Abstract
OBJECTIVE
The aim of the present study was to analyze the effect Cissus quadrangularis plant petroleum ether extract on the development of long bones during the intra-uterine developmental stage in rats.
METHODS
Pregnant rats (n=12) were randomly assigned into either a control group (n=6) or a Cissus quadrangularis treatment (n=6) group. Pregnant rats in the Cissus quadrangularis group were treated with Cissus quadrangularis petroleum ether extract at a dose of 500 mg/kg body weight from gestation day 9 until delivery. The animals in the control group received an equal volume of saline. Newborn pups were collected from both groups for alizarin red S - alcian blue staining to differentiate ossified and unossified cartilage. The ossified cartilage (bone) was morphometrically analyzed using Scion image software.
RESULTS
Morphometric analysis revealed that the percentage of the total length of ossified cartilage (bone) in pups born to treated dams was significantly higher (P<0.001– 0.0001) than that of the control group.
CONCLUSION
The results of the present study suggest that maternal administration of Cissus quadrangularis petroleum ether extract during pregnancy can stimulate the development of fetal bone growth during the intra-uterine developmental period.
Keywords: Estrogens, Phytoestrogens, Ossification, Alizarin red-Alcian blue
INTRODUCTION
There is increasing evidence that the intra-uterine environment influences the risk of developing chronic diseases such as cardiovascular disease, diabetes, and osteoporosis later in life.1 This is thought to occur through fetal programming, which refers to the ability of changes in environmental factors (e.g., nutrition, stress, and exposure to toxins) at critical periods during development to permanently alter the structure, physiology, or metabolism of the body.1 Exposure to ethanol in utero has a number of effects on the developing skeleton, while these effects do not appear to normalize after birth.2 Cissus quadrangularis (CQ) is a weed plant that is used commonly in India and Sri Lanka to hasten the fracture healing process.3–5 Leaf, stem, and root extracts from this plant are used in the management of various ailments.6–14 Phytochemical analysis of Cissus quadrangularis revealed a high content of ascorbic acid, carotene, phytosterol substances, and calcium,15 and there are reports of the presence of β-sitosterol, δ-amyrin, and δ-amyrone.16 All of these components have potentially different metabolic and physiological effects.17–19 Phytoestrogens, which are widely present in different plants in our environment, seem to have actions similar to estrogen on bone cells in vitro.20,21 Estrogen receptors have been detected in bone cells, both in osteoblasts and osteoclasts,22–24 suggesting the direct action of estrogens on these bone cells. Several studies have shown that estrogens can modulate bone cell physiology in vitro by a direct estrogen receptor-mediated mechanism.25–26 This evidence implicates a direct effect of estrogen on the skeleton and alternatively on bone tissue turnover. Although several excellent reviews have documented the beneficial effects of phytoestrogens on humans and laboratory animals,20,21 none have reported on the role of phytoestrogens on fetal rat bone ossification.
In our previous studies, we have demonstrated the effect of an ethanol extract of CQ on the ossification of fetal long bones and the thicknesses of cortical and trabecular bones of neonatal pups treated during days 9 through 21 of gestation.18,19 In our quest to identify and isolate the active principles of the CQ extract, we have more effectively extracted specific chemical compounds from the plant using different organic solvents, and have tested these extracts for their osteostimulant role in vitro. We have observed that CQ petroleum ether extract has a pronounced effect on osteoblasts (unpublished data); however, it is still unknown whether this CQ petroleum ether extract similarly affects the development of bone in utero. Therefore, it is reasonable to study the role of this extract, which is believed to extract the phytogenic steroids in the plant, on the development of fetal bones. Considering the paucity of available data concerning the effect of medicinal plants on the ossification of fetal bone, we aimed to systematically evaluate the in vivo effect of CQ petroleum ether extract on the skeletons of neonatal pups treated during the gestation period.
MATERIALS AND METHODS
Plant material and extraction
The stem of Cissus quadrangularis was collected from the Nalgonda District of Andhra Pradesh in India, identified, and authenticated by a botanist. A voucher specimen was deposited in the Pharmacology Department of Manipal University. The fleshy stems (2.5 kg) were washed, cut into small pieces, air-dried, and crushed into powder. The stem powder was exhaustively extracted with 95% ethanol using a Soxhlet apparatus, and a extract yield of 225 g was obtained. The total ethanol extract was concentrated in a vacuum, dissolved in water, and then partitioned with petroleum ether to obtain a petroleum ether extract at a yield of 18.2 g.
