Abstract
Background and Purpose
Methods to increase recruitment into acute stroke trials are needed. The purposes of this study were to evaluate the safety and acceptability of initiating acute stroke trials during early helicopter evacuation and to test an intervention to facilitate informed consent.
Methods
A randomized, controlled trial was done with patients with acute stroke who were transferred by helicopter to the University of Iowa Hospitals and Clinics from February 2007 to January 2008. The intervention to be evaluated was the use of fax and a telephone call to the patient/surrogate ahead of helicopter arrival at the outside emergency department. The aim was to improve the rate of subsequent consent (primary outcome) for a pilot trial of a potentially beneficial, low-risk medical intervention (ranitidine) to prevent aspiration pneumonitis. Consenting eligible patients received the infusion during the flight to University of Iowa Hospitals and Clinics.
Results
One hundred patients were enrolled. Consent rate was 54% in the intervention group and 50% in the control group (P=0.69). However, the consent rate was higher (69%) when prearrival communications between the coinvestigator and potential subjects were successful (P=0.04). This approach resulted in an average gain of 59 minutes as compared with initiating recruitment on arrival to University of Iowa Hospitals and Clinics.
Conclusions
Enrollment into stroke intervention trials is feasible during helicopter transportation from a community hospital emergency department to a tertiary stroke center. This underused resource may improve trial efficiency by enabling and expediting participation of remote populations currently excluded from research. Consent rates might be further improved by communication strategies that are more successful in reaching patients at outside emergency departments.
Keywords: acute stroke, air ambulances, clinical trials, emergency medical services, helicopter, interhospital, nonurban, randomized controlled trials, rural
Progress in acute stroke management depends on the completion of clinical trials testing interventions applicable to large numbers of patients.1,2 The efficiency of recruiting patients in acute stroke trials needs to be improved.3 Participation in research protocols is particularly problematic for the 25% of Americans living in nonurban areas4 or those who are distant from research centers.5 These patients often arrive to a community hospital before being transferred for further care. By the time they arrive at a research tertiary care center, it may be either too late to qualify for a research protocol6 or enrollment is delayed. A differential recruitment of patients based on their place of residence could challenge the fairness of a trial7 as well as future generalization of those results.5,8 Strategies are needed to enable earlier access to trials for those patients living at long distances from a stroke center.
We previously proposed extending acute research to remote patients by enrolling them at the local hospital emergency department (ED) by tertiary hospital-based air medical personnel functioning as coinvestigators.9 We intended to test the feasibility and safety of this approach by conducting an acute stroke pilot trial through the early interhospital helicopter transfer of patients with ischemic stroke and intracranial hemorrhage from local EDs to the tertiary stroke center. Specifically, we hypothesized that a strategy of establishing advanced contact between a coinvestigator and the subject/ surrogate before the helicopter arrived might help compensate for the short time of personal interaction for obtaining informed consent available at the outside ED. As a vehicle to test our hypothesis, we used a 2-stage trial in which we tested an advanced notification strategy aiming to improve informed consent for a subsequent pilot trial of a low-risk medical intervention. We chose a single dose of intravenous ranitidine as a safe intervention with a plausible effect in preventing aspiration pneumonia and chemical pneumonitis (APCP) by raising the stomach pH at early stages.10 APCP is a serious complication that triples the risk of dying after stroke.11 Because ranitidine is administered in a way consistent to most stroke therapies, it was the best compromise among safety, ethics, and future generalizability for this model trial. We named that vehicle trial AIRDOC (Antacids In Flight Reduce Disability and Overcome Complications).
Methods
Study Type
This was a double randomized, controlled trial of an advanced contact intervention to facilitate subsequent informed consent for a low-risk pharmacological intervention (Figure 1).
Figure 1.
