18 secretion through p38 and ERK MAPK activation in primary human keratinocytes. J Immunol. 2005;175:1776–84. doi: 10.4049/jimmunol.175.3.1776. [DOI] [PubMed] [Google Scholar]
- 58.Befus AD, Mowat C, Gilchrist M, Hu J, Solomon S, Bateman A. Neutrophil defensins induce histamine secretion from mast cells: mechanisms of action. J Immunol. 1999;163:947–53. [PubMed] [Google Scholar]
- 59.Niyonsaba F, Someya A, Hirata M, Ogawa H, Nagaoka I. Evaluation of the effects of peptide antibiotics human beta-defensins-1/-2 and LL-37 on histamine release and prostaglandin D(2) production from mast cells. Eur J Immunol. 2001;31:1066–75. doi: 10.1002/1521-4141(200104)31:4<1066::aid-immu1066>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 60.Chen X, Niyonsaba F, Ushio H, Hara M, Yokoi H, Matsumoto K, Saito H, Nagaoka I, Ikeda S, Okumura K, Ogawa H. Antimicrobial peptides human beta18 defensin (hBD)-3 and hBD-4 activate mast cells and increase skin vascular permeability. Eur J Immunol. 2007;37:434–44. doi: 10.1002/eji.200636379. [DOI] [PubMed] [Google Scholar]
- 61.Lillard JW, Jr, Boyaka PN, Chertov O, Oppenheim JJ, McGhee JR. Mechanisms for induction of acquired host immunity by neutrophil peptide defensins. Proc Natl Acad Sci USA. 1999;96:651–6. doi: 10.1073/pnas.96.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brogden KA, Heidari M, Sacco RE, Palmquist D, Guthmiller JM, Johnson GK, Jia HP, Tack BF, McCray PB. Defensin-induced adaptive immunity in mice and its potential in preventing periodontal disease. Oral Microbiol Immunol. 2003;18:95–9. doi: 10.1034/j.1399-302x.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- 63.Ritonja A, Kopitar M, Jerala R, Turk V. Primary structure of a new cysteine proteinase inhibitor from pig leucocytes. FEBS Lett. 1989;255:211–4. doi: 10.1016/0014-5793(89)81093-2. [DOI] [PubMed] [Google Scholar]
- 64.Zanetti M, Gennaro R, Romeo D. Cathelicidins: a novel protein family with a common proregion and a variable C-terminal antimicrobial domain. FEBS Lett. 1995;374:1–5. doi: 10.1016/0014-5793(95)01050-o. [DOI] [PubMed] [Google Scholar]
- 65.Zaiou M, Nizet V, Gallo RL. Antimicrobial and protease inhibitory functions of the human cathelicidin (hCAP18/LL-37) prosequence. J Invest Dermatol. 2003;120:810–6. doi: 10.1046/j.1523-1747.2003.12132.x. [DOI] [PubMed] [Google Scholar]
- 66.Yamasaki K, Schauber J, Coda A, Lin H, Dorschner RA, Schechter NM, Bonnart C, Descargues P, Hovnanian A, Gallo RL. Kallikrein-mediated proteolysis regulates the antimicrobial effects of cathelicidins in skin. FASEB J. 2006;20:2068–80. doi: 10.1096/fj.06-6075com. [DOI] [PubMed] [Google Scholar]
- 67.Sorensen OE, Follin P, Johnsen AH, Calafat J, Tjabringa GS, Hiemstra PS, Borregaard N. Human cathelicidin, hCAP-18, is processed to the antimicrobial peptide LL-37 by extracellular cleavage with proteinase 3. Blood. 2001;97:3951–9. doi: 10.1182/blood.v97.12.3951. [DOI] [PubMed] [Google Scholar]
- 68.Zelezetsky I, Pontillo A, Puzzi L, Antcheva N, Segat L, Pacor S, Crovella S, Tossi A. Evolution of the primate cathelicidin. Correlation between structural variations and antimicrobial activity. J Biol Chem. 2006;281:19861–71. doi: 10.1074/jbc.M511108200. [DOI] [PubMed] [Google Scholar]
- 69.Cowland JB, Johnsen AH, Borregaard N. hCAP-18, a cathelin/pro-bactenecin-like protein of human neutrophil specific granules. FEBS Lett. 1995;368:173–6. doi: 10.1016/0014-5793(95)00634-l. [DOI] [PubMed] [Google Scholar]
- 70.Frohm M, Agerberth B, Ahangari G, Stahle-Backdahl M, Liden S, Wigzell H, Gudmundsson GH. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J Biol Chem. 1997;272:15258–63. doi: 10.1074/jbc.272.24.15258. [DOI] [PubMed] [Google Scholar]
