Abstract
Elevated TNFα levels are associated with insulin resistance, but the molecular mechanisms linking cytokine signaling to impaired insulin function remain elusive. We previously demonstrated a role for Akt in insulin regulation of PKCβII alternative splicing through phosphorylation of SRp40, a required mechanism for insulin-stimulated glucose uptake. We hypothesized that TNFα attenuated insulin signaling by dephosphorylating Akt and its targets via ceramide-activated protein phosphatase (CAPP). Western blot analysis of L6 cell lysates demonstrated impaired insulin–stimulated phosphorylation of Akt, SRp40, and GSK3β in response to TNFα and the short chain C6 ceramide analog. TNFα increased serine/threonine phosphatase activity of PP1 in response to C6, but not insulin, suggesting a ceramide-specific effect. Myriocin, an inhibitor of de novo ceramide synthesis, blocked stimulation of the PP1 activity. Ceramide species measurement by LC-MS showed consistent increases in C24:1 and C16 ceramides. Effects of TNFα and C6 on insulin–stimulated phosphorylation of GSK3β were prevented by myriocin and tautomycin, a PP1 inhibitor, further implicating a de novo ceramide-PP1 pathway. Alternative splicing assays demonstrated that TNFα abolished insulin-mediated inclusion of the PKCβII exon. Collectively, our work demonstrates a role for PP1-like CAPP in mediating TNFα effects blocking insulin phosphorylation cascades involved in glycogen metabolism and alternative splicing.
Keywords: TNFα, Ceramide activated protein phosphatase (CAPP), Akt, SR proteins, PKCβII, alternative splicing
Introduction
Several lines of evidence indicate that tumor necrosis factor alpha (TNFα1), a pro-inflammatory cytokine, plays a role in insulin resistance. TNFα expression is increased in insulin resistant individuals and infusing humans with TNFα induces insulin resistance (1–3). Most animal models of insulin resistance produce significantly higher levels of TNFα, and the TNFα knockout mouse demonstrated increased insulin sensitivity and improved lipid metabolism (2, 4). Furthermore, cells treated with TNFα also present with impaired insulin signaling: decreased insulin receptor tyrosine kinase activity, increased serine phosphorylation of insulin receptor substrate 1, decreased GLUT4 translocation and abolishment of insulin mediated Akt phosphorylation (4–7).
Ceramide, a sphingolipid second messenger, is also linked to insulin resistance (2). Ceramide is generated via hydrolysis of sphingomyelin by sphingomyelinase or by the de novo pathway via condensation of palmitoyl CoA and serine (8). Ceramide is elevated in insulin responsive tissues of diabetic animals (9) and inhibits a number of kinases that are stimulated by insulin, including protein kinase B (PKBβ/Akt) (10–13) and protein kinase C (7, 14, 15). It is also known to activate atypical PKCs (16, 17). TNFα activates both de novo and hydrolysis pathways of ceramide generation (18), and ceramide mimics the effects of TNFα on insulin signaling (19–22) and apoptosis (23). Furthermore, when ceramide production is suppressed, TNFα-induced insulin resistance is reversed (24). However, the molecular mechanisms defining ceramide-induced alterations in insulin signaling remain unclear. Ceramide-activated protein phosphatases (CAPPs), including PP1 and PP2A, are allosterically activated by ceramide (25, 26). CAPPs have been shown to inhibit Akt by dephosphorylation of serines (27) and to modulate key cellular processes including exocytosis, alternative pre-mRNA splicing and glycogen metabolism (28–30). Hence, we investigated whether a ceramide-activated protein phosphatase could act on Akt and subsequent downstream substrates including SR proteins involved in alternative splicing of PKCβII mRNA in skeletal muscle cells.
PKCβ is a component of the serine/threonine kinase superfamily with roles in insulin signaling related to glucose transport and glycogen metabolism (31–33). It is a conventional PKC with two splice variants, βI and βII, that have distinct cellular functions. Insulin regulates alternative splicing of the protein kinase CβII (PKCβ) pre-mRNA (34–36). The regulation occurs by the phosphorylation of serine/arginine “SR” rich proteins under the control of Akt (37). The regulation is impaired in insulin resistant states and diabetes (35).
This study was designed to define the molecular link between acute inflammatory cytokines, the activation of CAPPs, the ceramide species involved, phosphorylation status of Akt and SR proteins and TNFα effects on insulin stimulated PKCβII splicing. To investigate this, L6 rat myotubes were treated with TNFα and C6 ceramide, a short chain ceramide analogue, and examined for insulin dependent phosphorylation of Akt, SRp40, and GSK3β. Inhibition of de novo ceramide generation blocked PP1, but not PP2A activation by TNFα. C16 and C24:1 ceramides were the major species increased by TNFα, and there was a significant reduction in insulin-mediated PKCβII exon inclusion in cells treated with the cytokine. Collectively, the results suggested that TNFα caused alterations in insulin signaling in a ceramide-dependent mechanism by activating CAPPs, which resulted in Akt and SRp40 dephosphorylation and decreased PKCβII splicing.
Materials and Methods
Cell culture
L6 rat skeletal muscle cells (obtained from Dr. Amira Klip, Hospital for Sick Children, Toronto, Canada) were grown in α-minimum essential medium (α-MEM) supplemented with 10% fetal bovine serum (FBS) (Cellgro) to 50–60% confluency. Penicillin G and streptomycin sulfate (100U/100 μg) were added and the cells were incubated at 5% CO2, 37°C, and 95% humidity. Cells were induced to differentiate by changing medium to α-MEM supplemented with 2% FBS for approximately 8 days. The extent of cellular differentiation was established by observation of multi-nucleation of >70% of cells. For phosphatase assays, cells were placed in HEPES buffered saline (HeBS) with 0.1% bovine serum albumin (BSA) prior to treatments. For western blot analysis, cells remained in α-MEM. TNFα was added to a final concentration of 15–150 ng/ml for 30 minutes. C6 ceramide (20 μM) was added for 2 hours. Insulin was added at a final concentration of 10 nM for 30 minutes. Myriocin (5 nM), an inhibitor of de novo ceramide synthesis, was added for 1 hour prior to TNFα treatment. Tautomycin (10 nM), an inhibitor of PP1, was added 1 hour prior to TNFα treatment.
Immunoprecipitation
Culture dishes with differentiated cells (100 mm) were washed with Ca2+/Mg2+-free PBS and cells mechanically detached in buffer (Tris HCl pH 7.4 50mM; NaCl, 150 mM; EDTA 1 mM; NaF, 10 mM; Triton X 100, 1% SDS, 0.1%; Na deoxycholate, 1%) with a cocktail of antiproteases (Leupeptin, 20 μg/ml; aprotinin, 10 μg/ml; PMSF, 0.1 mM; DTT, 1 mM). The lysate was centrifuged at 17,000 x g for 20 min at 4°C. The supernatant (0.5 ml) was used for immunoprecipitation by adding 25 μl A/G sepharose, rotating the suspension 30 min at 4°C. SRp40 antibody was then added and samples rotated overnight at 4°C. The suspension was centrifuged at 2000 x g for 1 min and the pellet washed 2x in Tris-buffered saline with Tween (TBST). After addition of Laemmli buffer, samples were separated on SDS-PAGE (38). Proteins were detected using anti-rabbit or anti-mouse IgG antibodies conjugated to horseradish peroxidase and enhanced chemiluminescense reagents. Controls include protein A and IgG (pre-immune sera).
Western blotting
Culture dishes with differentiated cells were washed with Ca2+/Mg2+-free PBS and cells mechanically detached in buffer (Tris HCL pH 7.4 50 mM; NaCl, 150 mM; EDTA 1 mM; NaF, 10 mM; Triton X 100, 1%; SDS, 0.1%; Na deoxycholate, 1%) with a coctail of antiproteases (leupeptin, 20 μg/ml; aprotinin, 10 μg/ml; PMSF, 0.1 mM; DTT, 1mM). The lysate was centrifuged at 17,000 x g for 20 min at 4°C. Cell lysates in Laemmli buffer (50 μg) were separated via 12.5% SDS-polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were electrophoretically transferred to nitrocellulose membranes and blocked with phosphate buffered saline with 0.1% Tween 20 and 4% non-fat dried milk. Membranes were washed and incubated with the following antibodies: phospho-Akt (serine 473, Cell Signaling); phospho-GSK3β (serine 9, Cell Signaling); Mab104, a monoclonal antibody against the phosphoepitope (RS domain) of SR proteins; phospho-Akt substrate, detects phosphorylation of Akt substrates at their Akt motifs (R-X-R-X-X-S/T), SRp40, phospho-IRS-1 (serine 616, Cell Signaling) or IRS-1 (Cell Signaling). Equal loading or identification of SR proteins was verified by stripping and reprobing membranes with β-actin or SRp40. Following incubation with secondary anti-rabbit or anti-mouse IgG-HRP, detection was performed using enhanced chemiluminesence (Pierce). Results were verified in 2–6 separate experiments.
Phosphatase assays
Cells washed 3 times in ice-cold HeBS were scraped into 1ml lysis buffer (50 mmol/l Tris, pH7.4, 150 mmol/l NaCl, 1 mmol/l PMSF, 10 μg/ml aprotinin, and 10 μg/ml leupeptin) and sonicated. Lysates were centrifuged at 400,000 × g for 10 min at 4°C. Up to 250 μl of supernatants were passed through 10 ml Sephadex G-25 columns to eliminate ATP and free phosphate. Serine/threonine phosphatase activities in these fractions were determined by measuring generation of free phosphate from phosphopeptide RRA(pT)VA using the molybdate-malachite green-phosphate complex assay (Promega). The peptide substrate was used in the absence of Ca2+ and Mg2+, which are required for the activity of PP2B and 2C. Assays were carried out using 10 μl of lysate in 10 μl of a Ca2+ and Mg2+-deficient reaction buffer (final concentration 50 mmol/l imidazole [pH 7.2], 0.2 mmol/l EGTA, 0.02% 2-mercaptoethanol, 0.1 mg/ml BSA, and 100 μmol/l phosphopeptide substrate), 5 μl phosphopeptide and 25 ul phosphate free water with the addition of either okadaic acid (20 nm or 0.2 nm) to distinguish between PP1 and PP2A activities. Reactions were carried out for 15 minutes in a 37°C shaker water bath. To stop the reaction, molybdate dye was added and free phosphate measured by OD at 630 nm. A standard curve was generated to determine free phosphate concentrations. Results were corrected for total protein in cell fractions by the Bradford method using a BSA standard curve and specific activity was calculated. Experiments were repeated a minimum of 2 times and reactions were carried out in duplicate or triplicate. Buffer plus peptide was used as a blank control.
Ceramide mass spectrometry
Cells were treated, and collected for phosphatase assay and approximately ½ of the total cell pellet was reserved for ceramide mass-spec analysis. The pellet was stored at −80°C. Ceramide molecular species analyses were performed by the Lipidomics Core Facility at the Medical University of South Carolina. Briefly, cell pellets were extracted into a one-phase neutral organic solvent system and analyzed by a Surveyor/TSQ 7000 LC/MS system. Qualitative analysis of ceramides was performed by a Parent Ion scan of a common fragment ion characteristic for a particular class of ceramides (39). The results are presented as mean ± SD of picomoles of ceramide species per μg of protein in the sample.
Alternative splicing of PKCβII
L6 cells were plated at 40–50% confluency in 6-well plates and differentiation to myotubes was initiated for 24 hrs prior to transfection of the PKCβII reporter minigene (2μg) mixed with 10μl of Lipofectamine™ (Invitrogen) reagent for 4 hours (35). After 36 hrs, fused myotubes (>70%) were pre-treated for 30 min with TNFα or inhibitors then insulin was added for 30 min. The minigene was evaluated for exon inclusion as follows. Total RNA was obtained using Trizol™ reagent (Invitrogen). One μg (total RNA) was reverse transcribed using oligo dT and the Superscript II™ preamplification kit. 1μl of the RT reaction was amplified using SD and SA specific primers and Platinum Taq™ DNA polymerase. After 25 cycles (95° 30 sec melt and 68°C 1 min anneal/extension), the PCR reaction is examined on PAGE followed by silver staining. As a control, β-actin was amplified (35).
Results
TNFα and Ceramide Altered Insulin-Dependent Phosphorylation Cascades
The activation of Akt via PI3-kinase is a central step in insulin action. Data from our lab has demonstrated that SRp40 is a direct target for phosphorylation and activation by Akt (37). SRp40 phosphorylation by PI3-kinase dependent pathways is required for the splicing of PKCβII in insulin-dependent cells (35, 36). It has not been demonstrated whether TNFα via de novo ceramide synthesis activates a serine/threonine protein phosphatase activity that targets Akt and SRp40. This could represent another mechanism for TNFα to attenuate insulin action in skeletal muscle in addition to other known pathways that inhibit IRS-1 function (40). To examine the potential role of the ceramide pathway, L6 myotubes were pre-treated for 30 minutes with TNFα or the short chain ceramide analogue, D-e-C6 ceramide, and the phosphorylation states of Akt and SRp40 were analyzed by Western immunoblot. Insulin treatment increased Akt phosphorylation on Serine 473 while TNFα pre-treatment attenuated the action of insulin and Akt was less phosphorylated. C6 ceramide mimicked the effects of TNFα but had a much more potent effect in attenuating insulin effects (Figure 1a). Similar results were obtained when mAb104 was used to detect phosphorylation at the RS domain of SRp40 immunoprecipitated from L6 myotubes (Figure 1b). Since SRp40 is also an Akt substrate (37), insulin regulated phosphorylation patterns at the Akt substrate motifs of SRp40 were also analyzed in L6 myotubes (Figure 1c). TNFα and C6 ceramide reduced insulin stimulated phosphorylation of SRp40 at its Akt motifs. This effect was not observed with dihydro-C6 ceramide, an inactive ceramide analog, suggesting that the ceramide effects are not due to non-specific effects of lipid treatment. Therefore, both ceramide and TNFα treatment resulted in the decreased phosphorylation of Akt and SRp40.
Figure 1. TNFα and C6 ceramide blocked insulin-mediated phosphorylation of Akt and SRp40.
(a) L6 cells were pre-treated with TNFα (150ng/ml, 30 minutes) or C6 ceramide (20uM, 2 hours) prior to insulin (I) (10nM, 30 minutes) addition as indicated. Western blot analysis was conducted with phospho-Akt antibody (serine 473). The membrane was stripped and re-probed with β-actin to ensure equal protein loading. (b) L6 cells underwent similar treatments as above except that TNFα was 15 ng/ml. Lysates were immunoprecipitated with SRp40 antibody, proteins separated and membranes were probed first with Mab104 followed by SRp40 antibody. (c) L6 cells were treated as above with the addition of a dihydroC6 treatment (20uM, 2 hours) or as a pre-treatment followed by insulin as a control. Membrane was probed with phosphoAkt substrate antibody, stripped and re-probed with SRp40 antibody to confirm identity of the protein. Each experiment was repeated a minimum of three times with similar results. The bar graphs summarize results from 3 experiments. The asterisk indicates a significant difference (p < 0.05, t-test compared to insulin effect.)
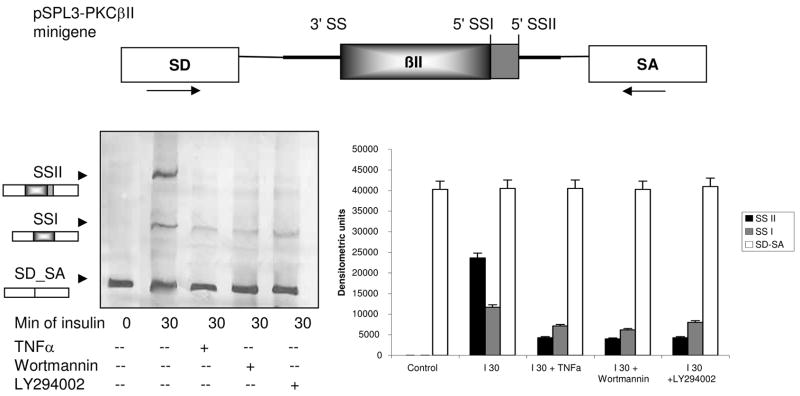
TNFα Blocked PKCβII Exon Inclusion
Previous work in our lab has demonstrated that phosphorylated SRp40 is required for PKCβII exon inclusion (36), and insulin is responsible for this phosphorylation via Akt activity. To test the functional relevance of TNFα-mediated decrease in phosphorylation of Akt and SRp40, alterations in insulin induced PKCβII exon inclusion were assessed utilizing a heterologous splicing minigene. Our data demonstrate that insulin promoted inclusion of the PKCβII exon. Two splice sites were activated. Activation of splice site II (SSII) was blocked by TNFα and the PI3Kinase inhibitors. Splice site I usage was partially blocked which may reflect the stronger role of SRp40 on splice site II selection (35). Importantly, TNFα pre-treatment blocked insulin effects on PKCβII SSII activation to the same extent as blocking Akt activation via the PI3K/PDK pathway with LY294002 or wortmannin (Figure 2).
Figure 2. TNFα blocked insulin induced PKCβII exon inclusion.
A heterologous minigene constructed by inserting the PKCβII genomic fragment into a multi-cloning site between the splice site donor (SD) and splice site acceptor (SA) in pSPL3 vector was used as a reporter for alternative splicing as described (35). PCR primers corresponding to the arrows in the upper diagram were used to detect insulin-mediated inclusion of the PKCβII exon at both 5′ splice sites within 30 minutes; both splice variants encode PKCβII. Insulin effects were blocked by TNFα (15 ng/ml) similar to the two PI3Kinase/Akt inhibitors: Wortmannin (100 nM) and LY294002 (10 μM). The densitometric scan summarizes of three separate experiments.
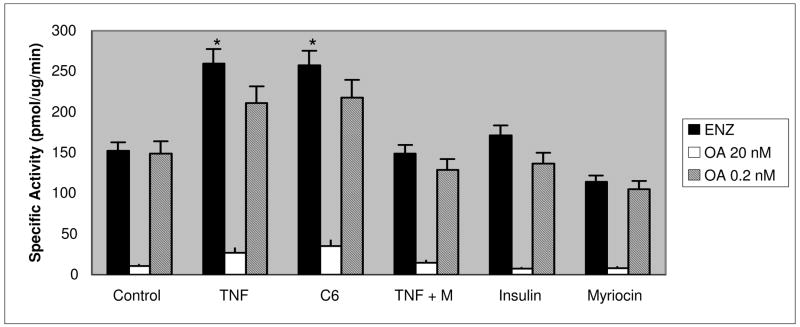
TNFα Increased Ceramide Activated Phosphatase Activity in Skeletal Muscle Cells
TNFα increases ceramide production via the de novo and sphingomyelinase pathways (2, 18). PP1 and PP2A are ceramide-activated protein phosphatases (CAPPs) (25). Although increases in ceramides are associated with insulin resistance (10, 19–21, 24), the activation of serine/threonine protein phosphatases in skeletal muscle by cytokines has not been examined. To further determine if the aberrant phosphorylation patterns noted in the immunoblot analyses above were consistent with ceramide-activated protein phosphatase activity, we examined L6 myotubes for protein phosphatase activity following TNFα, insulin, and C6 ceramide treatment along with an inhibitor of de novo ceramide synthesis. Short term TNFα treatment increased protein phosphatase specific activity approximately 2-fold (p < 0.05, Student’s t-test). C6-ceramide treatment mimicked this within 2 hours, while insulin treatment showed no increase in protein phosphatase activity. To distinguish between PP1-type and PP2A-type activities, okadaic acid was added to the phosphatase assay reaction with concentrations that inhibit both (20 nM) or PP2A specifically (0.2 nM). In L6 cells, high dose okadaic acid led to nearly complete abolishment of phosphatase activity, while the lower dose led to little or no inhibition indicating that the predominant phosphatase activated by TNFα and C6 ceramide was PP1 (p < 0.05) (Figure 3). Exposure of L6 cells to myriocin, an inhibitor of serine palmitoyltransferase in the de novo pathway (41), significantly suppressed activation of the phosphatase activity induced by TNFα to levels comparable to controls in L6 cells suggesting that TNFα activated de novo ceramide synthesis which, in turn activated PP1 activity in L6 cells.
Figure 3. TNFα and C6 ceramide stimulated a PP1-like serine/threonine phosphatase activity.
L6 cells were treated with TNFα (15 or 150 ng/nl) for 30 minutes, C6 ceramide (20 μM) for 2 hours insulin (10 nM) for 30 minutes or myriocin (M) (5nM) for 30 minutes. In the phosphatase assay wells, samples were treated with no inhibitor, okadaic acid 20 nM or okadaic acid 0.2 nM. Free phosphate was measured by optical density at 630 nm. Results are mean ±SEM of specific activity from 2–6 separate experiments assayed in duplicate. The asterisk indicates a significant difference from control (p < 0.05, t-test).
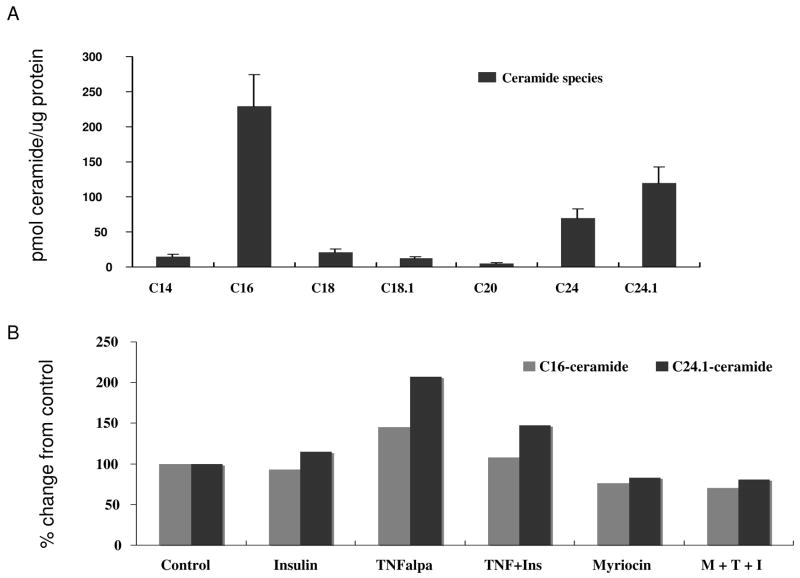
TNFα increased ceramide levels via the de novo sphingolipid pathway in L6 cells
To determine if intracellular ceramide levels were increased as a result of treatment with TNFα, cell pellets were assayed for ceramide content by liquid chromatography-mass spectrometery. In L6 cells, C16 and C24:1 ceramides were the predominant species detected (Figure 4A). Both species were increased by TNFα treatment, but only elevations of C24:1 ceramide (35–40%) were noted in the presence of insulin. This increase was blunted by pre-treatment with myriocin. Insulin treatment alone did not significantly increase C24:1 ceramide levels (Figure 4B). The levels of ceramide noted correspond to the decrease in protein phosphorylation noted in Figure 1 and increase in protein phosphatase activity.
Figure 4. TNFα stimulated de novo synthesis of ceramide.
(A) L6 cell pellets were analyzed via liquid chromatography-mass spectrometry to determine all the known cellular ceramide species. The predominant species detected in 6 different experiments were C16 and C24:1 ceramide. (B) The change from control for C16 and C24:1 ceramide following TNFα (T) pretreatment followed by insulin (I) or in the presence of myriocin (M) as determined by LC-MS. Shown is the mean of 2 representative experiments.
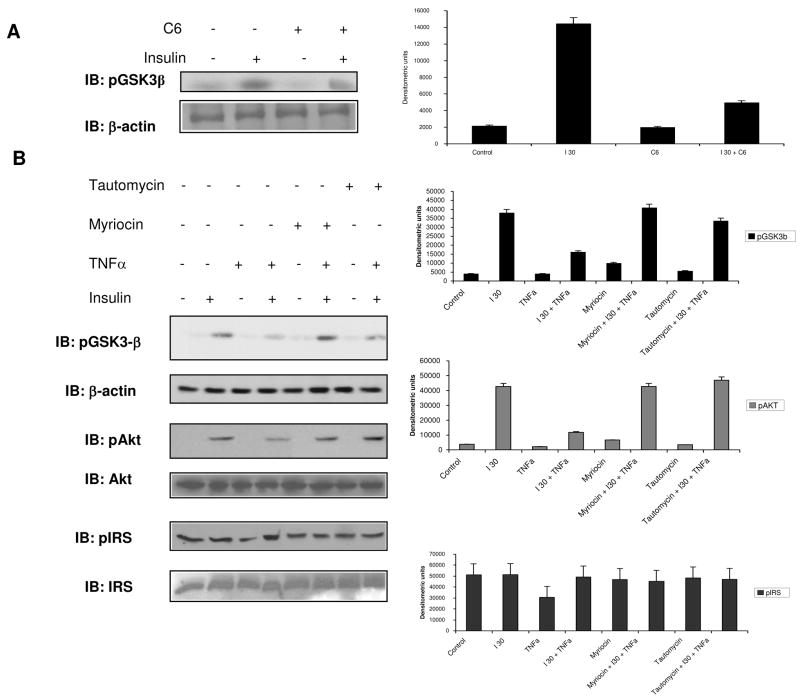
Ceramide Impaired Phosphorylation of Glycogen Synthase Kinase 3-β and Akt
In order to further characterize the mechanisms of TNFα-induced insulin resistance, we examined L6 cells for phosphorylation/dephosphorylation of glycogen synthase kinase 3-β (GSK3β). GSK3β is also an Akt substrate, which would make it a potential target of CAPPS activated by TNFα. As expected, insulin increased the phosphorylation of GSK3β and C6 ceramide attenuated this (Figure 5A). The attunation was not as great as noted with Akt in Figure 1, and this may be due to the subcellular partitioning of C6 and its ability to interact with phosphatases. The phosphorylation of GSK3β is an inactivating phosphorylation that leads to downstream glycogen production. When phosphoGSK3β was evaluated in the presence of TNFα, insulin’s effect on phosphorylation was blunted. When de novo ceramide synthesis was blocked by myriocin or when PP1 was inhibited by tautomycin, TNFα inhibition of insulin stimulated GSK3β phosphorylation was largely reversed (Figure 5B). The effect of C6 ceramide was more robust than TNFα in this case and may reflect the short time course used in these experiments to demonstrate activation of CAPPs. A similar reversal of TNFα activity was also noted on pAkt by myriocin and tautomycin (Figure 5B). IRS-1 phosphorylation was not significantly increased by TNFα and neither myriocin nor tautomycin treatment had any effect suggesting IRS-1 phosphorylation associated with insulin resistance was not occurring in this scenario (42, 43).
Figure 5. Ceramide Activated Protein Phosphatase inhibits GSK3β phosphorylation and behaves in a de novo ceramide-dependent, PP1 manner.
(A) L6 cells were treated as previously described. Membrane was probed with phospho-GSK3β antibody (serine 9) and reprobed with β-actin to ensure equal loading. (B) L6 cells were treated with myriocin (50 nM) for 1 hour, myriocin 1 hour then TNFα (150 ng/ml) for 30 minutes, tautomycin (10 nM) for 1 hour, or tautomycin for 1 hour then TNFα for 30 minutes prior to insulin (10 nM) for 30 minutes. Membrane was probed with phospho-GSK3β antibody before re-probing with phospho-Akt and β actin antibodies. A separate membrane was probed or phospho-IRS and IRS. Densitometric scans summarize data from three separate experiments.
Discussion
There is an abundance of correlative evidence indicating that TNFα causes insulin resistance. The molecular mechanisms are not well defined since TNFα has multiple effects on the insulin receptor and IRS-1 phosphorylation (4, 6, 43, 44). It also alters the IKK/NF-kB/I-kB complex (45). There are also reports of impaired PP1, glycogen synthase activity, and insulin-stimulated glucose uptake in L6 cells following TNFα treatment (46, 47). The molecular details of these pathways have not been demonstrated. The current studies expand the pathways involved in TNFα signaling to CAPPs, which, in this case, are PP1-like in nature.
We first investigated the effects of TNFα and ceramide on the insulin-induced phosphorylation state of Akt, a central controller of insulin action, and SRp40, an Akt2 target important for insulin regulation of alternative splicing of the PKCβ gene. Ceramide analogues have been shown to diminish Akt phosphorylation in brown adipocytes and skeletal muscle (12, 48). However, whether this was due to a serine/threonine protein phosphatase activity was not shown, nor were the increases in endogenous ceramide species. The effects of C6 ceramide treatment on Akt phosphorylation strongly suggest that its change in phosphorylation might be due to a protein phosphatase activity. To evaluate this, the substrate system was extended to other potential targets in the insulin signaling cascade, SRp40, also a substrate for Akt. Its phosphorylation was evaluated using the monoclonal antibody, mAb 104, which recognizes phosphoepitopes in “RS”, arginine-serine domains (49) as well as with the Akt substrate antibody. TNFα and C6 ceramide modulated insulin effects on SRp40 phosphorylation. C6 ceramide pretreatment attenuated insulin’s effect on Akt, SRp40, and GSK3β phosphorylation.
We linked TNFα’s effects in skeletal muscle to PKCβII alternative splicing since aberrant mRNA splicing is associated with many disease states including type2 diabetes (37). PKCβII splicing is regulated by insulin via PI3-kinase/Akt phosphorylation of SRp40 (35–37). TNFα abolished insulin-stimulated PKCβII exon inclusion in L6 myotubes. This is consistent with the inhibition of Akt activation and decreased SRp40 phosphorylation anticipated by LY294002 (36).
We characterized the protein phosphatase activity that TNFα stimulated presuming that it could be modified by ceramides. Previous studies suggested that protein phosphatases could be mediators of TNFα induced insulin resistance (47), and TNFα is known to be increased in skeletal muscle of diabetic subjects (50). Earlier studies identified a PP2A activity and showed that ceramide analogues mimicked the TNFα effect on MAPK inhibition and PP2A activation (46, 47). The phosphatase substrate presented to the enzyme in this study is different and thought to be more specific for PP2A, however, using differential doses of okadaic acid to distinguish between the two activities in the assay, it is clear that a PP1 activity was modified by TNFα in a manner consistent with CAPPS activation. Recently, a PP2A activity was found to dephosphorylate Akt (48, 51). However, ceramides allosterically activate both PP1 and PP2A (25, 28, 52). In the present case, a PP1-type of activity seems most likely since no inhibitory effects on CAPPS were observed with insulin but TNFα activation was blocked with myriocin treatment, suggesting a specific de novo ceramide pathway distinct from other phosphatases reported. The inhibition of alternative splicing via TNFα also supports our belief that a PP1 activity is relevant since PP1, not PP2A, has been shown to be involved in alternative splicing (53, 54). Nuclear inhibitor of PP1 (NIPP1) a major nuclear PP1-binding protein, co-localizes with splicing factors in the nucleus, and only PP1 activity has been shown to dephosphorylate SR proteins (28).
We were interested in determining the specific ceramide species responsible for the insulin resistant state induced by TNFα. TNFα treatment was associated with an increase in C16 and C24:1 ceramides, the major species detected in L6 myotubes, suggesting that these species regulated the PP1 activity. However, only C24:1 ceramide levels but not C16 ceramide levels remained elevated in the presence of insulin. Insulin resistance is induced via de novo ceramide synthesis by treating C2C12 skeletal muscle and primary rat islets cells with palmitate (10, 55), however, the species was not identified. In addition, treating 3T3-L1 adipocytes with both C2 and C6 ceramide analogs, causes increases in various markers of deficient insulin signaling such as insulin stimulated glucose uptake (56). These studies do not rule out the additional involvement of the sphingomyelinase pathway since this also increases ceramide levels and it has been demonstrated that TNFα inhibits insulin signaling in 3T3L1 adipocytes and myeloid 32D cells via activation of sphingomyelinase (19).
Previous work in L6 myotubes demonstrates that insulin increases a PP1 activity while concomitantly decreasing PP2A (57, 58). The difference noted may be explained by evidence of combinatorial control among serine/threonine phosphatases that confers functional specificity (30). It is not clear specifically which PP1 heterodimer is activated by insulin and whether this PP1 is ceramide-activated (46). Furthermore, the allosteric modulation of PP1 by ceramide may change its substrate specificity, subcellular localization, and metabolic consequences.
Finally, the ability of tautomycin and myriocin to inhibit a PP1 phosphatase and ceramide synthesis, respectively, clearly reversed the phosphorylation state of GSK3β and Akt. It could be argued that TNFα induced phosphorylation of IRS-1 at Ser 312 via PKCζ activation could be the cause for inhibition of Akt phosphorylation (59), and this possibility is not ruled out. However, we saw no effect of TNFα or myriocin and tautomycin on IRS-1 Ser 616, another phosphorylation site associated with the development of peripheral insulin resistance (42) suggesting that this sight is not a target for CAPP or TNFα phosphorylation and the effect described here is different..
In summary, this study has identified an additional molecular mechanism for cellular TNFα-induced insulin resistance which involves the de novo generation of ceramide that activates a PP1 activity capable of dephosphorylating both kinases and splicing factors. Although further characterization of the phosphatase binding protein are in order, this study has identified the species of ceramides increased by TNFα and an additional signaling pathway relevant to the cytokine in skeletal muscle cells which explains some of the actions of TNFα in insulin resistance..
Acknowledgments
This work was supported by NIH NIDDK 54393 (D.R.C.) and the Department of Veteran’s Affairs Medical Research Service (D.R.C. and N.A.P.). We thank Karen D. Corbin for assistance with experiments and manuscript preparation.
Support: This work was supported by NIH NIDDK 54393 (D.R.C.) and the Department of Veteran’s Affairs Medical Research Service (D.R.C. and N.A.P.).
Footnotes
DISCLOSURE STATEMENT: The authors have nothing to disclose.
NIH Statement: “This is an un-copyedited author manuscript copyrighted by the The Endocrine Society. This may not be duplicated or reproduced, other than for personal use or within the rule of “Fair Use of Copyrighted Materials” (section 107, Title 17, U.S. Code) without permission of the copyright owner, The Endocrine Society. From the time of acceptance following peer review, the full text of this manuscript is made freely available by The Endocrine Society at http:/www.endojournals.org/. The final copy edited article can be found at http:/www.endojournals.org/. The endocrine Society disclaims any responsibility or liability for errors or omissions in this version of the manuscipt or in any version derived from it by the N.I.H. or other parties. The citation of this article must include the following information: author(s), article title, journal title, year of publication and DOI.”
Abbreviations Used: CAPP, ceramide activated protein phosphatase; GSK3β, glycogen synthase kinase 3β; PI3 Kinase, phosphatidylinositol 3-kinase; PKC, protein kinase C; SR, serine/arginine rich; SRp40, serine/arginine rich protein 40; PP1, protein phosphatase 1; PP2A, protein phosphatase 2A, TNFα, tumor necrosis factor α.
References
- 1.Xu H, Uysal KT, Becherer JD, Arner P, Hotamisligil GS. Altered tumor necrosis factor-alpha (TNF-alpha) processing in adipocytes and increased expression of transmembrane TNF-alpha in obesity. Diabetes. 2002;51:1876–83. doi: 10.2337/diabetes.51.6.1876. [DOI] [PubMed] [Google Scholar]
- 2.Borst SE. The role of TNF-alpha in insulin resistance. Endocrine. 2004;23:177–82. doi: 10.1385/ENDO:23:2-3:177. [DOI] [PubMed] [Google Scholar]
- 3.Moller DE. Potential role of TNF-alpha in the pathogenesis of insulin resistance and type 2 diabetes. Trends Endocrinol Metab. 2000;11:212–7. doi: 10.1016/s1043-2760(00)00272-1. [DOI] [PubMed] [Google Scholar]
- 4.Hotamisligil GS. The role of TNFalpha and TNF receptors in obesity and insulin resistance. J Intern Med. 1999;245:621–5. doi: 10.1046/j.1365-2796.1999.00490.x. [DOI] [PubMed] [Google Scholar]
- 5.Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science. 1996;271:665–8. doi: 10.1126/science.271.5249.665. [DOI] [PubMed] [Google Scholar]
- 6.Peraldi P, Hotamisligil GS, Buurman WA, White MF, Spiegelman BM. Tumor necrosis factor (TNF)-alpha inhibits insulin signaling through stimulation of the p55 TNF receptor and activation of sphingomyelinase. J Biol Chem. 1996;271:13018–22. doi: 10.1074/jbc.271.22.13018. [DOI] [PubMed] [Google Scholar]
- 7.Rosenzweig T, Braiman L, Bak A, Alt A, Kuroki T, Sampson SR. Differential effects of tumor necrosis factor-alpha on protein kinase C isoforms alpha and delta mediate inhibition of insulin receptor signaling. Diabetes. 2002;51:1921–30. doi: 10.2337/diabetes.51.6.1921. [DOI] [PubMed] [Google Scholar]
- 8.Perry DK, Hannun YA. The role of ceramide in cell signaling. Biochim Biophys Acta. 1998;1436:233–43. doi: 10.1016/s0005-2760(98)00145-3. [DOI] [PubMed] [Google Scholar]
- 9.Hegarty BD, Furler SM, Ye J, Cooney GJ, Kraegen EW. The role of intramuscular lipid in insulin resistance. Acta Physiol Scand. 2003;178:373–83. doi: 10.1046/j.1365-201X.2003.01162.x. [DOI] [PubMed] [Google Scholar]
- 10.Schmitz-Peiffer C, Craig DL, Biden TJ. Ceramide generation is sufficient to account for the inhibition of the insulin-stimulated PKB pathway in C2C12 skeletal muscle cells pretreated with palmitate. J Biol Chem. 1999;274:24202–10. doi: 10.1074/jbc.274.34.24202. [DOI] [PubMed] [Google Scholar]
- 11.Straczkowski M, Kowalska I, Nikolajuk A, Dzienis-Straczkowska S, Kinalska I, Baranowski M, Zendzian-Piotrowska M, Brzezinska Z, Gorski J. Relationship between insulin sensitivity and sphingomyelin signaling pathway in human skeletal muscle. Diabetes. 2004;53:1215–21. doi: 10.2337/diabetes.53.5.1215. [DOI] [PubMed] [Google Scholar]
- 12.Stratford S, Hoehn KL, Liu F, Summers SA. Regulation of insulin action by ceramide: dual mechanisms linking ceramide accumulation to the inhibition of Akt/protein kinase B. J Biol Chem. 2004;279:36608–15. doi: 10.1074/jbc.M406499200. [DOI] [PubMed] [Google Scholar]
- 13.Summers SA, Garza LA, Zhou H, Birnbaum MJ. Regulation of insulin-stimulated glucose transporter GLUT4 translocation and Akt kinase activity by ceramide. Mol Cell Biol. 1998;18:5457–64. doi: 10.1128/mcb.18.9.5457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kajimoto T, Shirai Y, Sakai N, Yamamoto T, Matsuzaki H, Kikkawa U, Saito N. Ceramide-induced apoptosis by translocation, phosphorylation, and activation of protein kinase Cdelta in the Golgi complex. J Biol Chem. 2004;279:12668–76. doi: 10.1074/jbc.M312350200. [DOI] [PubMed] [Google Scholar]
- 15.Becker KP, Kitatani K, Idkowiak-Baldys J, Bielawski J, Hannun YA. Selective inhibition of juxtanuclear translocation of protein kinase C betaII by a negative feedback mechanism involving ceramide formed from the salvage pathway. J Biol Chem. 2005;280:2606–12. doi: 10.1074/jbc.M409066200. [DOI] [PubMed] [Google Scholar]
- 16.Kajita K, Mune T, Kanoh Y, Natsume Y, Ishizawa M, Kawai Y, Yasuda K, Sugiyama C, Ishizuka T. TNFalpha reduces the expression of peroxisome proliferator-activated receptor gamma (PPARgamma) via the production of ceramide and activation of atypical PKC. Diabetes Res Clin Pract. 2004;66 Suppl 1:S79–83. doi: 10.1016/j.diabres.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 17.Wang G, Silva J, Krishnamurthy K, Tran E, Condie BG, Bieberich E. Direct binding to ceramide activates protein kinase Czeta before the formation of a pro-apoptotic complex with PAR-4 in differentiating stem cells. J Biol Chem. 2005;280:26415–24. doi: 10.1074/jbc.M501492200. [DOI] [PubMed] [Google Scholar]
- 18.Dbaibo GS, El-Assaad W, Krikorian A, Liu B, Diab K, Idriss NZ, El-Sabban M, Driscoll TA, Perry DK, Hannun YA. Ceramide generation by two distinct pathways in tumor necrosis factor alpha-induced cell death. FEBS Lett. 2001;503:7–12. doi: 10.1016/s0014-5793(01)02625-4. [DOI] [PubMed] [Google Scholar]
- 19.Kanety H, Hemi R, Papa MZ, Karasik A. Sphingomyelinase and ceramide suppress insulin-induced tyrosine phosphorylation of the insulin receptor substrate-1. J Biol Chem. 1996;271:9895–7. doi: 10.1074/jbc.271.17.9895. [DOI] [PubMed] [Google Scholar]
- 20.Long SD, Pekala PH. Lipid mediators of insulin resistance: ceramide signalling down-regulates GLUT4 gene transcription in 3T3-L1 adipocytes. Biochem J. 1996;319(Pt 1):179–84. doi: 10.1042/bj3190179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelpe CL, Moore PC, Parazzoli SD, Wicksteed B, Rhodes CJ, Poitout V. Palmitate inhibition of insulin gene expression is mediated at the transcriptional level via ceramide synthesis. J Biol Chem. 2003;278:30015–21. doi: 10.1074/jbc.M302548200. [DOI] [PubMed] [Google Scholar]
- 22.Wang CN, O’Brien L, Brindley DN. Effects of cell-permeable ceramides and tumor necrosis factor-alpha on insulin signaling and glucose uptake in 3T3-L1 adipocytes. Diabetes. 1998;47:24–31. doi: 10.2337/diab.47.1.24. [DOI] [PubMed] [Google Scholar]
- 23.Kolesnick R, Hannun YA. Ceramide and apoptosis. Trends Biochem Sci. 1999;24:224–5. doi: 10.1016/s0968-0004(99)01408-5. author reply 227. [DOI] [PubMed] [Google Scholar]
- 24.Grigsby RJ, Dobrowsky RT. Inhibition of ceramide production reverses TNF-induced insulin resistance. Biochem Biophys Res Commun. 2001;287:1121–4. doi: 10.1006/bbrc.2001.5694. [DOI] [PubMed] [Google Scholar]
- 25.Chalfant CE, Kishikawa K, Mumby MC, Kamibayashi C, Bielawska A, Hannun YA. Long chain ceramides activate protein phosphatase-1 and protein phosphatase-2A. Activation is stereospecific and regulated by phosphatidic acid. J Biol Chem. 1999;274:20313–7. doi: 10.1074/jbc.274.29.20313. [DOI] [PubMed] [Google Scholar]
- 26.Wolff RA, Dobrowsky RT, