Abstract
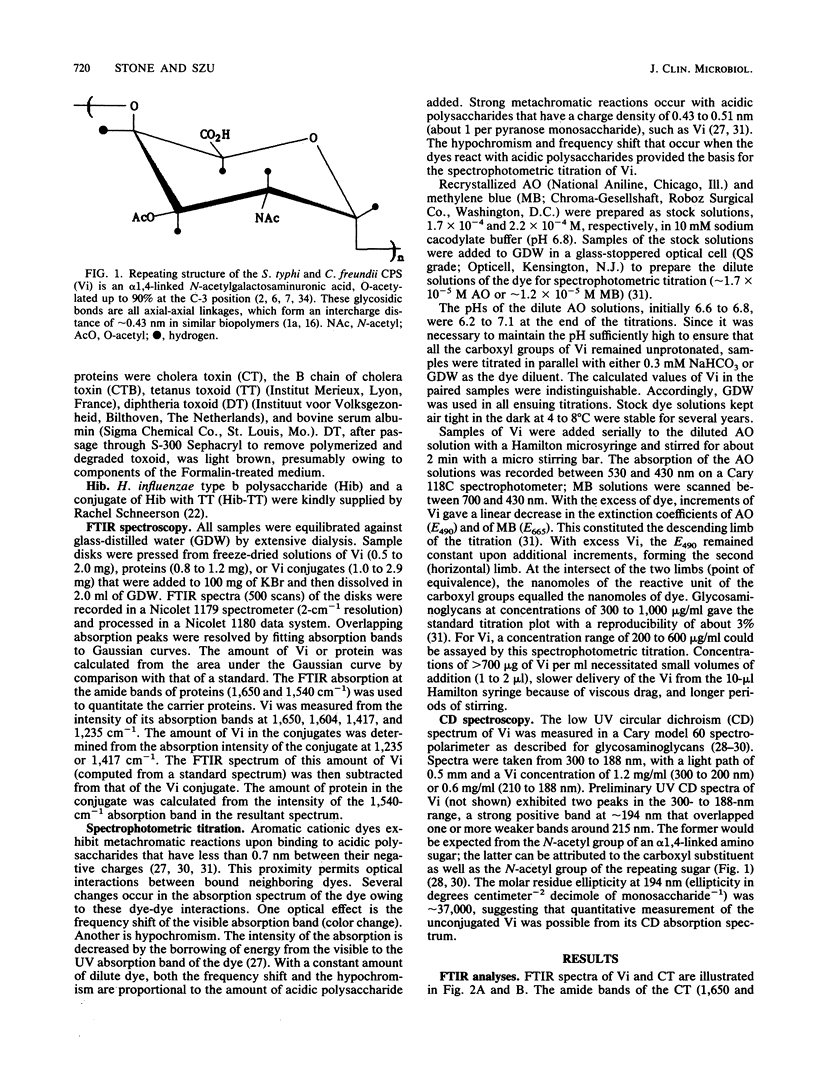
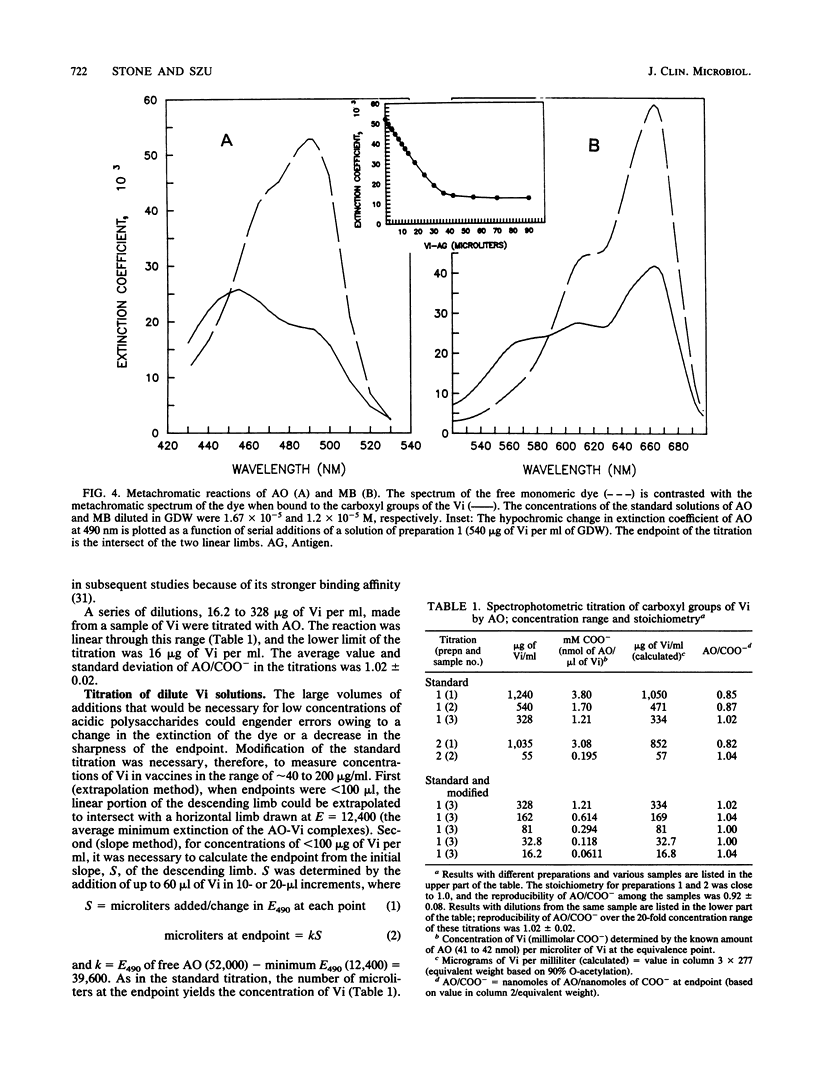
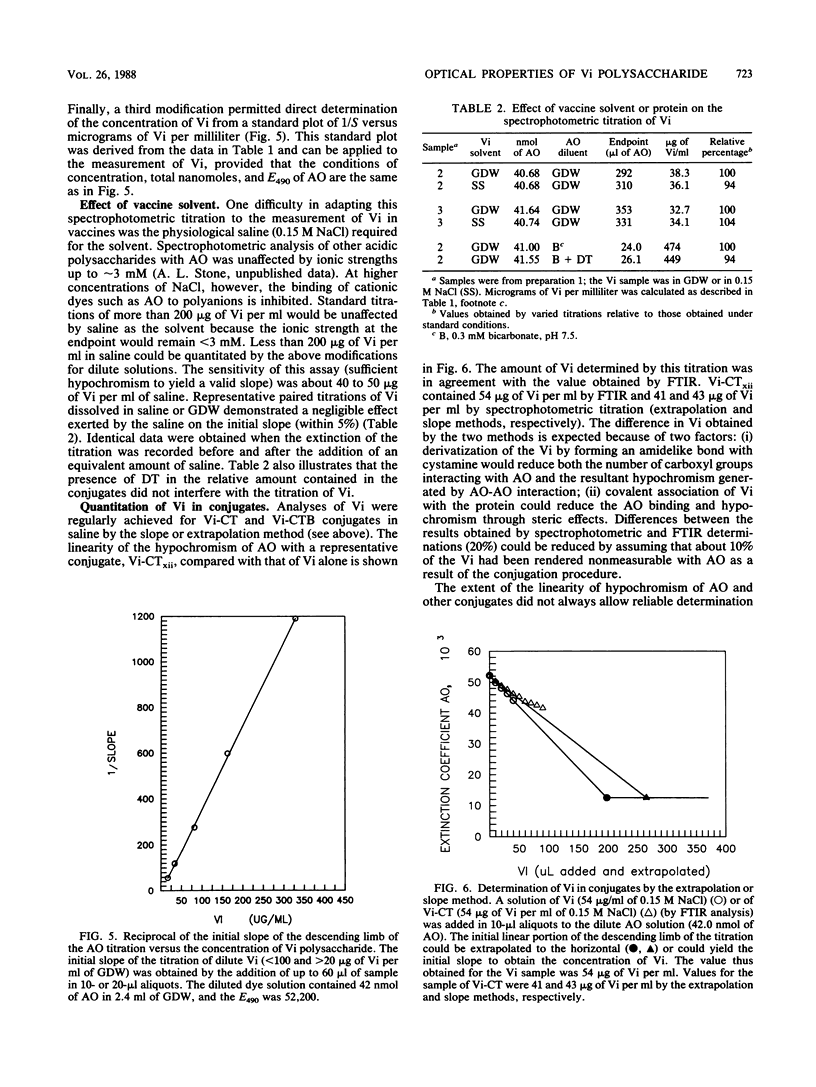
The capsular polysaccharide of Salmonella typhi and of Citrobacter freundii (Vi) is a linear homopolymer of alpha 1,4-linked N-acetylgalactosaminuronic acid, variably O-acetylated at the C-3 position. Vaccines composed of Vi confer protection against typhoid fever with an efficacy of about 70%; Vi has recently been conjugated to proteins to increase its immunogenicity and effectiveness (I.L. Acharya, R. Tapa, V.L. Gurubacharya, M.B. Shrestha, C.U. Lowe, D.D. Bryla, R. Schneerson, J.B. Robbins, T. Crampton, B. Trollfors, M. Cadoz, D. Schulz, and J. Armand, N. Engl. J. Med. 317:1101-1104, 1987; K.P. Klugman, I. Gilbertson, H.J. Kornhof, J.B. Robbins, R. Schneerson, D. Schulz, M. Cadoz, and J. Armand, Lancet ii:1165-1169, 1987; S.C. Szu, A.L. Stone, J.D. Robbins, R. Schneerson, and J.B. Robbins, J. Exp. Med. 166:1510-1524, 1987). Vi, however, cannot be measured by conventional colorimetric methods. Two optical techniques were adapted to quantitate Vi in vaccines. The first, Fourier-transformed infrared spectroscopy, was performed on salt-free, freeze-dried samples. The intensities of the absorbance peaks of Vi were proportional to the amount of Vi within the range of 0.25 to 2.0 mg. The amount of Vi was determined from integrated absorptions at the 1,235- or 1,417-cm-1 band. The second technique, spectrophotometric titration, was more sensitive than the Fourier-transformed infrared spectroscopy and could be performed on dilute solutions. The metachromatic effect of the reaction between the aromatic cationic dye acridine orange and the carboxyl groups of Vi was quantitative within +/- 2% in the range of 20 to 700 micrograms of Vi per ml. The accuracy of the titration of Vi in the vaccines was within +/- 8%. These two methods may be applicable to measure other capsular polysaccharides in vaccines.