Chemicals
Alizarin red S, alcian blue 8GX, and paraformaldehyde were purchased from Sigma Chemicals (USA). Potassium hydroxide and glycerol were obtained from Merck (India).
Animals
Twelve 3-month-old female Wistar rats weighing approximately 225 g were housed in the Central Animal Research Facility of Manipal University. The rats were housed in sanitized polypropylene cages containing sterile paddy husk as bedding. The animals were maintained under controlled conditions at a temperature of 23 ± 2 °C, humidity of 50 ± 5%, and a 12-h light– dark cycle. All animals were allowed free access to water and fed on a commercial diet. All of the conducted studies were approved by the Institutional Animal Ethical Committee (No.IAEC/KMC/06/2006–2007), Kasturba Medical College, Manipal, according to the prescribed guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), according to prescribed guidelines of the Government of India.
In order to impregnate the test rats and obtain a known gestational day, female rats in an estrous cycle were mated with young healthy male rats and subjected to a vaginal smear test after 12 hours. Detection of sperm in the vaginal smear was considered day 0 of pregnancy.
Acute toxicity study
Acute toxicity was measured on fasting rats. Animals were divided into groups of 10 each and orally given 500, 1000, 1500, 2500, 3000, 3500, 4000, 4500, and 5000 mg/kg body weight of CQ. The rats were continuously observed for 2 hours, checked regularly at one hour interval up to 6 hours, and daily thereafter for 30 days. Mortality, if any, was recorded.27
Experimental protocol
Pregnant rats were divided into control and CQ-treated groups (n=6 in each group). Pregnant rats in the treated group were treated with CQ petroleum ether extract of 500 mg/kg/day from gestation day 9 until delivery. The control group received an equal volume of saline.
At the end of the experiment, i.e., the day of birth, all the pups born to control and treated mothers were collected.
Under anesthesia, the skin, viscera, and adipose tissue were carefully removed from the newborn pups. All the eviscerated pups were fixed in 95% ethanol for 5 days and then processed for alizarin red S - alcian blue double staining to view the entire skeleton.28
Measurement of ossified skeleton (bone) and total skeleton length
The alizarin red- alcian blue-stained limbs were photographed with scales to measure the ossified skeleton length and the total lengths of the forelimb and hindlimb bones. The length of the ossified skeleton (bone) was measured using Scion image software for Windows (NIH software). The software was calibrated and the length was measured as a grey scale image (BMP) of the original JPEG image. The lengths of the humerus, radius, ulna, femur, tibia, and fibula were measured (n=6 limbs in each group), and the percentages of the total lengths of the mineralized skeletons in the control and treated groups were calculated.
Statistical analysis
Data are expressed as mean ± SEM (standard errors of mean). Data were analyzed by Student’s t-test. Probability (P) values less than 0.05 were considered significant.
RESULTS
The oral administration of Cissus quadrangularis plant petroleum ether extract did not cause mortality or any signs of clinical abnormality in the treated pregnant rats.
The Scion image data revealed a significant difference in the extent of ossification of the bones of the treated group compared to the control group.
The effect of CQ petroleum ether extract on the ossification of the humerus
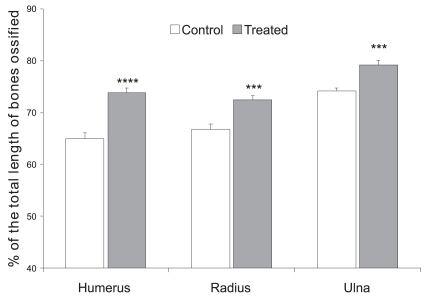
The scion image data concerning the length of ossified skeleton revealed a significant difference in the extent of ossification of the humerus in treated compared to control pups. The mean length of the ossified humerus in the control pups was 3.97 ± 0.06 mm (n=6; 64.97 ± 1.09% of total length of the humerus), while the mean length of the ossified humerus in pups born to CQ-treated mothers was 4.36 ± 0.04 mm (n=6;73.84 ± 0.89% of total length of the humerus (P<0.0001, Student’s t-test; Figures 1 and 2).
Figure 1.
