Abstract
We have developed an animal model in Swiss Webster mice to identify mechanisms by which prenatal exposure to cocaine results in persistent alterations in brain structure and function. Clinical data suggests that children who demonstrate the largest impairments in prenatal brain growth, which are positively correlated with the highest level of prenatal cocaine exposure, are more likely to demonstrate selective impairment in postnatal brain growth, as well as postnatal impairments in motor function, attention and language skills. We conducted neuroanatomic studies to identify the postnatal evolution of structural changes in the primary somatosensory (SI) cortex of the developing mouse brain following prenatal exposure to cocaine. Our previous work, and that of others, provides evidence that many of the processes underlying corticogenesis are disrupted by gestational exposure of the developing mouse brain to cocaine, and that from the earliest phases of corticogenesis that there is an imprecision in the development of cortical lamination. We performed morphometric comparisons between the brains of animals prenatally exposed to varying amounts of cocaine with vehicle and malnutrition controls on postnatal (P) days P9 and P50. We found that on P50, but not P9, the relative number of cortical neurons in S1 is significantly less in cocaine exposed animals as compared with controls. The significant decrease in the number of cells in cocaine exposed animals on P50 is evident as a decreased density of cells restricted to the infragranular compartment (layers 5 and 6). Those changes are not seen in malnourished animals.
Taken together our findings support the conclusion that cocaine-induced alterations in SI cortical cytoarchitectonics are in part a consequence of altered postnatal survival of infragranular cortical neurons, which are lost during the interval between P9 and P50. Determining whether a similar process is evident in a subset of humans following in utero cocaine exposure is a high priority for future clinical brain imaging studies, because analogous structural changes could impact the brain function and behavioral repertoire of infants and children following significant prenatal exposures.
Keywords: Cocaine, prenatal, gestation, brain growth, cortical lamination, cytoarchitecture, morphometry, DiI, mouse, drug abuse
INTRODUCTION
In 1996 the National Pregnancy and Health Survey published by the National Institute on Drug Abuse estimated that each year in the United States 5.5% of all expectant mothers, that is approximately 221,000 pregnant women, used an illicit drug at least once during their pregnancy (National Institute on Drug Abuse 1995a). That and subsequent studies have estimated that between 20 and 25% of all babies in America today are born to women who smoked cigarettes while pregnant (National Institute on Drug Abuse 1995b); and that 15–20% of all children in America were exposed to some level of alcohol in utero. Despite an extensive literature examining the ill-effects of prenatal drug exposure on neurodevelopmental outcomes, studies on the structural development of the brains of these exposed children are scarce. While it is generally appreciated that prenatal exposure to cocaine impairs human fetal brain growth, identifying the direct contribution prenatal drug exposure independently contributes to decreased head circumference in newborns has been difficult, in part due to the confounding effect of a multitude of additional risk factors that are associated with maternal cocaine abuse (LaGasse et al. 1998; Lester 1998). Despite the complexity of such confounding variables, some investigators (Zuckerman et al. 1989), using large, statistically powerful samples of pregnant women followed prospectively with serial urine drug screening in addition to historic data conclusively established that cocaine use during pregnancy induces fetal brain growth retardation in the children of these drug-abusing mothers.
Synthesis of the currently available clinical data leads to the following conclusions (reviewed in Malanga and Kosofsky 2003b): first, prenatal cocaine exposure independently results in impaired prenatal brain growth that is most profound following drug exposure throughout all three trimesters and at higher drug doses (Chasnoff et al. 1989). Second, infants who are exposed to cocaine in utero are at risk for alterations in postnatal behavior as assessed by the Brazelton scale (the NBAS, (Delaney-Black et al. 1996; Tronick et al. 1996)); more subtle measures of attention (Mayes et al. 1995; Jacobson et al. 1996); standardized cognitive measurement scales (e.g., the Bayley Scales of Infant Development (Chasnoff et al. 1992)); language development (Nulman et al. 1994; Mentis and Lundgren 1995), and motor systems maturation, including evidence of a syndrome of hypertonic tetraparesis (Chiriboga et al. 1993). Third, it is clearly not the case that every child exposed to cocaine in utero also demonstrates brain growth retardation or language delay, or inattention, or hypertonia; and even those children who experience significant prenatal cocaine exposure and demonstrate some may not exhibit all of these deficits. Nonetheless, it does appear that children who demonstrate the largest impairments in prenatal brain growth, which are positively correlated with the highest level of prenatal cocaine exposure (Mirochnick et al. 1995; Delaney-Black et al. 1996), are more likely to demonstrate selective impairment in postnatal brain growth, as well as postnatal impairments in motor function, attention and language skills (Azuma and Chasnoff 1993; Chiriboga et al. 1993; Nulman et al. 1994).
To understand how exposure of the fetus to drugs of abuse may result in these adverse outcomes, it is necessary to begin with consideration of how the drugs exert their biological effects. The pharmacological mechanism of action of cocaine is blockade of the reuptake of the catecholamine neurotransmitters norepinephrine (NE) and dopamine (DA) and the indoleamine serotonin (5-HT) into nerve terminals. In addition, cocaine, unlike other monoamine uptake inhibitors such as methylphenidate (Ritalin®) or the selective serotonin reuptake inhibitors (SSRIs) such as fluoxetine (Prozac®), exerts a local anesthetic effect through blockade of voltage-gated sodium channels (Gold et al. 1985). The central nervous system effects of cocaine are mediated through increased central dopaminergic and noradrenergic drive leading to CNS stimulation (Ritchie and Greene 1985). Peripherally, cocaine causes a catecholamine-induced increase in sympathetic tone leading to vasoconstriction, hypertension, and tachycardia.
The mechanisms by which maternal cocaine exposure may compromise fetal well-being have been studied using experimental animal models which have been developed to simulate gestational exposure to cocaine in humans. These models differ in terms of the route of administration, the dose of cocaine, the frequency with which the drug is administered as well as the gestational timing of the drug exposure. In particular, since most of the animal models pursued have been in rodents which deliver their offspring at a point approximating the transition from the second to third trimesters, the corresponding period of human gestation that has been most closely studied is equivalent to the first two thirds of intrauterine life. As the third trimester corresponds to the period of greatest human brain and body growth, it is not surprising that most animal models have not observed impairment in fetal brain or body growth following gestational cocaine exposure. Another factor is the effect of cocaine on maternal weight gain, which appears to be insignificant when the drug is administered intravenously, while a subset of animal models utilizing oral or non-intravenous parenteral (i.e., subcutaneous or intraperitoneal) cocaine administration have demonstrated some impairment of maternal weight gain at higher doses, which is reflected in part in impaired fetal body and brain growth. Whether the higher doses employed are clinically relevant remains a subject of debate in the field: many investigators have argued that to study the independent effects of cocaine requires administering the drug either intravenously or at a low enough dose that there is no concomitant malnutrition. Others have argued that because maternal malnutrition is usually encountered in drug-abusing mothers, and is therefore part of the clinical problem, the relative contribution of malnutrition and its potential interaction with direct effects of cocaine in altering fetal brain growth and subsequent behavior is an important, and clinically relevant, parameter to model.