- 71.Schauber J, Dorschner RA, Coda AB, Buchau AS, Liu PT, Kiken D, Helfrich YR, Kang S, Elalieh HZ, Steinmeyer A, Zugel U, Bikle DD, Modlin RL, Gallo RL. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J Clin Invest. 2007;117:803–11. doi: 10.1172/JCI30142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schauber J, Dorschner RA, Yamasaki K, Brouha B, Gallo RL. Control of the innate epithelial antimicrobial response is cell-type specific and dependent on relevant microenvironmental stimuli. Immunology. 2006;118:509–19. doi: 10.1111/j.1365-2567.2006.02399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zugel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–3. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 74.Braff MH, Di Nardo A, Gallo RL. Keratinocytes store the antimicrobial peptide cathelicidin in lamellar bodies. J Invest Dermatol. 2005;124:394–400. doi: 10.1111/j.0022-202X.2004.23443.x. [DOI] [PubMed] [Google Scholar]
- 75.Murakami M, Lopez-Garcia B, Braff M, Dorschner RA, Gallo RL. Postsecretory processing generates multiple cathelicidins for enhanced topical antimicrobial defense. J Immunol. 2004;172:3070–7. doi: 10.4049/jimmunol.172.5.3070. [DOI] [PubMed] [Google Scholar]
- 76.Braff MH, Hawkins MA, Di Nardo A, Lopez-Garcia B, Howell MD, Wong C, Lin K, Streib JE, Dorschner R, Leung DY, Gallo RL. Structure-function relationships among human cathelicidin peptides: dissociation of antimicrobial properties from host immunostimulatory activities. J Immunol. 2005;174:4271–8. doi: 10.4049/jimmunol.174.7.4271. [DOI] [PubMed] [Google Scholar]
- 77.Nizet V, Ohtake T, Lauth X, Trowbridge J, Rudisill J, Dorschner RA, Pestonjamasp V, Piraino J, Huttner K, Gallo RL. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414:454–7. doi: 10.1038/35106587. [DOI] [PubMed] [Google Scholar]
- 78.Rosenberger CM, Gallo RL, Finlay BB. Interplay between antibacterial effectors: a macrophage antimicrobial peptide impairs intracellular Salmonella replication. Proc Natl Acad Sci USA. 2004;101:2422–7. doi: 10.1073/pnas.0304455101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Iimura M, Gallo RL, Hase K, Miyamoto Y, Eckmann L, Kagnoff MF. Cathelicidin mediates innate intestinal defense against colonization with epithelial adherent bacterial pathogens. J Immunol. 2005;174:4901–7. doi: 10.4049/jimmunol.174.8.4901. [DOI] [PubMed] [Google Scholar]
- 80.Howell MD, Jones JF, Kisich KO, Streib JE, Gallo RL, Leung DY. Selective killing of vaccinia virus by LL-37: implications for eczema vaccinatum. J Immunol. 2004;172:1763–7. doi: 10.4049/jimmunol.172.3.1763. [DOI] [PubMed] [Google Scholar]
- 81.Lopez-Garcia B, Lee PH, Yamasaki K, Gallo RL. Anti-fungal activity of cathelicidins and their potential role in Candida albicans skin infection. J Invest Dermatol. 2005;125:108–15. doi: 10.1111/j.0022-202X.2005.23713.x. [DOI] [PubMed] [Google Scholar]
- 82.Dorschner RA, Lopez-Garcia B, Massie J, Kim C, Gallo RL. Innate immune defense of the nail unit by antimicrobial peptides. J Am Acad Dermatol. 2004;50:343–8. doi: 10.1016/j.jaad.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 83.Henzler Wildman KA, Lee DK, Ramamoorthy A. Mechanism of lipid bilayer disruption by the human antimicrobial peptide, LL-37. Biochemistry. 2003;42:6545–58. doi: 10.1021/bi0273563. [DOI] [PubMed] [Google Scholar]
- 84.Gutsmann T, Hagge SO, Larrick JW, Seydel U, Wiese A. Interaction of CAP18- derived peptides with membranes made from endotoxins or phospholipids. Biophys J. 2001;80:2935–45. doi: 10.1016/S0006-3495(01)76259-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tossi A, Scocchi M, Skerlavaj B, Gennaro R. Identification and characterization of a primary antibacterial domain in CAP18, a lipopolysaccharide binding protein from rabbit leukocytes. FEBS Lett. 1994;339:108–12. doi: 10.1016/0014-5793(94)80395-1. [DOI] [PubMed] [Google Scholar]
