Abstract
With improvements in survival among individuals diagnosed and treated for cancer there is an increasing recognition of the risk of long-term adverse effects of therapy. Second neoplasms represent one of the more serious late effects of treatment and are associated with a substantial level of morbidity and mortality. Survivors of childhood cancers, because of their potential longevity, are particularly at risk for this adverse outcome. The Childhood Cancer Survivor Study is a large cohort consisting of adult survivors of childhood cancer diagnosed and treated between 1970–1986. The CCSS has provided important new data to quantify radiation-associated risk for subsequent cancers including neoplasms of the breast, thyroid and central nervous system.
Keywords: Second Neoplasms, Childhood Cancer Survivors, Radiation
Introduction
The Office of Cancer Survivorship at the National Cancer Institute estimates that there are more than 11 million people in the U.S. who are alive following the diagnosis of cancer. Among this large survivor population, nearly two thirds are greater than five years beyond their cancer diagnosis. The increasing number of cancer survivors can be attributed to a number of factors, including more effective approaches to early detection and treatment.
During the past three to four decades the survival rates have increased markedly for many of the cancers diagnosed and treated during childhood and adolescence [1]. Recent population-based data from the U.S. document five-year survivorship if 79% for childhood cancer patients diagnosed between 1995–2001. Along with this success has come the realization that the therapies responsible for long-term survival of these patients also carry with them the potential for late-occurring adverse consequences. To varying degrees, it has been shown that long-term survivors are at risk of developing a spectrum of adverse outcomes including early death, second neoplasms, organ dysfunction such as cardiac, pulmonary, and endocrine, reduced growth and development, decreased fertility, impaired cognitive function, difficulties obtaining employment and insurance, and overall reduction in quality of life. Because of the young age at which these cancer patients are treated, and thus their potential longevity, the long-term effects of therapy are likely to have a much greater impact on their lives, families, and society at large, than the acute complications of therapy they have already endured.
Beginning in the mid- to late-1970s, reports from single institutions began to emerge documenting the potential risk of treatment-related late effects. The majority of earliest published reports reflect either case reports or case-series, and were limited by a small sample size and/or were derived from patient populations treated in a similar fashion. Thus, accurate quantification of exposure-specific risk was generally not possible. However, these initial observations provided direction for future collaborative research. Summarized in this report are data from the Childhood Cancer Survivor Study (CCSS) that address the topic of treatment-related second neoplasms among long-term survivors, with specific emphasis on risks associated with radiation treatment exposures.
The Childhood Cancer Survivor Study
The CCSS is a National Cancer Institute supported resource, consisting of a retrospective cohort of children and adolescents treated for childhood cancer at 26 collaborating institutions in the United States and Canada (see Appendix). Eligibility criteria for the cohort included: individuals diagnosed before age 21 years with leukemia, CNS cancers, Hodgkin disease, non-Hodgkin lymphoma, kidney cancers, neuroblastoma, soft tissue sarcomas, and bone tumors, with initial treatment between January 1, 1970 and December 31, 1986, and survival for at least five years from diagnosis. Details, including characteristics of this cohort and the study design, have been previously described [2]. Briefly, beginning in 1994 all participants (or parents of participants < 18 years of age) completed a self-administered baseline questionnaire or telephone interview. Subsequent update questionnaires/interviews have been administered every 2–3 years (study surveys are available at www.stjude.org/ccss). Treatment information, including surgical procedures, chemotherapy, and radiation therapy, was abstracted from medical records using a structured protocol. Data collected also included the dates of initiation and cessation of treatment for all chemotherapeutic agents, cumulative doses and routes of administration for 28 specific agents, and all surgical procedures. Records from radiation oncology departments (including treatment positions and diagrams) were centrally-reviewed at the CCSS Radiation Data Center at the University of Texas, M.D. Anderson Cancer Center for dosimetry assessment.
