Abstract
Acidocalcisomes are acidic organelles rich in calcium and phosphorus that have been conserved from bacteria to man. In parasitic protozoa acidocalcisomes possess enzymes that are absent or different from their mammalian counterparts and could be potential targets for chemotherapy, such as the vacuolar proton translocating pyrophosphatase, and the soluble inorganic pyrophosphatase, both of which are inhibited by pyrophosphate analogs (bisphosphonates). In addition, a number of drugs, including bisphosphonates, and diamidines appear to accumulate in these organelles and/or induce an increase in their numbers, potentially enhancing their toxicity. Bisphosphonates mechanism of action, however, is by inhibition of the isoprenoid pathway and more specifically the prenyl diphosphate synthases.
INTRODUCTION
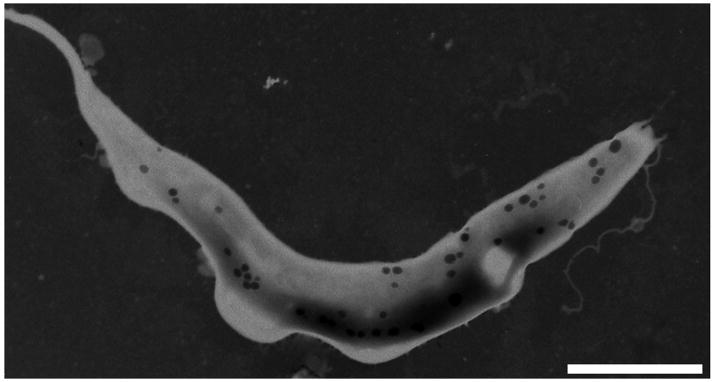
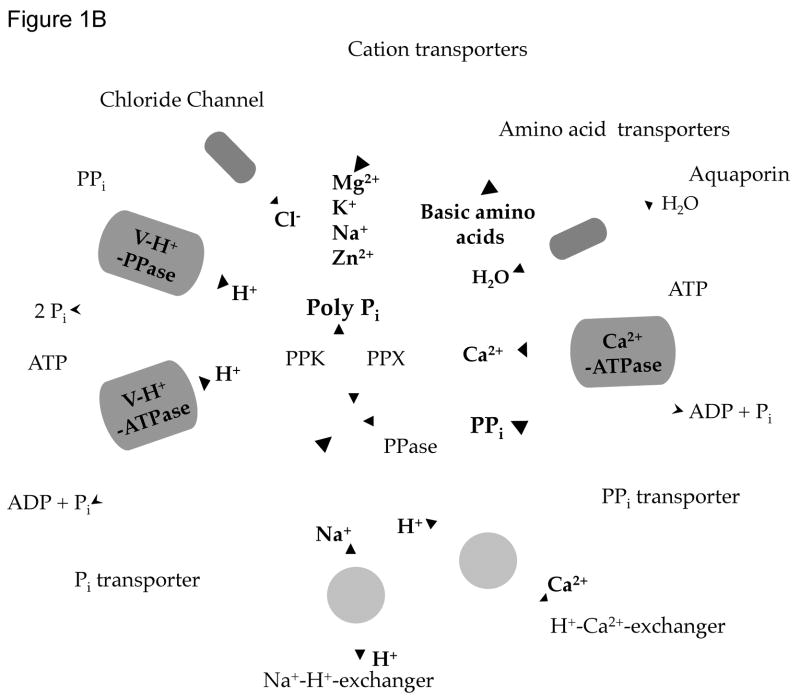
The acidocalcisome is a dense, acidic organelle (Fig. 1A) with a high concentration of phosphorus present as pyrophosphate and polyphosphate (poly P) complexed with calcium, and other cations. The acidocalcisome membrane contains a number of pumps (Ca2+-ATPase, V-H+-ATPase, H+-PPase), exchangers (Na+/H+, Ca2+/H+), and channels (aquaporins), while its matrix contains enzymes related to pyrophosphate and poly P metabolism [1] (Fig. 1B). Acidocalcisomes have been found in several pathogenic microorganisms [2] as well as in the green alga Chlamydomonas reinhardtii [3], and the slime mold Dictyostelium discoideum [4]. The identification of acidocalcisomes in bacteria [5, 6] and the finding that human platelet dense granules are similar to acidocalcisomes [7, 8], indicated that these are organelles have been conserved from bacteria to humans. Some of the potential functions of the acidocalcisome are the storage of cations and phosphorus, and its participation in pyrophosphate and polyphosphate metabolism, calcium homeostasis, maintenance of intracellular pH homeostasis, and osmoregulation [1]. The discovery of novel enzymes in this organelle that are absent from mammalian cells led to the finding of compounds (bisphosphonates) that produced radical cures in animal models of diseases caused by several parasites [9]. Further exploration of the structure and function of acidocalcisomes in protozoan parasite could lead to the identification of new targets for drug action.
Figure 1. Ultrastructure and composition of acidocalcisomes.
A. Transmission electron microscopy of a procyclic stage of Trypanosoma brucei showing the acidocalcisomes (dark granules). Bar = 3 μm. Reprinted with permission from ref. [87]. B. Schematic representation of an acidocalcisome. A H+ gradient is established by a vacuolar ATPase (V-H+-ATPase) and a vacuolar pyrophosphatase (V-H+-PPase). Ca2+ transport is driven by a Ca2+-ATPase. Other transporters include Na+/H+, and Ca2+/H+ exchangers, a Cl− channel, and a water channel or aquaporin. Transporters for basic amino acids, Pi, PPi, and cations are potentially present. The matrix is rich in PPi and polyphosphate (poly P) and enzymes involved in their metabolism (poly P kinase (PPK), exopolyphosphatase (PPX), and pyrophosphatase (PPase). Not all the enzymes and transporters are present in all acidocalcisomes.
POTENTIAL TARGET ENZYMES LOCATED IN THE ACIDOCALCISOME
Of the enzymes present in the acidocalcisomes, two have been found to be targets for drugs with in vitro and/or in vivo activity against different protozoan parasites: a vacuolar proton translocating pyrophosphatase (V-H+-PPase) and a soluble inorganic pyrophosphatase (PPase).
The V-H+-PPase activity has been detected in the following parasitic protozoa: Trypanosoma cruzi [10], T. brucei [11, 12], Leishmania donovani [13], L. amazonensis [14], Phytomonas françai [15], Leptomonas wallacei [16], Herpetomonas spp. [16, 17], Plasmodium spp. [18, 19], and Toxoplasma gondii [20, 21]. This enzyme also localizes to acidocalcisomes in all these species (Fig. 1B). The V-H+-PPase from T. cruzi functions in yeast [22]. The acidocalcisomal V-H+-PPase is K+-stimulated (type I), and can be used as a marker for acidocalcisome purification [3, 4, 10–13, 23]. Although it is not restricted to the acidocalcisome it is concentrated in this organelle. The T. cruzi V-H+-PPase is also found in the Golgi complex and in the plasma membrane [24]. The Plasmodium spp. V-H+-PPase is also localized in the digestive vacuole [19, 25, 26].