- 86.Nagaoka I, Hirota S, Niyonsaba F, Hirata M, Adachi Y, Tamura H, Heumann D. Cathelicidin family of antibacterial peptides CAP18 and CAP11 inhibit the expression of TNF-alpha by blocking the binding of LPS to CD14(+) cells. J Immunol. 2001;167:3329–38. doi: 10.4049/jimmunol.167.6.3329. [DOI] [PubMed] [Google Scholar]
- 87.Di Nardo A, Braff MH, Taylor KR, Na C, Granstein RD, McInturff JE, Krutzik S, Modlin RL, Gallo RL. Cathelicidin Antimicrobial Peptides Block Dendritic Cell TLR4 Activation and Allergic Contact Sensitization. J Immunol. 2007;178:1829–34. doi: 10.4049/jimmunol.178.3.1829. [DOI] [PubMed] [Google Scholar]
- 88.Taylor KR, Trowbridge JM, Rudisill JA, Termeer CC, Simon JC, Gallo RL. Hyaluronan fragments stimulate endothelial recognition of injury through TLR4. J Biol Chem. 2004;279:17079–84. doi: 10.1074/jbc.M310859200. [DOI] [PubMed] [Google Scholar]
- 89.De Y, Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J, Oppenheim JJ, Chertov O. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptorlike 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000;192:1069–74. doi: 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Koczulla R, von Degenfeld G, Kupatt C, Krotz F, Zahler S, Gloe T, Issbrucker K, Unterberger P, Zaiou M, Lebherz C, Karl A, Raake P, Pfosser A, Boekstegers P, Welsch U, Hiemstra PS, Vogelmeier C, Gallo RL, Clauss M, Bals R. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J Clin Invest. 2003;111:1665–72. doi: 10.1172/JCI17545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tokumaru S, Sayama K, Shirakata Y, Komatsuzawa H, Ouhara K, Hanakawa Y, Yahata Y, Dai X, Tohyama M, Nagai H, Yang L, Higashiyama S, Yoshimura A, Sugai M, Hashimoto K. Induction of keratinocyte migration via transactivation of the epidermal growth factor receptor by the antimicrobial peptide LL-37. J Immunol. 2005;175:4662–8. doi: 10.4049/jimmunol.175.7.4662. [DOI] [PubMed] [Google Scholar]
- 92.Tjabringa GS, Aarbiou J, Ninaber DK, Drijfhout JW, Sorensen OE, Borregaard N, Rabe KF, Hiemstra PS. The antimicrobial peptide LL-37 activates innate immunity at the airway epithelial surface by transactivation of the epidermal growth factor receptor. J Immunol. 2003;171:6690–6. doi: 10.4049/jimmunol.171.12.6690. [DOI] [PubMed] [Google Scholar]
- 93.Elssner A, Duncan M, Gavrilin M, Wewers MD. A novel P2X7 receptor activator, the human cathelicidin-derived peptide LL37, induces IL-1 beta processing and release. J Immunol. 2004;172:4987–94. doi: 10.4049/jimmunol.172.8.4987. [DOI] [PubMed] [Google Scholar]
- 94.Schittek B, Hipfel R, Sauer B, Bauer J, Kalbacher H, Stevanovic S, Schirle M, Schroeder K, Blin N, Meier F, Rassner G, Garbe C. Dermcidin: a novel human antibiotic peptide secreted by sweat glands. Nat Immunol. 2001;2:1133–7. doi: 10.1038/ni732. [DOI] [PubMed] [Google Scholar]
- 95.Rieg S, Garbe C, Sauer B, Kalbacher H, Schittek B. Dermcidin is constitutively produced by eccrine sweat glands and is not induced in epidermal cells under inflammatory skin conditions. Br J Dermatol. 2004;151:534–9. doi: 10.1111/j.1365-2133.2004.06081.x. [DOI] [PubMed] [Google Scholar]
- 96.Baechle D, Flad T, Cansier A, Steffen H, Schittek B, Tolson J, Herrmann T, Dihazi H, Beck A, Mueller GA, Mueller M, Stevanovic S, Garbe C, Mueller CA, Kalbacher H. Cathepsin D is present in human eccrine sweat and involved in the postsecretory processing of the antimicrobial peptide DCD-1L. J Biol Chem. 2006;281:5406–15. doi: 10.1074/jbc.M504670200. [DOI] [PubMed] [Google Scholar]