Among the CCSS cohort, second and subsequent neoplasms are initially ascertained through self-report via study questionnaires. Survivors, or their surrogate respondents, are asked to report the occurrence of any cancer (either a relapse of the initial childhood cancer or a new cancer), the institution where the subsequent diagnosis was made, and the treating physician. All positive responses are screened by the Co-Chair of the Second Malignancies Working Group, and those responses representing likely or possible second neoplasms are validated by requesting a copy of the pathology report. All pathology reports are centrally reviewed by the CCSS Pathology Center at the Cooperative Human Tissue Network in Columbus, OH. In the event that a pathology report can not be successfully obtained, the patient and/or surrogate response, death certificate, and/or other institutional records were reviewed to make a determination of whether the reported occurrence was a probably secondary neoplasm.
Quantification of Risk of Second Neoplasms
Various metrics can and are often used to quantify risk of second and subsequent neoplasms. Cumulative incidence estimates are calculated using time from diagnosis or some subsequent time point (i.e., five years) after childhood cancer diagnosis to the occurrence of the second neoplasm, while treating death as a competing risk event and censoring at the date of last contact [3]. Person-years of follow-up are calculated as the minimum time to second malignancy, death or last contact. Standard errors of cumulative incidence estimates are calculated and used to evaluate 95% confidence intervals (CI) [4]. To quantify risk of second malignant neoplasms, the standardized incidence ratios (SIR) and Excess absolute risk (EAR) are used. The SIR can only be calculated for diagnoses that are contained in the referent population cancer incidence data being utilized. Thus, in the U.S., non-melanoma skin cancers, most non-invasive cancer, and non-malignant tumors (i.e., those without an ICDO behavior code of 3), such as benign meningioma can not be included in SIR or EAR calculations. Typically in the U.S., SIR of observed to expected malignancies are calculated using the Surveillance, Epidemiology, and End Results (SEER) age-, sex- and race-specific incidence rates [5]. EAR is determined by subtracting the expected number of malignancies in the cohort from the observed number, dividing the difference by the person-years of follow-up, and multiplying by 1000. In analyses to determine the relative risk (RR) of developing a second neoplasm, one typically estimates the risk for host characteristics of interest (e.g., age at diagnosis, sex, race/ethnicity, etc.), as well as therapeutic exposures (e.g., radiation dose/site, chemotherapeutic agent, etc.) using a Poisson multivariable regression model with age as the timescale [6,7].
Overall Risk of Second Neoplasms in the CCSS Cohort
The initial assessment of second neoplasms in the CCSS cohort, published by Neglia et al. [8], demonstrated a cumulative incidence of second neoplasms (excluding non-melanoma skin cancers) of 3.2% at twenty years from original cancer diagnosis, which reflects an SIR of 6.38 (95% CI 5.67–7.13). Summarized in Figure 1 is the cumulative incidence, EAR, and SIR for second malignancies within specific diagnostics group of childhood cancers. Multivariate analyses, adjusting for any exposure to therapeutic doses of radiation, demonstrated that risk of a second neoplasm was significantly associated with female sex (p <0.001), childhood cancer diagnosed at a younger age (p <0.001), initial cancer diagnosis of Hodgkin disease (p <0.001) or soft tissue sarcoma (p=0.01), and exposure to alkylating agent chemotherapy (p=0.02).
Figure 1.
Second malignancies among the Childhood Cancer Survivor Study cohort (derived from Neglia, et al., 2001).
Assessment of risk according to type of second cancer demonstrated that the highest risks were seen for secondary tumors involving bone, breast, thyroid and central nervous system (Figure 2). Based upon these results, CCSS investigators initiated more in-depth studies of second tumors including breast, thyroid and central nervous system to further quantify risk associated with radiation therapy and other potential risk factors.
Figure 2.
Standardized Incidence Ratio by type of second malignancy (adapted from Neglia et al., 2001).