Our approach has been to study both phenomena independently and interactively. We commonly employ two doses of cocaine: 10 mg/kg (i.e., COC20) or 20 mg/kg (i.e., COC40) injected subcutaneously twice daily from embryonic (E) day E8 to E17, inclusive, a period that corresponds to the first and second trimesters of human gestation. Since mice are born on approximately E19, at a more premature stage than term newborn humans, as mentioned previously, rodent models do not allow for modeling of transplacental exposures corresponding to the third trimester of human gestation. All of our studies to date, which have been performed in Swiss Webster (SW) mice, have demonstrated that at the higher dose (40 mg/kg/day) there is associated maternal malnutrition and impaired fetal body and brain weight gain. Therefore, our COC40 group models both prenatal cocaine exposure as well as prenatal malnutrition. We compare this group in every experiment to animals which are similarly malnourished by nutritionally yoking one set of drug-free dams to the COC40 dams (saline pair-fed, or SPF40), which serve as negative controls, in that they are malnourished in the absence of cocaine. We also study in parallel animals that are treated with an intermediate dose of cocaine (20 mg/kg/day) that does not have significant effects on maternal weight gain or on offspring brain or body weight. Thus the COC20 group serves as a positive control, in that they are exposed to cocaine in the absence of maternal and fetal malnutrition. We anticipate seeing those effects that are due solely to cocaine in both the COC40 and COC20 offspring, with a dose-related effect possibly evident in the COC40 offspring. Those effects that are due to malnutrition we expect to see in the SPF40 and COC40 offspring. Effects that are interactive with cocaine and malnutrition we expect to be most dramatically evident in the COC40 offspring. While the pair-fed offspring are admittedly an imperfect control, as identified in our previous publications and by other investigators (Spear et al. 1989b; Spear 1993; Wilkins et al. 1998b), it is nevertheless the most appropriate nutritional control available for these studies. Another methodological control we and others utilize is fostering of newborn pups to surrogate, drug-free dams, thereby controlling for potential impairments in maternal care of the offspring consequent to chronic cocaine or malnutrition.
While there is a significant literature on the neurochemical (reviewed in Levitt 1998) and neurobehavioral (reviewed in Malanga and Kosofsky 2003a) impact of gestational drug exposure, this article reports for the first time the postnatal evolution of structural changes in the primary somatosensory neocortex of the developing mouse brain which we have identified following prenatal exposure to cocaine. Our previous work (Gressens et al. 1992; Kosofsky et al. 1994) provides evidence that many of the processes underlying corticogenesis are disrupted by gestational exposure of the developing mouse brain to cocaine, and that from the earliest phases of corticogenesis that there is an imprecision in the development of cortical lamination. By measuring the bi-parietal diameter of offspring (Wilkins et al. 1998a; 1998b; 1998c) we have established that there is a decrement in the cortical volume of newborns following prenatal exposure to cocaine at the highest doses we utilize (i.e., Coc40), findings coordinate with that observed in clinical studies of human babies born to cocaine-abusing mothers. To summarize the qualitative neuroanatomic findings from our mouse model (Gressens et al. 1992; Kosofsky et al. 1994): early brain growth is compromised, particularly evident as cortical thinning. The density of fascicles of radial glial processes is decreased without a change in the number of glial fibers per fascicle, and the normal timing of radial glial defasciculation is delayed. There is a disruption of the radial organization of the neuropil, with thinning of axonal-dendritic bundles and alteration of the final position of neurons, consequently resulting in imprecision of horizontal cortical lamination evident in tangentially discontinuous domains of disorganized cytoarchitecture which can be found throughout cortex of cocaine-exposed offspring.
To assess the validity of our preclinical model, data obtained from our mouse experiments must be compared with data from the clinical literature regarding the neuropathological effect of cocaine on the developing brain. Unfortunately, there have been no magnetic resonance (MR) morphometric studies, nor any neuropathological reports detailing the consequences of prenatal cocaine exposure on human brain development. However, our enthusiasm regarding the validity of our model is bolstered by neuropathological findings in primates (Lidow 1995; Ronnekleiv and Naylor 1995; Lidow and Song 2001b), in the rabbit (Jones et al. 1996), and in other species of rodents (Akbari et al. 1994) following transplacental cocaine exposure which demonstrate features consistent with the changes we have described in the mouse. In particular the findings in primates, the preclinical model most reflective of human neurobiology, and the model in which mechanisms underlying altered corticogenesis have been most extensively studied to date (Lidow 1995, 1998; Lidow and Song 2001b) underscores the importance of alterations in corticogenesis as being central to the neuroteratogenicity of cocaine, and the potential relevance of impaired brain growth as a marker for significant in utero drug exposure. In the studies reported here we have further analyzed the effect of prenatal cocaine versus malnutrition in contributing to alterations in the density and distribution of cells in primary somatosensory cortex (SI) of juvenile and adult mice.
METHODS
Gestational Cocaine Exposure
Adult timed-pregnant white Swiss-Webster dams were purchased (Taconic Labs) for all studies. Dams were housed individually with food and water available ad libitum in rooms with 12 hour light/dark cycles (7AM/7PM). Starting on day E6 the animals were handled for five minutes each morning, during which time the dams were weighed. For each experimental manipulation four pregnant dams of comparable weight were identified as an experimental group: two that received cocaine (COC40 or COC20), one that received saline with food available ad libitum (SAL), and one that was pair-fed to the COC40 dam (SPF40). On E7 all pregnant dams were placed on a liquid diet (Bio-Serv, #F1259SP). COC40, COC20 and SAL dams were allowed free access to the diet from embryonic day 7 (E7) until parturition. All dams were weighed and diet consumption was recorded daily for the COC and SAL dams from E7 through term. Gestational length was recorded for each gravid dam, and on the day of birth (P0) the total number of pups and the number of live pups per litter was recorded. On P0 each litter was fostered to an untreated, non-drug exposed surrogate black Swiss-Webster dam that had given birth within the preceding 24–72 hours. Two host black pups were mixed with 8 experimental white pups, normalizing litter size to ten. All pups were covered in host bedding in the dam's home cage prior to her reintroduction, facilitating acceptance of the surrogate pups. Pup weight and sex (by measuring anogenital distance) were recorded on post-natal day 1 (P1), at which time the 2 biological host pups were removed from the cage, normalizing litter size to eight. The gestational and offspring growth data reported here has been reported previously (Wilkins et al. 1998a, 1998b). The neuroanatomic data reported here is original, and was derived from siblings of animals comprising the previous report, which were sacrificed on P9 and P50 for the studies now reported.
The route of administration of cocaine for these experiments was subcutaneous (s.c.), which is the most frequently utilized method for prenatal exposure of drugs to fetal rodents; however, there are inherent advantages and disadvantages to this method. Intravenous dosing is most direct, but for recurrent drug administration placement of indwelling venous catheters would be required. Intraperitoneal dosing in pregnant dams presents the problem of potential for directly injecting the fetal sac, with consequent damage to the fetus, and/or sequestering of drug. Moreover, the direct actions of cocaine as an anesthetic, and in promoting uterine arterial vasoconstriction further confound interpretation regarding direct versus indirect effects after intraperitoneal administration. Subcutaneous administration of cocaine to pregnant dams leads to rapid and consistent absorption, with reproducible levels in maternal circulation and fetal brain and circulation within minutes (Dow-Edwards et al. 1989; Spear et al. 1989a; Collins et al. 1999). The major disadvantage of subcutaneous cocaine administration is that dermal lesions can develop at the injection site. These lesions may be uncomfortable for the animal and could potentially confound the experiment with the additional stress produced (Weinstock et al. 1992). We have found that by injecting each dam subcutaneously with a dilute cocaine solution at the base of the neck, where there is an extensive subcutaneous tissue plane, and by varying the exact placement of the injections we can minimize or avoid dermal lesions despite the twice daily serial injection regimen.