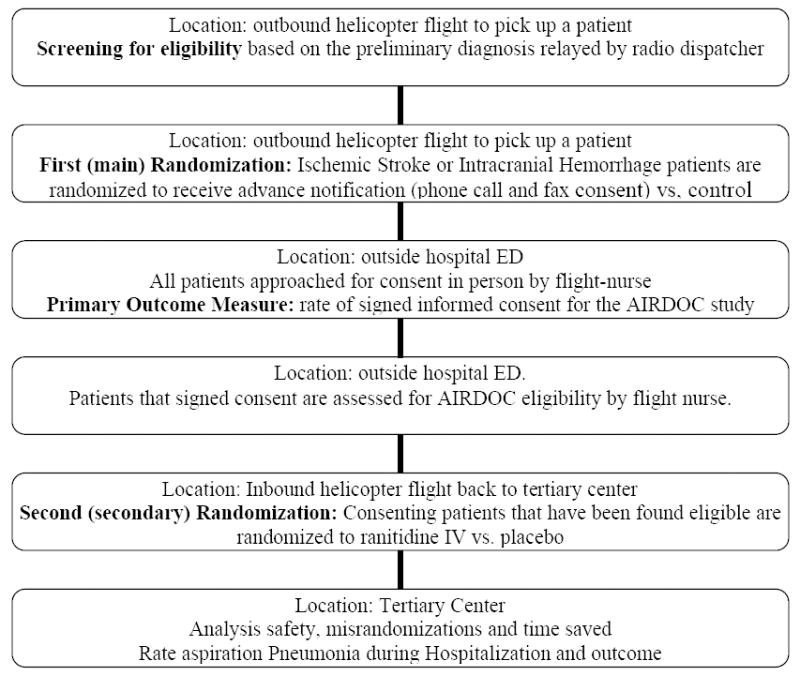
Outline of the Trial
Objectives
The primary objectives were (1) to test whether a strategy of advanced patient or surrogate notification would promote patient participation in stroke intervention trials performed during air medical transport; and (2) to test whether an intervention trial could be safely implemented during early helicopter evacuation of patients with either acute ischemic or hemorrhagic stroke using flight crews as coinvestigators. A secondary objective was to compare the rates of APCP during hospitalization between the ranitidine and placebo groups.
Study Setting and Organization
The study was performed during the helicopter transfer of patients with either acute ischemic stroke or intracranial hemorrhage between local hospital EDs located in several communities in Iowa and the tertiary stroke center at the University of Iowa Hospitals and Clinics (UIHC). All flights were done by the University of Iowa AirCare (AC), a University of Iowa-operated air ambulance service that transports patients to UIHC. The service consists of 2 different crews based in Iowa City and Waterloo, Iowa. Both AC crews are notified of the nature of their missions once they are airborne in order to avoid introducing disease biases in the aeronautical decision process. All 17 AC flight nurses and a flight physician became coinvestigators in this project. The coinvestigators are named in the Appendix. They all received certifications in the ethical aspects of research and the National Institutes of Health Stroke Scale (NIHSS), and they attended a half-day study initiation session that included rationale, safety aspects, study aims, and trial procedures. A notifying letter regarding the research procedures was mailed to all the ED directors in range of AC before starting the trial. This study was approved by the University of Iowa Institutional Review Board. The Institutional Review Board ruled that consent could be obtained at local sites by AC personnel because the potential subjects were considered to be patients of the UIHC.
Study Subjects
Subjects included all consecutive patients between February 2007 and January 2008 with a presumed ischemic stroke or intracranial hemorrhage for whom an air medical transfer to UIHC was requested through AC. Patients were diagnosed as having a possible stroke by the outside ED physician who requested transfer based on the available clinical, laboratory, and imaging data.
First-Stage Randomization
Subjects were randomized (1:1) to receive either the “advance notification” intervention or control during the outbound flight to pick up the patient. The flight nurse coinvestigator randomized the subject by opening a closed envelope stored in the aircraft. Randomization was done in blocks of 6 for each AC crew independently.
Communication Intervention
The intervention “advance notification” was an early information strategy aimed to facilitate subsequent informed consent. “Advanced notification” consisted of 2 complementary interventions: a fax with the informed consent documents and a telephone call. The informed consent documents used were approved by the University of Iowa Institutional Review Board. These documents were faxed from the LifeCom (a division of Air Methods Corporation) dispatch center to the patient or surrogate waiting at the outside ED at the request of the airborne outbound coinvestigator so the patient and surrogate could review the written materials in advance of the air crew arrival. The telephone call was made by the outbound investigator in-flight through the helicopter’s 800-MHz radio land phone system to the outside ED ahead of the helicopter crew’s arrival. In that call, the coinvestigator requested communication with the patient or his or her surrogate. The investigator introduced him- or herself to the patient/ surrogate and then followed a script that included introduction, purpose, a brief description of the study, and information that the consent had been faxed to their location. Subjects assigned to “no advance notification” did not receive the fax or phone call. Both “advance notification” and control groups were subsequently approached for informed consent for AIRDOC in person on arrival to the outside ED. Patients and surrogates in the intervention group were asked whether they actually received the intended fax/phone call.
Primary Outcome Measure
The main primary outcome measure was the proportion of patients who signed informed consent for the AIRDOC study. The null hypothesis was that there would be no differences in the rate of signing informed consent between the 2 groups.
