Abstract
Recent studies implicate specific PKC isoforms in the insulin-signaling cascade. Insulin activates PKCsα, βII, δ and ζ in several cell types. In addition, as will be documented in this review, certain members of the PKC family may also be activated and act upstream of PI3 and MAP kinases. Each of these isoforms has been shown one way or another either to mimic or to modify insulin-stimulated effects in one or all of the insulin-responsive tissues. Moreover, each of the isoforms has been shown to be activated by insulin stimulation or conditions important for effective insulin stimulation. Studies attempting to demonstrate a definitive role for any of the isoforms have been performed on different cells, ranging from appropriate model systems for skeletal muscle, liver and fat, such as primary cultures, and cell lines and even in vivo studies, including transgenic mice with selective deletion of specific PKC isoforms. In addition, studies have been done on certain expression systems such as CHO or HEK293 cells, which are far removed from the tissues themselves and serve mainly as vessels for potential protein–protein interactions. Thus, a clear picture for many of the isoforms remains elusive in spite of over two decades of intensive research. The recent intrusion of transgenic and precise molecular biology technologies into the research armamentarium has opened a wide range of additional possibilities for direct involvement of individual isoforms in the insulin signaling cascade. As we hope to discuss within the context of this review, whereas many of the long sought-after answers to specific questions are not yet clear, major advances have been made in our understanding of precise roles for individual PKC isoforms in mediation of insulin effects. In this review, in which we shall focus our attention on isoforms in the conventional and novel categories, a clear case will be made to show that these isoforms are not only expressed but are importantly involved in regulation of insulin metabolic effects.
Keywords: Insulin signaling, Conventional and novel PKCs, Tyrosine phosphorylation
Introduction
The binding of insulin to its receptor initiates a cascade of events leading to its many biological effects. The first step in this cascade is activation of the insulin receptor intrinsic tyrosine kinase, which phosphorylates endogenous substrate proteins, primarily members of the insulin receptor substrate (IRS) family [1]. Tyrosine phosphorylated motifs in these substrates serve as docking sites for the recruitment and activation of a number of signaling proteins, including phosphatidylinositol 3 (PI3) kinase and mitogen activated protein (MAP) kinase. Activation of these elements may then lead to stimulation of additional enzymes, among which are certain members of the protein kinase C (PKC) family of serine–threonine kinases. Recent studies implicate specific PKC isoforms in the insulin-signaling cascade [2–14]. Insulin activates PKCs α, βII, δ and ζ in several cell types including cell lines of skeletal muscle [11,12,15]. In addition, as will be documented in this review, certain members of the PKC family may also be activated and act upstream of PI3 and MAP kinases.
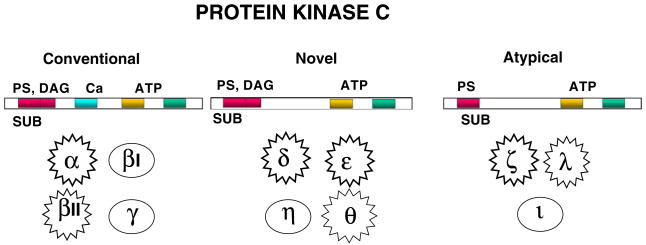
The PKC family plays important roles many intracellular signaling events, cell growth and differentiation [16–20]. It is composed of a number of individual isoforms which belong to three distinct categories-conventional, novel and atypical- based upon their structurally distinct N-terminal regulatory domains. The basic PKC structure of the conventional and novel categories is composed of the N-terminal regulatory domains (that contain an autoinhibitory pseudosubstrate domain and two membrane-targeting modules termed C1 and C2), and a highly conserved C-terminal catalytic domain (that contains the C3 and C4 motifs required for ATP/substrate binding and catalytic activity). The conventional isoforms (cPKCs—α, βI, βII, γ) contain two membrane-targeting regions, designated C1 and C2. The C1 domain can bind PMA (or endogenously generated DAG). The interfacing of the C1 region with PMA or DAG promotes PKC binding to membranes [21,22]. The C2 domain contains a motif found in many proteins that participate in membrane trafficking and signal transduction. C2 domains of cPKC isoforms bind anionic phospholipids in a calcium-dependent manner due to the presence of several calcium-binding residues. The novel isoforms (nPKCs—δ, ε, η and θ) also have similar N-terminal regulatory regions but differ in that the C2 domain lacks the calcium-binding side chains. Hence, nPKCs are maximally activated by DAG/PMA independent of calcium. It was recently reported the C2 domain of PKCδ (a novel PKC) possesses a phosphotyrosine binding motif [14], a finding of especial significance regarding activation of certain PKCs (as described below). The atypical isoforms (aPKCs—ζ and ι/λ) are the third PKC isoform subfamily. aPKCs lack a calcium-sensitive C2 domain and also do not bind DAG or PMA. Consequently, aPKCs are activated by a distinct set of phospholipid cofactors as well as by stimulus-induced phosphorylation events (described in recent reviews [23,24]).
Several of the PKC isoforms are alternatively spliced in addition to PKCβI and βII, where splicing is regulated by insulin [3]. There are alternatively spliced isoforms of PKCδ, θ, η and ς predicted from EST databases [25–27]. The importance of these more recently described isoforms in insulin action has not been described, to date, but the fact that some of the isoforms can encode up to 12 different splice variants with potentially unique cell functions opens new options for PKC in signaling pathways.
The major insulin-responsive tissues-skeletal muscle, liver and adipose tissue—express PKC isoforms from each of the categories, and the total number in each of these cells is in the range of 6–8 isoforms. These include conventional PKCs α, βI and βII, novel PKCs δ, ε and θ, and atypical ζ or λ. Each of these isoforms has been shown one way or another either to mimic or to modify insulin-stimulated effects in one or all of the insulin-responsive tissues. Moreover, each of the isoforms has been shown to be activated by insulin stimulation or conditions important for effective insulin stimulation. Studies attempting to demonstrate a definitive role for any of the isoforms have been performed on different cells, ranging from appropriate model systems for skeletal muscle, liver and fat, such as primary cultures, and cell lines and even in vivo studies, including transgenic mice with selective deletion of specific PKC isoforms, to certain expression systems such as CHO or HEK293 cells, which are far removed from the tissues themselves and serve mainly as vessels for potential protein–protein interactions. Thus, a clear picture for many of the isoforms remains elusive in spite of over two decades of intensive research. The recent intrusion of transgenic and precise molecular biology technologies into the research armamentarium has opened a wide range of additional possibilities for direct involvement of individual isoforms in the insulin signaling cascade. As we hope to discuss within the context of this review, whereas many of the long sought-after answers to specific questions are not yet clear, major advances have been made in our understanding of precise roles for individual PKC isoforms in mediation of insulin effects. We hope that this review, in which we shall focus our attention on isoforms in the conventional and novel categories, a clear case will be made to show that these isoforms are importantly involved in regulation of insulin metabolic effects. The readers are referred to recent reviews regarding the atypical isoforms for a different perspective from that which we will offer here [23,24].
Mechanisms of activation
Intracellular Ca2+
The conventional and novel PKC isoforms (α, βII, δ, ε and θ) are expressed in each of the insulin-responsive tissues and the presence of binding sites for Ca2+ (conventional), DAG and phosphatidyl serine and fatty acids makes their activation by insulin, through its ability to liberate DAG and Ca2+, a virtual certainty. As pointed out in an earlier review [28], PKC was initially defined as a proteolytically activated kinase and was subsequently found to be dependent on Ca2+ and phospholipids in vitro. Indeed, in the presence of DAG, the concentration of Ca2+ necessary for activation of cPKCs is in the physiological range. There is evidence for and against the possibility that insulin induces an increase in intracellular [Ca2+] [29–33]. In newborn rat brown adipocytes it was shown that the insulin-induced elevation of intracellular [Ca2+] was PI3 kinase dependent [34]. On the other hand, it was shown that glucose transport induced by means other than insulin appeared to involve Ca2+ activation of cPKC [35]. It has been noted that insulin resistance appears to be associated with increased levels of intracellular Ca2+, and that measures that increase intracellular Ca2+, thereby presumably increasing cPKC activity, in adipocytes and skeletal muscle can down-regulate insulin-stimulated glucose transport (see review [36]). The extent to which intracellularly released Ca2+ may be an independent means of PKC activation or an important co-factor requires additional study and evaluation. cPKC are activated by localized membrane changes in Ca2+ concentration [28], hence, large increases in intracellular Ca2+ are not necessary for their activation in vivo.
Serine phosphorylation by PDK
All of the kinases require prior phosphorylation by the phosphoinositide dependent kinase 1, PDK1 [37], and PKCβII has been shown to act as a PDK2 activity in some cases [38]. This phosphorylation occurs on the catalytic domain or activation loop. There is the potential for two other auto-phosphorylations in the C-terminal domain that are required for intracellular targeting of the kinases [37].
Whereas these isoforms have been shown by numerous groups to be activated by insulin [6,12,15,39–42], certain considerations have restricted recognition of their role as mediators of insulin action. A major impediment has been the assumption that these isoforms are almost exclusively activated by endogenously generated DAG. As DAG is released in large part by products of Phospholipase C activity, it is further assumed that these isoforms, in particular PKCα and PKCδ, are not activated by insulin by pathways other than via Phospholipase C. And, as especially the conventional and novel PKC isoforms expressed in a given tissue can be stimulated by each of the various co-factors, it has been difficult to evaluate selective involvement of a given isoform in a specific process.
The conventional concept of PKC activation is that the enzymes, when quiescent, are located in the cytoplasm and upon activation are translocated to their sites of action, such as the plasma membrane, nucleus or other cell organelles. As pointed out in a recent superb review on PKCδ activation [43], “the receptor-driven, lipid cofactor-dependent mechanism for PKC activation involving membrane-associated anchoring proteins does not adequately explain the PKC-dependent phosphorylation of proteins in non-membrane compartments”. There is now a body of evidence that certain PKC isoforms may act as lipid-independent enzymes when tyrosine-phosphorylated (probably by members of the Src family of tyrosine kinases). Thus, as stated in the Steinberg review, the model for activation of certain conventional and novel PKC isoforms must be broadened to include additional factors that influence PKCδ enzymology. The review by Steinberg presents a detailed and comprehensive discussion the sequential serine–threonine phosphorylations that prime and regulate the activation sequence and trafficking of PKC isoforms. In addition, the importance and potential role of tyrosine phosphorylation at various sites is analyzed in the context of the description of a modification of the current thinking of PKC activation. As it is beyond the scope of the current review to discuss these points in detail, we strongly recommend the Steinberg review for a comprehensive analysis of PKC activation. In brief, the conventional concept is derived from studies on cPKC isoforms (which reside in a closed/inactive conformation, with the autoinhibitory pseudosubstrate domain occluding the substrate-binding pocket) in the soluble fraction of quiescent cells. cPKCs poorly interact with membranes in the “resting state” (i.e., in the absence of calcium or DAG). With promotion of phosphoinositide hydrolysis and Ins(1,4,5)P3 generation by specific agonists, intracellular calcium is mobilized and binds to the C2 domain and increases its affinity for membranes. This initial association of cPKC with membranes facilitates the interaction of the C1 domain with DAG (the other product of phosphoinositide hydrolysis). C1/C2 domain engagement with membranes promotes a conformational change that expels the autoinhibitory pseudosubstrate domain from the substrate-binding pocket and facilitates the PKC-mediated phosphorylation of membrane substrates. With the exception of the C2 domain-mediated effects of calcium, nPKC isoform activation for the most part follows a similar mechanism. For cPKC and nPKC isoforms, translocation to membranes generally is considered the major criterion for activation.
Tyrosine phosphorylation as a means of activation
Steinberg summarizes the data pertaining to the importance of tyrosine phosphorylation of PKCδ in its activation and substrate targeting. Tyrosine phosphorylation of PKCδ has been reported for responses of salivary gland cells in response to carbachol [44,45], COS-7 cells in response to H2O2 [46,47], and, in addition, skeletal muscle cells in response to insulin [12]. Mouse, rat and human PKCδ contain 19, 21 and 20 tyrosine residues respectively. Multiple sites for tyrosine phosphorylation of PKCδ have been identified in its catalytic and regulatory domains as well as in the hinge region. In contrast with the sites for Ser/Thr phosphorylation, these tyrosine residues are not conserved across PKC family members. Tyrosine phosphorylation, while thought to be a relatively specific regulatory mechanism for PKCδ [48], may be a common regulatory mechanism for the entire family of PKC enzymes. No uniform pattern or consequence of PKCδ tyrosine phosphorylation can be extracted from the published literature, since the catalytic activity of tyrosine-phosphorylated PKCδ is variably described as decreased, increased, or even altered with regard to substrate specificity and cofactor requirements [45,46,49–51]. In fact, the precise configuration of tyrosine residues phosphorylated on PKCδ depends upon the nature of the particular stimulus and dictates the functional properties of the enzyme. For example, tyrosine phosphorylation of the catalytic domain (in cells treated with H2O2) increases the kinase activity of PKCδ, whereas phosphotyrosines in its regulatory domain (in cells treated with PMA or PDGF) influence the cellular actions of PKCδ without influencing kinase activity.