The cocaine doses used was 10 mg/kg or 20 mg/kg delivered in a concentration of 2 mg/ml, calculated as the weight of acid salt, dissolved in normal saline; twice daily from E8 to E17 inclusive; given at 7AM and 7PM. The doses were initially determined by review of the literature: other laboratories (Spear et al. 1989a; Dow-Edwards et al. 1990; Bilitzke and Church 1992) had identified that higher daily doses were associated with fetal wastage (tissue edema, intracranial bleeds, malformations) and maternal compromise (seizures or death). Collins et al. (1999) demonstrated that subcutaneous injection of cocaine, 20 mg/kg twice daily in pregnant rats from E8 through E21 delivered levels which peaked in dams at 60 minutes post-administration, and in pups at 120 minutes post-administration, comparable to peak levels obtained by human cocaine users smoking free base cocaine. We have reported similar findings using the same dosing regimen in our mouse model, in which fetal cocaine concentrations were approximately 2 to 3-fold less than dams, with greater brain:plasma cocaine ratio, and delayed clearance of cocaine and its metabolites in embryos versus dams (Wilkins et al. 1998a).
Quantitative Laminar and Cellular Analysis of Nissl-Stained Material - Image Analysis
We have developed methods for semi-automated, computer-assisted analysis of cortical thickness, cell number, and cell distribution of sections from brains of post-natal day 9 (P9) and 50 (P50) mice, facilitating quantitative neuroanatomical data acquisition and analysis. A description of the method with identification of the procedures we followed from experimental material to analyzed data follows.
Tissue Processing
Animals were sacrificed from multiple litters from each prenatal treatment group on P9 or on P50 and processed for histology. Animals were anesthetized with sodium pentobarbital, perfused transcardially with PBS followed by 4% paraformaldehyde (PFA) in 0.1M PB, brains dissected and post-fixed in 4% PFA for 4 hours, then placed overnight in cryoprotectant (2% DMSO/20% Glycerol in 0.1M PB). Frozen coronal sections (40 microns) were cut on a sliding microtome and mounted on gelatin coated slides. Every sixth section was processed (i.e., spacing between adjacent stained sections is 240 microns). Air dried sections were stained for Nissl-substance using freshly filtered 0.1% cresyl violet acetate (pH=7.4 with 0.3% acetic acid and 0.2% sodium acetate).
Imaging Hardware
An MCID system (M4 MicroComputer Imaging Device, Imaging Research Incorporated, St. Catherine’s, Ontario) was used for data analysis. This software directed a computerized Ludl XY motor controller stage on a Leitz DM RBE microscope. Data was collected with a Dage 72 high-resolution black-and-white camera; captured through a video camera digitizing interface; and analyses of optical density on captured images were performed in a semi-automated manner with a CSS Laboratories 486/66 microcomputer equipped with an ATI Graphics Ultra+ card and Matrox 640 Image Processing Boards. Data and computer interfaces were visualized with a NEC MultiSync 5FG Image Monitor and a NEC MultiSync 4FG Host Monitor, respectively. Using this system we were able to digitize images at a resolution of 640×480 pixels.
Image Acquisition and Analysis
A coronal level at the rostral end of the dorsal hippocampus, corresponding to the posteromedial barrel sub-field of primary somatosensory cortex (SI), was identified and the stained section from that level was placed in the computer-driven microscope. This area was chosen because it is the most highly laminated cortical structure found in the rodent brain, allowing for more reproducible identification of cytoarchitectonic borders. The investigators conducting the analysis (J-Q. R. and E.T.) were blinded to the prenatal treatment group, and selected the sections to be analyzed based on the gross neuroanatomic criterion outlined above. This unbiased sampling strategy insured that the measurements made reflect the seemingly random “patchy” distribution of the cocaine-induced disruptions of cortical lamination we have observed.
Low Magnification Laminar Analysis (5X)
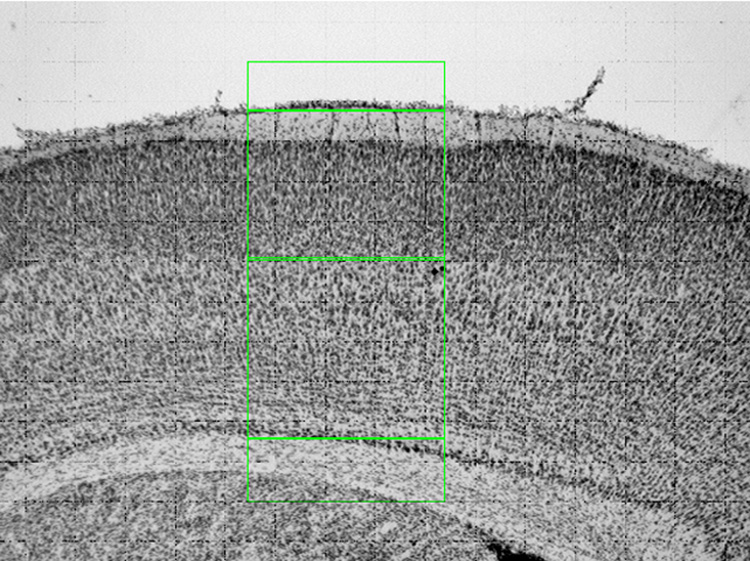
For the laminar analysis a single black-and-white video-captured image was aligned with the pial surface of the section parallel to the upper border and the grey matter-white matter junction parallel to the lower border of the video screen, such that a full thickness sector through SI was obtained in a single image. This same exercise was performed on the contralateral side of the same tissue section, yielding two images per brain for analysis, data from which the mean values for each animal was calculated as the average of both hemispheres The software-assisted measurement of cortical layers was performed by placing lines at the top of the pial surface, at the layer 4/5 border, and at the grey-white junction (see Figure 1). By calculating the calibrated distances between these lines, we tabulated the linear extent of neocortex (layers 1–6), as well as of the supragranular (layers 1–4; top) and infragranular (layers 5–6; bottom) compartments, and of the total cortical thickness for the sector of SI identified in each hemisphere at the relevant level. Of note, the dense layer 4 granule cells that typify SI are discerned in rodent as early as postnatal day 5, facilitating the laminar analysis of P9 and P50 mouse SI neocortex.
Figure 1.
Low magnification (5X) laminar analysis performed on the brain from a P9 mouse pup. The full thickness of primary somatosensory (SI) neocortex is displayed, with the supragranular and infragranular compartments identified in green overlay. The data derived using this protocol to analyze multiple fields in SI from brains of animals from each treatment group (P9: SAL= 9 animals, SPF 40= 10, COC 20 = 9, COC 40 = 8. P50: SAL= 10 animals, SPF 40 = 11, COC 20 = 10, COC 40 = 10) is shown in Table 2.
High Magnification Cellular Analysis (20X)
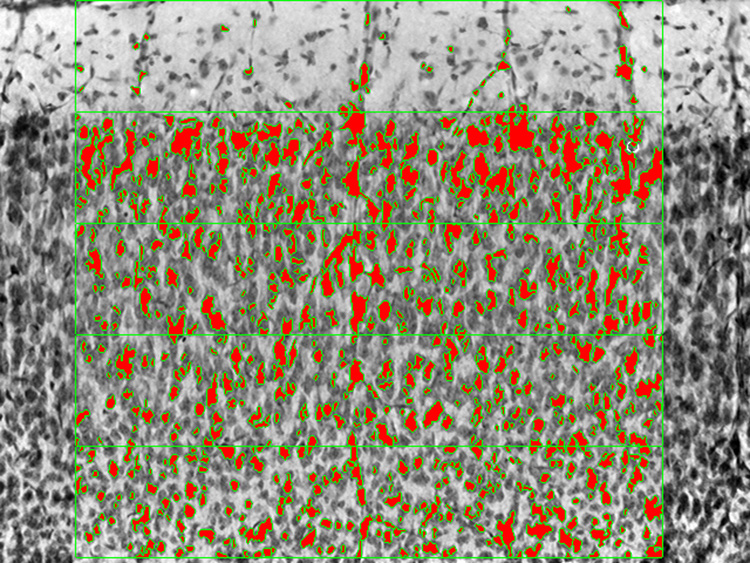
For the analysis of cell number and distribution, the same sector of SI from the same section was then viewed at 20X. Color filters were used to achieve optimal image contrast, and shading correction software (MCID, Imaging Research Inc.) was employed to obtain the most evenly illuminated image. The video image was aligned as for low-power analysis and was divided from the pial surface inward (i.e., top-to-bottom) into 5 parallel contiguous bins approximately 94 microns high and 500 microns wide creating an image overlay (see Figure 2). An optical density threshold was set by eye at a level which distinguished labeled cells from nonspecific background staining. The software additionally allowed us to specify the minimum size of an object (3 microns by 3 microns) to be counted as a cell. An auto-scan software feature allowed the digitized image to be analyzed, counting the number of elements in each bin of sufficient size and optical density to be identified as labeled cells. After the most superficial image and its data file was saved, the microscope stage was automatically adjusted one video field downward (480 microns) and the analysis was repeated for the middle and deep cortical layers. This same exercise was performed on the contralateral side of the same tissue section, and the mean values for each animal were calculated as the average of data derived from both hemispheres.