Screening for AIRDOC
Those patients who signed informed consent were subsequently screened for AIRDOC. The screening process included a review of available records and a neurological assessment using the NIHSS by the flight nurse coinvestigator. Inclusion criteria for AIRDOC included (1) age ≥18 years; (2) presumed ischemic stroke or intracranial hemorrhage <12 hours old; (3) NIHSS score ≥1 point; (4) negative pregnancy test (women <50 years); (5) patient had already been considered for recombinant tissue plasminogen activator; (6) intubation to protect the airway if appropriate; and (7) prestroke modified Rankin Scale score 0 to 1. Exclusion criteria included: (1) time onset uncertain or >12 hours; (2) transferred specifically to receive recombinant tissue plasminogen activator at UIHC; (3) nonstroke etiology; (4) temperature >37.8°C; (5) systolic blood pressure <100 mm Hg; (6) allergy to ranitidine; (7) white blood cell count >10 000; (8) blood glucose <60 or >300 mg/dL; (9) current need for antibiotics; (10) terminal illness or expected survival <3 months; and (11) prisoner or institutionalized individual.
Second-Stage Randomization and Pharmacological Intervention (AIRDOC)
Those consenting patients found to be eligible for AIRDOC were randomized 1:1 to receive either a 250-mL intravenous infusion of 50 mg ranitidine or a similarly looking normal saline. Investigators and subjects were both blinded to the treatment assignment by the Pharmacy Department at UIHC using a block randomization of 12 by the AC team. The study kits containing drug infusion bags were stored at each AC headquarters and carried on every helicopter mission in anticipation of a possible randomization. The study drug infusion was initiated at the outside hospital and continued during the inbound flight to UIHC.
AIRDOC Outcomes
Time spent in the consent/screening process and the difference between times of starting consent at outside ED and time of arrival at UIHC were recorded. Reasons for not obtaining consent and adverse effects or complications of the study medication were documented and also recorded by flight nurses. Nurse coinvestigators from the Stroke Service at UIHC invited all subjects approached for consent at the outside ED to participate in a survey about their experience. Those subjects who gave consent were also followed for outcomes during their hospitalization at UIHC. The presence of APCP by Fine’s criteria,12 final diagnosis, subtype of stroke,13 and the presence of misrandomizations and disposition at discharge were recorded. Disposition and Barthel Index at 3 months were obtained through a phone call.
Safety Monitoring
A specialist in infectious diseases at the University of Iowa (Patricia L. Winokur, MD) was the safety monitor. She periodically reviewed the records for the safety of the subjects.
Statistical Analyses
The intention-to-treat methodology was used in the primary outcome analysis. Fisher exact test was used to compare the rates between the control and intervention groups. Using the rate of successful informed consent in the TOAST study,14 we estimated that 40% of the approached subjects/surrogates would give consent. We calculated that 100 subjects/surrogates (50 per group) would have to be randomized to detect a benefit of the intervention of a 30% increase in the consent rate with 80% power using a 2-sided 5% level test of significance. The secondary (AIRDOC) outcome, rate of APCP, was compared with Fisher exact test. All statistical analyses were performed using SAS Version 9.1 (SAS Institute Inc, Cary, NC).
Results
Figure 2 shows the results of the first randomization testing the effectiveness of an advanced notification strategy in promoting subsequent signed informed consent.
Figure 2.
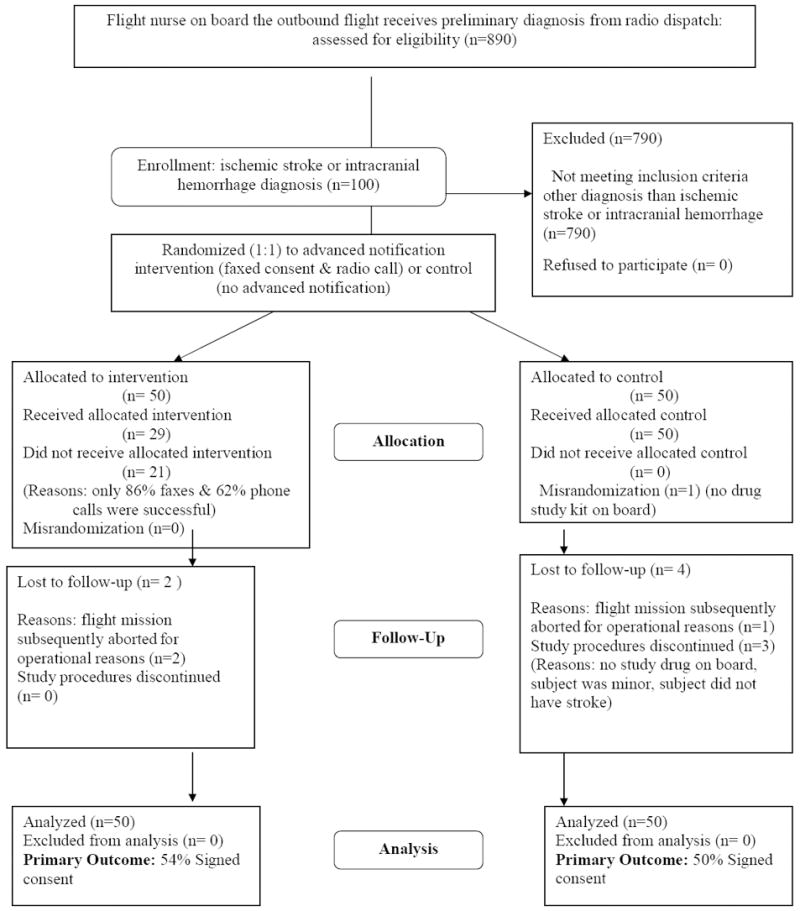
Results of the first randomization testing the effectiveness of advanced notification in promoting subsequent signed informed consent.
One hundred patients were randomized: 50 to advanced notification and 50 to no advanced notification (Figure 2). Ninety-four potential subjects were subsequently approached in person for the AIRDOC study at the outside ED. The 6 patients lost to follow-up included 3 cases in which the flight was aborted (2 for operational reasons, one due to symptoms being resolved), 2 cases in which the investigator stopped the study procedures on ED arrival after realizing obvious ineligibility (one subject was <18 years, one due to obvious nonstroke symptoms), and one case in which the coinvestigator opened the randomization envelope before realizing there was no study medication on board. Among patients assigned to the advance notification, the fax and phone call were successful in reaching its destination in 43 (86%) and 31 (62%) cases, respectively. Reasons for failure were technical for the radio phone (noise in the communication) and logistic (ED did not deliver the fax on time). The total number of patients signing informed consent was 52 (52%). Consent was obtained in 27 (54%) patients in the intervention group and in 25 (50%) patients in the control group (P=0.69). A post hoc analysis, however, showed that among those 29 patients who actually received both the phone and fax intervention, the consent rate was higher (69%) than among those not exposed to both fax and phone call (45%, P=0.03). The consent rate was 68% among those patients who actually received a phone call as compared with 46% among those who did not receive it (P=0.04). The consent rate was 53% among those patients who actually received a fax as compared with 52% among those who did not receive it (P=0.87).
Those 52 patients who gave consent for AIRDOC had a mean age of 63.9 years (SD, 13.3). Fifty-six percent were male, and their mean and median NIHSS scores were 5.9 and 2 points, respectively. The final diagnoses were large artery atherosclerosis stroke (8), cardioembolism (7), lacunar stroke (3), ischemic unknown (4), spontaneous intracerebral hemorrhage (7), subarachnoid hemorrhage (5), transient ischemic attack (4), venous thrombosis (one), traumatic intracerebral hemorrhage (5), and “other”(8; brain tumor, Bell’s palsy, migraine, myocardial infarction, hyponatremia, transient global amnesia, uncertain, missing). Current regulations prohibit data collection in nonconsented patients.
Reasons for not obtaining informed consent at the outside ED were surrogate refusal (n=15), patient refusal (n=10), insufficient time (n=8), relative not located (n=7), aborting consent process due to obvious ineligibility (n=5), flight aborted (n=2), and opposition from a local neurologist in a patient with a brain herniation (n=1). None of the outside ED physicians involved were opposed to the study or interfered with the coinvestigators’ consent and screening functions. The total average time spent at the outside ED by the air crews, including patient care activities, was 27 minutes (SD, 5.9). Average time spent specifically in the consent/screening process was 14.6 minutes (SD, 9.08). The average time “gained” through this new research mechanism (difference between the time of starting consent at an outside ED and the time of arrival at UIHC) was 59 minutes (SD, 16.5). This calculation does not take into account the additional time delay that would occur on arrival to the tertiary center to the start of a consent process, so we are likely underestimating the time gained through this mechanism.
Figure 3 shows the results for the secondary randomization testing the feasibility of initiating stroke trials at the outside ED during interhospital transfer. Twenty-seven of those consenting patients (52%) were subsequently found eligible in the screening process to be randomized to AIRDOC. Of those, 5 were ultimately considered to be misrandomizations. In one case, the investigator mistakenly opened the seal of the study kit despite acknowledging ineligibility due to symptoms beyond 12 hours, although the patient did not receive the infusion. The other 4 patients were noted to have exclusion criteria by the investigator (3 had symptoms beyond 12 hours and one had a NIHSS score of 0) but received study infusions in-flight. All these misrandomizations occurred in the first half of the study. No side effects or complications attributable to ranitidine were noted. There was no difference in the rate of APCP between the ranitidine and placebo groups (P=0.29). The final diagnoses after hospitalization of those 27 patients were ischemic stroke/transient ischemic attack (18), intracranial hemorrhage (4), and other (5; traumatic hemorrhage, Bell’s palsy, uncertain transient ischemic attack, complicated migraine, transient global amnesia).
Figure 3.
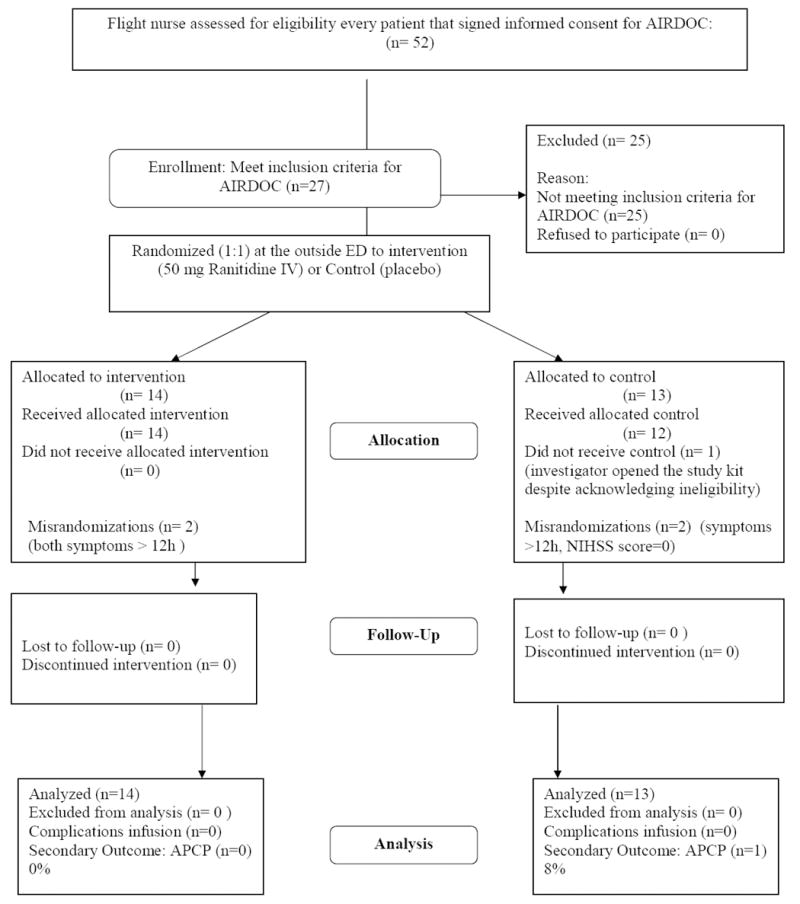
Results of the second randomization testing the feasibility of initiating stroke trials during inter-hospital transfer.
Supplemental Table I, available online at http://stroke.ahajournals.org, shows the results of a survey done on arrival to UIHC that asked patients or surrogates questions about the AIRDOC recruitment process and perceptions about the trial. Most patients had a positive impression of the process, although they stressed the importance of having face-to-face interaction when being approached for studies. No barriers to this research were identified at the outside EDs.
Discussion
We succeeded in implementing an acute stroke intervention trial during helicopter interhospital transport of patients with stroke from EDs in community hospitals to a regional comprehensive stroke center. Despite the time pressures, flight personnel were able to incorporate all the necessary clinical trial procedures and obtain informed consent at rates comparable to traditional hospital-based stroke trials14 without compromising the flight mission or generating opposition from physicians or staff at local EDs. Considerable time was gained. This strategy expands the potential reach of clinical research centers, and it is particularly important for patients living in remote or rural areas.
We envision that future stroke trials could include a cadre of air medical transportation personnel as coinvestigators, particularly in those centers with a dispersed population. This strategy has the potential to improve the recruitment efficiency of the clinical trial.3 Because a fraction of subjects will be treated through a “real-life” remote scenario based on a local ED diagnosis, it also may permit generalization of the trial results to most clinical settings. In addition, it may allow the design of smaller (and more feasible) trials because the magnitude of the efficacy of a stroke interventions is time-dependent,15 so expediting patient participation might result in a smaller sample size requirement.2 In addition to improving the efficiency and applicability of ongoing stroke trials, this approach would permit testing ancillary care interventions that are specifically applied during the helicopter flight.9 The relevance of this proposed mechanism is supported by the fact that 500 000 helicopter medical missions are flown each year in the United States.16,17 Although the breakdown by specific diagnoses is not available, we performed a survey among directors of Air Medical Transportation Services in March 2008 and estimated that ischemic and hemorrhagic stroke missions comprise 12% (95% CI, 5 to 19) of this total number (Ahmed A, Leira EC, unpublished data).
This approach also meets the current goal of ethical research that protects patient safety, including the use of standard written informed consent forms. At the same time, it avoids other issues related to research such as waiving18 or deferring19 consent in patients deemed incompetent. Because the legal transfer of care for these patients takes place when the flight crew arrives at the outside ED, our approach requires only approval of one Institutional Review Board, therefore avoiding the hurdle of requiring regulatory oversight from multiple institutions. This strategy involves a handful of specialized tertiary hospital-based flight personnel, which simplifies the training and certification procedures and lessens the variability in performance throughout the steps of a trial. Because these coinvestigators are exposed to many patients in their capture area, they are more likely to become experienced with the study procedures, which may increase the quality of the data as well as maintain enthusiasm for the trial among investigators.
We were pleased that the absolute rate of informed consent was high considering the novelty of this environment. It was comparable to that achieved in the tertiary care center-based TOAST trial.14 Our tactic of using the “downtime” of the outbound flight to inform the patient or subject by fax and phone about the ongoing study showed promise. It may reduce the rush of signing forms for a study by allowing more time for the subject and surrogate to consider participation. Unfortunately, such primary intervention did not improve the rate of signed informed consent in an intention-to-treat analysis. A secondary target population analysis suggests that those negative results were likely due to the large number of subjects in the intention-to-treat group who failed to receive the intended intervention. The phone call seemed more helpful than the fax in promoting consent but was often unsuccessful due to noise. Our experience shows that technical hurdles need to be addressed. Surveyed patients and surrogates generally did not object to the advanced notification and found it useful. This experience suggests promise for using more sophisticated advance communication strategies with better compliance during the outbound flight, which should be tested prospectively.
Prehospital stroke trials conducted by paramedics have been a successful initiative to accelerate research in large metropolitan areas.19 Implementation of such a strategy in dispersed remote populations20 may be difficult. Rural first responders or paramedics are often volunteers and have less training and exposure to stroke cases compared with their urban counterparts.21,22 It would be an overwhelming task to maintain the necessary skills and certifications to be successful coinvestigators for a large and dispersed workforce. The interhospital strategy we are proposing occurs in a different setting than prehospital trials but provides the first opportunity of intervention in dispersed areas with minimal logistical burden and reasonable safety. Unlike the prehospital approach, the interhospital setting would allow recruitment in a broader number of research protocols because patients will already have a CT scan, basic laboratory tests, and a preliminary diagnosis by an outside physician.9
The dispersion of resources would also be a barrier for research at those multiple small hospitals that do not have enough volume of patients to be proficient in performing a stroke trial.23 Certifying every ED physician in the catchment area of a comprehensive center also faces large logistical and regulatory hurdles. Telemedicine-assisted24 clinical trials could be an option for small hospitals ED, but potential barriers such as liability and lack of a personally obtained written informed consent would have to be addressed.25
We are aware of the constraints of application of this research. Although we used a consent form that stated all the possible risks of ranitidine, including life-threatening reactions, it could be argued that the rate of informed consent would have been lower for interventions with a higher risk profile. Another limitation is the procedural mistakes that occurred. These few mistakes were not surprising when we consider that flight personnel were new to clinical research and that they underwent only a single initial training session. Such issues can be overcome with training. Our observation that errors occurred in the first half of the study suggests a learning curve from the point of the investigators. Other limitations are the reliability of the stroke diagnosis from the outside ED physicians26 and a potential bias toward requesting helicopter transfer for more severe strokes.9 It is realistic to expect that patients with stroke mimics might be admitted into ischemic or hemorrhagic study protocols conducted through this setting. Our ischemic stroke mimic rates were comparable to previous series of patients considered for acute treatment.27 Current “ship and drip” systems that treat remote patients28 often rely on local ED ischemic stroke diagnosis, which inevitably results in the treatment of a number of stroke mimics.27 Similarly, enrolling a few stroke mimics through this approach is also a methodological strength, because it reproduces a “real-world” scenario that will permit generalization of future results to remote settings where the intervention is administered based on the outside ED diagnosis.8
In summary, we found that the helicopter interhospital transportation setting is a potentially valuable, underused resource that might enable and expedite participation in acute stroke trials for remote populations currently excluded from research. This approach, which may significantly improve stroke clinical trials efficiency and fairness, might be extrapolated to other acute time-dependent emergencies that can be diagnosed at smaller hospital EDs.
Table I.
Survey of patients and relatives approached for consent (n=60).
| Gender | Male (n=35) Female (n=25) |
| Race | White (n=58) Black (n=2) |
| Ethnicity | Non-Hispanic (n=59) Hispanic (n=1) |
| Survey completed by | Patient (n=50) spouse (n=8) parent (n=1) niece (n=1) |
| If you where reached by phone at the outside ED, what was your reaction? | Very pleasant (n=5)-somewhat pleasant (n=6)-neither pleasant nor upsetting (n=1)-somewhat upsetting (n=2)-very upsetting (n=0) |
| If you where reached by phone at the outside ED, did you find it useful? | Very informative(n=7)-somewhat informative (n=5)-neutral (n=1)-not very informative (n=0)-not informative at all (n=1) |
| If you where given the fax consent forms at the outside ED, did you find it useful? | Very informative(n=10)-somewhat informative (n=1)-neutral (n=3)-not very informative (n=0)-not informative at all (n=0) |
| Did you have time to read the faxed materials? | Yes (n=9)-somewhat (n=2)-no (n=1)-N/A (n=1) |
| If at an outside ED again, how would you prefer to be contacted? | Phone call (n=5)-fax (n=2)-brochures at outside ED (n=7)- “other” (Talk about it in person) (n=34) |
| Did you sign informed consent for AIRDOC? | Yes (n=47)-No (n=13) |
| If no, specify reason | Worried about risks (n=1)-Unfamiliar with aircrew (n=0)- interaction aircrew (n=0)-Not enough time (n=1)-wording consent form (n=0)-not enough time (n=0)-advice physician (n=1)-advice local ED (n=1)-mistrust research (n=1)-“other” (n=9) |
| Specify “other” | Cannot remember process (n=3), don’t like study medications (n=2), Did not qualify, need better ways communicate, unconscious, don’t want to bother while in helicopter |
| Suggestions | “Suggest color brochure”, “not sure, want local doctor to decide” |
N/A indicates not applicable.
Acknowledgments
We thank LifeCom (Omaha, Nebr) for their assistance by faxing the consent forms.
Source of Funding Funded in part by the Mentored Clinical Research Scholar Program at Iowa National Institutes of Health Grant 5K12RR017700-04 (Allyn Mark, MD, Principal Investigator).
Appendix
List of Coinvestigators
AC1 (Iowa City): T. Toycen, M. Kiger, P. Doser, D. Miller, J. Lipcamon, K. Dillard, M. Dillard, R. Ogren, S. Schultz, and A. Ahmed. AC2 (Waterloo): C. Niemeyer, C. Ratchford, A. Schott, D. Bagenstos, L. Burrage, M. Farmer, and K. Baerenwald.
Footnotes
Presented in part at the International Stroke Conference, New Orleans, February 2008.
Disclosures None.
References
- 1.Marler JR. NINDS clinical trials in stroke: lessons learned and future directions. Stroke. 2007;38:3302–3307. doi: 10.1161/STROKEAHA.107.485144. [DOI] [PubMed] [Google Scholar]
- 2.Fisher M, Albers GW, Donnan GA, Furlan AJ, Grotta JC, Kidwell CS, Sacco RL, Wechsler LR. Enhancing the development and approval of acute stroke therapies: Stroke Therapy Academic Industry roundtable. Stroke. 2005;36:1808–1813. doi: 10.1161/01.STR.0000173403.60553.27. [DOI] [PubMed] [Google Scholar]
- 3.Elkins JS, Khatabi T, Fung L, Rootenberg J, Johnston SC. Recruiting subjects for acute stroke trials: a meta-analysis. Stroke. 2006;37:123–128. doi: 10.1161/01.STR.0000195149.44390.aa. [DOI] [PubMed] [Google Scholar]
- 4.US Bureau of the Census. [April 2008];Urban and Rural Population 2000. Available at: www.census.gov.
- 5.Baquet CR, Commiskey P, Daniel MC, Mishra SI. Recruitment and participation in clinical trials: socio-demographic, rural/urban, and health care access predictors. Cancer Detect Prev. 2006;30:24–33. doi: 10.1016/j.cdp.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hacke W, Brott T, Caplan L, Meier D, Fieschi C, von KR, Donnan G, Heiss WD, Wahlgren NG, Spranger M, Boysen G, Marler JR. Thrombolysis in acute ischemic stroke: controlled trials and clinical experience. Neurology. 1999;53:S3–S14. [PubMed] [Google Scholar]
- 7.Emanuel EJ, Wendler D, Grady C. What makes clinical research ethical? JAMA. 2000;283:2701–2711. doi: 10.1001/jama.283.20.2701. [DOI] [PubMed] [Google Scholar]
- 8.Leira EC, Hess DC, Torner JC, Adams HP., Jr Rural urban differences in acute stroke practices: a modifiable disparity. Arch Neurol. 2008;65:887–891. doi: 10.1001/archneur.65.7.887. [DOI] [PubMed] [Google Scholar]
- 9.Leira EC, Lamb DL, Nugent AS, Ahmed A, Grimsman KJ, Clarke WR, Adams HP., Jr Feasibility of acute clinical trials during aerial interhospital transfer. Stroke. 2006;37:2504–2507. doi: 10.1161/01.STR.0000239661.07675.9d. [DOI] [PubMed] [Google Scholar]
- 10.Gouda BB, Lydon AM, Badhe A, Shorten GD. A comparison of the effects of ranitidine and omeprazole on volume and pH of gastric contents in elective surgical patients. Eur J Anaesthesiol. 2004;21:260–264. doi: 10.1017/s0265021504004028. [DOI] [PubMed] [Google Scholar]
- 11.Hilker R, Poetter C, Findeisen N, Sobesky J, Jacobs A, Neveling M, Heiss WD. Nosocomial pneumonia after acute stroke: implications for neurological intensive care medicine. Stroke. 2003;34:975–981. doi: 10.1161/01.STR.0000063373.70993.CD. [DOI] [PubMed] [Google Scholar]
- 12.Fine MJ, Hough LJ, Medsger AR, Li YH, Ricci EM, Singer DE, Marrie TJ, Coley CM, Walsh MB, Karpf M, Lahive KC, Kapoor WN. The hospital admission decision for patients with community-acquired pneumonia. Results from the pneumonia Patient Outcomes Research Team cohort study. Arch Intern Med. 1997;157:36–44. [PubMed] [Google Scholar]