Radiation-associated Secondary Thyroid Cancer
Utilizing a nested case-control study design, CCSS investigators conducted a detailed investigation of risk factors associated with the occurrence of a subsequent thyroid cancer [9]. The case series was derived from 72 pathologically-confirmed cases of thyroid cancer ( 78% papillary, 15% follicular, 7% other/unspecified). The control series consisted of survivors free of thyroid cancer and with an intact thyroid gland. A stratified random selection procedure, considering sex, age at diagnosis of initial cancer, and follow-up interval, was applied using a case:control ratio of 1:4. A minimum latency period of five years was applied for calculation of radiation exposure to the thyroid gland, with separate doses calculated for the left and right lobes of the thyroid, as well as the pituitary gland. Doses absorbed by organs, included those both within radiation therapy field and those resulting from scatter, were calculated. Any radiation exposure to the thyroid gland (versus no exposure) was associated with a 2.6-fold increased risk subsequent thyroid cancer (95% CI 1.1–7.1). Observed risk of subsequent thyroid malignancy was dependent on estimated radiation dose to the thyroid, but not in a linear dose-dependent fashion (Figure 3). Rather, the risk pattern fit a linear exponential dose-response model, consistent with the cell-killing hypothesis. In this analysis, radiation doses above 30 Gy to the thyroid gland were associated with a diminished risk of subsequent thyroid cancer compared to lower doses of radiation. While radiation exposure was the main focus of nested case-control study, it is noteworthy that no statistically significant associations were identified with exposure to chemotherapeutic agents.
Figure 3.
Risk of secondary thyroid malignancy according to dose of radiation exposure to the thyroid gland (adapted from Sigurdson et al., 2005).
Radiation-associated Secondary Breast Cancer
Within the past 15 years there has been numerous published report began to appear documenting an excess occurrence of breast cancer among young women treated with chest radiation during the pediatric or adolescent time period [11–23]. As described above, this excess risk has been documented within the CCSS cohort. Because of the characteristics of the CCSS, it has been possible to further investigate not only the magnitude of the long-term risk, but also begin to gain a greater understanding of the potential modifiers of risk.
An analysis of the 6068 women in the CCSS cohort focused on identification of risk factors for breast cancer [24]. A total of 111 confirmed cases of breast cancer, among 95 survivors, were included in the assessment of potential risk factors including primary cancer diagnosis, radiation and chemotherapy, age at diagnosis of initial childhood cancer, follow-up time since initial diagnosis, menstrual and reproductive history, and family history of cancer. The median age at diagnosis of breast cancer was 35 years (range 20–49 years), with an median interval between initial cancer diagnosis and breast cancer of 19 years (range 6–29 years). Among the survivors diagnosed with a subsequent breast cancer, the most common initial cancer diagnosis was Hodgkin disease (68%), followed by bone sarcoma (9%), and soft-tissue sarcoma (8%). Seventy-eight percent of survivors with breast cancer had received prior radiation therapy involving the chest, compared with only 20% of females survivors who had not developed breast cancer.
Risk of breast cancer among female survivors, relative to expected rates within the general population, are presented in Table 1. Statistically significantly elevated standardized incidence ratios were observed among those exposed to chest radiation for all initial diagnoses combined, Hodgkins disease, bone and soft-tissue sarcoma, non-Hodgkin lymphoma, and Wilms tumor. The highest excess was seen among survivors of Hodgkin disease following chest radiation, where the cumulative incidence of breast cancer was estimated to be 12.9% at 40 years of age. For survivors without radiation exposure to the chest, the incidence of breast cancer was highest among survivors of soft-tissue sarcoma (3.3% at 40 years of age).
Table 1.
Standardized Incidence Ratios (SIR) and 95% confidence intervals for breast cancer among childhood cancer survivors exposed and not exposed to chest radiation (adapted from Kenny et al., 2004).
| Primary Diagnosis | Chest RT | No Chest RT | ||
|---|---|---|---|---|
| SIR | 95% CI | SIR | 95% CI | |
| All Diagnoses | 24.7 | 19.3 – 31.0 | 4.8 | 2.9 – 7.4 |
| Hodgkin Disease | 26.3 | 20.2 – 33.7 | - | |
| Bone | 19.4 | 3.9 – 56.5 | 6.7 | 2.7 – 13.8 |
| Soft tissue sarcoma | 20.4 | 2.3 – 73.6 | 7.6 | 2.8 – 16.6 |
| Non-Hodgkin | 16.3 | 3.3 – 47.7 | 4.2 | 0.1 – 23.3 |
| Wilms | 45.8 | 5.2 – 165.4 | 9.5 | 0.1 – 53.0 |
| Leukemia | - | 2.7 | 0.5 – 7.8 | |
| CNS | - | 3.4 | 0.4 – 12.3 | |
Investigation of modifying factors, after adjustment for radiation exposure to the chest, identified family history of breast cancer and history of thyroid disease to infer an increased risk for breast cancer. Pelvic radiation exposure was found to be independently associated with a statistically significantly decreased risk of breast cancer.