- 97.Landgraf P, Sieg F, Wahle P, Meyer G, Kreutz MR, Pape HC. A maternal bloodborne factor promotes survival of the developing thalamus. FASEB J. 2005;19:225–7. doi: 10.1096/fj.04-1789fje. [DOI] [PubMed] [Google Scholar]
- 98.Steffen H, Rieg S, Wiedemann I, Kalbacher H, Deeg M, Sahl HG, Peschel A, Gotz F, Garbe C, Schittek B. Naturally processed dermcidin-derived peptides do not permeabilize bacterial membranes and kill microorganisms irrespective of their charge. Antimicrob Agents Chemother. 2006;50:2608–20. doi: 10.1128/AAC.00181-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lai YP, Peng YF, Zuo Y, Li J, Huang J, Wang LF, Wu ZR. Functional and structural characterization of recombinant dermcidin-1L, a human antimicrobial peptide. Biochem Biophys Res Commun. 2005;328:243–50. doi: 10.1016/j.bbrc.2004.12.143. [DOI] [PubMed] [Google Scholar]
- 100.Harder J, Schroder JM. RNase 7, a novel innate immune defense antimicrobial protein of healthy human skin. J Biol Chem. 2002;277:46779–84. doi: 10.1074/jbc.M207587200. [DOI] [PubMed] [Google Scholar]
- 101.Lehrer RI, Szklarek D, Barton A, Ganz T, Hamann KJ, Gleich GJ. Antibacterial properties of eosinophil major basic protein and eosinophil cationic protein. J Immunol. 1989;142:4428–34. [PubMed] [Google Scholar]
- 102.Domachowske JB, Dyer KD, Bonville CA, Rosenberg HF. Recombinant human eosinophil-derived neurotoxin/RNase 2 functions as an effective antiviral agent against respiratory syncytial virus. J Infect Dis. 1998;177:1458–64. doi: 10.1086/515322. [DOI] [PubMed] [Google Scholar]
- 103.Domachowske JB, Dyer KD, Adams AG, Leto TL, Rosenberg HF. Eosinophil cationic protein/RNase 3 is another RNase A-family ribonuclease with direct antiviral activity. Nucleic Acids Res. 1998;26:3358–63. doi: 10.1093/nar/26.14.3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hooper LV, Stappenbeck TS, Hong CV, Gordon JI. Angiogenins: a new class of microbicidal proteins involved in innate immunity. Nat Immunol. 2003;4:269–73. doi: 10.1038/ni888. [DOI] [PubMed] [Google Scholar]
- 105.Madsen P, Rasmussen HH, Leffers H, Honore B, Dejgaard K, Olsen E, Kiil J, Walbum E, Andersen AH, Basse B, et al. Molecular cloning, occurrence, and expression of a novel partially secreted protein “psoriasin” that is highly upregulated in psoriatic skin. J Invest Dermatol. 1991;97:701–12. doi: 10.1111/1523-1747.ep12484041. [DOI] [PubMed] [Google Scholar]
- 106.Hoffmann HJ, Olsen E, Etzerodt M, Madsen P, Thogersen HC, Kruse T, Celis JE. Psoriasin binds calcium and is upregulated by calcium to levels that resemble those observed in normal skin. J Invest Dermatol. 1994;103:370–5. doi: 10.1111/1523-1747.ep12395202. [DOI] [PubMed] [Google Scholar]
- 107.Martinsson H, Yhr M, Enerback C. Expression patterns of S100A7 (psoriasin) and S100A9 (calgranulin-B) in keratinocyte differentiation. Exp Dermatol. 2005;14:161–8. doi: 10.1111/j.0906-6705.2005.00239.x. [DOI] [PubMed] [Google Scholar]
- 108.Tavakkol A, Zouboulis CC, Duell EA, Voorhees JJ. A retinoic acid-inducible skin-specific gene (RIS-1/psoriasin): molecular cloning and analysis of gene expression in human skin in vivo and cultured skin cells in vitro. Mol Biol Rep. 1994;20:75–83. doi: 10.1007/BF00996356. [DOI] [PubMed] [Google Scholar]
- 109.Glaser R, Harder J, Lange H, Bartels J, Christophers E, Schroder JM. Antimicrobial psoriasin (S100A7) protects human skin from Escherichia coli infection. Nat Immunol. 2005;6:57–64. doi: 10.1038/ni1142. [DOI] [PubMed] [Google Scholar]
- 110.Ward PP, Conneely OM. Lactoferrin: role in iron homeostasis and host defense against microbial infection. Biometals. 2004;17:203–8. doi: 10.1023/b:biom.0000027693.60932.26. [DOI] [PubMed] [Google Scholar]