Tyrosine phosphorylation of certain other PKC isoforms in response to insulin may play an important role in both regulation of the activity state and their targeting to specific substrates. The lack of understanding and knowledge of this mechanism could be responsible for the controversy regarding the involvement of so-called “DAG sensitive” PKCs in insulin signaling [23,24]. It has been shown in several models of skeletal muscle that an insulin-induced increase in activity of PKCs α, βII, δ and ς is associated with phosphorylation on tyrosine [12,15]. In current studies, we (unpublished) have found that PKCε is also tyrosine phosphorylated and activated in response to insulin. Src tyrosine kinase has been shown to be involved in insulin and other growth factor signaling in several cell types, including skeletal muscle [52–60,42–49]. Moreover, in the case of PKCs α and δ, insulin-induced tyrosine phosphorylation is apparently mediated by the Src family of tyrosine kinases, if not Src tyrosine kinase itself ([31] and unpublished). Currently, the level of knowledge of potential tyrosine phosphorylation sites in PKCα and other PKC isoforms is quite limited, and to our knowledge, studies on their importance have yet to be performed. Hence, the identification of the tyrosine residues and the mechanisms underlying their phosphorylation by insulin may help to shed more light on the role of the different isoforms in insulin signaling.
With this background, we shall proceed to review the literature with regard to the possible roles of specific PKC isoforms in various stages of insulin signaling in skeletal muscle, liver and fat tissues. In the discussion that follows, we will attempt as well to differentiate between studies performed on cells that represent model systems for insulin signaling (skeletal muscle cells, hepatocytes, fat cells) in vivo and in vitro, and those cells or systems utilized primarily for “protein–protein interactions” and various molecular perturbations. We will first discuss the relevant conventional and then the novel isoforms.
Conventional PKC isoforms
PKCα
A number of studies have shown that insulin stimulates PKCα in insulin-responsive cells such as skeletal muscle, liver and fat [41,61,15,62–64]. The clarification of the relation between stimulation by insulin and a role in the insulin cascade leading to glucose regulation, however, has been more elusive. Investigators have utilized approaches and cell systems that provide means to attribute a definitive role for PKCα in insulin signaling. As the PKCs are serine–threonine kinases, and phosphorylation of serine and threonine residues on IR and IRS1 are known mechanisms for down-regulation of these proteins, it is not surprising that IR and IRS1 were among the first sites for investigation. Equally not surprising were findings that PKCα expressed in cells co-expressing either IR or IRS1 (3T3ir, HEK293, COSIR) induced their phosphorylation. Thus, the idea has developed and persisted that PKCα might in fact play a role in development of insulin resistance [64–69,54–58]. Nonetheless, a role in insulin-induced effects has not been verified.
A possible role for insulin-stimulated PKCα in insulin effects was suggested in a study on the possible role of PKC isoforms in TNF-α regulation of insulin effects in mouse skeletal muscle in primary culture [15]. PKCα was found to be constitutively associated with IRS1 but not IR. Insulin stimulation induced tyrosine phosphorylation associated with an increase in activity of PKCα, and caused PKCα to dissociate from IRS1. (Opposite effects were obtained with regard to PKCδ. This will be discussed subsequently.) Effects of insulin on PKCα were independent of PI3K. Interestingly, TNF-α also induced tyrosine phosphorylation and an increase in activity of PKCα. However, association of PKCα with IRS1 was increased over basal levels, and the insulin effects to induce tyrosine phosphorylation and stimulation of PKCα and its dissociation from IRS1 were abrogated by TNF-α. The findings that both insulin and TNF-α induced phosphorylation of tyrosine and yet caused opposite effects suggest that a different tyrosine site may be involved.
These results are consistent with a study on skeletal muscles and adipocytes in transgenic mice in which the PKCα gene was deleted [70]. Here, it was found that insulin signaling to IRS1-dependent PI3K, PKB and downstream processes, glucose transport and activation of ERK, were enhanced in tissues from PKCα KO mice. In adipocytes and skeletal muscles of wild-type mice and rats, insulin increased PKCα activity independent of PI3K. Thus, whereas PKCα is not required for insulin-stimulated glucose transport, PKCα is activated by insulin at least partly independently of PI3K and serves mainly as a physiological feedback inhibitor of insulin signaling. The mechanism of this feedback inhibition was not speculated upon.
In a recent study [64] on 3T3 fibroblasts overexpressing human insulin receptor (3T3-hIR), it was shown that insulin increased association of PKCα with IRS-1, along with an a 30 kDa protein (called 14-3-3 epsilon). Overexpression of this 14-3-3 epsilon reduced “IRS-1-bound PKCα activity” but not the amount of IRS-1-PKCα co-precipitate and increased IR and IRS-1 tyrosine phosphorylation. Conversely, knockdown of 14-3-3 epsilon produced the opposite effects. The authors suggested that the presence of 14-3-3 epsilon modulates PKCα activity. While not yet confirmed for classical insulin responsive tissues, these observations appear to support a role for PKCα as a negative regulator of IR signaling.
We suggest the following concept regarding the role of PKCα in insulin signaling. PKCα serves as a constitutively active inhibitory regulator of the insulin cascade through its association with IRS1. On stimulation with insulin, PKCα is dissociated from IRS1, thus releasing this protein from its down-regulated state. This, in a sense, opens the “gate” for transmission of the insulin signal. After about 10 min, PKCα re-associates with IRS1 to restore down-regulation of IRS1. Conditions that maintain or strengthen the PKCα-IRS1 association (such as TNF-α) reduce or inhibit insulin signaling via IRS1 and could account for certain aspects of insulin resistance. The ultimate effect of PKCα will depend on the sites of tyrosine phosphorylation in response to the various conditions and its modulation by TNFalpha/ceramide activated protein phosphatase activated protein phosphatases [71].
PKCβII
PKCβ was one of the earliest isoforms recognized in insulin signaling [5,72]. These early studies relied upon chromatographic elution profiles and specific activation by phospholipids, diacylglycerol and calcium [72–75]. In some cases, phorbol ester desensitization was taken as an indication of a role for specific PKC isoforms in insulin action [76]. We now know that this paradigm is difficult, at best, to use for a variety of factors. The primary one is that only the protein is temporarily down-regulated and its resynthesis occurs within minutes of insulin treatment. Also, subcellular localization of cPKC’s is important for their activity and phorbol esters target specific pools of the enzyme to unphysiological sites as suggested [72]. The activation of PKCβ isoforms is regulated by phospholipids, diacylglycerol, calcium, phospholipids and phosphoinositides as well as phorbol esters and they translocate to unique subcellular sites following activation. As mentioned earlier, all of the kinases require prior phosphorylation by the phosphoinositide dependent kinase 1, PDK1 [77], and PKCβII has been shown to act as a PDK2 activity in some cases [38] for activation of the catalytic domain. This phosphorylation is followed by two autophosphorylations within the C-terminal domain on most isoforms. When objective sequence alignments are carried out, five variable regions within the conventional PKC gene encode isoform specificity [78,79]. The last variable region (V5) of PKCβ consists of 50–52 amino acids that are alternatively spliced and results in transcripts encoding two proteins [74,80]. The regulation of exon inclusion by insulin produces PKCβII which further contributes to full effects on insulin signaling as shown with experiments that alter splicing, inhibit PKCβII activity, down-regulate PKCβII levels and overexpression of the active isoform [42,81,82].
Although there is a great deal of redundancy in PKC isoform function as demonstrated by the knockout animal studies [83], more refined experimental approaches have demonstrated that PKCβII has distinct functions from PKCβI. For example, the insulin receptor is phosphorylated by PKCβII in an IRS-1-dependent manner [84]. This results in down-regulation of the receptor tyrosine kinase activity. The functional involvement of this interaction is unknown but thought to be important with regard to the development of insulin resistance. PKCβII contains a unique instability element that destabilizes βII but not βI mRNA in the presence of high extracellular glucose [85–87]. Thus, the splicing of PKCβII may represent the active form of an alternatively spliced protein with its default product, PKCβI, acting in a permissive or non-specific manner. Early studies focused on the role of PKCβII activity in L6 myotubes. A dominant-negative acting PKCβII construct was developed to show that PKCβII but not βI was required for glucose uptake [4]. The specific PKβII inhibitor, CG53353 supports this conclusion. Demonstrating a role for PKCβII in cultured myotubes (both primary and cell line) insulin responsiveness was confirmed by other laboratories [12] including Sampson’s lab, Formisano’s lab (personal communication) and laboratories working on intact muscle showing a requirement for PKCβII in insulin stimulated glucose uptake [88,89].
This difference in function between the two isoforms is critical for demonstrating the importance of splicing regulation and it exists for a number of other functions of PKCβII including the fact that PKCβII binds specifically to β-actin [90]. Additionally, PKCβII was found at different subcellular locations in cardiac myocytes [91], Stebbins and Mochly-Rosen found that it binds specifically to RACK1 in vitro through the V5 region [92]. It is also known to impact signaling pathways in a specific manner as shown with its specific activation of the MAPK pathway [93]. The phosphorylation of TLS-FUS, a nuclear transcription factor that also functions in splicing, demonstrates the versatility of PKCβII functions [94,95]. The observation that PKCβII acts to phosphorylate Akt on Ser473 and acts as a PDK2 (phosphoinositide dependent kinase type 2) activity [96], underscores the role of PKCβII in signaling pathways.
The discovery that insulin regulated the alternative splicing of PKCβII (see section on Regulation of PKC below) was a serendipitous observation which resulted from the use of a ribonuclease protection assay using a probe that crossed the exon–exon boundary between the last common region the fifth variable variable region encoding PKCβI [81].
The original rationale for the design was to determine which isoform predominated in muscle cells. Thus, the protected fragment could distinguish either PKCβI or PKCβII. Insulin time courses in BC3H1 myocytes and later in L6 cells demonstrated that the switch from PKCβI to PKCβII isoforms was rapidly regulated within 10–15 min with protein synthesis accompanying this. The switch to PKCβII has now bee observed in virtually every cell type that responds to insulin including hepatocytes, adipocytes, brain and fibroblasts. This suggested an additional signaling pathway to the nucleus to regulate alternative splicing was functioning to replenish the enzyme which turned over fairly rapidly during insulin action. The involvement of a PI3-kinase dependent pathway was hypothesized using the specific inhibitor, LY294002, and overexpression of the p85 dominant negative regulatory subunit to block alternative splicing [10,86].
It is common knowledge that these tools also block insulin effects on glucose transport [97,98], but the link to PKCβII splicing was not easily bridged as PKCβII activity is required for full insulin effects on glucose uptake [4]. However, we (D.R.C. lab) were able to identify a splicing protein that was potentially involved in insulin action, SRp40, and show that it also mediated insulin effects on splicing via this pathway. This was the first time that the phosphorylation of a splicing factor by a signaling cascade had been identified. Since then, activation of the MAPK pathway, stress pathways and activation of the CaMkinase have been linked to splicing events.
PI3-kinase also modulates the activity of newly synthesized PKCβII via PDK1. The phosphorylation of PKCβII at Serine residues in its activation loop are crucial for its activity [99]. This phosphorylation has been demonstrated in intact skeletal muscle following insulin stimulation [89]. The kinase is also phosphorylated on tyrosine residues [12]. This has the potential for recruiting proteins with phosphotyrosine binding domains, but this has not yet been demonstrated. Thus, like PKCδ, PKCβII may have scaffold functions in insulin signaling.
The knockout animals for PKCβ are an interesting contrast. Like other PKC knockout models they do not demonstrate a phenotype that is diabetic or insulin resistant which may underscore the redundancy of PKC functions in vivo [83]. However, skeletal muscle overexpresses novel glucose transporters that may bypass normal PKCβII functions in regard to trafficking/sorting/docking of the Glut4 vesicles (Cooper, D.R., unpublished observation) and they may demonstrate increased insulin sensitivity, reflecting the effect of both PKCβI + II on feedback to the IR or SHP2 [83,100]. The PKCβ knockout mouse was developed on a C57Bl/6 background which is prone to obesity and insulin resistance as it ages. The fact that these animals do not develop diabetes is interesting since they are smaller and leaner than their controls (Cooper, D.R., unpublished observation) [101].
Novel PKC isoforms
PKCδ
As mentioned in the Introduction, PKCδ is one of the most widely studied of the PKC isoforms, particularly with regard to the importance of tyrosine phosphorylation in response to diFFerent stimuli and ligands in a wide variety of cells. A role for this isoform in insulin signaling, however, has only recently begun to be defined. Whereas, there is ample evidence that insulin stimulates PKCδ, the conventional concept that activation of this isoform (as well as others of the conventional and novel categories) is dependent on DAG released via phospholipase C has hindered understanding of its possible function. In addition, investigators have relied on translocation of PKCδ from cytosolic to membrane fractions as the major indication of PKCδ stimulation (see [102]). This approach overlooks the possibilities of translocation of PKCδ to specific substrates rather than simply to the plasma membrane. Moreover, it does not take into account either the frequently reported high levels of PKCδ in the plasma membrane under basal or “resting (i.e., non-insulin stimulated) conditions or the alterations in activation state as a consequence of insulin-induced PKCδ tyrosine phosphorylation.