Radiation-associated Secondary CNS Tumors
Employing a nested case-control study design, CCSS investigators evaluated risk factors for the occurrence of new primary neoplasms of the central nervous system, with a specific interest in a dose-response pattern of radiation exposure [25]. This report included assessment of 116 survivors who developed a subsequent CNS tumor, each compared to four individually matched control subjects (matched on age at original cancer diagnosis and sex). The median interval between original cancer diagnosis and development of a subsequent glioma or subsequent meningioma was 9 years and 17 years, respectively. Radiation exposure was statistically significantly associated with risk of a subsequent CNS tumor (OR=6.8 for glioma and OR=9.9 for meningioma), with a linear dose-response pattern. However, the pattern differed substantially for glioma compared to meningioma, with slopes of 0.33 and 1.06 per Gy, respectively. Younger age at radiation exposure was an independent risk factor for a secondary glioma.
Summary
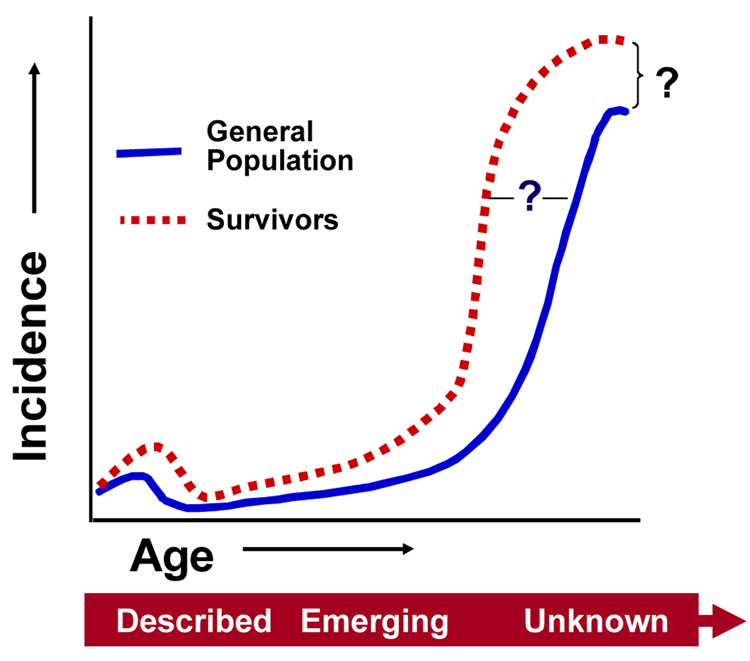
While the body of literature documenting the increased risk of second and subsequent cancers among pediatric cancer survivors is substantial, there still remains many unknowns about the very long-term effects (Figure 4). Answers to the questions of (1) to what degree will cancer treatment exposures in childhood impact upon an earlier onset of adult malignancies and (2) how many new malignancies ultimately result from treatments for pediatric malignancies. Ongoing surveillance and study of exposed population will, with time, provide this important information.
Figure 4.
Unanswered questions regarding risk of subsequent malignancies among childhood cancer survivors.
In looking forward, it is clear that efforts are being made, where possible, to limit exposure to radiation and/or chemotherapy in newly diagnosed pediatric cancer populations. Moreover, with the continuing technological advances being realized new options are available to reduce exposures to non-cancer tissues, with the anticipated result being a reduction in long-term iatrogenic risks. Rigorous, systematic study of exposed populations will be required to document the impact of these efforts, while also investigating other potential risk factors that may operate either independently or through interaction to modify risk of radiation-associated late effects.
Acknowledgement
This work was supported by a grant from the National Cancer Institute (U24 CA55727).