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acharya I. L., Lowe C. U., Thapa R., Gurubacharya V. L., Shrestha M. B., Cadoz M., Schulz D., Armand J., Bryla D. A., Trollfors B. Prevention of typhoid fever in Nepal with the Vi capsular polysaccharide of Salmonella typhi. A preliminary report. N Engl J Med. 1987 Oct 29;317(18):1101–1104. doi: 10.1056/NEJM198710293171801. [DOI] [PubMed] [Google Scholar]
- Atkins E. D., Nieduszynski I. A., Mackie W., Parker K. D., Smolko E. E. Structural components of alginic acid. II. The crystalline structure of poly-alpha-L-guluronic acid. Results of x-ray diffraction and polarized infrared studies. Biopolymers. 1973;12(8):1879–1887. doi: 10.1002/bip.1973.360120814. [DOI] [PubMed] [Google Scholar]
- CLARK W. R., McLAUGHLIN J., WEBSTER M. E. An aminohexuronic acid as the principal hydrolytic component of the Vi antigen. J Biol Chem. 1958 Jan;230(1):81–89. [PubMed] [Google Scholar]
- Eskola J., Peltola H., Takala A. K., Käyhty H., Hakulinen M., Karanko V., Kela E., Rekola P., Rönnberg P. R., Samuelson J. S. Efficacy of Haemophilus influenzae type b polysaccharide-diphtheria toxoid conjugate vaccine in infancy. N Engl J Med. 1987 Sep 17;317(12):717–722. doi: 10.1056/NEJM198709173171201. [DOI] [PubMed] [Google Scholar]
- Gotschlich E. C., Rey M., Etienne J., Sanborn W. R., Triau R., Cvjetanović B. The immunological responses observed in field studies in Africa with group A meningococcal vaccines. Prog Immunobiol Stand. 1971;5:485–491. [PubMed] [Google Scholar]
- Jennings H. J. Capsular polysaccharides as human vaccines. Adv Carbohydr Chem Biochem. 1983;41:155–208. doi: 10.1016/s0065-2318(08)60058-x. [DOI] [PubMed] [Google Scholar]
- Klugman K. P., Gilbertson I. T., Koornhof H. J., Robbins J. B., Schneerson R., Schulz D., Cadoz M., Armand J. Protective activity of Vi capsular polysaccharide vaccine against typhoid fever. Lancet. 1987 Nov 21;2(8569):1165–1169. doi: 10.1016/s0140-6736(87)91316-x. [DOI] [PubMed] [Google Scholar]