As a serine–threonine kinase, PKCδ might be expected to function primarily as a negative regulator of IR signaling, particularly in view of the role of serine phosphorylation on activities of IR and IRS [103–105]. And, indeed, many studies have shown that activated PKCδ readily serine phosphorylates these important proteins. Thus, PKCδ, although presumably requiring co-expression of IRS-1, inhibits the tyrosine kinase activity of the insulin receptor in human kidney embryonic cells [103]. In addition, PKCδ has been overexpressed in CHO cells and, when activated by phorbol esters found to increase serine phosphorylation of IRS-1 [100,106]. In the same report, it was shown in a study on H4IIE hepatoma cells that expression of either constitutively active PKCδ, or of wild type PKCδ followed by phorbol ester stimulation, inhibited tyrosine phosphorylation of IRS-1 in response to insulin.
We have recently found that insulin induces phosphorylation of IRS-1 on Ser 307 (Jacob, Zick, Brodie and Sampson-submitted for publication), a suggested site for phosphorylation by PKCs activated by phorbol esters [107,108]. These results support the notion that insulin-activated PKCδ serves as a negative regulator of IR signaling. It would thus appear that the positive regulation by PKCδ of IR signaling might involve the intervention of another protein. This could be provided by Src tyrosine kinase, which is induced by insulin to associate with both IR and PKCδ, and has been shown to up catalyze insulin-induced IR tyrosine phosphorylation [60]. Cross-talk between PKCδ and STK has been reported in a number of systems, and it is possible that a positive interaction between these two signaling proteins might increase IR signaling.
Stimulation of PKCδ by insulin in adipocytes has been both affirmed [109–111], and denied [102]. As these studies relied on insulin-induced translocation, the differences might best be explained by the differences in cell type, the former being rat adipocytres and the latter 3T3-L1 adipocytes. On the other hand, a clear stimulation of PKCδ by insulin was reported in NIH3T3 fibroblasts [62].
A possible role of PKCδ in insulin-induced gene transcription in hepatocytes was suggested as a result of studies showing translocation of PKCδ from the cytosol to the membrane fraction by insulin in H4IIE (H4) rat hepatoma cells, along with a reduction in gene transcription in response to insulin in these cells that had been pre-treated with PMA to down-regulate PKCδ [112].
The role of PKCδ in insulin signaling in hepatocytes is unclear. On the one hand, in a recent report [113] it was concluded that PKCδ (as well as isoforms α, ε or ς) was not stimulated by insulin and apparently not involved in insulin effects on primary rat hepatocytes. In this study, the investigators relied on DAG release and translocation of PKC to the plasma membrane as indicators of PKC stimulation. As we discussed these mechanisms may not be sufficient to determine PKC stimulation. In current (unpublished) studies utilizing PKCδ tyrosine phosphorylation levels and direct measurements of PKSδ activity, we have found evidence that this isoform may play a critical role in insulin-induced glucogenesis in hepatocytes. The possibility of PKCδ as a positive regulator of IR signaling was firmly introduced by several studies. In one, performed on NIH3T3 fibroblasts, PKCδ (as well as other isoforms) was found to be involved in insulin-induced internalization and transporting of IR [62]. In studies on mammalian skeletal muscle cells in primary culture, it was shown that insulin induced tyrosine phosphorylation of PKCδ via a PI3K-independent mechanism, thus obviating the dependence of activation on released DAG [12]. This pathway was subsequently shown to involve activation of Src tyrosine kinase [52]. It was also reported that insulin-induced GLUT4 translocation and glucose uptake was abrogated by inhibition of PKCδ, either pharmacologically or by overexpression of a kinase dead dominant negative PKCδ [11]. Moreover, overexpression of WTPKCδ in the primary skeletal muscle cells increased GLUT4 translocation and glucose uptake in the absence of insulin stimulation. In another study, it was reported that insulin induces PKCδ to associate directly with IR and that this isoform plays an essential role in insulin-induced tyrosine phosphorylation and internalization of IR [13]. In all of these and subsequent studies on skeletal muscle cells, interaction of IR occurred exclusively with PKCδ and not with any other PKC isoform (α, βII, ε or ς). On the other hand, in HIRcB cells, a rat fibroblast cell line overexpressing human IR, IR association with PKCβI and βII as well as δ was reported [114].
PKCε
Protein kinase Cε, like PKC βII and δ has both transduction and modulating effects on insulin action. Its activation by insulin was noted early on in rat skeletal muscle tissues and adipocytes, as well as in HIRC-B cells and BC3H-1 myocytes [41,115–117]. Like PKCβII, PKCε is also resistant to phorbol ester-induced down-regulation in HIRC-B fibroblasts, suggesting that PKCε resides in a specialized compartment in cells. A potential role for PKCε as a transducer of insulin action was shown by its overexpression in NIH-3T3 fibroblasts where the translocation of GLUT1 transporters to plasma membrane was greatly enhanced compared to (untransfected) cells where no translocation of GLUT1 was detected [42]. The expression of PKCε was shown to be depressed in muscle from Zucker obese insulin resistant rats [118]. Ceramide, a product of TNF-α signaling, inhibits PKCε/ERK interactions, and it also blocks IGF-1 induced PKCε association with RAF-1, an upstream kinase of ERK. This suggests that the anti-mitogenic actions of ceramide could limit the ability of PKCε to form a signaling complex with Raf-1 and ERK [119]. Ceramide also inhibited PKCδ translocation [120]. This would further suggest that these PKCs acted as transducers of signaling.
As a modulator of insulin action, however, PKCε has been shown to increase the inhibitory effect of TNF-α on insulin signaling [121]. This was supported by the observation that the inhibition of insulin receptor kinase was not increased by other PKC isoforms. The mechanism is still unclear. A reduction of insulin sensitivity was also shown in rats fed a high fat diet which is associated with translocation of PKCε [122]. Regarding effects of PKC on glycogen synthesis, FFA treatment of C2C12 myotubes leads to inhibition of glycogen synthesis [122], presumably through ceramide. This was attributed to PKCε translocation since the effects of oleate treatment were most obvious on this isoform. However, overexpression of wild-type PKCε had no significant effects on glycogen synthesis in untreated C2C12 myotubes in these studies. This could be due to the isoform being expressed in the wrong subcellular compartment. Other studies found that the inhibition of insulin-stimulated glycogen synthesis correlated with changes in PKCε distribution in denervated rat soleus muscle [123].
In liver it has been shown that increased DAG appears to activate PKCε. This leads to reduced insulin-stimulated tyrosine phosphorylation of IRS-2. Thus, as in skeletal muscle with regard to PKCθ, there is reduced IRS-2 associated PI3-kinase activity as a result of which there is decreased activation of hepatic glycogen synthase and reduced phosphorylation of foxo1 resulting in increased hepatic gluconeogenesis [124–126].
PKCθ
Although most PKC isoforms have a dual action in stimulating insulin signaling and feeding back to induce insulin resistance in disease, the experimental evidence for PKCθ suggest that it is a negative regulator of insulin signaling in both skeletal muscle and adipocytes. Early reports suggested that PKCθ had a unique tissue distribution and it is the predominant isoform in skeletal muscle [127], activated by insulin [41], but further studies on PKCθ have shown that abundance of expression does not always correlate with a role in a specific tissue. The physiological role of PKCθ is still being examined. Increases in PKC θ expression accompany the differentiation of skeletal muscle cells to myotubes in culture [128]. This probably correlates with the potential role for PKCθ in development and maturation of the neuromuscular junctions [129]. This induction of PKCθ is not the case with human myoblasts derived from diabetic subjects and expression of PKCθ is decreased in skeletal muscle myotubes from type 2 diabetics compared to non-diabetic subjects [61]. In rat L6 myotubes, the overexpression of PKCθ was not associated with changes in glucose transport activity (basal or insulin stimulated). The decrease in activity, however, was associated with decreased glycogen synthase activity [128]. This association with glycogen synthesis was confirmed in C2C12 cells with fatty acids [130]. Other groups have reported that PKCθ immunoreactive protein increased in white muscle (but not red muscle) in fructose-induced insulin resistant rats [131]. This suggested that PKCθ activation acted as an inhibitor of insulin action. Other models of insulin resistance and diabetes have agreed with the findings in human and diabetic cells. In skeletal muscle from diabetic Goto-Kakazaki rats, acute hyperglycemia activated PDK-1, PKCα/β, PKCδ and PKCς, but not PKCθ or Akt/PKB. In these studies, PKC phosphorylation but not PKCθ levels was examined [132]. Thus, it is possible that PKCθ expression was depressed in this model and would be in agreement with diabetic human skeletal muscle [128]. In skeletal muscle from obese, insulin resistant patients, the decrease in PKCθ (as well as PKCμ and −η) expression was also noted [133]. PKCς levels were not altered in these patients.
Several studies indicate that intracellular accumulation of DAG in skeletal muscle activates PKCθ, which can phosphorylate IRS-1 on serine307 residues. This leads to decreased insulin stimulated tyrosine phosphorylation of IRS-1 and reduced IRS-1 that is associated with PI3-kinase activation resulting in reduced insulin stimulated glucose transport [134,135]. In contrast, PKC-θ inactivation prevented fat-induced defects in insulin signaling and glucose transport in skeletal muscle [136]. In addition, free fatty acid (FFA) has the ability to activate PKCθ which can further activate NF-κB [137]. There appear to be several mechanisms by which PKCθ could be inducing insulin resistance. In adipocytes, FFA activation of the isoform was believed to activate IKK and JNK which mediate IRS-1 serine phosphorylation and degradation [138]. This was demonstrated using a PKC inhibitor, however, and was inferred indirectly in rats fed high fat diets. There were similar findings for PKCθ in insulin resistance in this study. Further studies in skeletal muscle myotubes (C2C12) have shown that palmitate activated PKCθ, and PKCθ was involved in activation of NF-κB [139]. Other PKC isoforms such as βII, δ and ε were not activated. This links FFA and PKCθ with a proinflammatory state since TNF-α expression was also increased.
Additional studies on animal models
Various animal models have been used to attempt to further clarify the role of certain PKC isoforms in insulin-related events. In one study [132], normal or diabetic (Goto–Kakizaki–GK) rats were infused with glucose followed by a continuous infusion of saline or insulin. Under euglycemic and hyperglycemic conditions, basal and insulin-stimulated PKCς activity was not altered. Interestingly, basal PKCς activity was increased under hyperglycemic conditions in GK and Wistar rats. The glucose effects were not specific to PKCς, because an increase in phosphorylation of PKCα/β and PKCδ, but not PKCθ, via a PI 3-kinase-protein kinase B/Akt-independent mechanism was also observed.
Glucose uptake and oxidative stress were examined in adipocytes of type 2 diabetic animals [140]. Adipocytes were isolated either from C57Bl/6J mice that were raised on a high-fat diet (HF) and developed obesity and insulin resistance, or from control animals. The activity of PKCδ increased twofold in HF adipocytes compared with control adipocytes and was further activated by H2O2. The results indicated that increased glucose intake in HF adipocytes increases oxidative stress, which in turn promotes the activation of PKCδ, and that these consequential events may be responsible, at least in part, for development of HF diet-induced insulin resistance in the fat tissue.
PKCδ has also been implicated in fatty acid induced hepatic insulin resistance [141]. Studies were done on rats in the basal state and during hyperinsulinemic clamps. Free fatty acid infusion induced hepatic PKCδ translocation from the cytosolic to membrane fraction of hepatic cells. The authors concluded that the progressive insulin resistance induced by FFA in the liver is associated with a progressive increase in hepatic PKCδ translocation.
In an early study on hepatocytes of both normal and streptozotocin-induced diabetic animals [142], it was reported that streptozotocin increased levels of the PKCβII and α, with no change in the level of PKCς. Diabetes induction also appeared to have elicited the translocation of PKCβII and PKCα to the membrane fraction. The level of PKCε, which was noted only in membrane fractions, was also increased upon induction of diabetes.
In another study on obese (fa/fa) Zucker rats [118], levels of PKC α, β and ε mRNA, protein, and enzyme activity in soleus muscle were decreased compared to soleus from Zucker lean control (fa/-) animals. Increases in membrane-associated PKCθ, PKCα, PKCβ and PKCε were also observed in rat gastrocnemius muscles after insulin treatment in vivo [41]. In skeletal muscles from the high-fat-fed rat, the ratio of particulate to cytosolic PKC ε in red muscles from fat-fed rats was increased nearly sixfold, suggesting chronic activation. In contrast, the amount of cytosolic PKCθ was down-regulated to 45% of control, while the ratio of particulate to cytosolic levels increased, suggesting a combination of chronic activation and down-regulation [143]. The link between insulin resistance and PKC was investigated further by assessing the effects of the thiazolidinedione insulin-sensitizer BRL-49653 on PKC isoenzymes in muscle. BRL-49653 increased the recovery of nPKC isoenzymes in cytosolic fractions of red muscle from fat-fed rats, reducing their apparent activation and/or down-regulation, whereas PKC in control rats was unaffected [143].
The sand rat (Psammomys obesus) is an animal model of nutritionally induced diabetes. It was reported that several PKC isoforms (α, ε and ς), representing all three subclasses of PKC, are overexpressed in the skeletal muscle of diabetic animals of this species [144]. In diabetes prone animals of this species, the number of insulin receptors was found to decrease. These data suggest that overexpression of PKCε may be causally related to the development of insulin resistance in these animals, possibly by increasing the degradation of IRs. Studies have also been performed on this animal model of nutritionally induced type 2 diabetes mellitus, in which physical exercise can prevent the progression of the disease [145]. It was reported that 4 weeks of physical exercise improved insulin-signaling responses in these animals and that this was associated with an increase in association of PKCδ with IR. The authors concluded that this mechanism may be involved in the preventive effect of exercise on type 2 diabetes mellitus in these animals.