Figure 2.
High magnification (20X) image of the same region of SI neocortex from the same animal shown in Figure 1, in which the upper cortical layers are evident and the 5 superficial-most horizontal bins are identified (green overlay). Individual elements of sufficient size and optical density to meet the threshold criterion were identified (red overlay) in an automated fashion. The quantitation of cells using this protocol to analyze multiple fields in SI was performed on the identical animals from each treatment group subjected to the low magnification analysis. The data from this visual analysis are reported as the relative mean cell count averaged across animals within a treatment group (Figure 3) and as the relative mean density of cells averaged across P50 animals within a treatment group (Figure 4).
DiI Labeling
The lipophilic, long-chain dialkylcarbocyanine dye, DiI (Molecular Probes, Eugene, OR), was placed in the thalamus to retrogradely label corticothalamic and other corticofugal axons and their cells of origin and to anterogradely label thalamocortical axons. DiI (3H-Indolium, 2-[3-(1,3-dihydro-3,3-dimethyl-1-octadecyl-2H-indol-2-ylidene)-1-propenyl]-3,3-dimethyl-1-octadecyl-perchlorate) crystals 100–150 microns in diameter were manually deposited into the brain under a surgical microscope: brains were block cut in the coronal plane at the level of the thalamus and DiI crystals were inserted through the exposed surface. 6–12 brains from P9 mouse pups from 2–3 litters were used from each prenatal treatment group. After dye placement, brains were returned to PFA fixative and stored at room temperature for 1–2 months to allow for dye diffusion. Brains were embedded in agarose and sectioned in the coronal plane on a Vibratome at a thickness of 100–200 µm. Serial sections were collected in 0.1M phosphate buffer; pH 7.4, wet-mounted on glass slides and examined under a Leitz DM RBE microscope fitted with rhodamine filters. Representative sections from each prenatal treatment group were photographed. Additional sections were stained with a fluorescent nuclear stain (Hoechst 33285; Sigma, St.Louis, Mo.) to determine the location of the retrogradely labeled cortical neurons with respect to architectonic subdivisions of the developing cerebral cortex. Coronal sections at approximately the same levels as utilized for the nissl-stained material were analyzed using an approach similar to the high magnification analysis outlined above, though the parallel contiguous bins approximately 100 microns high and 500 microns wide were identified using a calibrated eyepiece reticle. As the number of retrogradely labeled cells varied from animal to animal, data was reported as the percentage of the total labeled cells counted contained in each bin.
Data Analysis
Mean cell counts per bin and total cell counts were averaged across multiple animals from each treatment group. ANOVAs (Statistica) were compared by treatment group for cortical thickness, and cell count, the latter calculated for the full cortical thickness and for each bin separately. Significant main effects of prenatal treatment on cortical thickness and cell counts (P<0.05) were explored with post-hoc pairwise comparisons (Newman-Keuls or Dunnett’s test). Values from 8–12 animals from 8–12 litters per prenatal treatment condition were averaged for each measurement reported at each time point. By measuring and averaging values from 14–20 fields (one or both hemispheres from each animal) our SEM values were less than 10% (typically about 5%), and there was only slight incremental improvement in the SEM by averaging additional fields or animals (data not shown). All experimental procedures were reviewed and approved by the Massachusetts General Hospital Subcommittee on Research Animal Care and conformed to NIH guidelines.
RESULTS
Maternal/Litter Characteristics
Data regarding the effects of cocaine exposure at various doses, and malnutrition in our prenatal exposure model as previously published (Wilkins et al. 1998a) are summarized in Table 1. These data were previously compiled from the dams and siblings of the animals utilized for these studies, which were sacrificed on P9 or P50 for the original neuroanatomic studies reported below. Of note, cocaine administration at the highest dose that we employ in this model (40 mg/kg/day), and malnutrition compromise maternal weight gain but does not alter the length of gestation or the number of viable pups delivered per litter, and therefore does not appear to be frankly teratogenic.
Table 1.
Summary of Offspring: Litter and Growth Parameters†
| COC40 | COC20 | SPF40 | SAL | |
|---|---|---|---|---|
| % Gestational Wt Gain, E8-E18 | 25.1 ± 1.2#** | 33.5 ± 1.3 | 15.8 ± 1.9 ** | 37.6 ± 0.8 |
| Total Food Intake (g), E8-E16 | 157 ± 7.4** | 179 ± 5.9 | NA | 205 ± 7.6 |
| Gestational Length (days) | 19.0 ± 0.2 | 19.2 ± 0.5 | 18.9 ± 0.2 | 18.3 ± 0.2 |
| Litter Size at P0 (# of pups) | 11.4 ± 0.5 | 11.5 ± 1.0 | 10.9 ± 0.4 | 12.6 ± 0.5 |
| P1 Wt (g)§ | 1.3 ± .06** | 1.7 ± .07 | 1.2 ± .03** | 1.6 ± .03 |
| P9 Wt§ | 4.4 ± .13** | 5.6 ± .18 | 4.4 ± .14** | 5.0 ± .11 |
| P50 Wt Males | 29.7 ± 1.3* | 34.9 ± 1.5 | 29.5 ± 1.1** | 34.2 ± .98 |
| P1 BPD (in)§ | .278 ± .004** | .303 ± .004 | .268 ± .003** | .292 ± .002 |
| P9 BPD§ | .405 ± .003** | .438 ± .005 | .403 ±.004** | .429 ± .003 |
| P50 BPD Males | .529 ± .001 | .538 ± .006 | .533 ± .008 | .531 ± .005 |
Adapted from Wilkins et al., Neurotoxicol Teratol 20, 1998
Data expressed as means ± S.E.M.
Males and females are combined on P1 and P9
p <.05; treatment group smaller that SAL controls (Dunnettt)
p <.01; treatment group smaller than SAL controls (Dunnettt)
Postnatal Growth
Data regarding the effects of drug exposure at various cocaine doses, and malnutrition on newborn (P1), and postnatal (P9 and P50) weight and biparietal diameter as previously published (Wilkins et al. 1998a) are shown in Table 1. Cocaine administration at the highest dose that we employe (40 mg/kg/day) and malnutrition compromise postnatal brain growth through P9; however, there is a “catch up” phenomenon in the radial brain growth of COC40 males evident by P50. It should be noted that like head circumference in the human, biparietal diameter in rodents is proportional to cortical volume; however, neither one of these values directly measures brain volume.