In earlier work, it was found that some pyrophosphate analogs, bisphosphonates (containing a non-hydrolyzable P-C-P, rather that a P-O-P, backbone) as well as imidodiphosphate (containing a non-hydrolyzable P-N-P group), were inhibitors of a plant (mung bean, Vigna radiata L.) V-H+-PPase [27]. A more extensive investigation of the structural aspects of the effectiveness of bisphosphonates as competitive inhibitors of this enzyme was reported later [28]. More recently the results of a three-dimensional quantitative structure-activity relationship (3D-QSAR) comparative molecular field analysis (ConMFA) of the activity of 18 bisphosphonates and imidodiphosphate in the inhibition of a mung beam (Vigna radiata L.) V-H+-PPase was reported [29], and it was shown that the activities of the V-H+-PPase inhibitors could be predicted to within about a factor of two. Several of the compounds investigated were active against the parasite enzymes [10, 11, 13, 20, 30]. One of the best known inhibitors of the V-H+-PPase, aminomethylenediphosphonate (AMDP) [31], was able to impair intracellular replication of T. gondii in tissue culture cells exerting little or no effect on host cell invasion [20, 30]. Some of the treated parasites had ultrastructural alterations compatible with acidocalcisome disruption [30].
The vacuolar soluble pyrophosphatase (VSP1) present in Trypanosoma brucei, is essential for growth of bloodstream forms in their mammalian host and is located in acidocalcisomes [32] (Fig. 1B). Depending on the nature of its divalent metal ion cofactor, this soluble enzyme can act either as a pyrophosphatase (PPase; with +Mg2+) or as a short-chain polyphosphatase (PPX; with +Zn2+). It was found that the exopolyphosphatase (tripolyphosphatase) activity (in the presence of Zn2+, which is abundant in acidocalcisomes) of TbVSP1 was inhibited by bisphosphonates [33]. The inhibition of the recombinant TbVSP1 by a panel of 81 bisphosphonates was reported [33]. The IC50 values for enzyme inhibition were found to vary from 2 to 850 μM. In general the most active compounds contained both a single aromatic ring and a hydrogen bond donor feature. Thirteen of the most potent compounds were tested in vivo in a mouse model of T. brucei infection. The most active compound in vivo provided a 40% protection from death with no apparent side effects, suggesting that further development of such compounds might be of interest [33].
ROLE OF THE ACIDOCALCISOME IN THE MECHANISM OF ACTION OF BISPHOSPHONATES
Bisphosphonates are used to treat a variety of bone resorption diseases including osteoporosis, Paget’s disease, hypercalcemia caused by malignancy, and tumor metastases in bone [34]. Bisphosphonates have also been shown to have activity against different protozoan parasites in vitro and in vivo [9].
Selective action on bone is based on the binding of the bisphosphonate moiety to the bone mineral [34]. It has been postulated that the acidocalcisomes are equivalent in composition to the bone mineral and that accumulation of bisphosphonates in these organelles, as they do in bone mineral, facilitates their antiparasitic action [35].
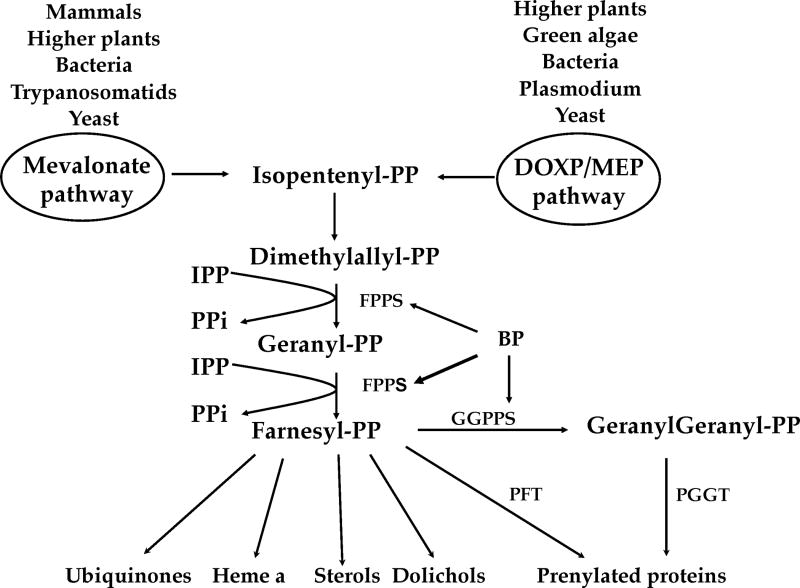
The primary target for the nitrogen-containing or amino-bisphosphonates is though to be the isoprenoid pathway at the level of the enzyme farnesyl diphosphate synthase (FPPS) [36–41] (Fig. 2). By inhibiting this enzyme, bisphosphonates inhibit the formation of farnesyl diphosphate (FPP), a compound used in protein prenylation of proteins like Ras, Rho and Rap [42–44], and in the production of dolichols, ubiquinones, heme a, and sterols. FPPS inhibition results, in addition to decreased prenylation of proteins and generation of FPP derivatives, in the accumulation of isopentenyl diphosphate (IPP), a known γδ Tcell activator [45]. These alterations lead to apoptotic cell death [46–48]. Recent work has shown that bisphosphonates can also target other enzymes of the isoprenoid pathway, like for example geranylgeranyl diphosphate synthase (GGPPS) [49] (Fig. 2) and that they can target multiple sites in prenyltransferases [50]. Interestingly, some bisphosphonates are also able to inhibit the activity of T. cruzi hexokinase, an enzyme that in contrast to the mammalian enzyme, is inhibited by PPi [51, 52]
Figure 2. Overview of the pathway for isoprenoid synthesis.
The DOXP/MEP pathway, present in higher plants, green algae, some bacteria, Plasmodium spp. and yeast as well as the Mevalonate pathway, present in mammals, higher plants, some bacteria, trypanosomatids, and yeast, generate isopentenyl diphosphate (IPP), which isomerizes to dimethylallyl diphosphate (DMAPP). The farnesyl diphosphate synthase (FPPS) catalizes the reaction of DMAPP with IPP to generate geranyl diphosphate (GPP), which incorporates another IPP to generate farnesyl diphosphate (FPP). FPP is the precursor for ubiquinones, heme a, sterols, dolichols and geranylgeranyl diphosphate (GGPP) through the action of GGPP synthase. Bisphosphonates (BP) inhibit the short chain prenyl transferases (FPPS and GGPPS).