A recent study detailed a number of specific genes that underwent changes in expression in fat of the Fat-specific Insulin Receptor Knock-out (FIRKO) mouse [146]. Among the 48 affected genes was PKCδ, which underwent down-regulation in fat from FIRKO mice, thus indicating its possible involvement in insulin signaling in fat.
Regulation of PKC expression
Stimulation of conventional and novel PKCs by phorbol esters is known to induce their rapid degradation. This has been studied for the fate of PKCδ, and it was shown that activation by phorbol esters targets PKCδ for degradation by the ubiquitin–proteosome system [147]. The rapid involvement of PKCδ in early upstream insulin signaling, and possibly downstream events, suggests that there is high degree of utilization of this isoform and a potential requirement for rapid regulation of expression and protein levels. A few studies have investigated certain aspects of this problem. It was reported that insulin induces a rapid (5–20 min) increase in PKCα, β, ζ and ε RNA in 3T3 L1 adipocytes and B3CH1 myocytes, but the mechanism—whether de novo transcription, translation, or decreased degradation—was not elucidated [148,149]. Moreover, data on PKCδ RNA expression were not presented. In a recent study on PKCδ expression in primary cultured mouse keratinocytes, it was shown that TNF-α induces an increase in PKCδ mRNA within 30 min [150]. Thus, insulin might induce a rapid increase in PKCδ gene transcription. In a recent study, we examined the effects of insulin on PKCδ levels and expression in several model systems of skeletal muscle including primary cultures of rat and mouse, L8 and L6 rat cell lines, C2C12 mouse cell line, and mouse skeletal muscle in vivo [151]. Insulin stimulation increased PKCδ protein levels in whole cell lysates of each of the model systems. This effect was not due to an inhibition by insulin of the rate of PKCδ protein degradation. In fact, insulin increases the rate of degradation of PKCδ. Insulin also increased 35S-methionine incorporation into PKCδ within 5–15 min. Pretreatment of cells with transcription or translation inhibitors abrogated the insulin-induced increase in PKCδ protein levels. We also found that insulin rapidly increased the level of PKCδ RNA, an effect abolished by inhibitors of transcription. The insulin-induced increase in PKCδ expression was not reduced by inhibition of either PI3 Kinase or MAP kinase, indicating that these signaling mechanisms are not involved, consistent with insulin activation of PKCδ. Studies on cells transfected with the PKCδ promoter demonstrate that insulin activated the promoter within 5 min. It appears, therefore, that the expression of PKCδ may be regulated in a rapid manner during the course of insulin action in skeletal muscle and raise the possibility that PKCδ may be an immediate early response gene activated by insulin. Interestingly, insulin also increases PKCδ protein levels in rat and mouse hepatocytes, but the mechanism(s) are as yet not clear. Whether other insulin-sensitive PKC isoforms are similarly regulated by insulin is not certain. Unpublished studies suggest that fairly rapid insulin-induced increases in PKCα and PKCε levels may also occur in skeletal muscle, but there may also be an eFFect of insulin to inhibit their degradation as well to induce their de novo synthesis.
The regulation of PKCβII by insulin is complex since both post-transcriptional and post-translational regulation occurs via the PI3-kinase pathway. The regulation of the post-transcriptional splicing involves insulin activation of PI3-kinase/Akt and the phosphorylation of downstream splicing factors [10,152,153]. The involvement of additional splicing kinases is also evolving since Akt appears to be a pivotal kinase in the regulation of serine-arginine rich splicing factor phosphorylation (Jiang K, Corbin, K.D., Watson, J.E., Hagiwara, M., Patel, N.A., Cooper, D.R. submitted for publication). The Akt-directed phosphorylation of splicing factors can act in a dual manner. It can cause the factor to act as an enhancer of splicing as is the case for SRp40 [153], or the possibility exists that phosphorylation of factors may cause them to repress exon selection as might be the case with factors that repress selection of the PKCβI exon.
Conclusions and future perspectives
In this survey of literature pertaining to the various conventional and novel PKC isoforms expressed in insulin-responsive tissues, we have taken the approach that if a given protein is expressed and if its activity is changed by insulin, then there is likely to be a chance that it may “do something”. We have also attempted to broaden the concept of “activation” from the old idea of translocation to the membrane, to include interaction with its putative substrate as well as alteration in tyrosine phosphorylation state. This latter approach has been of great importance in furthering the understanding of the role of PKCδ in numerous processes in a variety of cells as recently reviewed in detail [43]. Tyrosine phosphorylation of PKC isoforms has the ability to utilize the enzyme as a docking protein, like IRS, for various other proteins. This could be especially important in the alternative signaling pathway involving c-cbl and TC10 [154] where the caveolae contain multiple PKC binding proteins that have not been characterized [155]. These proteins could play some role in endocytosis/exocytosis or fatty acid transport. Utilizing this approach, we can conclude that each of the PKC isoforms has one or more roles in the insulin signaling cascade, if not via direct activation in response to insulin then by activation via conditions that modify insulin-induced effects.
Several new themes are suggested. With the realization of alternative splicing as a means of regulating signaling pathways it is possible that splice variants of the PKC isoforms will be recognized in modulating certain compartment specific actions of insulin signaling. Moreover, the findings that insulin may rapidly regulate the expression and abundance of these isoforms at both the RNA and protein levels via pre- and post-transcription mechanisms as well as by altering rates of degradation raise interesting possibilities regarding the mechanisms for maintaining PKC availability in response to insulin release. Also, the importance of tyrosine phosphorylation, particularly but not only of PKCδ, cannot be ignored. This brings into play the importance of protein tyrosine phosphatases and their potential role in tissue insulin resistance. It also reminds us of the importance of serine/threonine phosphatases modulated by insulin that can potentially modulate the PKCs and their substrates.
The fact that so many substrates, such as Akt, transcription factors, structural proteins, components of caveolae, IRS, IR, are phosphorylated by PKCs, and their ability to bind to actin and regulate its association with the GLUT vesicles, points out the regulatory nature of these kinases at the signaling level (Figs. 1 and 2).
Fig. 1.
Schematic diagram of the different categories of PKC isoforms
Fig. 2.
Schematic diagram of PKC isoforms expressed in insulin-responsive tissues and potential substrates in the insulin signaling cascade.
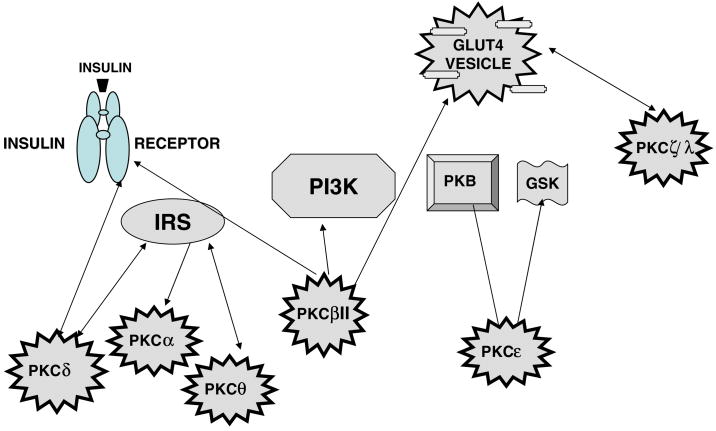
The potential substrates for the various isoforms are summarized in Fig. 3 and Table 1.
Fig. 3.
Summary diagram of PKC isoforms with their documented substrates in insulin-responsive tissues. The documented relations of isoform–substrate interactions for the various isoforms are: PKCα—IRS(−1); PKCβII—IR, PKB(Akt), MARCKS, GLUT4 transporter; PKCδ—IR, IRS-1, PKB; PKCε—PKB, GSK3; PKCθ—IRS-1; (PKCς—glucose transporter).
Table 1.
Current studies addressing PKC isoforms in insulin action
| PKC isoform | Potential substrate/site of action | Effect | Linked to | Reference |
|---|---|---|---|---|
| PKCα | Insulin receptor IRS | Insulin resistance, ▼glucose uptake | Mouse model, cultured cells | [15,62,64,65,67–70,104,156,157] |
| PKCβ (I + II) | MAPK, unknown | Mitogenic effects, no effect on ISGT, insulin resistance | Cultured cells, mouse model | [83,118,158,159] |
| PKCβI | Insulin receptor | Insulin resistance | Mouse model, cultured cells | [42,63,65,83,84] |
| PKCβII | Cytoskeleton, MARCKS, IR, glucose transporter vesicle trafficking | ▲Glucose uptake | Cultured cells, rat models, human muscle cells | [3,10,12,35,42,81,82,160–162] |
| PKCδ | Insulin receptor, IRS signaling | ▲ Glucose uptake, IR endocytosis* | Skeletal muscle, cultured cells | [13,42,62,104] |
| PKCε | Unknown (GSK3) | ▲ Glucose uptake, insulin resistance | Cultured cells | [42,143] (unpublished) |
| PKCθ | Insulin receptor tissue sensitivity | Insulin resistance | Cultured cells, human skeletal muscle | [104,128,137,143,163–165] |
| PKCζ/λ | IRS, ERC vesicles, Akt, glucose transporter vesicle | Insulin resistance, ▲ glucose uptake, no effect on ISGT | Cultured cells | [12,14,159,164,166,167] |
Immediately obvious is the potential for a given isoform to interact with more than one substrate and for a given potential substrate to be influenced by more than one PKC isoform. Moreover, it is clear that, whereas the atypical PKC isoforms (λ/ς) have received major emphasis in the insulin signaling literature, the remaining isoforms reviewed here appear to play no less an important role in various steps in the cascade. There thus remains a major challenge to be able to define more precisely the mechanisms underlying the targeting of specific PKC isoforms to specific substrates by insulin, or by conditions that may influence insulin action. As each of the PKC isoforms is tyrosine phosphorylated by insulin stimulation ([12,52] and our unpublished findings), it is not unreasonable to assume that, as is the case for PKCδ in other systems in response to different stimuli, phosphorylation of specific tyrosine sites may play an important role in this targeting. The use of different PKC isoforms with specific tyrosine site mutations may provide a useful strategy for clarifying individual roles in insulin signaling.
Acknowledgments
The authors acknowledge the following sources of support. Supported in part by the Russell Berrie Foundation and D-Cure, Diabetes Care in Israel, the Chief Scientist’s Office of the Israel Ministry of Health, and by the Sorrell Foundation. SRS is the incumbent of the Louis Fisher Chair in Cellular Pathology. Supported by the Medical Research Service of the Department of Veterans Affairs and NIH DK 54393.