Morphometric Analysis of SI
We performed a computer-assisted quantitative analysis of laminar thickness (Figure 1) and of cellular distribution and number (Figure 2–Figure 4) in primary somatosensory neocortex (SI) from P9 male and female animals, and from P50 male animals. Data from the lower magnification (5X) laminar analysis (Table 2; Figure 1) demonstrated that on post-natal day 9 (P9) the total cortical thickness and thickness of the supragranular compartment (i.e., cortical layers I–IV), but not the infragranular compartment, was significantly smaller in animals exposed to higher doses (COC40) of cocaine during gestation compared to both vehicle (SAL) and pair-fed nutritional controls (SPF40). Similarly, on P9 the total cortical thickness and supragranular cortical thickness were significantly smaller in animals exposed to both intermediate (COC20) and higher doses (COC40) of cocaine during gestation compared to SAL controls. Supragranular thickness, but not total cortical thickness, was smaller in pair-fed SPF40 brains compared to SAL controls. In brains from adult (P50) mice, the thickness of the supragranular compartment, the infragranular compartment, and the total cortical thickness was significantly less in COC40, COC20 and SPF40 animals compared with SAL controls. In addition, the total cortical thickness of COC20, but not COC40, brains on P50 was found to be significantly smaller than SPF40; the majority of this effect appears to be mediated by decreased thickness of the infragranular compartment, as the thickness of the supragranular compartment was not significantly different from SPF40 controls.
Figure 4.
Relative density of cells in primary somatosensory cortex of P50 offspring. Cell density was derived by dividing the total number of cells measured under high (20X) magnification by the thickness of the total, infragranular, or supragranular cortex measured under low (5X) magnification normalized to the thickness of the same compartment in SAL animals; i.e., Density = Cell Count / (Thickness[Prenatal Treatment]/ Thickness[SAL]). There was a significant main effect of prenatal treatment evident only in the infragranular compartment on P50 (F(9,80) = 2.32; p<0.05) * Newman-Keuls test, p<0.05 vs. SPF40 and SAL.
Table 2.
Summary of Offspring: Cytoarchitectonic Data
| COC40 | COC20 | SPF40 | SAL | |
|---|---|---|---|---|
| P9 Offspring: | n=8 | n=9 | n=10 | n=9 |
| Supragranular | 406.6±9.6§*+ | 440.4±41.1* | 447.6±34.8* | 497.3±37.7 |
| Infragranular | 623.8±30.8 | 665.6±42.7 | 661.1±56.1 | 661.3±41.7 |
| Total | 1032.5±31.1*+ | 1084.0±42.0* | 1122.2±61.6 | 1159.3±51.9 |
| P50 Offspring: | n=10 | n=10 | n=11 | n=10 |
| Supragranular | 499.7±32.2* | 485.5±29.5* | 515.2±30.8* | 552.9±22.3 |
| Infragranular | 595.8±27.3* | 563.5±36.4*+ | 601.5±27.2* | 638.9±35.5 |
| Total | 1105.5±45.9* | 1049.0±55.9*+ | 1116.7±40.4 * | 1191.8±48.1 |
SI cortical thickness in microns; data expressed as means ± S.E.M.
P<0.05 compared to SAL controls
P<0.05 compared to SPF controls
The higher magnification (20X) quantitative cellular analysis (Figure 2 and Figure 3) revealed that there was no significant difference between prenatal treatment groups in the total number of cells present in the cortex on P9; however, the total number of cortical cells was decreased in adult (P50) cocaine-exposed animals (COC40 and COC20) compared to controls (SPF40 and SAL). To better identify whether the decreased number of cells in P50 cocaine-exposed offspring was homogeneously distributed or was a result of a more selective loss of cells in particular compartments, an analysis of cell density related to cortical position was performed. As shown in Figure 4, the density of cells in the supragranular cortical compartment did not differ between COC40, COC20 and control animals; however, there was a significant decrease in density of cells in the infragranular cortical compartment in cocaine-exposed offspring compared to controls. Of note, differences in total cortical cell number evident in Figure 3 were no longer evident as decreases in total cell density of Figure 4 after normalizing decreased cell number to the decreased cortical width in the COC40 and COC20 animals.
Figure 3.
Relative number of primary somatosensory cortical cells at post-natal days 9 (P9) and 50 (P50) in brains of COC40, COC20, SPF40, and SAL animals. At P50, but not at P9, there were significantly fewer cortical cells in the brains of COC40 and COC20 mice than in either vehicle (SAL) or nutritional (SPF40) controls. On P9 there was no significant effect of prenatal treatment on relative cortical cell number (F(3,82) = 1.8; p=0.12); on P50 there was a significant main effect of prenatal treatment on relative cortical cell number (F(6,74) = 4.0, p<0.01). *Newman-Keuls test, p<0.05 vs. SPF40 and SAL at P50.
Cell Labeling
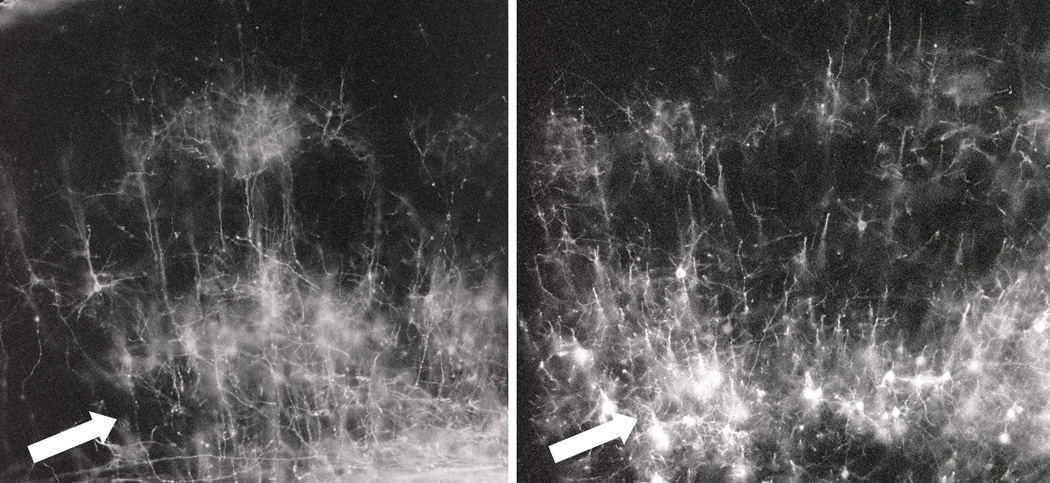
To further pursue the observation of decreased precision of cell distribution in SI cortex of cocaine-exposed animals, we conducted a retrograde DiI labeling study of cortical projection neurons (i.e., cortico-cortical, cortico-thalamic, cortico-striatal and cortico-spinal pyramidal cells) in SI of P9 mice from each prenatal treatment group. As shown in Figure 5 and Figure 6, cells from which descending projections originate were significantly displaced to deeper cortical positions in the brains of COC40 and COC20 animals compared to SAL and SPF40 controls, but the differences were subtle, and only significant at one cortical position (Figure 6: P<0.05 in Bin 9).
Figure 5.
Low magnification (10X) image of representative sections through SI cortex from SAL (left) and COC40 (right) animals. Sections were collected, wet-mounted, and viewed under rhodamine fluorescence 4–8 weeks following insertion of DiI crystals into the VPL/VPM thalamus of fixed brains from P9 animals of each treatment group. Note the relatively dispersed pattern of neuronal cell bodies in the infragranular cortex (layers V–VI, open arrows) of the COC40 specimen compared to the same strata of the SAL animal.
Figure 6.
Counts of DiI-labeled cells in brains of P9 mice from each prenatal treatment group. Cortical projection neurons from which descending projections originate were displaced to deeper cortical positions in the brains of COC40 and COC20 animals compared to SAL and SPF40 controls. *Newman-Keuls test, p<0.05 for Bin 9/Layer V–VI.
DISCUSSION
Summary
The results reported here are descriptive in nature, but the quantitative differences that we have found can be summarized as follows:
On P9 the supragranular and total cortical thickness of S1 was less in cocaine exposed animals as compared with controls, a finding which persists to adulthood. Similar findings were found in malnourished animals.
On P9 the distribution of DiI labeled cells was displaced slightly deeper in the cocaine exposed animals as compared with controls. Those changes were not evident in malnourished animals.
On P9 the relative number of cortical neurons in S1 was not different in cocaine exposed animals as compared with controls.
On P50 the relative number of cortical neurons in S1 was significantly less in cocaine exposed animals as compared with controls. The significant decrease in the number of cells in cocaine exposed animals on P50 was evident as a decreased density of cells restricted to the infragranular compartment (layers 5 and 6). Those changes were not seen in malnourished animals.
Taken together our findings support the conclusion that cocaine-induced alterations in SI cortical cytoarchitectonics were in part a consequence of altered postnatal survival of infragranular cortical neurons, which appear to have been lost during the interval between P9 and P50.
Prenatal Malnutrition vs. Cocaine
As reported by others, prenatal malnutrition can result in early as well as persistent decreases in cortical thickness. Studies have identified that such changes are not attributable to decreased cell number, but rather increased cell density, presumably due to decreased neuronal connections and resulting neuropil (Dobbing 1974; Escobar 1974). The finding of decreased cortical thickness on P9 and P50 in cocaine-exposed animals may therefore be attributable to the effects of malnutrition and/or cocaine. However, the fact that the significant decrease in the number of infragranular neurons evident on P50 in cocaine-exposed animals was not seen in animals solely exposed to malnutrition implies that such changes are specifically due to cocaine. Of note, the changes evident in SPF40 animals may be attributable to malnutrition and/or stress. As is evident in Table 1, food deprivation of the SPF40 dams induces less gestational weight gain than is evident in the COC40 dams with which they are pair fed, suggesting that imposed malnutrition additionally subjects the SPF40 dams to stress (for discussion, see Wilkins et al. 1998b).
Mechanistic Implications of our findings
Neurogenesis
The data that we have reported here utilizing our model identifies no significant effect of prenatal treatment on P9 cell numbers in SI, suggesting that the total numbers of neurons generated was not altered as a result of prenatal cocaine exposure or malnutrition. This is consistent with direct measurements of the bromodeoxyuridine (BrdU) labeling index that we have performed in cortical (medial prefrontal cortex) and subcortical (ganglionic eminence) structures which on E15 were comparable in animals from the four prenatal treatment groups we employ (Crandall et al. 2004). That is not to say that prenatal cocaine exposure does not alter the timing and pattern of neurogenesis: in fact, more detailed studies of the cell cycle in a primate model of prenatal cocaine exposure (Lidow and Song 2001a) reveals that there are significant periodic fluctuations in cell production within the fetal pseudo-stratified ventricular epithelium (PVE), with acute inhibition and subsequent augmentation of neurogenesis following a cocaine injection. However, despite such cocaine-induced alterations in the progression of proliferating neuronal precursors through the cell cycle, that study reported that there was no significant cocaine-induced alteration in the numbers of neurons generated. The extent to which cocaine-induced fluctuations in dopamine concentration within the proliferating cell compartments results in such changes, as has been reported in the striatum (Ohtani et al. 2003), is a topic of active research.
Neuronal Migration
While the total number of neurons generated following prenatal cocaine exposure was not altered, tritiated thymidine neuronal birth dating studies from our laboratory (Gressens et al. 1992) and others (Lidow et al. 2001; Lidow and Song 2001a, 2001b) have reported that cells born synchronously are more widely dispersed across multiple cortical layers. This has led to the speculation that cortical migration may be altered following prenatal cocaine exposure, leading to the hypothesis that impaired migration may contribute to the impaired precision of cortical cytoarchitectonics reported in our mouse model and Dr. Lidow’s primate model. We have found direct evidence for a delay in the tangential migration of GABAergic neurons from the ganglionic eminence to the cerebral cortex of cocaine-exposed, but not malnourished E15 mice (Crandall et al. 2004). Taken together these data support the conclusion that prenatal exposure to cocaine can induce alterations in both the radial and tangential migration of cortical neurons. The fact that in the primate model prenatal cocaine administration during E40-E102 but not E40-E70 nor E103-160 induces alterations in neuronal migration which results in cells aberrantly located in the infragranular compartment and in the white matter (Lidow et al. 2001) suggests that there may be a direct link between the significant periodic fluctuations in cell production within the fetal PVE during the period E70-102 that influence the subsequent imprecision of neuronal migration.
Neuronal Survival
One question that remains is whether neurons that are delayed in their migration, or which get ‘trapped’ at positions deeper in the cortex or white matter, undergo cell death in increased numbers following prenatal cocaine exposure, and if so, whether such exaggerated cell death is immediate or delayed. While we have not directly studied cell death in our model, our data showing comparable cell numbers on P9 does not support early cell death as being operative in this effect. In contrast, such early cocaine-induced cell death has been identified in the primate fetal cerebral wall on E66 (He et al. 1999), including the proliferative region (i.e., PVE), the migratory zone (i.e., intermediate zone), and the evolving cortex (i.e., cortical plate). The loss of infragranular neurons between P9 and P50 evident in cocaine-exposed mice which we have reported suggests that while exaggerated cell death is occurring, in our model this is a very late phenomenon. This is unlike what has been reported in the primate (He et al. 1999) and the explanation for such differences is not readily apparent. The potential mechanisms mediating such delayed cell death in our model may involve elimination of cells which are misplaced or altered in their pattern of connectivity. What is provocative is that such mechanisms appear to be operative days to weeks following the delay and imprecision of fetal neuronal migration we have previously reported (Gressens et al. 1992; Kosofsky et al. 1994; Crandall et al. 2004). Moreover, as evidenced by our P9 DiI data, the ‘misplaced’ cells in the cocaine-exposed animals that do send descending collateral axons to subcortical targets such as the internal capsule and thalamus are located at slightly deeper positions than controls. While this period of presumed delayed cell death corresponds in part to the period of synaptic stabilization and pruning, some of which is mediated by dopaminergic mechanisms (Gerdeman et al. 2003), we are presently unable to identify the cause.
Comparison of our results and quantitative methods and those of others
As mentioned previously, some of these same changes have been seen in the primate, where the loss of cells is even more dramatic. Of note the decreased number of cells in the primate is evident at two months postnatally (Lidow 1995), an age at which the structural maturation of neocortex is similar to our P9 mice, but not exactly comparable. In that model the loss of cortical neurons was found throughout the cortical mantle: we have restricted our analysis to S1 and cannot comment on the extent to which similar changes may be present in other areas, nor have we made statements about volumetric differences in these brains. While the limitations of our quantitative methods, which provide only relative differences, need to be acknowledged, there is no reason to believe that there would be any systematic methodological variation evident in the cocaine exposed animals as compared with the malnourished or saline controls. Therefore, although we did not employ stereological methods but rather provide relative differences between animals from the various prenatal treatment groups, they nonetheless demonstrate important differences between such animals.
Potential clinical implications of our findings, and the need for clinical validation
The extent to which the finding reported here can be generalized may be limited by the species studied, and by the route, dose, frequency, and timing of cocaine administration. Whether the factors that contributed to the magnitude and nature of the alterations in cortical cytoarchitecture described are operating in the human fetus exposed to cocaine in utero is a critically important question. To test such hypotheses in a clinical setting, improved neuroimaging tools will be needed. High resolution semi-automated MR morphometric techniques that are currently being developed (Fischl et al. 2002) could perhaps identify alterations in cortical volume, thickness, and with higher field strength ultimately lamination in drug-exposed human children. Utilizing sophisticated MR analytic methods, Bookstein et al. (Bookstein et al. 2001) identified alterations in the size, shape and contour of the corpus callosum of adults with FAS and FAE as compared with controls, which may provide a marker for the alcohol-exposed phenotype. Given the loss of cortical neurons and presumably cortico-cortical connections in cocaine-exposed animals that we and others have reported there is an urgent need to perform a comparable analysis on brain images obtained from cocaine-exposed children, with strict attention to the potential additive or synergistic effects of concomitant alcohol exposure, which is commonly co-abused by pregnant individuals addicted to cocaine. By performing such MR morphometric analyses in individuals in whom longitudinal clinical data has been obtained, including analysis of the potential contribution of such confounders, the extent to which the types of neuropathological changes that we have reported may contribute to some of neurobehavioral deficits in drug-exposed humans may be determined.
ACKNOWLEDGMENTS
The authors are grateful to Dr. Pradeep Bhide for his helpful comments in the preparation of this manuscript. This work was supported by grants from the National Institute on Drug Abuse (DA015429 (CJM); DA000354 (BEK), DA008648 (BEK)) and an award from the William Randolph Hearst Fund (CJM).
REFERENCES
- Akbari HM, Whitaker-Azmitia PM, Azmitia EC. Prenatal cocaine decreases the trophic factor S-100Band induced microcephaly: reversal by postnatal 5-HT1Areceptor agonist. Neuroscience Letters. 1994;170:141–144. doi: 10.1016/0304-3940(94)90259-3. [DOI] [PubMed] [Google Scholar]
- Azuma SD, Chasnoff IJ. Outcome of children prenatally exposed to cocaine and other drugs: A path analysis of three-year data. Pediatrics. 1993;92:396–402. [PubMed] [Google Scholar]
- Bilitzke PJ, Church MW. Prenatal cocaine and alcohol exposures affect rat behavior in a stress test (the Porsolt swim test) Neurotoxicology and Teratology. 1992;14:359–364. doi: 10.1016/0892-0362(92)90043-a. [DOI] [PubMed] [Google Scholar]
- Bookstein FL, Sampson PD, Streissguth AP, Connor PD. Geometric morphometrics of corpus callosum and subcortical structures in the fetal-alcohol-affected brain. Teratology. 2001;64(1):4–32. doi: 10.1002/tera.1044. [DOI] [PubMed] [Google Scholar]
- Chasnoff IJ, Griffith DR, Freier C, Murray J. Cocaine/polydrug use in pregnancy: two-year follow-up. Pediatrics. 1992;89:284–289. [PubMed] [Google Scholar]
- Chasnoff IJ, Griffith DR, MacGregor S, Dirkes K, Burns KA. Temporal patterns of cocaine use in pregnancy. Journal of the American Medical Association. 1989;261:1741–1744. [PubMed] [Google Scholar]
- Chiriboga CA, Bateman DA, Brust JCM, Hauser WA. Neurologic findings in neonates with intrauterine cocaine exposure. Pediatric Neurology. 1993;9:115–119. doi: 10.1016/0887-8994(93)90045-e. [DOI] [PubMed] [Google Scholar]
- Chiriboga CA, Vibbert M, Malouf R, Suarez M, Abrams E, Heagarty M, Brust JCM, Hauser WA. Neurological outcome in children exposed in utero to cocaine: Children who outgrow cerebral palsy. CNS Abstracts: Annals of Neurology. 1993;34:459. [Google Scholar]
- Collins LM, Pahl JA, Meyer JS. Distribution of cocaine and metabolites in the pregnant rat and fetus in a chronic subcutaneous injection model. Neurotoxicology and Teratology. 1999;21:639–646. doi: 10.1016/s0892-0362(99)00037-9. [DOI] [PubMed] [Google Scholar]
- Crandall JE, Hackett HE, Tobet SA, Kosofsky BE, Bhide PG. Cocaine exposure decreases GABA neuron migration from the ganglionic eminence to the cerebral cortex in embryonic mice. Cerebral Cortex. 2004 doi: 10.1093/cercor/bhh027. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney-Black V, Covington C, Ostrea E, Jr, Romero A, Baker D, Tagle M-T, Nordstrom-Klee B, Silvestre MA, Angelilli ML, Hack C, Long J. Prenatal cocaine and neonatal outcome: Evaluation of dose-response relationship. Pediatrics. 1996;98:735–740. [PubMed] [Google Scholar]
- Dobbing J. Cravioto J, Hambraeus L, Vahlqvist B. Symposia of the Swedish Nutrition Foundation XII: Early Malnutrition and Mental Development. Uppsala: Almqvist & Wiksell; 1974. Prenatal nutrition and neurological development; pp. 96–110. [Google Scholar]
- Dow-Edwards D, Fico TA, Osman M, Gamagaris Z, Hutchings DE. Comparison of oral and subcutaneous routes of cocaine administration on behavior, plasma drug concentration and toxicity in female rats. Pharmacology Biochemistry and Behavior. 1989;33:167–173. doi: 10.1016/0091-3057(89)90446-2. [DOI] [PubMed] [Google Scholar]
- Dow-Edwards DL, Freed LA, Fico TA. Structural and functional effects of prenatal cocaine exposure in adult rat brain. Brain Res Dev Brain Res. 1990;57(2):263–268. doi: 10.1016/0165-3806(90)90052-z. [DOI] [PubMed] [Google Scholar]
- Escobar A. Cravioto J, Hambraeus L, Vahlqvist B. Symposia of the Swedish Nutrition Foundation XII: Early Malnutrition and Mental Development. Uppsala: Almqvist & Wiksell; 1974. Cytoarchitectonic derangement in the cerebral cortex of the undernourished rat; pp. 55–60. [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Gerdeman GL, Partridge JG, Lupica CR, Lovinger DM. It could be habit forming: drugs of abuse and striatal synaptic plasticity. Trends Neurosci. 2003;26(4):184–192. doi: 10.1016/S0166-2236(03)00065-1. [DOI] [PubMed] [Google Scholar]
- Gold MS, Washton AM, Dackis CA. Kozel NJ, Adams EH. Cocaine use in America: epidemiologic and clinical perspectives. Washington, D.C.: NIDA Research Monograph #61; 1985. Cocaine abuse: neurochemistry, phenomenology, and treatment; pp. 130–150. [PubMed] [Google Scholar]
- Gressens P, Kosofsky BE, Evrard P. Cocaine-induced disturbances of corticogenesis in the developing murine brain. Neurosci Lett. 1992;140(1):113–116. doi: 10.1016/0304-3940(92)90694-3. [DOI] [PubMed] [Google Scholar]
- He N, Song ZM, Lidow MS. Cocaine induces cell death within the primate fetal cerebral wall. Neuropathol Appl Neurobiol. 1999;25(6):504–512. doi: 10.1046/j.1365-2990.1999.00211.x. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Jacobson JL, Sokol RJ, Martier SS, Chiodo LM. New evidence for neurobehavioral effects of in utero cocaine exposure. The Journal of Pediatrics. 1996;129:581–589. doi: 10.1016/s0022-3476(96)70124-5. [DOI] [PubMed] [Google Scholar]
- Jones L, Fischer I, Levitt P. Nonuniform alteration of dendritic development in the cerebral cortex following prenatal cocaine exposure. Cerebral Cortex. 1996;6:431–445. doi: 10.1093/cercor/6.3.431. [DOI] [PubMed] [Google Scholar]
- Kosofsky BE, Wilkins AS, Gressens P, Evrard P. Transplacental cocaine exposure: a mouse model demonstrating neuroanatomic and behavioral abnormalities. J Child Neurol. 1994;9(3):234–241. doi: 10.1177/088307389400900303. [DOI] [PubMed] [Google Scholar]
- LaGasse LL, Van Vorst RF, Brunner SM, Lester BM. Effects of in utero exposure to cocaine and/or opiates on infants' reaching behavior. Ann N Y Acad Sci. 1998;846:405–407. [PubMed] [Google Scholar]
- Lester BM. The Maternal Lifestyles Study. Ann N Y Acad Sci. 1998;846:296–305. [PubMed] [Google Scholar]
- Levitt P. Prenatal effects of drugs of abuse on brain development. Drug Alcohol Depend. 1998;51(1–2):109–125. doi: 10.1016/s0376-8716(98)00070-2. [DOI] [PubMed] [Google Scholar]
- Lidow MS. Prenatal cocaine exposure adversely affects development of the primate cerebral cortex. Synapse. 1995;21(4):332–341. doi: 10.1002/syn.890210408. [DOI] [PubMed] [Google Scholar]
- Lidow MS. Nonhuman primate model of the effect of prenatal cocaine exposure on cerebral cortical development. Ann N Y Acad Sci. 1998;846:182–193. [PubMed] [Google Scholar]
- Lidow MS, Bozian D, Song ZM. Cocaine affects cerebral neocortical cytoarchitecture in primates only if administered during neocortical neuronogenesis. Brain Res Dev Brain Res. 2001;128(1):45–52. doi: 10.1016/s0165-3806(01)00139-0. [DOI] [PubMed] [Google Scholar]
- Lidow MS, Song ZM. Effect of cocaine on cell proliferation in the cerebral wall of monkey fetuses. Cereb Cortex. 2001a;11(6):545–551. doi: 10.1093/cercor/11.6.545. [DOI] [PubMed] [Google Scholar]
- Lidow MS, Song ZM. Primates exposed to cocaine in utero display reduced density and number of cerebral cortical neurons. J Comp Neurol. 2001b;435(3):263–275. doi: 10.1002/cne.1028. [DOI] [PubMed] [Google Scholar]
- Malanga CJ, Kosofsky BE. Does drug abuse beget drug abuse? Behavioral analysis of addiction liability in animal models of prenatal drug exposure. Brain Res Dev Brain Res. 2003a;147(1–2):47–57. doi: 10.1016/j.devbrainres.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Malanga CJ, Kosofsky BE. Charney DS, Nestler EJ, Bunney BS. Neurobiology of Mental Illness. New York: Oxford University Press; 2003b. Effects of Drugs of Abuse on Brain Development. [Google Scholar]
- Mayes LC, Bornstein MH, Chawarska K, Granger RH. Information processing and developmental assessments in 3-month-old infants exposed prenatally to cocaine. Pediatrics. 1995;95:539–545. [PubMed] [Google Scholar]
- Mentis M, Lundgren K. Effects of prenatal exposure to cocaine and associated risk factors on language development. Journal of Speech and Hearing Research. 1995;38:1303–1318. doi: 10.1044/jshr.3806.1303. [DOI] [PubMed] [Google Scholar]
- Mirochnick M, Frank DA, Cabral H, Turner A, Zuckerman B. Relation between meconium concentration of the cocaine metabolite benzoylecgonine and fetal growth. J Pediatr. 1995;126(4):636–638. doi: 10.1016/s0022-3476(95)70367-5. [DOI] [PubMed] [Google Scholar]
- National Institute on Drug Abuse. National Institutes of Health, Department of Health and Human Services. Washington, D.C.: U.S. Government Printing Office; 1995a. National Pregnancy and Health Survey. A National Institute on Drug Abuse report. [Google Scholar]
- National Institute on Drug Abuse. NIDA Research Findings. 1. Vol. 10. Bethesda, MD: 1995b. NIDA survey provides first national data on drug use during pregnancy. [Google Scholar]
- Nulman I, Rovet J, Altmann D, Bradley C, Einarson T, Koren G. Neurodevelopment of adopted children exposed in utero to cocaine. Canadian Medical Association Journal. 1994;151:1592–1597. [PMC free article] [PubMed] [Google Scholar]
- Ohtani N, Goto T, Waeber C, Bhide PG. Dopamine modulates cell cycle in the lateral ganglionic eminence. J Neurosci. 2003;23(7):2840–2850. doi: 10.1523/JNEUROSCI.23-07-02840.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie JM, Greene NM. Gilman AG, Goodman LS, Rall TW, Murad F. The Pharmacological Basis of Therapeutics. New York: Macmillan; 1985. Cocaine; pp. 309–310. [Google Scholar]
- Ronnekleiv OK, Naylor BR. Chronic cocaine exposure in the fetal rhesus monkey: consequences for early development of dopamine neurons. Journal of Neuroscience. 1995;15:7330–7343. doi: 10.1523/JNEUROSCI.15-11-07330.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. Missing pieces of the puzzle complicate conclusions about cocaine's neurobehavioral toxicity in clinical populations: importance of animal models. Neurotoxicology and Teratology. 1993;15:307–309. doi: 10.1016/0892-0362(93)90031-i. [DOI] [PubMed] [Google Scholar]
- Spear LP, Frambes NA, Kirstein CL. Fetal and maternal brain and plasma levels of cocaine and benzoylecgonine following chronic subcutaneous administration of cocaine during gestation in rats. Psychopharmacology. 1989a;97:427–431. doi: 10.1007/BF00439542. [DOI] [PubMed] [Google Scholar]
- Spear LP, Kirstein CL, Bell J, Yoottanasumpun V, Greenbaum R, O'Shea J, Hoffmann H, Spear NE. Effects of prenatal cocaine exposure on behavior during the early postnatal period. Neurotoxicol Teratol. 1989b;11(1):57–63. doi: 10.1016/0892-0362(89)90086-x. [DOI] [PubMed] [Google Scholar]
- Tronick EZ, Frank DA, Cabral H, Mirochnick M, Zuckerman B. Late dose-response effects of prenatal cocaine exposure on newborn neurobehavioral performance. Pediatrics. 1996;98(1):76–83. [PMC free article] [PubMed] [Google Scholar]
- Weinstock M, Matlina E, Maor GI, Rosen H, McEwen BS. Prenatal stress selectively alters the reactivity of the hypothalamic- pituitary adrenal system in the female rat. Brain Res. 1992;595(2):195–200. doi: 10.1016/0006-8993(92)91049-k. [DOI] [PubMed] [Google Scholar]
- Wilkins AS, Genova LM, Posten W, Kosofsky BE. Transplacental cocaine exposure. 1: A rodent model. Neurotoxicol Teratol. 1998a;20(3):215–226. doi: 10.1016/s0892-0362(97)00125-6. [DOI] [PubMed] [Google Scholar]
- Wilkins AS, Jones K, Kosofsky BE. Transplacental cocaine exposure. 2: Effects of cocaine dose and gestational timing. Neurotoxicol Teratol. 1998b;20(3):227–238. doi: 10.1016/s0892-0362(97)00127-x. [DOI] [PubMed] [Google Scholar]
- Wilkins AS, Marota JJ, Tabit E, Kosofsky BE. Transplacental cocaine exposure. 3: Mechanisms underlying altered brain development. Neurotoxicol Teratol. 1998c;20(3):239–249. doi: 10.1016/s0892-0362(97)00128-1. [DOI] [PubMed] [Google Scholar]
- Zuckerman B, Frank DA, Hingson R, Amaro H, Levenson SM, Kayne H, Parker S, Vinci R, Aboagye K, Fried LE, et al. Effects of maternal marijuana and cocaine use on fetal growth. N Engl J Med. 1989;320(12):762–768. doi: 10.1056/NEJM198903233201203. [see comments] [DOI] [PubMed] [Google Scholar]