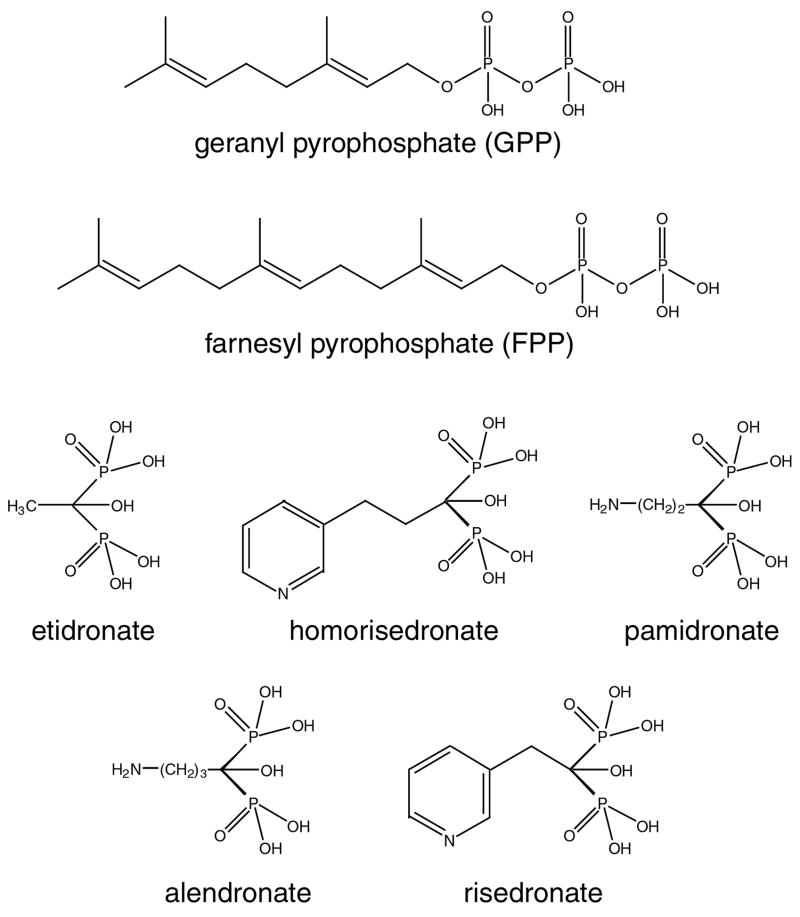
Nitrogen-containing bisphosphonates (Fig. 3) were first found to be effective in the inhibition of T. cruzi in vitro and in vivo without toxicity to the host cell [53]. Later, a series of bisphosphonates was tested on the growth of T. gondii, T. b. rhodesiense, L. donovani and P. falciparum in vitro showing that bisphosphonates could effectively inhibit the growth of these parasites [35]. The bisphosphonate risedronate (Fig. 3) was shown to inhibit Cryptosporidium parvum growth in vivo using a xenograft model [54].
Figure 3. Structure of GPP, FPP, and different bisphosphonates commercially available for the treatment of bone resorption diseases.
The use of another bisphosphonate, pamidronate (Fig. 3), resulted in the radical cure of experimental cutaneous leishmaniasis in mice [55]. Pamidronate was also active in vivo against L. donovani by intravenous administration [56]. Risedronate (Fig. 3) had a 50% effective dosage of five 2.6 mg/kg of body weight intraperitoneal doses against L. donovani-infected mice.
In vivo testing against T. gondii in mice showed that risedronate can significantly increase the survival of mice infected by this parasite [57]. In vitro testing of risedronate in T. cruzi showed that it had selective antiproliferative effects against the intracellular amastigotes, and at 100 μM, was able to prevent completely the development of T. cruzi infection of murine muscle heart or Vero cells, and to cure cultures which were already infected [58]. In vivo testing of bisphosphonates against T. cruzi has shown that risedronate can significantly increase the survival of mice infected by T. cruzi [57, 59].
The effect of a series of 102 bisphosphonates on the inhibition of growth of Entamoeba histolytica and Plasmodium falciparum in vitro was also determined [60]. The most active compounds (IC50 4–9 μM) against E. histolytica were nitrogen-containing bisphosphonates with relatively large aromatic side chains. Five bisphosphonates were selected and screened for their ability to delay the development of amebic liver abscess formation in an E. histolytica infected hamster model and 2 compounds were found to decrease liver abscess formation at 10 mg/kg ip with little or no effect on normal liver mass [60]. With P. falciparum, the most active compounds were n-alkyl bisphosphonates (Fig. 4). Five compounds were selected for in vivo investigation in a Plasmodium berghei ANKA Balb/c mouse suppressive test. The most active compound caused an 80% reduction in parasitemia with no overt toxicity [60].
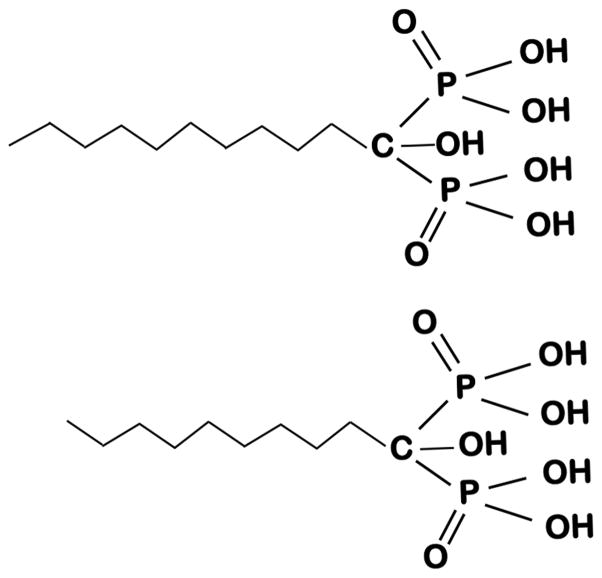
Figure 4. Structure of n-alkyl bisphosphonates effective against Apicomplexan parasites.
The figure shows the structure of compounds with 9 and 10-carbon chain.
The activity of 60 bisphosphonates against the replication of T. gondii in vitro and of three of the most active compounds, in vivo has been investigated [61]. The two most active compounds found were n-alkyl bisphosphonates containing long (n = 9 or 10) hydrocarbon chains (Fig. 4), not the nitrogen-containing species used in bone resorption therapy. The three most active compounds found in vitro were tested in vivo in a Smith-Webster mouse model and the two most active bisphosphonates were found to provide up to an 80% protection from death, a considerable improvement over that found previously with nitrogen-containing bisphosphonates [61]. This effect may originate in the much higher therapeutic indices of these alkyl bisphosphonates, as deduced from in vitro assays using LD50 values for growth inhibition of a human cell line.
Alkyl bisphosphonates (Fig. 4) were also shown to be potent inhibitors of T. cruzi amastigotes growth in vitro [62]. Overall, these results indicate that alkyl bisphosphonates are promising compounds for further development as agents against parasite growth, in vivo [61, 62], especially against Apicomplexan parasites [60, 61].
There is strong evidence that the main target of the most active bisphosphonates in protozoan parasites is the isoprenoid biosynthesis pathway enzyme farnesyl diphosphate synthase (FPPS): (1) There is excellent correlation between inhibition of the enzyme in T. cruzi [63], T. brucei [65], T. gondii [65], and L. major [66] and growth of these parasites in vitro; (2) In vitro “rescue’ experiments showed reversal of risedronate-induced growth inhibition of T. b. rhodesiense by GGPP, FPP, or farnesol [67]; (3) Molecular modeling and structure-activity investigations of enzyme and in vitro growth inhibition data in T. brucei resulted in similar pharmacophores [64]; (4) a T. gondii strain engineered to overexpress FPPS required considerably higher levels of bisphosphonates to achieve 50% growth inhibition, while the IC50 for atovaquone (which does not inhibit FPPS) remained the same in the overexpressing strain [65]; (5) Promastigotes of L. major overexpressing FPPS were highly resistant to risedronate and the degree of resistance correlated with the increase in enzyme activity [68]; (7) RNAi experiments in T. brucei has shown that FPPS is an essential enzyme thus validating it as a target for chemotherapeutic agents [65].