References
- 1.White MF. The insulin signalling system and the IRS proteins. Diabetologia. 1997;40(Suppl 2):2–17. doi: 10.1007/s001250051387. [DOI] [PubMed] [Google Scholar]
- 2.Acevedo-Duncan M, Cooper DR, Standaert ML, Farese RV. Immunological evidence that insulin activates protein kinase C in BC3H-1 myocytes. FEBS Lett. 1989;244:174–176. doi: 10.1016/0014-5793(89)81186-x. [DOI] [PubMed] [Google Scholar]
- 3.Chalfant CE, Mischak H, Watson JE, Winkler BC, Goodnight J, Farese RV, Cooper DR. Regulation of alternative splicing of protein kinase C beta by insulin. J Biol Chem. 1995;270:13326–13332. doi: 10.1074/jbc.270.22.13326. [DOI] [PubMed] [Google Scholar]
- 4.Chalfant CEKY, Ohno S, Konno Y, Fisher AA, Bisnauth LD, Watson JE, Cooper DR. A carboxy-terminal deletion mutant of protein kinase C beta II inhibits insulin-stimulated 2-deoxyglucose uptake in L6 rat skeletal muscle cells. Mol Endocrinol. 1996;10:273–1281. doi: 10.1210/mend.10.10.9121494. [DOI] [PubMed] [Google Scholar]
- 5.Cooper DR, Konda TS, Standaert ML, Davis JS, Pollet RJ, Farese RV. Insulin increases membrane and cytosolic protein kinase C activity in BC3H-1 myocytes. J Biol Chem. 1987;262:3633–3639. [PubMed] [Google Scholar]
- 6.Cooper DR, Watson JE, Hernandez H, Yu B, Standaert ML, Ways DK, Arnold TT, Ishizuka T, Farese RV. Direct evidence for protein kinase C involvement in insulin-stimulated hexose uptake. Biochem Biophys Res Commun. 1992;188:142–148. doi: 10.1016/0006-291x(92)92361-z. [DOI] [PubMed] [Google Scholar]
- 7.Cooper DR, Hernandez H, Kuo JY, Farese RV. Insulin increases the synthesis of phospholipid and diacylglycerol and protein kinase C activity in rat hepatocytes. Arch Biochem Biophys. 1990;276:486–494. doi: 10.1016/0003-9861(90)90749-o. [DOI] [PubMed] [Google Scholar]
- 8.Farese RV, Standaert ML, Arnold T, Yu B, Ishizuka T, Hoffman J, Vila M, Cooper DR. The role of protein kinase C in insulin action. Cell Signal. 1992;4:133–143. doi: 10.1016/0898-6568(92)90077-l. [DOI] [PubMed] [Google Scholar]
- 9.Ishizuka T, Cooper DR, Arnold T, Hernandez H, Farese RV. Downregulation of protein kinase C and insulin-stimulated 2-deoxyglucose uptake in rat adipocytes by phorbol esters, glucose, and insulin. Diabetes. 1991;40:1274–1281. doi: 10.2337/diab.40.10.1274. [DOI] [PubMed] [Google Scholar]
- 10.Patel NA, Chalfant CE, Watson JE, Wyatt JR, Dean NM, Eichler DC, Cooper DR. Insulin regulates alternative splicing of protein kinase C beta II through a phosphatidylinositol 3-kinase-dependent pathway involving the nuclear serine/arginine-rich splicing factor, SRp40, in skeletal muscle cells. J Biol Chem. 2001;276:22648–22654. doi: 10.1074/jbc.M101260200. [DOI] [PubMed] [Google Scholar]
- 11.Braiman L, Alt A, Kuroki T, Ohba M, Bak A, Tennenbaum T, Sampson SR. Protein kinase Cdelta mediates insulin-induced glucose transport in primary cultures of rat skeletal muscle. Mol Endocrinol. 1999;13:2002–2012. doi: 10.1210/mend.13.12.0393. [DOI] [PubMed] [Google Scholar]
- 12.Braiman L, Sheffi-Friedman L, Bak A, Tennenbaum T, Sampson SR. Tyrosine phosphorylation of specific protein kinase C isoenzymes participates in insulin stimulation of glucose transport in primary cultures of rat skeletal muscle. Diabetes. 1999;48:1922–1929. doi: 10.2337/diabetes.48.10.1922. [DOI] [PubMed] [Google Scholar]
- 13.Braiman L, Alt A, Kuroki T, Ohba M, Bak A, Tennenbaum T, Sampson SR. Insulin induces specific interaction between insulin receptor and PKC in primary cultured skeletal muscle. Mol Endocrinol. 2001;15:565–574. doi: 10.1210/mend.15.4.0612. [DOI] [PubMed] [Google Scholar]
- 14.Braiman L, Alt A, Kuroki T, Ohba M, Bak A, Tennenbaum T, Sampson SR. Activation of protein kinase C zeta induces serine phosphorylation of VAMP2 in the GLUT4 compartment and increases glucose transport in skeletal muscle. Mol Cell Biol. 2001;21:7852–7861. doi: 10.1128/MCB.21.22.7852-7861.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenzweig T, Braiman L, Bak A, Alt A, Kuroki T, Sampson SR. Differential effects of tumor necrosis factor-alpha on protein kinase C isoforms alpha and delta mediate inhibition of insulin receptor signaling. Diabetes. 2002;51:1921–1930. doi: 10.2337/diabetes.51.6.1921. [DOI] [PubMed] [Google Scholar]
- 16.Buchner K. Protein kinase C in the transduction of signals toward and within the cell nucleus. Eur J Biochem. 1995;228:211–221. [PubMed] [Google Scholar]
- 17.Nishizuka Y. The family of protein kinase C for signal transduction. JAMA. 1989;262:1826–1833. [PubMed] [Google Scholar]
- 18.Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumor promotion. Nature. 1984;308:693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- 19.Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986;233:305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- 20.Nishizuka Y. The molecular heterogeneity of PKC and its implications for cellular regulation. Nature. 1988;334:661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- 21.Cho W. Membrane targeting by C1 and C2 domains. J Biol Chem. 2001;276:32407–32410. doi: 10.1074/jbc.R100007200. [DOI] [PubMed] [Google Scholar]
- 22.Hurley JH, Misra S. Signaling and subcellular targeting by membrane-binding domains. Annu Rev Biophys Biomol Struct. 2000;29:49–79. doi: 10.1146/annurev.biophys.29.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirai T, Chida K. Protein kinase Czeta (PKCzeta): activation mechanisms and cellular functions. J Biochem (Tokyo) 2003;133:1–7. doi: 10.1093/jb/mvg017. [DOI] [PubMed] [Google Scholar]
- 24.Farese RV. Function and dysfunction of aPKC isoforms for glucose transport in insulin-sensitive and insulin-resistant states. Am J Physiol Endocrinol Metab. 2002;283:E1–E11. doi: 10.1152/ajpendo.00045.2002. [DOI] [PubMed] [Google Scholar]
- 25.Ueyama T, Ren Y, Ohmori S, Sakai K, Tamaki N, Saito N. cDNA cloning of an alternative splicing variant of protein kinase C delta (PKC deltaIII), a new truncated form of PKCdelta, in rats. Biochem Biophys Res Commun. 2000;269:557–563. doi: 10.1006/bbrc.2000.2331. [DOI] [PubMed] [Google Scholar]
- 26.Thanaraj TA, Stamm S, Clark F, Riethoven JJ, Le TV, Muilu J. ASD: the alternative splicing database. Nucleic Acids Res. 2006:D64–D69. doi: 10.1093/nar/gkh030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stamm S, Riethoven JJ, Le TV, Gopalakrishnan C, Kumanduri V, Tang Y, Barbosa-Morais NL, Thanaraj TA. ASD: a bioinformatics resource on alternative splicing. Nucleic Acids Res. 2006:D46–D55. doi: 10.1093/nar/gkj031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gschwendt M. Protein kinase C delta. Eur J Biochem. 1999;259:555–564. doi: 10.1046/j.1432-1327.1999.00120.x. [DOI] [PubMed] [Google Scholar]
- 29.Naitoh T, Kobayashi S, Kimura I, Kimura M. 1956 Intracellular Ca2+ and Mg2+ regulation for insulin-stimulated glucose uptake into mouse diaphragm muscles. Jpn J Pharmacol. 1991:241–244. doi: 10.1254/jjp.56.241. [DOI] [PubMed] [Google Scholar]
- 30.Foot EA, Leighton B. Effects of calcium antagonists on insulin-mediated glucose metabolism in skeletal muscle. Diabetes. 1943:73–79. doi: 10.2337/diab.43.1.73. [DOI] [PubMed] [Google Scholar]
- 31.Walaas SI, Horn RS, Adler A, Albert KA, Walaas O. 220 Insulin increases membrane protein kinase C activity in rat diaphragm. FEBS Lett. 1987:17311–17318. doi: 10.1016/0014-5793(87)80837-2. [DOI] [PubMed] [Google Scholar]
- 32.Terada S, Muraoka I, Tabata I. 1994 Changes in [Ca2+]i induced by several glucose transport-enhancing stimuli in rat epitrochlearis muscle. J Appl Physiol. 2003:1813–1820. doi: 10.1152/japplphysiol.00780.2002. [DOI] [PubMed] [Google Scholar]
- 33.Espinosa A, Estrada M, Jaimovich E. 182 IGF-I and insulin induce different intracellular calcium signals in skeletal muscle cells. J Endocrinol (Aug) 2004:339–352. doi: 10.1677/joe.0.1820339. [DOI] [PubMed] [Google Scholar]
- 34.Lorenzo M, Teruel T, Hernandez R, Kayali AG, Webster NJ. 278 PLCgamma participates in insulin stimulation of glucose uptake through activation of PKCzeta in brown adipocytes. Exp Cell Res (Aug) 2002:15146–15157. doi: 10.1006/excr.2002.5570. [DOI] [PubMed] [Google Scholar]
- 35.Khayat ZA, Tsakiridis T, Ueyama A, Somwar R, Ebina Y, Klip A. Rapid stimulation of glucose transport by mitochondrial uncoupling depends in part on cytosolic Ca2+ and cPKC. Am J Physiol. 1998;275:C1487–C1497. doi: 10.1152/ajpcell.1998.275.6.C1487. [DOI] [PubMed] [Google Scholar]
- 36.McCarty MF. PKC-mediated modulation of L-type calcium channels may contribute to fat-induced insulin resistance. Med Hypotheses. 2006;66:824–831. doi: 10.1016/j.mehy.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 37.Toker A, Newton AC. Akt/protein kinase B is regulated by auto-phosphorylation at the hypothetical PDK-2 site. J Biol Chem. 2000;275:8271–8274. doi: 10.1074/jbc.275.12.8271. [DOI] [PubMed] [Google Scholar]
- 38.Dong LQ, Liu F. PDK2: the missing piece in the receptor tyrosine kinase signaling pathway puzzle. Am J Physiol Endocrinol Metab. 2005:E187–E196. doi: 10.1152/ajpendo.00011.2005. [DOI] [PubMed] [Google Scholar]
- 39.Ishizuka T, Yamamoto M, Kajita K, Nagashima T, Yasuda K, Miura K, Cooper DR, Farese RV. Insulin stimulates novel protein kinase C in rat adipocytes. Biochem Biophys Res Commun. 1992;183:814–820. doi: 10.1016/0006-291x(92)90556-z. [DOI] [PubMed] [Google Scholar]
- 40.Yu B, Standaert M, Arnold T, Hernandez H, Watson J, Ways K, Cooper DR, Farese RV. Effects of insulin on diacylglycerol/protein kinase-C signalling and glucose transport in rat skeletal muscles in vivo and in vitro. Endocrinology. 1992;130:3345–3355. doi: 10.1210/endo.130.6.1597146. [DOI] [PubMed] [Google Scholar]
- 41.Yamada K, Avignon A, Standaert ML, Cooper DR, Spencer B, Farese RV. Effects of insulin on the translocation of protein kinase C-theta and other protein kinase C isoforms in rat skeletal muscles. Biochem J. 1995;308:177–180. doi: 10.1042/bj3080177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cooper DR, Watson JE, Patel N, Illingworth P, Acevedo-Duncan M, Goodnight J, Chalfant CE, Mischak H. Ectopic expression of protein kinase CbetaII, -delta, and -epsilon, but not -betaI or -zeta, provide for insulin stimulation of glucose uptake in NIH-3T3 cells. Arch Biochem Biophys. 1999;372:69–79. doi: 10.1006/abbi.1999.1472. [DOI] [PubMed] [Google Scholar]
- 43.Steinberg SF. Distinctive activation mechanisms and functions for protein kinase Cdelta. Biochem J. 2004:15449–15459. doi: 10.1042/BJ20040704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soltoff SP, Toker A. Carbachol, substance P, and phorbol ester promote the tyrosine phosphorylation of protein kinase Cδ in salivary gland epithelial cells. J Biol Chem. 1995;270:13490–13495. doi: 10.1074/jbc.270.22.13490. [DOI] [PubMed] [Google Scholar]
- 45.Benes C, Soltoff SP. Modulation of PKCdelta tyrosine phosphorylation and activity in salivary and PC-12 cells by Src kinases. Am J Physiol Cell Physiol. 2001;280:C1498–C1510. doi: 10.1152/ajpcell.2001.280.6.C1498. [DOI] [PubMed] [Google Scholar]