- Käyhty H., Karanko V., Peltola H., Mäkelä P. H. Serum antibodies after vaccination with Haemophilus influenzae type b capsular polysaccharide and responses to reimmunization: no evidence of immunologic tolerance or memory. Pediatrics. 1984 Nov;74(5):857–865. [PubMed] [Google Scholar]
- Leontein K., Lindberg B., Lönngren J., Carlo D. J. Structural studies of the capsular polysaccharide from Streptococcus pneumoniae type 12A. Carbohydr Res. 1983 Apr 1;114(2):257–266. doi: 10.1016/0008-6215(83)88192-0. [DOI] [PubMed] [Google Scholar]
- Liau D. F., Melly M. A., Hash J. H. Surface polysaccharide from Staphylococcus aureus M that contains taurine, D-aminogalacturonic acid, and D-fucosamine. J Bacteriol. 1974 Sep;119(3):913–922. doi: 10.1128/jb.119.3.913-922.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy S. V., Melly M. A., Harris T. M., Hellerqvist C. G., Hash J. H. The repeating sequence of the capsular polysaccharide of Staphylococcus aureus M. Carbohydr Res. 1983 Jun 16;117:113–123. doi: 10.1016/0008-6215(83)88080-x. [DOI] [PubMed] [Google Scholar]
- Peltola H., Käyhty H., Sivonen A., Mäkelä H. Haemophilus influenzae type b capsular polysaccharide vaccine in children: a double-blind field study of 100,000 vaccinees 3 months to 5 years of age in Finland. Pediatrics. 1977 Nov;60(5):730–737. [PubMed] [Google Scholar]
- Robbins J. B., Austrian R., Lee C. J., Rastogi S. C., Schiffman G., Henrichsen J., Mäkelä P. H., Broome C. V., Facklam R. R., Tiesjema R. H. Considerations for formulating the second-generation pneumococcal capsular polysaccharide vaccine with emphasis on the cross-reactive types within groups. J Infect Dis. 1983 Dec;148(6):1136–1159. doi: 10.1093/infdis/148.6.1136. [DOI] [PubMed] [Google Scholar]