Percentages of total lengths of humerus, radius, and ulna ossified in pups born to control and treated (petroleum ether extract of CQ, 500 mg/kg/day) mothers. Note that there are significant increases in the lengths of the ossified humerus, (**** P<0.0001, Student’s t-test), radius, and ulna (*** P<0.001 Student’s t-test) in pups born to CQ-treated mothers
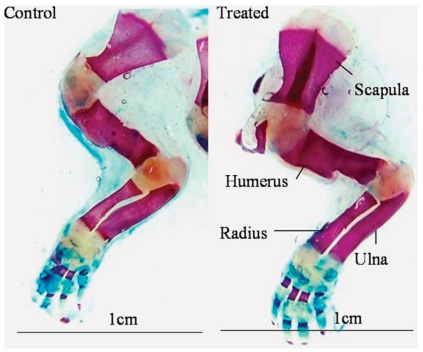
Figure 2.
Photograph of Alizarin red S and alcian blue-stained forelimbs showing the ossified and unossified portions of bones in pups born to control or CQ petroleum ether extract-treated mothers. Note the increased lengths of the humerus, radius, and ulna in the treated group. Scale bar = 1 cm in both photographs
Effect of the CQ extract on the ossification of the radius and ulna
The lengths of the ossified radius and ulna were found to be 3.58 ± 0.02 mm (n=6; 66.73 ± 1.09% of total length) and 4.42 ± 0.03 mm (n=6; 74.16 ± 0.61% of total length), respectively, in the control pups. The lengths of the ossified radius and ulna of pups born to treated mothers were found to be significantly increased compared to the control pups (Radius: 3.64 ± 0.04 mm, n=6; 72.49 ± 0.79% of total length (P<0.001); Ulna: 4.68 ± 0.05 mm, n=6; 79.14 ± 0.97% of total length (P<0.001, Student’s t-test; Figures 1 and 2).
The effect of the CQ extract on the ossification of the femur
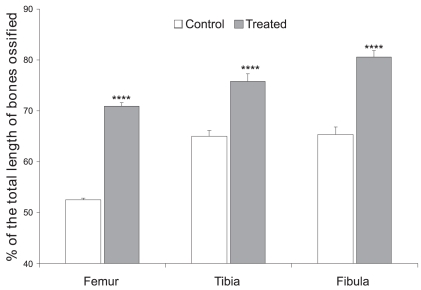
The length of the ossified femur was 3.06 ± 0.02 mm (n=6; 52.52 ± 0.35% of total length) in the control pups and 3.66 ± 0.04 mm in pups born to CQ-treated mothers (n=6; 70.85 ± 0.70% of total length, P<0.0001 Student’s t-test, Figures 3 and 4).
Figure 3.
Percentages of total lengths of femur, tibia, and fibula ossified in pups born to control or CQ petroleum ether extract-treated mothers. Note there is a significant increase in the lengths of the ossified femur, tibia, and fibula (**** P<0.0001, Student’s t-test)
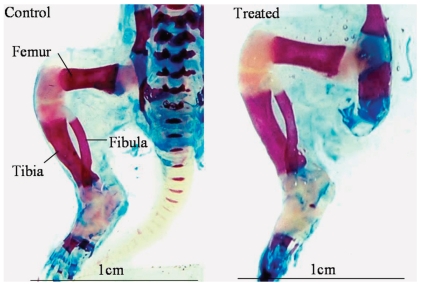
Figure 4.
Photograph of Alizarin red S and alcian blue-stained pup hindlimbs showing the ossified and unossified portions of bones in pups born to control or CQ petroleum ether extract-treated mothers. Note the increased lengths of the femur, tibia, and fibula in the treated group. Scale bar = 1cm in both photographs
The effect of the extract on the ossification of the tibia and fibula
The lengths of the ossified tibia and fibula were 3.77 ± 0.14 mm (n=6; 64.97 ± 1.17% of total length) and 3.41 ± 0.04 mm (n=6; 65.34 ± 1.41% of total length), respectively, in the control group. The lengths of the ossified tibia and fibula of in pups born to the treated mothers were found to be significantly increased compared to control pups (Tibia: 4.17 ± 0.07 mm, n=6; 75.77 ± 1.52% of total length (P<0.0001, Student’s t-test); Fibula: 4.12 ± 0.05 mm, n=6; 80.55 ± 1.21% of total length (P<0.0001, Student’s t- test; Figures 3 and 4).