- 46.Konishi H, Tanaka M, Takemura Y, Matsuzaki H, Ono Y, Kikkawa U, Nishizuka Y. Activation of protein kinase C by tyrosine phosphorylation in response to H2O2. Proc Natl Acad Sci USA. 1997;94:11233–11237. doi: 10.1073/pnas.94.21.11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Konishi H, Yamauchi E, Taniguchi H, Yamamoto T, Matsuzaki H, Takemura Y, Ohmae K, Kikkawa U, Nishizuka Y. Phosphorylation sites of protein kinase C delta in H2O2-treated cells and its activation by tyrosine kinase in vitro. Proc Natl Acad Sci USA. 2001;98:6587–6592. doi: 10.1073/pnas.111158798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Benes CH, Wu N, Elia AE, Dharia T, Cantley LC, Soltoff SP. The C2 domain of PKCdelta is a phosphotyrosine binding domain. Cell. 2005;121:271–280. doi: 10.1016/j.cell.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 49.Li W, Mischak H, Yu JC, Wang LM, Mushinski JF, Heidaran MA, Pierce JH. Tyrosine phosphorylation of protein kinase C-delta in response to its activation. J Biol Chem. 1994;269:2349–2352. [PubMed] [Google Scholar]
- 50.Denning MF, Dlugosz AA, Threadgill DW, Maguson T, Yuspa SH. Activation of the epidermal growth factor receptor signal transduction pathway stimulates tyrosine phosphorylation of protein kinase C. J Biol Chem. 1996;271:5325–5331. doi: 10.1074/jbc.271.10.5325. [DOI] [PubMed] [Google Scholar]
- 51.Acs P, Beheshti M, Szallasi Z, Li L, Yuspa SH, Blumberg PM. Effect of a tyrosine 155 to phenylalanine mutation of protein kinase cdelta on the proliferative and tumorigenic properties of NIH 3T3 fibroblasts. Carcinogenesis. 2000;21:887–891. doi: 10.1093/carcin/21.5.887. [DOI] [PubMed] [Google Scholar]
- 52.Rosenzweig T, Aga-Mizrachi S, Bak A, Sampson SR. Src tyrosine kinase regulates insulin-induced activation of protein kinase C (PKC) delta in skeletal muscle. Cell Signal. 2004;16:1299–1308. doi: 10.1016/j.cellsig.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 53.Arbet-Engels C, Tartare-Deckert S, Eckhart W. C-terminal Src kinase associates with ligand-stimulated insulin-like growth factor-I receptor. J Biol Chem. 1999;274:5422–5428. doi: 10.1074/jbc.274.9.5422. [DOI] [PubMed] [Google Scholar]
- 54.Boney CM, Sekimoto H, Gruppuso PA, Frackelton AR., Jr Src family tyrosine kinases participate in insulin-like growth factor I mitogenic signaling in 3T3-L1 cells. Cell Growth Differ. 2001;12:379–386. [PubMed] [Google Scholar]
- 55.Kaburagi Y, Satoh S, Tamemoto H, Yamamoto-Honda R, Tobe K, Veki K, Yamauchi T, Kono-Sugita E, Sekihara H, Aizawa S, Cushman SW, Akanuma Y, Yazaki Y, Kadowaki T. Role of insulin receptor substrate-1 and pp60 in the regulation of insulin-induced glucose transport and GLUT4 translocation in primary adipocytes. J Biol Chem. 1997;272:25839–25844. doi: 10.1074/jbc.272.41.25839. [DOI] [PubMed] [Google Scholar]
- 56.Muller G, Wied S, Frick W. signaling in adipocytes Cross talk of pp125(FAK) and pp59(Lyn) non-receptor tyrosine kinases to insulin-mimetic. Mol Cell Biol. 2000;20:4708–4723. doi: 10.1128/mcb.20.13.4708-4723.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perrotti N, Taylor SI, Richert ND, Rapp UR, Pastan IH, Roth J. Immunoprecipitation of insulin receptors from cultured human lymphocytes (IM-9 cells) by antibodies to pp60src. Science. 1985;227:761–763. doi: 10.1126/science.3918346. [DOI] [PubMed] [Google Scholar]
- 58.Sun XJ, Pons S, Asano T, Myers MGJ, Glasheen E, White MF. The Fyn tyrosine kinase binds Irs-1 and forms a distinct signaling complex during insulin stimulation. J Biol Chem. 1996;271:10583–10587. doi: 10.1074/jbc.271.18.10583. [DOI] [PubMed] [Google Scholar]
- 59.White MF, Werth DK, Pastan I, Kahn CR. Phosphorylation of the solubilized insulin receptor by the gene product of the Rous sarcoma virus, pp60src. J Cell Biochem. 1984;26:169–179. doi: 10.1002/jcb.240260305. [DOI] [PubMed] [Google Scholar]
- 60.Yu KT, Werth DK, Pastan IH, Czech MP. src kinase catalyzes the phosphorylation and activation of the insulin receptor kinase. J Biol Chem. 1985;260:5838–5846. [PubMed] [Google Scholar]
- 61.Bandyopadhyay G, Standaert ML, Zhao LYB, Yu B, Avignon A, Galloway L, Karnam P, Moscat J, Farese RV. Activation of protein kinase C (alpha, beta, and zeta) by insulin in 3T3/L1 cells. Transfection studies suggest a role for PKC-zeta in glucose transport. J Biol Chem. 1997;272:2551–2558. doi: 10.1074/jbc.272.4.2551. [DOI] [PubMed] [Google Scholar]
- 62.Formisano P, Oriente F, Miele C, Caruso M, Auricchio R, Vigliotta G, Condorelli G, Beguinot F. In NIH-3T3 fibroblasts, insulin receptor interaction with specific protein kinase C isoforms controls receptor intracellular routing. J Biol Chem. 1998;273:13197–13202. doi: 10.1074/jbc.273.21.13197. [DOI] [PubMed] [Google Scholar]
- 63.Condorelli G, Vigliotta G, Trencia A, Maitan MA, Caruso M, Miele C, Oriente F, Santopietro S, Formisano P, Beguinot F. Protein kinase C (PKC)-alpha activation inhibits PKC-zeta and mediates the action of PED/PEA-15 on glucose transport in the L6 skeletal muscle cells. Diabetes. 2001;50:1244–1252. doi: 10.2337/diabetes.50.6.1244. [DOI] [PubMed] [Google Scholar]
- 64.Oriente F, Andreozzi F, Romano C, Perruolo G, Perfetti A, Fiory F, Miele C, Beguinot F, Formisano P. Protein kinase C-alpha regulates insulin action and degradation by inter acting with insulin receptor substrate-1 and 14-3-3epsilon. J Biol Chem. 2005 doi: 10.1074/jbc.M508570200. [DOI] [PubMed] [Google Scholar]
- 65.Chin JE, Dickens M, Tavare JM, Roth RA. Overexpression of protein kinase C isoenzymes alpha, beta I, gamma, and epsilon in cells overexpressing the insulin receptor. Effects on receptor phosphorylation and signaling. J Biol Chem. 1993;268:6338–6347. [PubMed] [Google Scholar]
- 66.Chin JE, Liu F, Roth RA. Activation of protein kinase C alpha inhibits insulin-stimulated tyrosine phosphorylation of insulin receptor substrate-1. Mol Endocrinol. 1994;8:51–58. doi: 10.1210/mend.8.1.7512195. [DOI] [PubMed] [Google Scholar]
- 67.Liu F, Roth RA. Insulin-stimulated tyrosine phosphorylation of protein kinase C alpha: evidence for direct interaction of the insulin receptor and protein kinase C in cells. Biochem Biophys Res Commun. 1994;200:1570–1577. doi: 10.1006/bbrc.1994.1630. [DOI] [PubMed] [Google Scholar]
- 68.Liu F, Roth RA. Identification of serines-1035/1037 in the kinase domain of the insulin receptor as protein kinase C alpha mediated phosphorylation sites. FEBS Lett. 1994;352:389–392. doi: 10.1016/0014-5793(94)00996-1. [DOI] [PubMed] [Google Scholar]
- 69.Danielsen AG, Liu F, Hosomi Y, Shii K, Roth RA. Activation of protein kinase C alpha inhibits signaling by members of the insulin receptor family. J Biol Chem. 1995;270:21600–21605. doi: 10.1074/jbc.270.37.21600. [DOI] [PubMed] [Google Scholar]
- 70.Leitges M, Plomann M, Standaert ML, Bandyopadhyay G, Sajan MP, Kanoh Y, Farese RV. Knockout of PKC alpha enhances insulin signaling through PI3K. Mol Endocrinol. 2002;16:847–858. doi: 10.1210/mend.16.4.0809. [DOI] [PubMed] [Google Scholar]
- 71.Obeid LM, Hannun YA. Ceramide, stress, and a “LAG” in aging. Sci Aging Knowledge Environ (Oct) 2003:1E27. doi: 10.1126/sageke.2003.39.pe27. [DOI] [PubMed] [Google Scholar]
- 72.Cooper DR, de Ruiz G, Fanjul LF, Mojsilovic L, Standaert ML, Pollet RJ, Farese RV. Insulin but not phorbol ester treatment increases phosphorylation of vinculin by protein kinase C in BC3H-1 myocytes. FEBS Lett. 1987;214:122–126. doi: 10.1016/0014-5793(87)80025-x. [DOI] [PubMed] [Google Scholar]
- 73.Ohno S, Kawasaki H, Imajoh S, Suzuki K, Inagaki M, Yokokura H, Sakoh T, Hidaka H. 325 Tissue-specific expression of three distinct types of rabbit protein kinase C. Nature. 1987 Jan 8;:14161–14166. doi: 10.1038/325161a0. [DOI] [PubMed] [Google Scholar]
- 74.Ono Y, Kikkawa U, Ogita K, Fujii T, Kurokawa T, Asaoka Y, Sekiguchi K, Ase K, Igarashi K, Nishizuka Y. 236 Expression and properties of two types of protein kinase C: alternative splicing from a single gene. Science (May) 1987:291116–291120. doi: 10.1126/science.3576226. [DOI] [PubMed] [Google Scholar]
- 75.Marais RM, Parker PJ. 182 Purification and characterisation of bovine brain protein kinase C isotypes alpha, beta and gamma. Eur J Biochem (Jun) 1987:1129–1137. doi: 10.1111/j.1432-1033.1989.tb14809.x. [DOI] [PubMed] [Google Scholar]
- 76.Blackshear PJ. Approaches to the study of protein kinase C involvement in signal transduction. Am J Med Sci. 1988:231–240. doi: 10.1016/s0002-9629(15)40866-3. [DOI] [PubMed] [Google Scholar]
- 77.Toker A, Newton AC. Cellular signaling: pivoting around PDK-1. Cell. 2000:13185–13188. doi: 10.1016/s0092-8674(00)00110-0. [DOI] [PubMed] [Google Scholar]
- 78.Coussens L, Rhee L, Parker PJ, Ullrich A. Alternative splicing increases the diversity of the human protein kinase C family. DNA (1987 Oct) 2006:389–394. doi: 10.1089/dna.1987.6.389. [DOI] [PubMed] [Google Scholar]
- 79.Coussens L, Parker PJ, Rhee L, Yang-Feng TL, Chen E, Waterfield MD, Francke U, Ullrich A. 233 Multiple, distinct forms of bovine and human protein kinase C suggest diversity in cellular signaling pathways. Science (Aug) 1986:22859–22866. doi: 10.1126/science.3755548. [DOI] [PubMed] [Google Scholar]
- 80.Ono Y, Kurokawa T, Fujii T, Kawahara K, Igarashi K, Kikkawa U, Ogita K, Nishizuka Y. 206 Two types of complementary DNAs of rat brain protein kinase C. Heterogeneity determined by alternative splicing. FEBS Lett (Oct) 1986:6347–6352. doi: 10.1016/0014-5793(86)81010-9. [DOI] [PubMed] [Google Scholar]
- 81.Chalfant CE, Watson JE, Bisnauth LD, Kang JB, Patel N, Obeid LM, Eichler DC, Cooper DR. Insulin regulates protein kinase CbetaII expression through enhanced exon inclusion in L6 skeletal muscle cells. A novel mechanism of insulin- and insulin-like growth factor-i-induced 5″; splice site selection. J Biol Chem. 1998;273:910–916. doi: 10.1074/jbc.273.2.910. [DOI] [PubMed] [Google Scholar]
- 82.Chalfant CE, Ohno S, Konno Y, Fisher AA, Bisnauth LD, Watson JE, Cooper DR. A carboxy-terminal deletion mutant of protein kinase C beta II inhibits insulin-stimulated 2-deoxyglucose uptake in L6 rat skeletal muscle cells. Mol Endocrinol. 1996;10:1273–1281. doi: 10.1210/mend.10.10.9121494. [DOI] [PubMed] [Google Scholar]
- 83.Standaert ML, Bandyopadhyay G, Galloway L, Soto J, Ono Y, Kikkawa U, Farese RV, Leitges M. Effects of knockout of the protein kinase C beta gene on glucose transport and glucose homeostasis. Endocrinology. 1999;140:4470–4477. doi: 10.1210/endo.140.10.7073. [DOI] [PubMed] [Google Scholar]
- 84.Bossenmaier B, Mosthaf L, Mischak H, Ullrich A, Haring HU. Protein kinase C isoforms beta 1 and beta 2 inhibit the tyrosine kinase activity of the insulin receptor. Diabetologia. 1997;40:863–866. doi: 10.1007/s001250050761. [DOI] [PubMed] [Google Scholar]
- 85.Patel NA, Chalfant CE, Yamamoto M, Watson JE, Eichler DC, Cooper DR. Acute hyperglycemia regulates transcription and post-transcriptional stability of PKCbetaII mRNA in vascular smooth muscle cells. FASEB J. 1999;13:103–113. doi: 10.1096/fasebj.13.1.103. [DOI] [PubMed] [Google Scholar]
- 86.Patel NA, Yamamoto M, Illingworth P, Mancu D, Mebert K, Chappell DS, Watson JE, Cooper DR. Phosphoinositide 3-kinase mediates protein kinase C beta II mRNA destabilization in rat A10 smooth muscle cell cultures exposed to high glucose. Arch Biochem Biophys. 2002;403:111–120. doi: 10.1016/S0003-9861(02)00208-4. [DOI] [PubMed] [Google Scholar]
- 87.Patel NA, Eichler DC, Chappell DS, Illingworth PA, Chalfant CE, Yamamoto M, Dean NM, Wyatt JR, Mebert K, Watson JE, Cooper DR. The protein kinase C beta II exon confers mRNA instability in the presence of high glucose concentrations. J Biol Chem. 2003;278:1149–1157. doi: 10.1074/jbc.M206797200. [DOI] [PubMed] [Google Scholar]
- 88.Wright DC, Craig BW, Fick CA, Lim KI. The effects of phospholipase C inhibition on insulin-stimulated glucose transport in skeletal muscle. Metabolism. 2002:271–273. doi: 10.1053/meta.2002.30500. [DOI] [PubMed] [Google Scholar]