- Robbins J. B. Vaccines for the prevention of encapsulated bacterial diseases: current status, problems and prospects for the future. Immunochemistry. 1978 Nov;15(10-11):839–854. doi: 10.1016/0161-5890(78)90117-7. [DOI] [PubMed] [Google Scholar]
- Robbins J. D., Robbins J. B. Reexamination of the protective role of the capsular polysaccharide (Vi antigen) of Salmonella typhi. J Infect Dis. 1984 Sep;150(3):436–449. doi: 10.1093/infdis/150.3.436. [DOI] [PubMed] [Google Scholar]
- Schneerson R., Barrera O., Sutton A., Robbins J. B. Preparation, characterization, and immunogenicity of Haemophilus influenzae type b polysaccharide-protein conjugates. J Exp Med. 1980 Aug 1;152(2):361–376. doi: 10.1084/jem.152.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneerson R., Robbins J. E., Chu C. Y., Sutton A., Schiffman G., Vann W. F. Semi-synthetic vaccines composed of capsular polysaccharides of pathogenic bacteria covalently bound to proteins for the prevention of invasive diseases. Prog Allergy. 1983;33:144–158. [PubMed] [Google Scholar]
- Schneerson R., Rodrigues L. P., Parke J. C., Jr, Robbins J. B. Immunity to disease caused by Hemophilus influenzae type b. II. Specificity and some biologic characteristics of "natural," infection-acquired, and immunization-induced antibodies to the capsular polysaccharide of Hemophilus influenzae type b. J Immunol. 1971 Oct;107(4):1081–1089. [PubMed] [Google Scholar]
- Smith E. J. Purification and properties of an acidic polysaccharide isolated from Achromobacter georgiopolitanum. J Biol Chem. 1968 Oct 10;243(19):5139–5144. [PubMed] [Google Scholar]
- Stone A. L. Aggregation of cationic dyes on acid polysaccharides. II. Quantitative parameters on metachromasy. Biochim Biophys Acta. 1967 Oct 9;148(1):193–206. doi: 10.1016/0304-4165(67)90294-2. [DOI] [PubMed] [Google Scholar]
- Stone A. L., Bradley D. F. Aggregation of cationic dyes on acid polysaccharides. I. Spectrophotometric titration with acridine orange and other metachromatic dyes. Biochim Biophys Acta. 1967 Oct 9;148(1):172–192. doi: 10.1016/0304-4165(67)90293-0. [DOI] [PubMed] [Google Scholar]
- Stone A. L. Optical rotary dispersion of mucopolysaccharides. 3. Ultraviolet circular dichroism and conformational specificity in amide groups. Biopolymers. 1971;10(4):739–751. doi: 10.1002/bip.360100411. [DOI] [PubMed] [Google Scholar]
- Szu S. C., Stone A. L., Robbins J. D., Schneerson R., Robbins J. B. Vi capsular polysaccharide-protein conjugates for prevention of typhoid fever. Preparation, characterization, and immunogenicity in laboratory animals. J Exp Med. 1987 Nov 1;166(5):1510–1524. doi: 10.1084/jem.166.5.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M. The analysis of lipopolysaccharide (endotoxin) in meningococcal polysaccharide vaccines by silver staining following SDS-polyacrylamide gel electrophoresis. J Biol Stand. 1986 Jan;14(1):25–33. doi: 10.1016/s0092-1157(86)80006-3. [DOI] [PubMed] [Google Scholar]
- WEBSTER M. E., CLARK W. R., FREEMAN M. E. Evidence for an aminohexuronic acid as hydrolytic product of Vi antigen. Arch Biochem Biophys. 1954 May;50(1):223–224. doi: 10.1016/0003-9861(54)90028-4. [DOI] [PubMed] [Google Scholar]
- WEBSTER M. E., SAGIN J. F., ANDERSON P. R., BREESE S. S., FREEMAN M. E., LANDY M. Studies on Vi antigen. IV. Physicochemical characterization of Vi antigens isolated from V form Enterobacteriaceae. J Immunol. 1954 Jul;73(1):16–22. [PubMed] [Google Scholar]
- WHITESIDE R. E., BAKER E. E. The Vi antigens of the Enterobacteriaceae. V. Serologic differences of Vi antigens revealed by deacetylation. J Immunol. 1961 May;86:538–542. [PubMed] [Google Scholar]
- WILLIAMSON A. R., ZAMENHOF S. The type-specific substance of Hemophilus influenzae, type d: the natural occurrence of glucosamine uronic acid. J Biol Chem. 1963 Jul;238:2255–2258. [PubMed] [Google Scholar]