DISCUSSION
This study evaluates the effect of CQ petroleum ether extract on the ossification of the fetal skeleton. Pups born to mothers treated with 500 mg/kg of CQ from gestation day 9 until delivery exhibited a significant increase in the lengths of the ossified parts of the skeleton in both forelimbs and hindlimbs. This study is an extension of our previous investigations on the effect of CQ extract on the development of the fetal skeleton in rats. In that study, we reported that the treatment of pregnant rats with a CQ ethanolic extract of 750 mg/kg/day enhanced the ossification of the fetal skeleton.18 The present study demonstrates, for the first time, that maternal treatment with petroleum ether extracts during gestation can dramatically influence the skeleton of the fetus.
In the present study, CQ was used at a dose of 500 mg/kg body weight based on our acute toxicity studies. This dose appears to be safe, as treated animals did not show any adverse effects; however, a dose-response study is essential to determining the minimum effective dose that shows a maximum effect.
The increased mineralization (ossification) observed in the present study could be attributed to the phytogenic steroid compounds isolated from this plant, which may have altered the maternal estrogen levels or reached the growing fetus through the placenta.15 Singh et. al.12 have reported the presence of several phytosteroids in the extract of CQ. It has been reported that such phytosteroids can pass through the placental barrier.30,31 This was observed in the study by Magliaccio et al.,31 wherein they altered the maternal estrogen levels by providing external steroidal hormones, and demonstrated alterations in the skeletons of neonatal pups. The bone growth observed in pups born to treated mothers in the present study may be due to proliferation and differentiation of chondrocytes. This may be regulated by phytogenic steroids probably present in the CQ petroleum ether extract, which may act as an analog of estrogen. It has been reported that estrogen stimulates proliferation and differentiation of the chondrocytes and thereby increases bone length.32
Animal experiments have demonstrated that hormones affect development during sensitive periods of early life and permanently program the structure and physiology of the bone tissues.33 It is well known that changes in estrogen levels can dramatically modify bone tissue turnover in both postmenopausal women34 and ovarectomized animals.35 In addition, long term exposure of adult animals to estradiol or diethylstilbestrol induces hyperplasia of bone tissue.22
These data strongly suggest that bone tissue directly responds to estrogen-like target tissues.
Further, transient neonatal exposure of female mice to estrogen has been shown to increase the bone mass in the animals during adulthood.26 Thus, these data strongly suggest that alterations of estrogen levels before puberty, especially in early phases of development, can dramatically influence skeletal maturation, including final peak bone density. Perturbation in the maternal estrogen level during pregnancy has been shown to alter the developing skeleton.31 Evidence indicates that osteoporosis can be triggered during the intrauterine growth period and can result in a defective skeleton for newborn offspring.33 In these cases, treatment with a petroleum ether extract may be useful in reversing/preventing the osteoporotic fractures and improving the bone mineral density.
In conclusion, the results of the present study provide evidence that CQ petroleum ether extract is a promising agent for treating intrauterine growth defects in the skeletal system. Further studies on the isolation and characterization of the active chemical constituents of CQ petroleum ether extract that are responsible for bone anabolic activity are warranted; however, further basic studies and clinical trials will be needed to substantiate the efficacy of Cissus quadrangularis.
ACKNOWLEDGEMENTS
We thank Dr. Narga Nair, Professor and Head, Department of Anatomy, Kasturba Medical College, Manipal University, Manipal, for her support of this study.