- 111.Baker HM, Baker EN. Lactoferrin and iron: structural and dynamic aspects of binding and release. Biometals. 2004;17:209–16. doi: 10.1023/b:biom.0000027694.40260.70. [DOI] [PubMed] [Google Scholar]
- 112.Valenti P, Antonini G. Lactoferrin: an important host defence against microbial and viral attack. Cell Mol Life Sci. 2005;62:2576–87. doi: 10.1007/s00018-005-5372-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Orsi N. The antimicrobial activity of lactoferrin: current status and perspectives. Biometals. 2004;17:189–96. doi: 10.1023/b:biom.0000027691.86757.e2. [DOI] [PubMed] [Google Scholar]
- 114.Cumberbatch M, Dearman RJ, Uribe-Luna S, Headon DR, Ward PP, Conneely OM, Kimber I. Regulation of epidermal Langerhans cell migration by lactoferrin. Immunology. 2000;100:21–8. doi: 10.1046/j.1365-2567.2000.00014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Heinzel R, Appelhans H, Gassen G, Seemuller U, Machleidt W, Fritz H, Steffens G. Molecular cloning and expression of cDNA for human antileukoprotease from cervix uterus. Eur J Biochem. 1986;160:61–7. doi: 10.1111/j.1432-1033.1986.tb09940.x. [DOI] [PubMed] [Google Scholar]
- 116.Thompson RC, Ohlsson K. Isolation, properties, and complete amino acid sequence of human secretory leukocyte protease inhibitor, a potent inhibitor of leukocyte elastase. Proc Natl Acad Sci USA. 1986;83:6692–6. doi: 10.1073/pnas.83.18.6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.McNeely TB, Dealy M, Dripps DJ, Orenstein JM, Eisenberg SP, Wahl SM. Secretory leukocyte protease inhibitor: a human saliva protein exhibiting antihuman immunodeficiency virus 1 activity in vitro. J Clin Invest. 1995;96:456–64. doi: 10.1172/JCI118056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hiemstra PS, Maassen RJ, Stolk J, Heinzel-Wieland R, Steffens GJ, Dijkman JH. Antibacterial activity of antileukoprotease. Infect Immun. 1996;64:4520–4. doi: 10.1128/iai.64.11.4520-4524.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tomee JF, Hiemstra PS, Heinzel-Wieland R, Kauffman HF. Antileukoprotease: an endogenous protein in the innate mucosal defense against fungi. J Infect Dis. 1997;176:740–7. doi: 10.1086/514098. [DOI] [PubMed] [Google Scholar]
- 120.Wingens M, van Bergen BH, Hiemstra PS, Meis JF, van Vlijmen-Willems IM, Zeeuwen PL, Mulder J, Kramps HA, van Ruissen F, Schalkwijk J. Induction of SLPI (ALP/HUSI-I) in epidermal keratinocytes. J Invest Dermatol. 1998;111:996–1002. doi: 10.1046/j.1523-1747.1998.00425.x. [DOI] [PubMed] [Google Scholar]
- 121.Hochstrasser K, Albrecht GJ, Schonberger OL, Rasche B, Lempart K. An elastase-specific inhibitor from human bronchial mucus. Isolation and characterization. Hoppe Seylers Z Physiol Chem. 1981;362:1369–75. doi: 10.1515/bchm2.1981.362.2.1369. [DOI] [PubMed] [Google Scholar]
- 122.Wiedow O, Schroder JM, Gregory H, Young JA, Christophers E. Elafin: an elastase-specific inhibitor of human skin. Purification, characterization, and complete amino acid sequence. J Biol Chem. 1990;265:14791–5. [PubMed] [Google Scholar]
- 123.Molhuizen HO, Alkemade HA, Zeeuwen PL, de Jongh GJ, Wieringa B, Schalkwijk J. SKALP/elafin: an elastase inhibitor from cultured human keratinocytes. Purification, cDNA sequence, and evidence for transglutaminase cross-linking. J Biol Chem. 1993;268:12028–32. [PubMed] [Google Scholar]
- 124.Simpson AJ, Maxwell AI, Govan JR, Haslett C, Sallenave JM. Elafin (elastasespecific inhibitor) has anti-microbial activity against gram-positive and gramnegative respiratory pathogens. FEBS Lett. 1999;452:309–13. doi: 10.1016/s0014-5793(99)00670-5. [DOI] [PubMed] [Google Scholar]
- 125.Nonomura K, Yamanishi K, Yasuno H, Nara K, Hirose S. Upregulation of elafin/SKALP gene expression in psoriatic epidermis. J Invest Dermatol. 1994;103:88–91. doi: 10.1111/1523-1747.ep12391802. [DOI] [PubMed] [Google Scholar]