- 89.Wright DC, Fick CA, Olesen JB, Craig BW. Evidence for the involvement of a phospholipase C–protein kinase C signaling pathway in insulin stimulated glucose transport in skeletal muscle. Life Sci. 2003:2361–2371. doi: 10.1016/s0024-3205(03)00256-x. [DOI] [PubMed] [Google Scholar]
- 90.Blobe GC, Khan WA, Halpern AE, Obeid LM, Hannun YA. Selective regulation of expression of protein kinase C beta isoenzymes occurs via alternative splicing. J Biol Chem. 1993:1510627–1510635. [PubMed] [Google Scholar]
- 91.Disatnik MH, Buraggi G, Mochly-Rosen D. Localization of protein kinase C isozymes in cardiac myocytes. Exp Cell Res. 1994:287–297. doi: 10.1006/excr.1994.1041. [DOI] [PubMed] [Google Scholar]
- 92.Stebbins EG, Mochly-Rosen D. Binding specificity for RACK1 resides in the V5 region of beta II protein kinase C. J Biol Chem. 2001:1029644–1029650. doi: 10.1074/jbc.M101044200. [DOI] [PubMed] [Google Scholar]
- 93.Yan SF, Lu J, Zou YS, Soh-Won J, Cohen DM, Buttrick PM, Cooper DR, Steinberg SF, Mackman N, Pinsky DJ, Stern DM. Hypoxia-associated induction of early growth response-1 gene expression. J Biol Chem. 1999:2115030–2115040. doi: 10.1074/jbc.274.21.15030. [DOI] [PubMed] [Google Scholar]
- 94.Perrotti D, Bonatti S, Trotta R, Martinez R, Skorski T, Salomoni P, Grassilli E, Lozzo RV, Cooper DR, Calabretta B. TLS/FUS, a pro-oncogene involved in multiple chromosomal translocations, is a novel regulator of BCR/ABL-mediated leukemogenesis. EMBO J. 1998:34442–34455. doi: 10.1093/emboj/17.15.4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Meissner M, Lopato S, Gotzmann J, Sauermann G, Barta A. Proto-oncoprotein TLS/FUS is associated to the nuclear matrix and complexed with splicing factors PTB, SRm160, and SR proteins. Exp Cell Res. 2003:15184–15195. doi: 10.1016/s0014-4827(02)00046-0. [DOI] [PubMed] [Google Scholar]
- 96.Kawakami Y, Nishimoto H, Kitaura J, Maeda-Yamamoto M, Kato RM, Littman DR, Leitges M, Rawlings DJ, Kawakami T. Protein kinase C betaII regulates Akt phosphorylation on Ser-473 in a cell type- and stimulus-specific fashion. J Biol Chem. 2004:1247720–1247725. doi: 10.1074/jbc.M408797200. [DOI] [PubMed] [Google Scholar]
- 97.Shepherd PR, Nave BT, O’Rahilly S. The role of phosphoinositide 3-kinase in insulin signalling. J Mol Endocrinol. 1996;17:175–184. doi: 10.1677/jme.0.0170175. [DOI] [PubMed] [Google Scholar]
- 98.Shepherd PR, Siddle K, Nave BT. Is stimulation of class-1 phosphatidylinositol 3-kinase activity by insulin sufficient to activate pathways involved in glucose metabolism. Biochem Soc Trans. 1997;25:978–981. doi: 10.1042/bst0250978. [DOI] [PubMed] [Google Scholar]
- 99.Toker A, Meyer M, Reddy KK, Falck JR, Aneja R, Aneja S, Parra A, Burns DJ, Ballas LM, Cantley LC. Activation of protein kinase C family members by the novel polyphosphoinositides PtdIns-3,4-P2 and PtdIns-3,4,5-P3. J Biol Chem. 1994;269:32358–32367. [PubMed] [Google Scholar]
- 100.Strack V, Krutzfeldt J, Kellerer M, Ullrich A, Lammers R, Haring HU. The protein–tyrosine–phosphatase SHP2 is phosphorylated on serine residues 576 and 591 by protein kinase C isoforms alpha, beta 1, beta 2, and eta. Biochemistry. 2002:15603–15608. doi: 10.1021/bi011327v. [DOI] [PubMed] [Google Scholar]
- 101.Leitges M, Schmedt C, Guinamard R, Davoust J, Schaal S, Stabel S, Tarakhovsky A. Immunodeficiency in protein kinase cbeta-deficient mice. Science. 1996;273:788–791. doi: 10.1126/science.273.5276.788. [DOI] [PubMed] [Google Scholar]
- 102.Frevert EU, Kahn BB. Protein kinase C isoforms epsilon, eta, delta and zeta in murine adipocytes: expression, subcellular localization and tissue-specific regulation in insulin-resistant states. Biochem J. 1996;316:865–871. doi: 10.1042/bj3160865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kellerer M, Haring HU. Pathogenesis of insulin resistance: modulation of the insulin signal at receptor level. Diabetes Res Clin Pract. 1995;28(Suppl):S173–S177. doi: 10.1016/0168-8227(95)01070-t. [DOI] [PubMed] [Google Scholar]
- 104.Kellerer M, Mushack J, Seffer E, Mischak H, Ullrich A, Haring HU. Protein kinase C isoforms alpha, delta and theta require insulin receptor substrate-1 to inhibit the tyrosine kinase activity of the insulin receptor in human kidney embryonic cells (HEK 293 cells) Diabetologia. 1998;41:833–838. doi: 10.1007/s001250050995. [DOI] [PubMed] [Google Scholar]
- 105.Strack V, Hennige AM, Krutzfeldt J, Bossenmaier B, Klein HH, Kellerer M, Lammers R, Haring HU. Serine residues 994 and 1023/25 are important for insulin receptor kinase inhibition by protein kinase C isoforms beta2 and theta. Diabetologia. 2000;43:443–449. doi: 10.1007/s001250051327. [DOI] [PubMed] [Google Scholar]
- 106.Greene MW, Morrice N, Garofalo RS, Roth RA. Modulation of human insulin receptor substrate-1 tyrosine phosphorylation by protein kinase Cdelta. Biochem J. 2004;378:105–116. doi: 10.1042/BJ20031493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mussig K, Staiger H, Fiedler H, Moeschel K, Beck A, Kellerer M, Haring HU. Shp2 is required for protein kinase C-dependent phosphorylation of serine 307 in insulin receptor substrate-1. J Biol Chem. 2005;280:32693–32699. doi: 10.1074/jbc.M506549200. [DOI] [PubMed] [Google Scholar]
- 108.Aguirre V, Werner ED, Giraud J, Lee YH, Shoelson SE, White MF. Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J Biol Chem. 2002;277:1531–1537. doi: 10.1074/jbc.M101521200. [DOI] [PubMed] [Google Scholar]
- 109.Ishizuka T, Cooper DR, Farese RV. Insulin stimulates the translocation of protein kinase C in rat adipocytes. FEBS Lett. 1989;257:337–340. doi: 10.1016/0014-5793(89)81565-0. [DOI] [PubMed] [Google Scholar]
- 110.Ishizuka T, Yamamoto M, Kajita K, Nagashima T, Taniguchi O, Wada H, Itaya S, Yasuda K. Phorbol ester and insulin stimulate protein kinase C isoforms in rat adipocytes. Diabetes Res Clin Pract. 1994;26:91–99. doi: 10.1016/0168-8227(94)90145-7. [DOI] [PubMed] [Google Scholar]
- 111.Ishizuka T, Nagashima T, Kajita K, Yamamoto M, Wada H, Itaya S, Yamada K, Miura A, Kanoh Y, Ishizawa M, Yasuda K. Acute effects of phorbol ester and insulin on insulin-induced glucose uptake and protein kinase C activation in rat adipocytes. Diabetes Res Clin Pract. 1997;37:49–52. doi: 10.1016/s0168-8227(97)00037-5. [DOI] [PubMed] [Google Scholar]
- 112.Reks SE, Smith PH, Messina JL, Weinstock RS. Translocation of PKC delta by insulin in a rat hepatoma cell line. Endocrine. 1998;8:161–167. doi: 10.1385/ENDO:8:2:161. [DOI] [PubMed] [Google Scholar]
- 113.Probst I, Beuers U, Drabent B, Unthan-Fechner K, Butikofer P. The diacylglycerol and protein kinase C pathways are not involved in insulin signalling in primary rat hepatocytes. Eur J Biochem. 2003;270:4635–4646. doi: 10.1046/j.1432-1033.2003.03853.x. [DOI] [PubMed] [Google Scholar]
- 114.Pillay TS, Xiao S, Keranen L, Olefsky JM. Regulation of the insulin receptor by protein kinase C isoenzymes: preferential interaction with beta isoenzymes and interaction with the catalytic domain of betaII. Cell Signal. 2004;16:97–104. doi: 10.1016/s0898-6568(03)00090-1. [DOI] [PubMed] [Google Scholar]
- 115.Farese RV, Standaert ML, Francois AJ, Ways K, Arnold TP, Hernandez H, Cooper DR. Effects of insulin and phorbol esters on subcellular distribution of protein kinase C isoforms in rat adipocytes. Biochem J. 1992;288:319–323. doi: 10.1042/bj2880319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhao L, Standaert ML, Cooper DR, Avignon A, Farese RV. Effects of insulin on protein kinase-C (PKC) in HIRC-B cells: specific activation of PKC epsilon and its resistance to phorbol ester-induced down-regulation. Endocrinology. 1994;135:2504–2510. doi: 10.1210/endo.135.6.7988438. [DOI] [PubMed] [Google Scholar]
- 117.Standaert ML, Avignon A, Arnold T, Saba-Siddique SI, Copper DR, Watson J, Zhou X, Galloway L, Farese RV. Insulin translocates PKC-epsilon and phorbol esters induce and persistently translocate PKC-beta 2 in BC3H-1 myocytes. Cell Signal. 1996:313–316. doi: 10.1016/0898-6568(96)00043-5. [DOI] [PubMed] [Google Scholar]
- 118.Cooper DR, Watson JE, Dao ML. Decreased expression of protein kinase-C alpha, beta, and epsilon in soleus muscle of Zucker obese (fa/fa) rats. Endocrinology. 1993;133:2241–2247. doi: 10.1210/endo.133.5.8404676. [DOI] [PubMed] [Google Scholar]
- 119.Bourbon NA, Yun J, Berkey D, Wang Y, Kester M. Inhibitory actions of ceramide upon PKC-epsilon/ERK interactions. Am J Physiol Cell Physiol. 2001:C1403–C1411. doi: 10.1152/ajpcell.2001.280.6.C1403. [DOI] [PubMed] [Google Scholar]
- 120.Sawai H, Okazaki T, Takeda Y, Tashima M, Sawada H, Okuma M, Kishi S, Umehara H, Domae N. Ceramide-induced translocation of protein kinase C-delta and -epsilon to the cytosol Implications in apoptosis. J Biol Chem. 1997;272:2452–2458. doi: 10.1074/jbc.272.4.2452. [DOI] [PubMed] [Google Scholar]
- 121.Kellerer M, Mushack J, Mischak H, Haring HU. Protein kinase C (PKC) epsilon enhances the inhibitory effect of TNF alpha on insulin signaling in HEK293 cells. FEBS Lett. 1997;418:119–122. doi: 10.1016/s0014-5793(97)01357-4. [DOI] [PubMed] [Google Scholar]
- 122.Schmitz-Peiffer C, Craig DL, Biden TJ. Ceramide generation is sufficient to account for the inhibition of the insulin-stimulated PKB pathway in C2C12 skeletal muscle cells pretreated with palmitate. J Biol Chem. 1999;274:24202–24210. doi: 10.1074/jbc.274.34.24202. [DOI] [PubMed] [Google Scholar]
- 123.Lin Y, Brady MJ, Wolanske K, Holbert R, Ruderman NB, Yaney GC. Alterations of nPKC distribution, but normal Akt/PKB activation in denervated rat soleus muscle. Am J Physiol Endocrinol Metab. 2002:E318–E325. doi: 10.1152/ajpendo.00390.2001. [DOI] [PubMed] [Google Scholar]
- 124.Samuel VT, Liu ZX, Qu X, Elder BD, Bilz S, Befroy D, Romanelli AJ, Shulman GI. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem. 2004;279:32345–32353. doi: 10.1074/jbc.M313478200. [DOI] [PubMed] [Google Scholar]
- 125.Neschen S, Morino K, Hammond LE, Zhang D, Liu ZX, Romanelli AJ, Cline GW, Pongratz RL, Zhang XM, Choi CS, Coleman RA, Shulman GI. Prevention of hepatic steatosis and hepatic insulin resistance in mitochondrial acyl-CoA:glycerol-sn-3-phosphate acyltransferase 1 knockout mice. Cell Metab. 2005;2:55–65. doi: 10.1016/j.cmet.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 126.Savage DB, Choi CS, Samuel VT, Liu ZX, Zhang D, Wang A, Zhang XM, Cline GW, Yu XX, Geisler JG, Bhanot S, Monia BP, Shulman GI. Reversal of diet-induced hepatic steatosis and hepatic insulin resistance by antisense oligonucleotide inhibitors of acetyl-CoA carboxylases 1 and 2. J Clin Invest. 2006 doi: 10.1172/JCI27300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Osada S, Mizuno K, Saido TC, Suzuki K, Kuroki T, Ohno S. A new member of the protein kinase C family, nPKC theta, predominantly expressed in skeletal muscle. Mol Cell Biol. 1992;12:3930–3938. doi: 10.1128/mcb.12.9.3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chalfant CE, Ciaraldi TP, Watson JE, Nikoulina S, Henry RR, Cooper DR. Protein kinase Ctheta expression is increased upon differentiation of human skeletal muscle cells: dysregulation in type 2 diabetic patients and a possible role for protein kinase Ctheta in insulin-stimulated glycogen synthase activity. Endocrinology. 2000;141:2773–2778. doi: 10.1210/endo.141.8.7591. [DOI] [PubMed] [Google Scholar]