REFERENCES
- 1.Barker DJP. Mothers, babies and health in later life. 2nd ed. Churchill Livingstone; Edinburgh: 1998. [Google Scholar]
- 2.Day NL, Leech SL, Richardson GA, Cornelius MD, Robles N, Larkby C. Prenatal alcohol exposure predicts continued deficits in offspring size at 14 years of age. Alcohol Clin Exp Res. 2002;26:1584–91. doi: 10.1097/01.ALC.0000034036.75248.D9. [DOI] [PubMed] [Google Scholar]
- 3.Deka DK, Lahon LC, Saikia J, Mukit A. Effect of Cissus quadrangularis in accelerating healing process of experimentally fractured radius-ulna of dog, a preliminary study. Indian J of Pharmacol. 1994;26:44–5. [Google Scholar]
- 4.Udupa KN, Prasad GC. Further studies on the effect of Cissus quadrangularis in accelerating fracture healing. Indian J Med Res. 1964;52:26–35. [PubMed] [Google Scholar]
- 5.Udupa KN, Prasad GC. Cissus quadrangularis in healing of fractures. A clinical study. J Indian Med Assoc. 1962;38:590–593. [PubMed] [Google Scholar]
- 6.Chidambara Murthy KN, Vanitha A, Mahadeva Swamy M, Ravishankar GA. Antioxidant and antimicrobial activity of Cissus quadrangularis. L J Med Food. 2003;6:99–105. doi: 10.1089/109662003322233495. [DOI] [PubMed] [Google Scholar]
- 7.Singh SP, Mishra N, Dixit KS, Singh N, Kohli RP. An experimental study of analgesic activity of Cissus quadrangularis. Indian J of Pharmacol. 1984;79:162–63. [Google Scholar]
- 8.Oliver-Bever B. Medicinal plants in tropical West Africa. II. Plants acting on the nervous system. J Ethnopharmacol. 1983;7:1–93. doi: 10.1016/0378-8741(83)90082-x. [DOI] [PubMed] [Google Scholar]
- 9.Jainu M, Devi CS. Gastroprotective action of Cissus quadrangularis extract against NSAID induced gastric ulcer: role of proinflammatory cytokines and oxidative damage. Chem Biol Interact. 2006;161:262–70. doi: 10.1016/j.cbi.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 10.Jainu M, Mohan KV, Devi CS. Protective effect of Cissus quadrangularis on neutrophil mediated tissue injury induced by aspirin in rats. J Ethnopharmacol. 2006;104:302–5. doi: 10.1016/j.jep.2005.08.076. [DOI] [PubMed] [Google Scholar]
- 11.Oben JE, Ngondi JL, Momo CN, Agbor GA, Sobgui CS. The use of a Cissus quadrangularis/Irvingia gabonensis combination in the management of weight loss: a double-blind placebo-controlled study. Lipids Health Dis. 2008;7:12. doi: 10.1186/1476-511X-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh G, Rawat P, Maurya R. Constituents of Cissus quadrangularis. Nat Prod Res. 2007;21:522–8. doi: 10.1080/14786410601130471. [DOI] [PubMed] [Google Scholar]
- 13.Panthong A, Supraditaporn W, Kanjanapothi D, Taesotikul T, Reutrakul V. Analgesic, anti-inflammatory and venotonic effects of Cissus quadrangularis Linn. J Ethnopharmacol. 2007;110:264–70. doi: 10.1016/j.jep.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 14.Jain A, Dixit J, Prakash D. Modulatory effects of Cissus quadrangularis on periodontal regeneration by bovine-derived hydroxyapatite in intrabony defects: exploratory clinical trial. J Int Acad Periodontol. 2008;10:59–65. [PubMed] [Google Scholar]
- 15.Sen SP. Study of the active constituents (ketosteroids) of Cissus quadrangularis. Indian J of Pharmacol. 1964;4:247. [Google Scholar]
- 16.Mehta M, Kaur N, Bhutani KK. Determination of marker constituents from Cissus quadrangularis Linn. and their quantitation by HPTLC and HPLC. Phytochem Anal. 2001;12:91–5. doi: 10.1002/pca.569. [DOI] [PubMed] [Google Scholar]
- 17.Shirwaikar A, Khan S, Malini S. Antiosteoporotic effect of ethanol extract of Cissus quadrangularis Linn. on ovariectomized rat. J Ethnopharmacol. 2003;89:245–50. doi: 10.1016/j.jep.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Potu BK, Rao MS, Swamy NVB, Kutty GN. Cissus quadrangularis plant extract enhances the ossification of fetal bones. Pharmacologyonline. 2007;1:63–70. [Google Scholar]
- 19.Rao MS, Potu BK, Swamy NVB, Kutty GN. Cissus quadrangularis plant extractenhances the development of cortical bone and trabeculae in the fetal femur. Pharmacologyonline. 2007;3:190–202. [Google Scholar]
- 20.Adlercreutz H, Mazur W. Phytoestrogens and Western diseases. Ann Med. 1997;29:95–120. doi: 10.3109/07853899709113696. [DOI] [PubMed] [Google Scholar]
- 21.Adlercreutz H. Phytooestrogens and cancer. Lancet Oncol. 2002;3:364–73. doi: 10.1016/s1470-2045(02)00777-5. [DOI] [PubMed] [Google Scholar]
- 22.Greenman DI, Delongchamp RR. Interactive response to DES in CH3 mice. Food Chem Toxicol. 1986;24:931–34. doi: 10.1016/0278-6915(86)90320-0. [DOI] [PubMed] [Google Scholar]
- 23.Eriksen EF, Colvard DS, Berg NJ, Graham ML, Mann KG, Spelberg TC, et al. Evidence of estrogen receptor in normal human osteoblast like cells. Science. 1988;241:84–6. doi: 10.1126/science.3388021. [DOI] [PubMed] [Google Scholar]
- 24.Gray TK, Flynn TC, Gray KM, Nabell LM. 17βestrodiol acts directly on the clonal osteoblastic cell line UMR-106. PNAS. 1987;84:6267–71. doi: 10.1073/pnas.84.17.6267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ernst M, Parker MG, Rodan GA. Functional estrogen receptor in osteoblastic cellsdemonstrated by transfection with a receptor gene containing an estrogen responseelement. Mol Endocrinol. 1991;5:1597–06. doi: 10.1210/mend-5-11-1597. [DOI] [PubMed] [Google Scholar]
- 26.Migliaccio S, Davis VL, Gibson MK, Gray KT, Korach KS. Estrogens modulate theresponsiveness of osteoblast like cells (ROS 17/2. 8) stably transfected with estrogen receptor. Endocrinology. 1992;130:2617–24. doi: 10.1210/endo.130.5.1572285. [DOI] [PubMed] [Google Scholar]
- 27.Ghosh MN. Fundamentals of Experimental Pharmacology. 2nd Ed. Calcutta, India: Scientific Book Agency; 1984. pp. 153–57. [Google Scholar]
- 28.Minoru I. Differential staining of cartilage and bone in fetal mouse skeleton by alcian blue and alizarin red s. Cong Anom. 1976;16:171–73. [Google Scholar]
- 29.Todaka E, Sakurai K, Fukata H, Miyagawa H, Uzuki M, Omori M, et al. Fetal exposure to phytoestrogens - the difference in phytoestrogens status between mother and fetus. Environ Res. 2005;99:195–203. doi: 10.1016/j.envres.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 30.Mustafa AM, Malintan NT, Seelan S, Zhan Z, Mohamed Z, Hassan J, et al. Phytoestrogens levels determination in the cord blood from Malaysia rural and urban populations. Toxicol and Appl Pharmacol. 2007;222:25–32. doi: 10.1016/j.taap.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 31.Migliaccio S, Newbold RR, Bullock BC, Jefferson WJ, Sutton FG, JR, Mclachlan JA, et al. Alterations of maternal estrogen levels during gestation affect the skeleton of female offspring. Endocrinology. 1996;137:2118–25. doi: 10.1210/endo.137.5.8612556. [DOI] [PubMed] [Google Scholar]
- 32.Chagin AS, Chrysis D, Takigawa M, Ritzen EM, Savendahl L. Locally produced estrogen promotes fetal rat metatarsal bone growth; an effect mediated through increased chondrocytes proliferation and decreased apoptosis. Journal of Endocrinology. 2006;188:193–203. doi: 10.1677/joe.1.06364. [DOI] [PubMed] [Google Scholar]
- 33.Cooper C, Javaid MK, Taylor P, Walker Bone K, Dennison E, Arden N. Fetal origin of osteoporotic fractures. Calcif Tissue Int. 2002;70:391–94. doi: 10.1007/s00223-001-0044-z. [DOI] [PubMed] [Google Scholar]
- 34.Lindsay R, Hart DM, Aikken JM, McDonald EB, Anderson JB, Clark AC. Long-term prevention of postmenopausal osteoporosis by estrogen: evidence of an increased bone mass after delayed onset of estrogen treatment. Lancet. 1976;1:1038–40. doi: 10.1016/s0140-6736(76)92217-0. [DOI] [PubMed] [Google Scholar]
- 35.Wronsky TJ, Cintron M, Doherty AL, Dann LM. Estrogen treatment prevents osteopenia and depresses bone turnover in ovariectomized rats. Endocrinology. 1988;123:681–86. doi: 10.1210/endo-123-2-681. [DOI] [PubMed] [Google Scholar]