- 126.Alkemade JA, Molhuizen HO, Ponec M, Kempenaar JA, Zeeuwen PL, de Jongh GJ, van Vlijmen-Willems IM, van Erp PE, van de Kerkhof PC, Schalkwijk J. SKALP/elafin is an inducible proteinase inhibitor in human epidermal keratinocytes. J Cell Sci. 1994;107(Pt 8):2335–42. doi: 10.1242/jcs.107.8.2335. [DOI] [PubMed] [Google Scholar]
- 127.Tanaka N, Fujioka A, Tajima S, Ishibashi A, Hirose S. Elafin is induced in epidermis in skin disorders with dermal neutrophilic infiltration: interleukin-1 beta and tumour necrosis factor-alpha stimulate its secretion in vitro. Br J Dermatol. 2000;143:728–32. doi: 10.1046/j.1365-2133.2000.03766.x. [DOI] [PubMed] [Google Scholar]
- 128.Pfundt R, Wingens M, Bergers M, Zweers M, Frenken M, Schalkwijk J. TNFalpha and serum induce SKALP/elafin gene expression in human keratinocytes by a p38 MAP kinase-dependent pathway. Arch Dermatol Res. 2000;292:180–7. doi: 10.1007/s004030050475. [DOI] [PubMed] [Google Scholar]
- 129.Harder J, Bartels J, Christophers E, Schroder JM. A peptide antibiotic from human skin. Nature. 1997;387:861. doi: 10.1038/43088. [DOI] [PubMed] [Google Scholar]
- 130.Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, Gallo RL, Leung DY. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151–60. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- 131.Harder J, Schroder JM. Psoriatic scales: a promising source for the isolation of human skin-derived antimicrobial proteins. J Leukoc Biol. 2005;77:476–86. doi: 10.1189/jlb.0704409. [DOI] [PubMed] [Google Scholar]
- 132.Christophers E, Henseler T. Contrasting disease patterns in psoriasis and atopic dermatitis. Arch Dermatol Res. 1987;279(Suppl):S48–S51. doi: 10.1007/BF00585919. [DOI] [PubMed] [Google Scholar]
- 133.Nomura I, Goleva E, Howell MD, Hamid QA, Ong PY, Hall CF, Darst MA, Gao B, Boguniewicz M, Travers JB, Leung DY. Cytokine milieu of atopic dermatitis, as compared to psoriasis, skin prevents induction of innate immune response genes. J Immunol. 2003;171:3262–9. doi: 10.4049/jimmunol.171.6.3262. [DOI] [PubMed] [Google Scholar]
- 134.Howell MD, Novak N, Bieber T, Pastore S, Girolomoni G, Boguniewicz M, Streib J, Wong C, Gallo RL, Leung DY. Interleukin-10 downregulates antimicrobial peptide expression in atopic dermatitis. J Invest Dermatol. 2005;125:738–45. doi: 10.1111/j.0022-202X.2005.23776.x. [DOI] [PubMed] [Google Scholar]
- 135.Rieg S, Steffen H, Seeber S, Humeny A, Kalbacher H, Dietz K, Garbe C, Schittek B. Deficiency of dermcidin-derived antimicrobial peptides in sweat of patients with atopic dermatitis correlates with an impaired innate defense of human skin in vivo. J Immunol. 2005;174:8003–10. doi: 10.4049/jimmunol.174.12.8003. [DOI] [PubMed] [Google Scholar]
- 136.de Jongh GJ, Zeeuwen PL, Kucharekova M, Pfundt R, van der Valk PG, Blokx W, Dogan A, Hiemstra PS, van de Kerkhof PC, Schalkwijk J. High expression levels of keratinocyte antimicrobial proteins in psoriasis compared with atopic dermatitis. J Invest Dermatol. 2005;125:1163–73. doi: 10.1111/j.0022-202X.2005.23935.x. [DOI] [PubMed] [Google Scholar]
- 137.Prado-Montes de Oca E, Garcia-Vargas A, Lozano-Inocencio R, Gallegos-Arreola MP, Sandoval-Ramirez L, Davalos-Rodriguez NO, Figuera LE. Association of beta-defensin 1 single nucleotide polymorphisms with atopic dermatitis. Int Arch Allergy Immunol. 2007;142:211–8. doi: 10.1159/000097023. [DOI] [PubMed] [Google Scholar]
- 138.Leung TF, Li CY, Liu EK, Tang NL, Chan IH, Yung E, Wong GW, Lam CW. Asthma and atopy are associated with DEFB1 polymorphisms in Chinese children. Genes Immun. 2006;7:59–64. doi: 10.1038/sj.gene.6364279. [DOI] [PubMed] [Google Scholar]