APPENDIX
The Childhood Cancer Survivor Study (CCSS) is a collaborative, multi-institutional project, funded as a resource by the National Cancer Institute, of individuals who survived five or more years after diagnosis of childhood cancer.
CCSS is a retrospectively ascertained cohort of 20,346 childhood cancer survivors diagnosed before age 21 between 1970 and 1986 and approximately 4,000 siblings of survivors, who serve as a control group. The cohort was assembled through the efforts of 26 participating clinical research centers in the United States and Canada. The study is currently funded by a U24 resource grant (NCI grant # U24 CA55727) awarded to St. Jude Children’s Research Hospital. Currently, we are in the process of expanding the cohort to include an additional 14,000 childhood cancer survivors diagnosed before age 21 between 1987 and 1999. For information on how to access and utilize the CCSS resource, visit www.stjude.org/ccss
| CCSS Institutions and Investigators | ||
|---|---|---|
| St. Jude Children’s Research Hospital, Memphis, TN | Leslie L. Robison, Ph.D.#‡, Melissa Hudson, M.D.*‡ Greg Armstrong, M.D. ‡, Daniel M. Green, M.D.‡ | |
| Children's Healthcare of Atlanta/Emory University Atlanta, GA | Lillian Meacham, M.D. *, Ann Mertens, Ph.D. ‡ | |
| Children's Hospitals and Clinics of Minnesota Minneapolis St. Paul, MN | Joanna Perkins, M.D.* | |
| Children’s Hospital and Medical Center, Seattle, WA | Douglas Hawkins, M.D.*, Eric Chow, M.D. ‡ | |
| Children’s Hospital, Denver, CO | Brian Greffe, M.D.* | |
| Children’s Hospital Los Angeles, CA | Kathy Ruccione, RN, MPH* | |
| Children’s Hospital, Oklahoma City, OK | John Mulvihill, M.D.‡ | |
| Children’s Hospital of Philadelphia, PA | Jill Ginsberg, M.D.*, Anna Meadows, M.D. ‡ | |
| Children’s Hospital of Pittsburgh, PA | Jean Tersak, M.D. *, | |
| Children’s National Medical Center, Washington, DC | Gregory Reaman, M.D.*, Roger Packer, M.D.‡ | |
| Cincinnati Children’s Hospital Medical Center | Stella Davies, M.D., Ph.D.‡ | |
| City of Hope- Los Angeles, CA | Smita Bhatia, M.D. *‡ | |
| Dana-Farber Cancer Institute/Children’s Hospital Boston, MA | Lisa Diller, M.D.*†, | †, |
| Fred Hutchinson Cancer Research Center, Seattle, WA | Wendy Leisenring, Sc.D.*‡ | |
| Hospital for Sick Children, Toronto, ON *‡ | Mark Greenberg, MBChB.*, Paul C. Nathan, M.D. | |
| International Epidemiology Institute, Rockville, MD | John Boice, Sc.D.‡ | |
| Mayo Clinic, Rochester, MN | Vilmarie Rodriguez, M.D. * | |
| Memorial Sloan-Kettering Cancer Center New York | Charles Sklar, M.D.*‡, Kevin Oeffinger, M.D.‡ | |
| Miller Children’s Hospital | Jerry Finklestein, MD † | |
| National Cancer Institute, Bethesda, MD | Roy Wu, Ph.D.†, Nita Sibel, M.D. †, Preetha Rajaraman, Ph.D. † | |
| Nationwide Children's Hospital, Columbus, Ohio | Amanda Termuhlen, M.D.*, Sue Hammond, M.D.‡ | |
| Riley Hospital for Children, Indianapolis, IN | Terry A. Vik, M.D.* | |
| Roswell Park Cancer Institute, Buffalo, NY | Martin Brecher, M.D. * | |
| St. Louis Children’s Hospital, MO | Robert Hayashi, M.D.* | |
| Stanford University School of Medicine, Stanford, CA ‡, | Neyssa Marina, M.D. *, Sarah S. Donaldson, M.D. | |
| Texas Children’s Hospital, Houston, TX | Zoann Dreyer, M.D.* | |
| University of Alabama, Birmingham, AL | Kimberly Whelan, M.D., MSPH* | |
| University of Alberta, Edmonton, AB | Yutaka Yasui, Ph.D.‡ | |
| University of California-Los Angeles, CA M.D. †‡ | Jacqueline Casillas, MD MSHS*, Lonnie Zeltzer, | |
| University of California-San Francisco, CA | Robert Goldsby, M.D.* | |
| University of Michigan, Ann Arbor, MI | Raymond Hutchinson, M.D.* | |
| University of Minnesota, Minneapolis, MN | Joseph Neglia, M.D., MPH‡*, | |
| University of Southern California | Dennis Deapen, Dr. P.H. ‡ | |
| UT-Southwestern Medical Center at Dallas, TX | Dan Bowers, M.D.* | |
| U.T.M.D. Anderson Cancer Center, Houston, TX Ph.D.‡ | Louise Strong, M.D.*‡, Marilyn Stovall, MPH, |
Institutional Principal Investigator
Member CCSS Steering Committee
Former Institutional Principal Investigator
Project Principal Investigator (U24 CA55727)
Footnotes
Dr. Robison has no relevant financial relationships or potential conflicts of interest related to the materials to be presented.