- 13.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE., III Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 14.Low molecular weight heparinoid, ORG 10172 (danaparoid), and outcome after acute ischemic stroke: a randomized controlled trial. The Publications Committee for the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) Investigators. JAMA. 1998;279:1265–1272. [PubMed] [Google Scholar]
- 15.Hacke W, Donnan G, Fieschi C, Kaste M, von KR, Broderick JP, Brott T, Frankel M, Grotta JC, Haley EC, Jr, Kwiatkowski T, Levine SR, Lewandowski C, Lu M, Lyden P, Marler JR, Patel S, Tilley BC, Albers G, Bluhmki E, Wilhelm M, Hamilton S. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363:768–774. doi: 10.1016/S0140-6736(04)15692-4. [DOI] [PubMed] [Google Scholar]
- 16.Association of Air Medical Services. [April 2008]; Available at: www.aams.org/Content/NavigationMenu/AboutAAMS/FrequentlyAskedQuestions/default.htm.
- 17.Hutton K, Sand C. Appropriateness of medical transport and access to care in acute stroke syndromes. Air Med J. 2005;24:220–221. doi: 10.1016/j.amj.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Bateman BT, Meyers PM, Schumacher HC, Mangla S, Pile-Spellman J. Conducting stroke research with an exception from the requirement for informed consent. Stroke. 2003;34:1317–1323. doi: 10.1161/01.STR.0000065230.00053.B4. [DOI] [PubMed] [Google Scholar]
- 19.Saver JL, Kidwell C, Eckstein M, Starkman S FAST-MAG Pilot Trial Investigators. Prehospital neuroprotective therapy for acute stroke: results of the Field Administration of Stroke Therapy-Magnesium (FAST-MAG) pilot trial. Stroke. 2004;35:e106–108. doi: 10.1161/01.STR.0000124458.98123.52. [DOI] [PubMed] [Google Scholar]
- 20.Messina JP, Shortridge AM, Groop RE, Varnakovida P, Finn MJ. Evaluating Michigan’s community hospital access: spatial methods for decision support. Int J Health Geogr. 2006;5:42. doi: 10.1186/1476-072X-5-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chng CL, Collins J, Eaddy S. A comparison of rural and urban emergency medical system (EMS) personnel: a Texas study. Prehosp Disaster Med. 2001;16:159–165. doi: 10.1017/s1049023x00025917. [DOI] [PubMed] [Google Scholar]
- 22.Stripe SC, Susman J. A rural–urban comparison of prehospital emergency medical services in Nebraska. J Am Board Fam Pract. 1991;4:313–318. [PubMed] [Google Scholar]
- 23.Burgin WS, Staub L, Chan W, Wein TH, Felberg RA, Grotta JC, Demchuk AM, Hickenbottom SL, Morgenstern LB. Acute stroke care in non-urban emergency departments. Neurology. 2001;57:2006–2012. doi: 10.1212/wnl.57.11.2006. [DOI] [PubMed] [Google Scholar]
- 24.Hess DC, Wang S, Hamilton W, Lee S, Pardue C, Waller JL, Gross H, Nichols F, Hall C, Adams RJ. REACH: clinical feasibility of a rural telestroke network. Stroke. 2005;36:2018–2020. doi: 10.1161/01.STR.0000177534.02969.e4. [DOI] [PubMed] [Google Scholar]
- 25.Dreezen I. Telemedicine and informed consent. Med Law. 2004;23:541–549. [PubMed] [Google Scholar]
- 26.Smith WS. Can emergency medicine physicians accurately identify iv t-PA eligible acute stroke patients? Neurocrit Care. 2007;7:101–102. doi: 10.1007/s12028-007-0083-z. [DOI] [PubMed] [Google Scholar]
- 27.Scott PA, Silbergleit R. Misdiagnosis of stroke in tissue plasminogen activator-treated patients: characteristics and outcomes. Ann Emerg Med. 2003;42:611–618. doi: 10.1016/s0196-0644(03)00443-8. [DOI] [PubMed] [Google Scholar]
- 28.Wang DZ, Rose JA, Honings DS, Garwacki DJ, Milbrandt JC. Treating acute stroke patients with intravenous tPA. The OSF stroke network experience. Stroke. 2000;31:77–81. doi: 10.1161/01.str.31.1.77. [DOI] [PubMed] [Google Scholar]