- 139.Yamasaki K, Di Nardo A, Bardan A, Murakami M, Ohtake T, Coda A, Dorschner RA, Bonnart C, Descargues P, Hovnanian A, Morhenn VB, Gallo RL. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med. 2007 doi: 10.1038/nm1616. [DOI] [PubMed] [Google Scholar]
- 140.Kostmann R. Infantile genetic agranulocytosis (agranulocytosis infantalis hereditaria): A new recessive lethal disease in man. Acta Paediatr Scand. 1956;45:1. [PubMed] [Google Scholar]
- 141.Putsep K, Carlsson G, Boman HG, Andersson M. Deficiency of antibacterial peptides in patients with morbus Kostmann: an observation study. Lancet. 2002;360:1144–9. doi: 10.1016/S0140-6736(02)11201-3. [DOI] [PubMed] [Google Scholar]
- 142.Dorschner RA, Pestonjamasp VK, Tamakuwala S, Ohtake T, Rudisill J, Nizet V, Agerberth B, Gudmundsson GH, Gallo RL. Cutaneous injury induces the release of cathelicidin anti-microbial peptides active against group A Streptococcus. J Invest Dermatol. 2001;117:91–7. doi: 10.1046/j.1523-1747.2001.01340.x. [DOI] [PubMed] [Google Scholar]
- 143.Schauber J, Dorschner R, Coda A, Buchau A, Liu P, Kiken D, Helfrich Y, Kang S, Elalieh H, Steinmeyer A, Zugel U, Bikle D, Modlin R, Gallo R. Injury 24 enhances TLR2 function and anitmicrobial peptide expression through a vitamin D-dependent machanism. J Clin Invest. 2007 doi: 10.1172/JCI30142. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sorensen OE, Cowland JB, Theilgaard-Monch K, Liu L, Ganz T, Borregaard N. Wound healing and expression of antimicrobial peptides/polypeptides in human keratinocytes, a consequence of common growth factors. J Immunol. 2003;170:5583–9. doi: 10.4049/jimmunol.170.11.5583. [DOI] [PubMed] [Google Scholar]
- 145.Cole AM, Shi J, Ceccarelli A, Kim YH, Park A, Ganz T. Inhibition of neutrophil elastase prevents cathelicidin activation and impairs clearance of bacteria from wounds. Blood. 2001;97:297–304. doi: 10.1182/blood.v97.1.297. [DOI] [PubMed] [Google Scholar]
- 146.Ortega MR, Ganz T, Milner SM. Human beta defensin is absent in burn blister fluid. Burns. 2000;26:724–6. doi: 10.1016/s0305-4179(00)00052-8. [DOI] [PubMed] [Google Scholar]
- 147.Milner SM, Ortega MR. Reduced antimicrobial peptide expression in human burn wounds. Burns. 1999;25:411–3. doi: 10.1016/s0305-4179(98)00192-2. [DOI] [PubMed] [Google Scholar]
- 148.Jacobsen F, Mittler D, Hirsch T, Gerhards A, Lehnhardt M, Voss B, Steinau HU, Steinstraesser L. Transient cutaneous adenoviral gene therapy with human host defense peptide hCAP-18/LL-37 is effective for the treatment of burn wound infections. Gene Ther. 2005;12:1494–502. doi: 10.1038/sj.gt.3302568. [DOI] [PubMed] [Google Scholar]
- 149.Steinstraesser L, Klein RD, Aminlari A, Fan MH, Khilanani V, Remick DG, Su GL, Wang SC. Protegrin-1 enhances bacterial killing in thermally injured skin. Crit Care Med. 2001;29:1431–7. doi: 10.1097/00003246-200107000-00022. [DOI] [PubMed] [Google Scholar]
- 150.Niyonsaba F, Ushio H, Nakano N, Ng W, Sayama K, Hashimoto K, Nagaoka I, Okumura K, Ogawa H. Antimicrobial Peptides Human beta-Defensins Stimulate Epidermal Keratinocyte Migration, Proliferation and Production of Proinflammatory Cytokines and Chemokines. J Invest Dermatol. 2006 doi: 10.1038/sj.jid.5700599. [DOI] [PubMed] [Google Scholar]
- 151.Heilborn JD, Nilsson MF, Kratz G, Weber G, Sorensen O, Borregaard N, Stahle-Backdahl M. The cathelicidin anti-microbial peptide LL-37 is involved in reepithelialization of human skin wounds and is lacking in chronic ulcer epithelium. J Invest Dermatol. 2003;120:379–89. doi: 10.1046/j.1523-1747.2003.12069.x. [DOI] [PubMed] [Google Scholar]
- 152.Oono T, Shirafuji Y, Huh WK, Akiyama H, Iwatsuki K. Effects of human neutrophil peptide-1 on the expression of interstitial collagenase and type I collagen in human dermal fibroblasts. Arch Dermatol Res. 2002;294:185–9. doi: 10.1007/s00403-002-0310-6. [DOI] [PubMed] [Google Scholar]
- 153.Conner K, Nern K, Rudisill J, O'Grady T, Gallo RL. The antimicrobial peptide LL-37 is expressed by keratinocytes in condyloma acuminatum and verruca vulgaris. J Am Acad Dermatol. 2002;47:347–50. doi: 10.1067/mjd.2002.122190. [DOI] [PubMed] [Google Scholar]
- 154.Howell MD, Wollenberg A, Gallo RL, Flaig M, Streib JE, Wong C, Pavicic T, Boguniewicz M, Leung DY. Cathelicidin deficiency predisposes to eczema herpeticum. J Allergy Clin Immunol. 2006;117:836–41. doi: 10.1016/j.jaci.2005.12.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Marchini G, Lindow S, Brismar H, Stabi B, Berggren V, Ulfgren AK, Lonne-Rahm S, Agerberth B, Gudmundsson GH. The newborn infant is protected by an innate antimicrobial barrier: peptide antibiotics are present in the skin and vernix caseosa. Br J Dermatol. 2002;147:1127–34. doi: 10.1046/j.1365-2133.2002.05014.x. [DOI] [PubMed] [Google Scholar]
- 156.Chronnell CM, Ghali LR, Ali RS, Quinn AG, Holland DB, Bull JJ, Cunliffe WJ, McKay IA, Philpott MP, Muller-Rover S. Human beta defensin-1 and -2 expression in human pilosebaceous units: upregulation in acne vulgaris lesions. J Invest Dermatol. 2001;117:1120–5. doi: 10.1046/j.0022-202x.2001.01569.x. [DOI] [PubMed] [Google Scholar]
- 157.Nagy I, Pivarcsi A, Koreck A, Szell M, Urban E, Kemeny L. Distinct strains of Propionibacterium acnes induce selective human beta-defensin-2 and interleukin- 8 expression in human keratinocytes through toll-like receptors. J Invest Dermatol. 2005;124:931–8. doi: 10.1111/j.0022-202X.2005.23705.x. [DOI] [PubMed] [Google Scholar]
- 158.Oono T, Huh WK, Shirafuji Y, Akiyama H, Iwatsuki K. Localization of human beta-defensin-2 and human neutrophil peptides in superficial folliculitis. Br J Dermatol. 2003;148:188–91. doi: 10.1046/j.1365-2133.2003.509915.x. [DOI] [PubMed] [Google Scholar]
- 159.Kreuter A, Hyun J, Skrygan M, Sommer A, Bastian A, Altmeyer P, Gambichler T. Ultraviolet A1-induced downregulation of human beta-defensins and interleukin-6 and interleukin-8 correlates with clinical improvement in localized scleroderma. Br J Dermatol. 2006;155:600–7. doi: 10.1111/j.1365-2133.2006.07391.x. [DOI] [PubMed] [Google Scholar]
- 160.Escher N, Spies-Weisshart B, Kaatz M, Melle C, Bleul A, Driesch D, Wollina U, von Eggeling F. Identification of HNP3 as a tumour marker in CD4+ and CD4- lymphocytes of patients with cutaneous T-cell lymphoma. Eur J Cancer. 2006;42:249–55. doi: 10.1016/j.ejca.2005.07.033. [DOI] [PubMed] [Google Scholar]
- 161.Gambichler T, Skrygan M, Huyn J, Bechara FG, Sand M, Altmeyer P, Kreuter A. Pattern of mRNA expression of beta-defensins in basal cell carcinoma. BMC Cancer. 2006;6:163. doi: 10.1186/1471-2407-6-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Schauer E, Trautinger F, Kock A, Schwarz A, Bhardwaj R, Simon M, Ansel JC, Schwarz T, Luger TA. Proopiomelanocortin-derived peptides are synthesized and released by human keratinocytes. J Clin Invest. 1994;93:2258–62. doi: 10.1172/JCI117224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cutuli M, Cristiani S, Lipton JM, Catania A. Antimicrobial effects of alpha-MSH peptides. J Leukoc Biol. 2000;67:233–9. doi: 10.1002/jlb.67.2.233. [DOI] [PubMed] [Google Scholar]
- 164.Oono T, Matsuura H, Morizane S, Yamasaki O, Iwatsuki K. A case of infectious eccrine hidradenitis. J Dermatol. 2006;33:142–5. doi: 10.1111/j.1346-8138.2006.00031.x. [DOI] [PubMed] [Google Scholar]
- 165.Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–32. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]