References
- 1.Ries LA, Melbert D, Krapcho M, et al., editors. [Accessed 18 Sept 2008];Bethesda, MD: National Cancer Institute; SEER cancer statistics review, 1975–2004. 2007 http://seer.cancer.gov/csr/1975_2004/.
- 2.Robison LL, Mertens AC, Boice JD, et al. Study design and cohort characteristics of the Childhood Cancer Survivor Study: a multi-institutional collaborative project. Med Pediatr Oncol. 2002;38:229–239. doi: 10.1002/mpo.1316. [DOI] [PubMed] [Google Scholar]
- 3.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 4.Marubini E, Valsecchi MG. Analysing Survival Data from Clinical Trials and Observational Studies. Chichester: Wiley; 1995. [Google Scholar]
- 5.National Cancer Institute. Cancer Surveillance Research Program. Surveillance, Epidemiology, and End Results (SEER) Program Public-Use CD-ROM
- 6.Breslow N, Day N. Statistical methods in cancer research, Volume II - The design and analysis of cohort studies. Lyon: IARC Scientific Publications; 1987. [PubMed] [Google Scholar]
- 7.Yasui Y, Liu Y, Neglia JP, et al. A methodological issue in the analysis of second-primary cancer incidence in long-term survivors of childhood cancers. Am J Epidemiol. 2003;158:1108–1113. doi: 10.1093/aje/kwg278. [DOI] [PubMed] [Google Scholar]
- 8.Neglia JP, Friedman DL, Yasui Y, et al. Second malignant neoplasms in five-year survivors of childhood cancer: childhood cancer survivor study. J Natl Cancer Inst. 2001;93:618–629. doi: 10.1093/jnci/93.8.618. [DOI] [PubMed] [Google Scholar]
- 9.Sigurdson AJ, Ronckers CM, Mertens AC, et al. Primary thyroid cancer after a first tumour in childhood (the Childhood Cancer Survivor Study): a nested case-control study. Lancet. 2005;365:2014–2023. doi: 10.1016/S0140-6736(05)66695-0. [DOI] [PubMed] [Google Scholar]
- 10.Bhatia S, Yasui Y, Robison LL, et al. High risk of subsequent neoplasms continues with extended follow-up of childhood Hodgkin's disease: report from the Late Effects Study Group. J Clin Oncol. 2003;21:4386–4394. doi: 10.1200/JCO.2003.11.059. [DOI] [PubMed] [Google Scholar]
- 11.Metayer C, Lynch CF, Clarke EA, et al. Second cancers among long-term survivors of Hodgkin's disease diagnosed in childhood and adolescence. J Clin Oncol. 2000;18:2435–2443. doi: 10.1200/JCO.2000.18.12.2435. [DOI] [PubMed] [Google Scholar]
- 12.Taylor AJ, Winter DL, Stiller CA, Murphy M, Hawkins MM. Risk of breast cancer in female survivors of childhood Hodgkin's disease in Britain: a population-based study. Int J Cancer. 2007;120:384–391. doi: 10.1002/ijc.22261. [DOI] [PubMed] [Google Scholar]
- 13.Travis LB, Hill D, Dores GM, et al. Cumulative absolute breast cancer risk for young women treated for Hodgkin lymphoma. J Natl Cancer Inst. 2005;97:1428–1437. doi: 10.1093/jnci/dji290. [DOI] [PubMed] [Google Scholar]