The farnesyl diphosphate synthase genes from T. cruzi [63], T. brucei [64], L. major [68], and T. gondii [65] have been cloned and their protein products purified and characterized biochemically. The tridimensional structures of T. brucei [69, 70], T. cruzi [71], P. berghei, and C. parvum [72] FPPSs have been solved, providing mechanistic insights that will have important implications for future drug design.
The reasons why alkyl-bisphosphonates have higher activity than nitrogen-containing bisphosphonates in Apicomplexan parasites is now becoming clear. In the Apicomplexans, the putative “FPPS” enzymes actually produce not only FPP, but much longer (C20, C25 and up) prenyl diphosphates [65]. Long chain bisphosphonates are able to block the TgFPPS active site (since it is bifunctional). Moreover, the availability of the closely related P. berghei and C. parvum X-ray structures [72] strongly suggests a structural explanation in that the Apicomplexans have a F to C, S substitution in the fifth aminoacid upstream of the first aspartate rich domain (FARM) region, enabling longer chain inhibitors to bind in the active site [65]. However, these inhibitors are expected to have a steric clash with the FF groups in the host cell FPPS, resulting in no FPPS inhibition [70]. Interestingly, it has been shown that inhibition of TgFPPS, which is a bifunctional enzyme generating longer chain isoprenoids (GGPP) [65], correlates better with inhibition of solanesyl diphosphate synthase from T. cruzi (TcSPPS), which is an enzyme that generates the 45-carbon solanesyl diphosphate (SPP, [73a]) than with inhibition of other FPPSs, that generate only FPP [73b).
In summary, there are several reasons for bisphosphonates to be good candidate drugs for treatment of parasitic disease. First, they have already been developed to treat other diseases and consequently have low toxicity; second, their structures are simple, so they are easy to synthesize; third, experimental results have shown that several bisphosphonates have excellent inhibitory activity against different parasites in vitro and in vivo.
OTHER DRUGS TARGETING THE ACIDOCALCISOME
Acidocalcisomes are also known by the names ‘volutin granules’ or ‘polyphosphate granules’ [1], and early work by Ormerod [74] proposed that they become more visible under light microscopy when cells are treated with drugs. For this reason they were also named as ‘chemotherapy granules’ [75]. Hawkins and Smiles in 1941 [76] were able to show accumulation of the fluorescent drug stilbamidine in trypanosome granules. Other drugs, like quinapyramine, suramin, hydroxystilbaminine [77], and acriflavine [76] were also found to concentrate in these granules. Interestingly, some of these drugs are first concentrated in the kinetoplast and nucleus, then diffuse to the cytosol, and finally concentrate in granules [76, 77]. Recent work on other diamidines such as DB75 (furamidine) and DB820, which are in phase III clinical trials against human African trypanosomiasis, revealed a similar pattern of accumulation, first in DNA-containing regions such as the nucleus and kinetoplast and later in acidocalcisomes [78]. However, the impact that acidocalcisome accumulation has on the mechanism of action of these compounds in not known [79].
Ketoconazole and terbinafine, two sterol biosynthesis inhibitors, were shown to induce the formation of numerous and diverse acidocalcisomes in promastigotes and amastigotes of L. amazonensis, which were enclosed by in larger compartments with access to endocytic tracers [80]. Naphthoimidazole compounds were found to decrease the electron density of acidocalcisomes of T. cruzi [81].
Azithromicin, a drug used against toxoplasmosis has also been shown to accumulate in acidic compartments within T. gondii tachyzoites [82]. Other chemotherapeutic agents used against malaria (e.g. chloroquine) have also been shown to accumulate in acidic compartments [83] and Na+/H+ exchangers such as monensin are used in the treatment of coccidiosis. Chloroquine accumulates in the acidocalcisomes of T. brucei, slows down growth in vivo and prolongs the survival time of infected mice [83].
CONCLUSIONS
In conclusion, acidocalcisomes are potential targets for the chemotherapy of protozoan parasitic diseases not only because they possess enzymes that are absent or different from their mammalian counterparts, but also because of their acidic characteristics, which allow them to accumulate basic drugs, potentially enhancing their toxicity.
Acknowledgments
Work in our laboratories was supported by grants from the National Institutes of Health (AI-68647 to RD and AI-68467 to SNJM).
References
- 1.Docampo R, de Souza W, Miranda K, Rohloff P, Moreno SN. Acidocalcisomes - conserved from bacteria to man. Nat Rev Microbiol. 2005;3:251–61. doi: 10.1038/nrmicro1097. [DOI] [PubMed] [Google Scholar]
- 2.Docampo R, Moreno SN. The acidocalcisome. Mol Biochem Parasitol. 2001;114:151–9. doi: 10.1016/s0166-6851(01)00246-8. [DOI] [PubMed] [Google Scholar]
- 3.Ruiz FA, Marchesini N, Seufferheld M, Govindjee Docampo R. The polyphosphate bodies of Chlamydomonas reinhardtii possess a proton-pumping pyrophosphatase and are similar to acidocalcisomes. J Biol Chem. 2001;276:46196–203. doi: 10.1074/jbc.M105268200. [DOI] [PubMed] [Google Scholar]
- 4.Marchesini N, Ruiz FA, Vieira M, Docampo R. Acidocalcisomes are functionally linked to the contractile vacuole of Dictyostelium discoideum. J Biol Chem. 2002;277:8146–53. doi: 10.1074/jbc.M111130200. [DOI] [PubMed] [Google Scholar]
- 5.Seufferheld M, Lea CR, Vieira M, Oldfield E, Docampo R. The H+-pyrophosphatase of Rhodospirillum rubrum is predominantly located in polyphosphate-rich acidocalcisomes. J Biol Chem. 2004;279:51193–202. doi: 10.1074/jbc.M406099200. [DOI] [PubMed] [Google Scholar]
- 6.Seufferheld M, Vieira MC, Ruiz FA, Rodrigues CO, Moreno SN, Docampo R. Identification of organelles in bacteria similar to acidocalcisomes of unicellular eukaryotes. J Biol Chem. 2003;278:29971–8. doi: 10.1074/jbc.M304548200. [DOI] [PubMed] [Google Scholar]
- 7.Smith SA, Mutch NJ, Baskar D, Rohloff P, Docampo R, Morrissey JH. Polyphosphate modulates blood coagulation and fibrinolysis. Proc Natl Acad Sci U S A. 2006;103:903–8. doi: 10.1073/pnas.0507195103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruiz FA, Lea CR, Oldfield E, Docampo R. Human platelet dense granules contain polyphosphate and are similar to acidocalcisomes of bacteria and unicellular eukaryotes. J Biol Chem. 2004;279:44250–7. doi: 10.1074/jbc.M406261200. [DOI] [PubMed] [Google Scholar]
- 9.Docampo R, Moreno SN. Bisphosphonates as chemotherapeutic agents against trypanosomatid and apicomplexan parasites. Curr Drug Targets Infect Disord. 2001;1:51–61. doi: 10.2174/1568005013343191. [DOI] [PubMed] [Google Scholar]
- 10.Scott DA, de Souza W, Benchimol M, Zhong L, Lu HG, Moreno SN, Docampo R. Presence of a plant-like proton-pumping pyrophosphatase in acidocalcisomes of Trypanosoma cruzi. J Biol Chem. 1998;273:22151–8. doi: 10.1074/jbc.273.34.22151. [DOI] [PubMed] [Google Scholar]
- 11.Rodrigues CO, Scott DA, Docampo R. Characterization of a vacuolar pyrophosphatase in Trypanosoma brucei and its localization to acidocalcisomes. Mol Cell Biol. 1999;19:7712–23. doi: 10.1128/mcb.19.11.7712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lemercier G, Dutoya S, Luo S, Ruiz FA, Rodrigues CO, Baltz T, Docampo R, Bakalara N. A vacuolar-type H+-pyrophosphatase governs maintenance of functional acidocalcisomes and growth of the insect and mammalian forms of Trypanosoma brucei. J Biol Chem. 2002;277:37369–76. doi: 10.1074/jbc.M204744200. [DOI] [PubMed] [Google Scholar]
- 13.Rodrigues CO, Scott DA, Docampo R. Presence of a vacuolar H+-pyrophosphatase in promastigotes of Leishmania donovani and its localization to a different compartment from the vacuolar H+-ATPase. Biochem J. 1999;340:759–66. [PMC free article] [PubMed] [Google Scholar]
- 14.Miranda K, Docampo R, Grillo O, Franzen A, Attias M, Vercesi A, Plattner H, Hentschel J, de Souza W. Dynamics of polymorphism of acidocalcisomes in Leishmania parasites. Histochem Cell Biol. 2004;121:407–18. doi: 10.1007/s00418-004-0646-4. [DOI] [PubMed] [Google Scholar]
- 15.Miranda K, Rodrigues CO, Hentchel J, Vercesi A, Plattner H, de Souza W, Docampo R. Acidocalcisomes of Phytomonas francai possess distinct morphological characteristics and contain iron. Microsc Microanal. 2004;10:647–55. doi: 10.1017/S1431927604040887. [DOI] [PubMed] [Google Scholar]
- 16.Moraes Moreira BL, Soares Medeiros LC, Miranda K, de Souza W, Hentschel J, Plattner H, Barrabin H. Kinetics of pyrophosphate-driven proton uptake by acidocalcisomes of Leptomonas wallacei. Biochem Biophys Res Commun. 2005;334:1206–13. doi: 10.1016/j.bbrc.2005.06.205. [DOI] [PubMed] [Google Scholar]
- 17.Soares Medeiros LC, Moreira BL, Miranda K, de Souza W, Plattner H, Hentschel J, Barrabin H. A proton pumping pyrophosphatase in acidocalcisomes of Herpetomonas sp. Mol Biochem Parasitol. 2005;140:175–82. doi: 10.1016/j.molbiopara.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 18.Luo S, Marchesini N, Moreno SN, Docampo R. A plant-like vacuolar H+-pyrophosphatase in Plasmodium falciparum. FEBS Lett. 1999;460:217–20. doi: 10.1016/s0014-5793(99)01353-8. [DOI] [PubMed] [Google Scholar]
- 19.McIntosh MT, Drozdowicz YM, Laroiya K, Rea PA, Vaidya AB. Two classes of plant-like vacuolar-type H+-pyrophosphatases in malaria parasites. Mol Biochem Parasitol. 2001;114:183–95. doi: 10.1016/s0166-6851(01)00251-1. [DOI] [PubMed] [Google Scholar]
- 20.Rodrigues CO, Scott DA, Bailey BN, De Souza W, Benchimol M, Moreno B, Urbina JA, Oldfield E, Moreno SN. Vacuolar proton pyrophosphatase activity and pyrophosphate (PPi) in Toxoplasma gondii as possible chemotherapeutic targets. Biochem J. 2000;349:737–45. doi: 10.1042/bj3490737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo S, Ruiz FA, Moreno SN. The acidocalcisome Ca2+-ATPase (TgA1) of Toxoplasma gondii is required for polyphosphate storage, intracellular calcium homeostasis and virulence. Mol Microbiol. 2005;55:1034–45. doi: 10.1111/j.1365-2958.2004.04464.x. [DOI] [PubMed] [Google Scholar]
- 22.Hill JE, Scott DA, Luo S, Docampo R. Cloning and functional expression of a gene encoding a vacuolar-type proton-translocating pyrophosphatase from Trypanosoma cruzi. Biochem J. 2000;351:281–8. doi: 10.1042/0264-6021:3510281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodrigues CO, Ruiz FA, Rohloff P, Scott DA, Moreno SN. Characterization of isolated acidocalcisomes from Toxoplasma gondii tachyzoites reveals a novel pool of hydrolyzable polyphosphate. J Biol Chem. 2002;277:48650–6. doi: 10.1074/jbc.M208990200. [DOI] [PubMed] [Google Scholar]
- 24.Martinez R, Wang Y, Benaim G, Benchimol M, de Souza W, Scott DA, Docampo R. A proton pumping pyrophosphatase in the Golgi apparatus and plasma membrane vesicles of Trypanosoma cruzi. Mol Biochem Parasitol. 2002;120:205–13. doi: 10.1016/s0166-6851(01)00456-x. [DOI] [PubMed] [Google Scholar]
- 25.Biagini GA, Bray PG, Spiller DG, White MR, Ward SA. The digestive food vacuole of the malaria parasite is a dynamic intracellular Ca2+ store. J Biol Chem. 2003;278:27910–5. doi: 10.1074/jbc.M304193200. [DOI] [PubMed] [Google Scholar]
- 26.Saliba KJ, Allen RJ, Zissis S, Bray PG, Ward SA, Kirk K. Acidification of the malaria parasite’s digestive vacuole by a H+-ATPase and a H+-pyrophosphatase. J Biol Chem. 2003;278:5605–12. doi: 10.1074/jbc.M208648200. [DOI] [PubMed] [Google Scholar]
- 27.Kim EJ, Zhen RG, Rea PA. Heterologous expression of plant vacuolar pyrophosphatase in yeast demonstrates sufficiency of the substrate-binding subunit for proton transport. Proc Natl Acad Sci U S A. 1994;91:6128–32. doi: 10.1073/pnas.91.13.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gordon-Weeks R, Parmar S, Davies TG, Leigh RA. Structural aspects of the effectiveness of bisphosphonates as competitive inhibitors of the plant vacuolar proton-pumping pyrophosphatase. Biochem J. 1999;337:373–7. [PMC free article] [PubMed] [Google Scholar]
- 29.Szabo CM, Oldfield E. An investigation of bisphosphonate inhibition of a vacuolar proton-pumping pyrophosphatase. Biochem Biophys Res Commun. 2001;287:468–73. doi: 10.1006/bbrc.2001.5617. [DOI] [PubMed] [Google Scholar]
- 30.Drozdowicz YM, Shaw M, Nishi M, Striepen B, Liwinski HA, Roos DS, Rea PA. Isolation and characterization of TgVP1, a type I vacuolar H+-translocating pyrophosphatase from Toxoplasma gondii. The dynamics of its subcellular localization and the cellular effects of a diphosphonate inhibitor. J Biol Chem. 2003;278:1075–85. doi: 10.1074/jbc.M209436200. [DOI] [PubMed] [Google Scholar]
- 31.Zhen RG, Baykov AA, Bakuleva NP, Rea PA. Aminomethylenediphosphonate: A potent type-specific inhibitor of both plant and phototrophic bacterial H+-pyrophosphatases. Plant Physiol. 1994;104:153–59. doi: 10.1104/pp.104.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lemercier G, Espiau B, Ruiz FA, Vieira M, Luo S, Baltz T, Docampo R, Bakalara N. A pyrophosphatase regulating polyphosphate metabolism in acidocalcisomes is essential for Trypanosoma brucei virulence in mice. J Biol Chem. 2004;279:3420–5. doi: 10.1074/jbc.M309974200. [DOI] [PubMed] [Google Scholar]
- 33.Kotsikorou E, Song Y, Chan JM, Faelens S, Tovian Z, Broderick E, Bakalara N, Docampo R, Oldfield E. Bisphosphonate inhibition of the exopolyphosphatase activity of the Trypanosoma brucei soluble vacuolar pyrophosphatase. J Med Chem. 2005;48:6128–39. doi: 10.1021/jm058220g. [DOI] [PubMed] [Google Scholar]
- 34.Rodan GA. Mechanisms of action of bisphosphonates. Annu Rev Pharmacol Toxicol. 1998;38:375–88. doi: 10.1146/annurev.pharmtox.38.1.375. [DOI] [PubMed] [Google Scholar]
- 35.Martin MB, Grimley JS, Lewis JC, Heath HT, 3rd, Bailey BN, Kendrick H, Yardley V, Caldera A, Lira R, Urbina JA, Moreno SN, Docampo R, Croft SL, Oldfield E. Bisphosphonates inhibit the growth of Trypanosoma brucei, Trypanosoma cruzi, Leishmania donovani, Toxoplasma gondii, and Plasmodium falciparum: a potential route to chemotherapy. J Med Chem. 2001;44:909–16. doi: 10.1021/jm0002578. [DOI] [PubMed] [Google Scholar]
- 36.van beek E, Lowik C, van der Pluijm G, Papapoulos S. The role of geranylgeranylation in bone resorption and its suppression by bisphosphonates in fetal bone explants in vitro: A clue to the mechanism of action of nitrogen-containing bisphosphonates. J Bone Miner Res. 1999;14:722–9. doi: 10.1359/jbmr.1999.14.5.722. [DOI] [PubMed] [Google Scholar]
- 37.van Beek E, Pieterman E, Cohen L, Lowik C, Papapoulos S. Nitrogen-containing bisphosphonates inhibit isopentenyl pyrophosphate isomerase/farnesyl pyrophosphate synthase activity with relative potencies corresponding to their antiresorptive potencies in vitro and in vivo. Biochem Biophys Res Commun. 1999;255:491–4. doi: 10.1006/bbrc.1999.0224. [DOI] [PubMed] [Google Scholar]
- 38.Keller RK, Fliesler SJ. Mechanism of aminobisphosphonate action: characterization of alendronate inhibition of the isoprenoid pathway. Biochem Biophys Res Commun. 1999;266:560–3. doi: 10.1006/bbrc.1999.1849. [DOI] [PubMed] [Google Scholar]
- 39.Bergstrom JD, Bostedor RG, Masarachia PJ, Reszka AA, Rodan G. Alendronate is a specific, nanomolar inhibitor of farnesyl diphosphate synthase. Arch Biochem Biophys. 2000;373:231–41. doi: 10.1006/abbi.1999.1502. [DOI] [PubMed] [Google Scholar]
- 40.Dunford JE, Thompson K, Coxon FP, Luckman SP, Hahn FM, Poulter CD, Ebetino FH, Rogers MJ. Structure-activity relationships for inhibition of farnesyl diphosphate synthase in vitro and inhibition of bone resorption in vivo by nitrogen-containing bisphosphonates. J Pharmacol Exp Ther. 2001;296:235–42. [PubMed] [Google Scholar]
- 41.Cromartie TH, Fisher KJ, Grossman JN. The discovery of a novel site of action of alendronate inhibition of the isoprenoid pathway. Pestic Biochem Physiol. 1999;63:114–26. [Google Scholar]
- 42.Green JR. Skeletal complications of prostate cancer: pathophysiology and therapeutic potential of bisphosphonates. Acta Oncol. 2005;44:282–92. doi: 10.1080/02841860510029644. [DOI] [PubMed] [Google Scholar]
- 43.Luckman SP, Hughes DE, Coxon FP, Graham R, Russell G, Rogers MJ. Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J Bone Miner Res. 1998;13:581–9. doi: 10.1359/jbmr.1998.13.4.581. [DOI] [PubMed] [Google Scholar]
- 44.Fisher JE, Rogers MJ, Halasy JM, Luckman SP, Hughes DE, Masarachia PJ, Wesolowski G, Russell RG, Rodan GA, Reszka AA. Alendronate mechanism of action: geranylgeraniol, an intermediate in the mevalonate pathway, prevents inhibition of osteoclast formation, bone resorption, and kinase activation in vitro. Proc Natl Acad Sci U S A. 1999;96:133–8. doi: 10.1073/pnas.96.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kunzmann V, Bauer E, Feurle J, Weissinger F, Tony HP, Wilhelm M. Stimulation of gammadelta T cells by aminobisphosphonates and induction of antiplasma cell activity in multiple myeloma. Blood. 2000;96:384–92. [PubMed] [Google Scholar]
- 46.Luckman SP, Coxon FP, Ebetino FH, Russell RG, Rogers MJ. Heterocycle-containing bisphosphonates cause apoptosis and inhibit bone resorption by preventing protein prenylation: evidence from structure-activity relationships in J774 macrophages. J Bone Miner Res. 1998;13:1668–78. doi: 10.1359/jbmr.1998.13.11.1668. [DOI] [PubMed] [Google Scholar]
- 47.Hughes DE, Wright KR, Uy HL, Sasaki A, Yoneda T, Roodman GD, Mundy GR, Boyce BF. Bisphosphonates promote apoptosis in murine osteoclasts in vitro and in vivo. J Bone Miner Res. 1995;10:1478–87. doi: 10.1002/jbmr.5650101008. [DOI] [PubMed] [Google Scholar]
- 48.Monkkonen H, Auriola S, Lehenkari P, Kellinsalmi M, Hassinen IE, Vepsalainen J, Monkkonen J. A new endogenous ATP analog (ApppI) inhibits the mitochondrial adenine nucleotide translocase (ANT) and is responsible for the apoptosis induced by nitrogen-containing bisphosphonates. Br J Pharmacol. 2006;147:437–45. doi: 10.1038/sj.bjp.0706628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Szabo CM, Matsumura Y, Fukura S, Martin MB, Sanders JM, Sengupta S, Cieslak JA, Loftus TC, Lea CR, Lee HJ, Koohang A, Coates RM, Sagami H, Oldfield E. Inhibition of geranylgeranyl diphosphate synthase by bisphosphonates and diphosphates: a potential route to new bone antiresorption and antiparasitic agents. J Med Chem. 2002;45:2185–96. doi: 10.1021/jm010412y. [DOI] [PubMed] [Google Scholar]
- 50.Guo RT, Cao R, Liang PH, Ko TP, Chang TH, Hudock MP, Jeng WY, Chen CK, Zhang Y, Song Y, Kuo CJ, Yin F, Oldfield E, Wang AH. Bisphosphonates target multiple sites in both cis- and trans-prenyltransferases. Proc Natl Acad Sci U S A. 2007;104:10022–7. doi: 10.1073/pnas.0702254104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hudock MP, Sanz-Rodriguez CE, Song Y, Chan JM, Zhang Y, Odeh S, Kosztowski T, Leon-Rossell A, Concepcion JL, Yardley V, Croft SL, Urbina JA, Oldfield E. Inhibition of Trypanosoma cruzi hexokinase by bisphosphonates. J Med Chem. 2006;49:215–23. doi: 10.1021/jm0582625. [DOI] [PubMed] [Google Scholar]
- 52.Sanz-Rodriguez CE, Concepcion JL, Pekerar S, Oldfield E, Urbina JA. Bisphosphonates as inhibitors of Trypanosoma cruzi hexokinase: kinetic and metabolic studies. J Biol Chem. 2007;282:12377–87. doi: 10.1074/jbc.M607286200. [DOI] [PubMed] [Google Scholar]
- 53.Urbina JA, Moreno B, Vierkotter S, Oldfield E, Payares G, Sanoja C, Bailey BN, Yan W, Scott DA, Moreno SN, Docampo R. Trypanosoma cruzi contains major pyrophosphate stores, and its growth in vitro and in vivo is blocked by pyrophosphate analogs. J Biol Chem. 1999;274:33609–15. doi: 10.1074/jbc.274.47.33609. [DOI] [PubMed] [Google Scholar]
- 54.Moreno B, Bailey BN, Luo S, Martin MB, Kuhlenschmidt M, Moreno SN, Docampo R, Oldfield E. 31P NMR of apicomplexans and the effects of risedronate on Cryptosporidium parvum growth. Biochem Biophys Res Commun. 2001;284:632–7. doi: 10.1006/bbrc.2001.5009. [DOI] [PubMed] [Google Scholar]
- 55.Rodriguez N, Bailey BN, Martin MB, Oldfield E, Urbina JA, Docampo R. Radical cure of experimental cutaneous leishmaniasis by the bisphosphonate pamidronate. J Infect Dis. 2002;186:138–40. doi: 10.1086/341074. [DOI] [PubMed] [Google Scholar]
- 56.Yardley V, Khan AA, Martin MB, Slifer TR, Araujo FG, Moreno SN, Docampo R, Croft SL, Oldfield E. In vivo activities of farnesyl pyrophosphate synthase inhibitors against Leishmania donovani and Toxoplasma gondii. Antimicrob Agents Chemother. 2002;46:929–31. doi: 10.1128/AAC.46.3.929-931.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Garzoni LR, Waghabi MC, Baptista MM, de Castro SL, Meirelles Mde N, Britto CC, Docampo R, Oldfield E, Urbina JA. Antiparasitic activity of risedronate in a murine model of acute Chagas’ disease. Int J Antimicrob Agents. 2004;23:286–90. doi: 10.1016/j.ijantimicag.2003.07.019. [DOI] [PubMed] [Google Scholar]
- 58.Garzoni LR, Caldera A, Meirelles Mde N, de Castro SL, Docampo R, Meints GA, Oldfield E, Urbina JA. Selective in vitro effects of the farnesyl pyrophosphate synthase inhibitor risedronate on Trypanosoma cruzi. Int J Antimicrob Agents. 2004;23:273–85. doi: 10.1016/j.ijantimicag.2003.07.020. [DOI] [PubMed] [Google Scholar]
- 59.Bouzahzah B, Jelicks LA, Morris SA, Weiss LM, Tanowitz HB. Risedronate in the treatment of murine Chagas’ disease. Parasitol Res. 2005;96:184–7. doi: 10.1007/s00436-005-1331-9. [DOI] [PubMed] [Google Scholar]
- 60.Ghosh S, Chan JM, Lea CR, Meints GA, Lewis JC, Tovian ZS, Flessner RM, Loftus TC, Bruchhaus I, Kendrick H, Croft SL, Kemp RG, Kobayashi S, Nozaki T, Oldfield E. Effects of bisphosphonates on the growth of Entamoeba histolytica and Plasmodium species in vitro and in vivo. J Med Chem. 2004;47:175–87. doi: 10.1021/jm030084x. [DOI] [PubMed] [Google Scholar]
- 61.Ling Y, Sahota G, Odeh S, Chan JM, Araujo FG, Moreno SN, Oldfield E. Bisphosphonate inhibitors of Toxoplasma gondi growth: in vitro, QSAR, and in vivo investigations. J Med Chem. 2005;48:3130–40. doi: 10.1021/jm040132t. [DOI] [PubMed] [Google Scholar]
- 62.Szajnman SH, Ravaschino EL, Docampo R, Rodriguez JB. Synthesis and biological evaluation of 1-amino-1,1-bisphosphonates derived from fatty acids against Trypanosoma cruzi targeting farnesyl pyrophosphate synthase. Bioorg Med Chem Lett. 2005;15:4685–90. doi: 10.1016/j.bmcl.2005.07.060. [DOI] [PubMed] [Google Scholar]
- 63.Montalvetti A, Bailey BN, Martin MB, Severin GW, Oldfield E, Docampo R. Bisphosphonates are potent inhibitors of Trypanosoma cruzi farnesyl pyrophosphate synthase. J Biol Chem. 2001;276:33930–7. doi: 10.1074/jbc.M103950200. [DOI] [PubMed] [Google Scholar]
- 64.Montalvetti A, Fernandez A, Sanders JM, Ghosh S, Van Brussel E, Oldfield E, Docampo R. Farnesyl pyrophosphate synthase is an essential enzyme in Trypanosoma brucei. In vitro RNA interference and in vivo inhibition studies. J Biol Chem. 2003;278:17075–83. doi: 10.1074/jbc.M210467200. [DOI] [PubMed] [Google Scholar]
- 65.Ling Y, Li ZH, Miranda K, Oldfield E, Moreno SN. The farnesyl diphosphate/geranylgeranyl diphosphate synthase of Toxoplasma gondii is a bifunctional enzyme and a molecular target of bisphosphonates. J Biol Chem. 2007;282:30804–30816. doi: 10.1074/jbc.M703178200. [DOI] [PubMed] [Google Scholar]
- 66.Sanders JM, Song Y, Chan JM, Zhang Y, Jennings S, Kosztowski T, Odeh S, Flessner R, Schwerdtfeger C, Kotsikorou E, Meints GA, Gomez AO, Gonzalez-Pacanowska D, Raker AM, Wang H, van Beek ER, Papapoulos SE, Morita CT, Oldfield E. Pyridinium-1-yl bisphosphonates are potent inhibitors of farnesyl diphosphate synthase and bone resorption. J Med Chem. 2005;48:2957–63. doi: 10.1021/jm040209d. [DOI] [PubMed] [Google Scholar]
- 67.Martin MB, Sanders JM, Kendrick H, de Luca-Fradley K, Lewis JC, Grimley JS, Van Brussel EM, Olsen JR, Meints GA, Burzynska A, Kafarski P, Croft SL, Oldfield E. Activity of bisphosphonates against Trypanosoma brucei rhodesiense. J Med Chem. 2002;45:2904–14. doi: 10.1021/jm0102809. [DOI] [PubMed] [Google Scholar]
- 68.Ortiz-Gomez A, Jimenez C, Estevez AM, Carrero-Lerida J, Ruiz-Perez LM, Gonzalez-Pacanowska D. Farnesyl diphosphate synthase is a cytosolic enzyme in Leishmania major promastigotes and its overexpression confers resistance to risedronate. Eukaryot Cell. 2006;5:1057–64. doi: 10.1128/EC.00034-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mao J, Gao YG, Odeh S, Robinson H, Montalvetti A, Docampo R, Oldfield E. Crystallization and preliminary X-ray diffraction study of the farnesyl diphosphate synthase from Trypanosoma brucei. Acta Crystallogr D Biol Crystallogr. 2004;60:1863–6. doi: 10.1107/S0907444904020633. [DOI] [PubMed] [Google Scholar]
- 70.Mao J, Mukherjee S, Zhang Y, Cao R, Sanders JM, Song Y, Zhang Y, Meints GA, Gao YG, Mukkamala D, Hudock MP, Oldfield E. Solid-state NMR, crystallographic, and computational investigation of bisphosphonates and farnesyl diphosphate synthase-bisphosphonate complexes. J Am Chem Soc. 2006;128:14485–97. doi: 10.1021/ja061737c. [DOI] [PubMed] [Google Scholar]
- 71.Gabelli SB, McLellan JS, Montalvetti A, Oldfield E, Docampo R, Amzel LM. Structure and mechanism of the farnesyl diphosphate synthase from Trypanosoma cruzi: implications for drug design. Proteins. 2006;62:80–8. doi: 10.1002/prot.20754. [DOI] [PubMed] [Google Scholar]
- 72.Vedadi M, Lew J, Artz J, Amani M, Zhao Y, Dong A, Wasney GA, Gao M, Hills T, Brokx S, Qiu W, Sharma S, Diassiti A, Alam Z, Melone M, Mulichak A, Wernimont A, Bray J, Loppnau P, Plotnikova O, Newberry K, Sundararajan E, Houston S, Walker J, Tempel W, Bochkarev A, Kozieradzki I, Edwards A, Arrowsmith C, Roos D, Kain K, Hui R. Genome-scale protein expression and structural biology of Plasmodium falciparum and related Apicomplexan organisms. Mol Biochem Parasitol. 2007;151:100–10. doi: 10.1016/j.molbiopara.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 73a.Ferella M, Montalvetti A, Rohloff P, Miranda K, Fang J, Reina S, Kawamukai M, Bua J, Nilsson D, Pravia C, Katzin A, Cassera MB, Aslund L, Andersson B, Docampo R, Bontempi EJ. A solanesyl-diphosphate synthase localizes in glycosomes of Trypanosoma cruzi. J Biol Chem. 2006;281:39339–48. doi: 10.1074/jbc.M607451200. [DOI] [PubMed] [Google Scholar]
- 73b.Szajman SH, Garcia Linares GE, Li ZH, Jiang C, Galizzi M, Bontempi EJ, et al. Synthesis and biological evaluation of 2-alkylaminoethyl-1,1-bisphosphonic acids against Trypanosma cruzi and Toxoplasma gondii targeting farnesyl diphosphate synthase. Bioorg Med Chem. 2008;16:3283–3290. doi: 10.1016/j.bmc.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ormerod WE. The study of volutin granules in trypanosomes. Trans R Soc Trop Med Hyg. 1961;55:313–32. doi: 10.1016/0035-9203(61)90100-6. [DOI] [PubMed] [Google Scholar]
- 75.Ormerod WE. A study of basophilic inclusion bodies produced by chemotherapeutic agents in trypanosomes. Br J Pharmacol Chemother. 1951;6:334–41. doi: 10.1111/j.1476-5381.1951.tb00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Macadam RF, Williamson J. Drug effects on the fine structure of Trypanosoma rhodesiense: acriflavine, ethidium and antrycide. Ann Trop Med Parasitol. 1974;68:291–9. doi: 10.1080/00034983.1974.11686951. [DOI] [PubMed] [Google Scholar]
- 77.Ormerod WE, Shaw JJ. A study of granules and other changes in phase-contrast appearance produced by chemotherapeutic agents in trypanosomes. Br J Pharmacol Chemother. 1963;21:259–72. doi: 10.1111/j.1476-5381.1963.tb01525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mathis AM, Bridges AS, Ismail MA, Kumar A, Francesconi I, Anbazhagan M, Hu Q, Tanious FA, Wenzler T, Saulter J, Wilson WD, Brun R, Boykin DW, Tidwell RR, Hall JE. Diphenyl furans and aza analogs: effects of structural modification on in vitro activity, DNA binding, and accumulation and distribution in trypanosomes. Antimicrob Agents Chemother. 2007;51:2801–10. doi: 10.1128/AAC.00005-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mathis AM, Holman JL, Sturk LM, Ismail MA, Boykin DW, Tidwell RR, Hall JE. Accumulation and intracellular distribution of antitrypanosomal diamidine compounds DB75 and DB820 in African trypanosomes. Antimicrob Agents Chemother. 2006;50:2185–91. doi: 10.1128/AAC.00192-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vannier-Santos MA, Martiny A, Lins U, Urbina JA, Borges VM, de Souza W. Impairment of sterol biosynthesis leads to phosphorus and calcium accumulation in Leishmania acidocalcisomes. Microbiology. 1999;145:3213–20. doi: 10.1099/00221287-145-11-3213. [DOI] [PubMed] [Google Scholar]
- 81.Menna-Barreto RF, Henriques-Pons A, Pinto AV, Morgado-Diaz JA, Soares MJ, De Castro SL. Effect of a beta-lapachone-derived naphthoimidazole on Trypanosoma cruzi: identification of target organelles. J Antimicrob Chemother. 2005;56:1034–41. doi: 10.1093/jac/dki403. [DOI] [PubMed] [Google Scholar]
- 82.Schwab JC, Cao Y, Slowik MR, Joiner K. Localization of azithromicin in Toxoplasma gondii. Antimicrob Agents Chemother. 1994;38:1620–7. doi: 10.1128/aac.38.7.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Coppens I, Baudhin P, Opperdoes FR, Courtoy PJ. Role of acidic compartments in Trypanosoma brucei, with special reference to low-density lipoprotein processing. Mol Biochem Parasitol. 1993;58:223–32. doi: 10.1016/0166-6851(93)90044-x. [DOI] [PubMed] [Google Scholar]
- 84.Fang J, Rohloff P, Miranda K, Docampo R. Ablation of a small transmembrane protein of Trypanosoma brucei (TbVTC1) involved in the synthesis of polyphosphate alters acidocalcisome biogenesis and function, and leads to a cytokinesis defect. Biochem J. 2007;407:161–170. doi: 10.1042/BJ20070612. [DOI] [PMC free article] [PubMed] [Google Scholar]