- 129.Hilgenberg L, Miles K. 108 Developmental regulation of a protein kinase C isoform localized in the neuromuscular junction. J Cell Sci (Jan) 1995:51–61. doi: 10.1242/jcs.108.1.51. [DOI] [PubMed] [Google Scholar]
- 130.Cazzolli R, Craig DL, Biden TJ, Schmitz-Peiffer C. Inhibition of glycogen synthesis by fatty acid in C2C12 muscle cells is independent of PKC-alpha, -epsilon, and -theta. AJP—Endocrinol Metab. 2002;282:E1204–E1213. doi: 10.1152/ajpendo.00487.2001. [DOI] [PubMed] [Google Scholar]
- 131.Donnelly R, Qu X. Mechanisms of insulin resistance and new pharmacological approaches to metabolism and diabetic complications. Clin Exp Pharmacol Physiol. 1998;25:79–87. doi: 10.1111/j.1440-1681.1998.tb02181.x. [DOI] [PubMed] [Google Scholar]
- 132.Steiler TL, Galuska D, Leng Y, Chibalin AV, Gilbert M, Zierath JR. Effect of hyperglycemia on signal transduction in skeletal muscle from diabetic Goto–Kakizaki rats. Endocrinology. 2003;144:5259–5267. doi: 10.1210/en.2003-0447. [DOI] [PubMed] [Google Scholar]
- 133.Itani SI, Zhou Q, Pories WJ, MacDonald KG, Dohm GL. Involvement of protein kinase C in human skeletal muscle insulin resistance and obesity. Diabetes. 2000;49:1353–1358. doi: 10.2337/diabetes.49.8.1353. [DOI] [PubMed] [Google Scholar]
- 134.Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106:171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yu C, Chen Y, Cline GW, Zhang D, Zong H, Wang Y, Bergeron R, Kim JK, Cushman SW, Cooney GJ, Atcheson B, White MF, Kraegen EW, Shulman GI. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem. 2002;277:50230–50236. doi: 10.1074/jbc.M200958200. [DOI] [PubMed] [Google Scholar]
- 136.Kim JK, Fillmore JJ, Sunshine MJ, Albrecht B, Higashimori T, Kim DW, Liu ZX, Soos TJ, Cline GW, O’Brien WR, Littman DR, Shulman GI. PKC-theta knockout mice are protected from fat-induced insulin resistance. J Clin Invest. 2004;114:823–827. doi: 10.1172/JCI22230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Griffin ME, Marcucci MJ, Cline GW, Bell K, Barucci N, Lee D, Goodyear LJ, Kraegen EW, White MF, Shulman GI. Free fatty acid-induced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signaling cascade. Diabetes. 1999;48:1270–1274. doi: 10.2337/diabetes.48.6.1270. [DOI] [PubMed] [Google Scholar]
- 138.Gao Z, Zhang X, Zuberi A, Hwang D, Quon MJ, Lefevre M, Ye J. Inhibition of insulin sensitivity by free fatty acids requires activation of multiple serine kinases in 3T3-L1 adipocytes. Mol Endocrinol. 2004;18:2024–2034. doi: 10.1210/me.2003-0383. [DOI] [PubMed] [Google Scholar]
- 139.Jove M, Laguna JC, Vazquez-Carrera M. Agonist-induced activation releases peroxisome proliferator-activated receptor beta/delta from its inhibition by palmitate-induced nuclear factor-kappaB in skeletal muscle cells. Biochim Biophys Acta. 2005;1734:52–61. doi: 10.1016/j.bbalip.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 140.Talior I, Yarkoni M, Bashan N, Eldar-Finkelman H. Increased glucose uptake promotes oxidative stress and PKC-delta activation in adipocytes of obese, insulin-resistant mice. Am J Physiol Endocrinol Metab. 2003;285:E295–E302. doi: 10.1152/ajpendo.00044.2003. [DOI] [PubMed] [Google Scholar]
- 141.Lam TK, Yoshii H, Haber CA, Bogdanovic E, Lam L, Fantus IG, Giacca A. Free fatty acid-induced hepatic insulin resistance: a potential role for protein kinase C-delta. Am J Physiol Endocrinol Metab. 2002;283:E682–E691. doi: 10.1152/ajpendo.00038.2002. [DOI] [PubMed] [Google Scholar]
- 142.Tang EY, Parker PJ, Beattie J, Houslay MD. Diabetes induces selective alterations in the expression of protein kinase C isoforms in hepatocytes. FEBS Lett. 1993;326:117–123. doi: 10.1016/0014-5793(93)81774-t. [DOI] [PubMed] [Google Scholar]
- 143.Schmitz-Peiffer C, Browne CL, Oakes ND, Watkinson A, Chisholm DJ, Kraegen EW, Biden TJ. Alterations in the expression and cellular localization of protein kinase C isozymes epsilon and theta are associated with insulin resistance in skeletal muscle of the high-fat-fed rat. Diabetes. 1997;46:169–178. doi: 10.2337/diab.46.2.169. [DOI] [PubMed] [Google Scholar]
- 144.Ikeda Y, Olsen GS, Ziv E, Hansen LL, Busch AK, Hansen BF, Shafrir E, Mosthaf-Seedorf L. Cellular mechanism of nutritionally induced insulin resistance in Psammomys obesus: overexpression of protein kinase Cepsilon in skeletal muscle precedes the onset of hyperinsulinemia and hyperglycemia. Diabetes. 2001;50:584–592. doi: 10.2337/diabetes.50.3.584. [DOI] [PubMed] [Google Scholar]
- 145.Heled Y, Shapiro Y, Shani Y, Moran DS, Langzam L, Braiman L, Sampson SR, Meyerovitch J. Physical exercise enhances protein kinase C delta activity and insulin receptor tyrosine phosphorylation in diabetes-prone psammomys obesus. Metabolism. 2003;52:1028–1033. doi: 10.1016/s0026-0495(03)00154-9. [DOI] [PubMed] [Google Scholar]
- 146.Bluher M, Patti ME, Gesta S, Kahn BB, Kahn CR. Intrinsic heterogeneity in adipose tissue of fat-specific insulin receptor knock-out mice is associated with differences in patterns of gene expression. J Biol Chem. 2004;233:1891–1901. doi: 10.1074/jbc.M404569200. [DOI] [PubMed] [Google Scholar]
- 147.Lu Z, Liu D, Hornia A, Devonish W, Pagano M, Foster DA. Activation of protein kinase C triggers its ubiquitination and degradation. Mol Cell Biol. 1998;18:839–845. doi: 10.1128/mcb.18.2.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Avignon A, Standaert ML, Yamada K, Mischak H, Spencer B, Farese RV. Insulin increases mRNA levels of protein kinase C-alpha and -beta in rat adipocytes and protein kinase C-alpha, -beta and -theta in rat skeletal muscle. Biochem J. 1995;308:181–187. doi: 10.1042/bj3080181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ishizuka T, Kajita K, Yamada K, Miura A, Kanoh Y, Ishizawa M, Wada H, Itaya S, Yamamoto M, Yasuda K, Nagata K, Okano Y. Insulin regulated PKC isoform mRNA in rat adipocytes. Diabetes Res Clin Pract. 1996;33:159–167. doi: 10.1016/0168-8227(96)01324-1. [DOI] [PubMed] [Google Scholar]
- 150.Suh KS, Tatunchak TT, Crutchley JM, Edwards LE, Marin KG, Yuspa SH. Genomic structure and promoter analysis of PKC-delta. Genomics. 2003;82:57–67. doi: 10.1016/s0888-7543(03)00072-7. [DOI] [PubMed] [Google Scholar]
- 151.Horovitz-Fried M, Cooper DR, Patel NA, Cipok M, Brand C, Bak A, Inbar A, Jacob AI, Sampson SR. Insulin rapidly upregulates protein kinase Cdelta gene expression in skeletal muscle. Cell Signal. 2006;18:183–193. doi: 10.1016/j.cellsig.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 152.Patel NA, Apostolatos HS, Mebert K, Chalfant CE, Watson JE, Pillay TS, Sparks J, Cooper DR. Insulin regulates protein kinase CbetaII alternative splicing in multiple target tissues: development of a hormonally responsive heterologous minigene. Mol Endocrinol. 2004:899–911. doi: 10.1210/me.2003-0391. [DOI] [PubMed] [Google Scholar]
- 153.Patel NA, Kaneko S, Apostolatos HS, Bae SS, Watson JE, Davidowitz K, Chappell DS, Birnbaum MJ, Cheng JQ, Cooper DR. Molecular and genetic studies imply Akt-mediated signaling promotes protein kinase CbetaII alternative splicing via phosphorylation of serine/arginine-rich splicing factor SRp40. J Biol Chem. 2005;8:14302–14309. doi: 10.1074/jbc.M411485200. [DOI] [PubMed] [Google Scholar]
- 154.Kanzaki M, Mora S, Hwang JB, Saltiel AR, Pessin JE. Atypical protein kinase C (PKCzeta/lambda) is a convergent downstream target of the insulin-stimulated phosphatidylinositol 3-kinase and TC10 signaling pathways. J Cell Biol. 2004;19(164):279–290. doi: 10.1083/jcb.200306152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Aboulaich N, Vainonen JP, Stralfors P, Vener AV. Vectorial proteomics reveal targeting, phosphorylation and specific fragmentation of polymerase I and transcript release factor (PTRF) at the surface of caveolae in human adipocytes. Biochem J. 2004;383:237–248. doi: 10.1042/BJ20040647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.De Fea K, Roth RA. Protein kinase C modulation of insulin receptor substrate-1 tyrosine phosphorylation requires serine 612. Biochemistry. 1997;36:12939–12947. doi: 10.1021/bi971157f. [DOI] [PubMed] [Google Scholar]
- 157.Bollag GE, Roth RA, Beaudoin J, Mochly-Rosen D, Koshland DEJ. Protein kinase C directly phosphorylates the insulin receptor in vitro and reduces its protein-tyrosine kinase activity. Proc Natl Acad Sci USA. 1986;83:5822–5824. doi: 10.1073/pnas.83.16.5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Formisano P, Oriente F, Fiory F, Caruso M, Miele C, Maitan MA, Andreozzi F, Vigliotta G, Condorelli G, Beguinot F. Insulin-activated protein kinase Cbeta bypasses Ras and stimulates mitogen-activated protein kinase activity and cell proliferation in muscle cells. Mol Cell Biol. 2000;20:6323–6333. doi: 10.1128/mcb.20.17.6323-6333.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bandyopadhyay G, Standaert ML, Galloway L, Moscat J, Farese RV. Evidence for involvement of protein kinase C (PKC)-zeta and noninvolvement of diacylglycerol-sensitive PKCs in insulin-stimulated glucose transport in L6 myotubes. Endocrinology. 1997;138:4721–4731. doi: 10.1210/endo.138.11.5473. [DOI] [PubMed] [Google Scholar]
- 160.Wright DC, Fick CA, Olesen JB, Craig BW. Evidence for the involvement of a phospholipase C–protein kinase C signaling pathway in insulin stimulated glucose transport in skeletal muscle. Life Sci. 2003;73:61–71. doi: 10.1016/s0024-3205(03)00256-x. [DOI] [PubMed] [Google Scholar]
- 161.Chappell DS, Watson JE, Cooper DR. Co-localization of PKCII and Glut4 in rat vascular smooth muscle cells after insulin treatment (Abstract) [Google Scholar]
- 162.Chappell DS, Watson JE, Cooper DR. Involvement of PKCII, MARCKS and actin in insulin-regulated GLUT4 translocation and glucose uptake in L6 skeletal muscle cells (Abstract) [Google Scholar]
- 163.Donnelly R, Reed MJ, Azhar S, Reaven GM. 135 Expression of the major isoenzyme of protein kinase-C in skeletal muscle, nPKC theta, varies with muscle type and in response to fructose-induced insulin resistance. Endocrinology (Dec) 1994:2369–2374. doi: 10.1210/endo.135.6.7988419. [DOI] [PubMed] [Google Scholar]
- 164.Kotani K, Ogawa W, Matsumoto M, Kitamura T, Sakaue H, Hino Y, Miyake K, Sano W, Akimoto K, Ohno S, Kasuga M. Requirement of atypical protein kinase clambda for insulin stimulation of glucose uptake but not for Akt activation in 3T3-L1 adipocytes. Mol Cell Biol. 1998;18:6971–6982. doi: 10.1128/mcb.18.12.6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Cazzolli R, Carpenter L, Biden TJ, Schmitz-Peiffer C. A role for protein phosphatase 2A-like activity, but not atypical protein kinase Czeta, in the inhibition of protein kinase B/Akt and glycogen synthesis by palmitate. Diabetes. 2001:2210–2218. doi: 10.2337/diabetes.50.10.2210. [DOI] [PubMed] [Google Scholar]
- 166.Liu YF, Paz K, Herschkovitz A, Alt A, Tennenbaum T, Sampson SR, Ohba M, Kuroki T, LeRoith D, Zick Y. Insulin stimulates PKCzeta-mediated phosphorylation of insulin receptor substrate-1 (IRS-1). A self-attenuated mechanism to negatively regulate the function of IRS proteins. J Biol Chem. 2001;276:14459–14465. doi: 10.1074/jbc.M007281200. [DOI] [PubMed] [Google Scholar]
- 167.Standaert ML, Galloway L, Karnam P, Bandyopadhyay G, Moscat J, Farese RV. Protein kinase C-zeta as a downstream effector of phosphatidylinositol 3-kinase during insulin stimulation in rat adipocytes. Potential role in glucose transport. J Biol Chem. 1997;272:30075–30082. doi: 10.1074/jbc.272.48.30075. [DOI] [PubMed] [Google Scholar]