- 14.Travis LB, Hill DA, Dores GM, et al. Breast cancer following radiotherapy and chemotherapy among young women with Hodgkin disease. JAMA. 2003;290:465–475. doi: 10.1001/jama.290.4.465. [DOI] [PubMed] [Google Scholar]
- 15.Cutuli B, Borel C, Dhermain F, et al. Breast cancer occurred after treatment for Hodgkin's disease: analysis of 133 cases. Radiother Oncol. 2001;59:247–255. doi: 10.1016/s0167-8140(01)00337-1. [DOI] [PubMed] [Google Scholar]
- 16.Wolden SL, Hancock SL, Carlson RW, Goffinet DR, Jeffrey SS, Hoppe RT. Management of breast cancer after Hodgkin's disease. J Clin Oncol. 2000;18:765–772. doi: 10.1200/JCO.2000.18.4.765. [DOI] [PubMed] [Google Scholar]
- 17.Yahalom J, Petrek JA, Biddinger PW, et al. Breast cancer in patients irradiated for Hodgkin's disease: a clinical and pathologic analysis of 45 events in 37 patients. J Clin Oncol. 1992;10:1674–1681. doi: 10.1200/JCO.1992.10.11.1674. [DOI] [PubMed] [Google Scholar]
- 18.Aisenberg AC, Finkelstein DM, Doppke KP, Koerner FC, Boivin JF, Willett CG. High risk of breast carcinoma after irradiation of young women with Hodgkin's disease. Cancer. 1997;79:1203–1210. doi: 10.1002/(sici)1097-0142(19970315)79:6<1203::aid-cncr20>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 19.Hancock SL, Tucker MA, Hoppe RT. Breast cancer after treatment of Hodgkin's disease. J Natl Cancer Inst. 1993;85:25–31. doi: 10.1093/jnci/85.1.25. [DOI] [PubMed] [Google Scholar]
- 20.Ng AK, Bernardo MV, Weller E, et al. Second malignancy after Hodgkin disease treated with radiation therapy with or without chemotherapy: long-term risks and risk factors. Blood. 2002 Sep. 15100(6):1989–1996. doi: 10.1182/blood-2002-02-0634. [DOI] [PubMed] [Google Scholar]
- 21.Wahner-Roedler DL, Nelson DF, Croghan IT, et al. Risk of breast cancer and breast cancer characteristics in women treated with supradiaphragmatic radiation for Hodgkin lymphoma: Mayo Clinic experience. Mayo Clin Proc. 2003;78:708–715. doi: 10.4065/78.6.708. [DOI] [PubMed] [Google Scholar]
- 22.Wolden SL, Lamborn KR, Cleary SF, Tate DJ, Donaldson SS. Second cancers following pediatric Hodgkin's disease. J Clin Oncol. 1998;16:536–544. doi: 10.1200/JCO.1998.16.2.536. [DOI] [PubMed] [Google Scholar]
- 23.Diller L, Medeiros Nancarrow C, Shaffer K, et al. Breast cancer screening in women previously treated for Hodgkin's disease: a prospective cohort study. J Clin Oncol. 2002;20:2085–2091. doi: 10.1200/JCO.2002.08.031. [DOI] [PubMed] [Google Scholar]
- 24.Kenney LB, Yasui Y, Inskip PD, et al. Breast cancer after childhood cancer: a report from the Childhood Cancer Survivor Study. Ann Intern Med. 2004;141:590–597. doi: 10.7326/0003-4819-141-8-200410190-00006. [DOI] [PubMed] [Google Scholar]
- 25.Neglia JP, Robison LL, Stovall M, et al. New primary neoplasms of the central nervous system in survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2006;98:1528–1537. doi: 10.1093/jnci/djj411. [DOI] [PubMed] [Google Scholar]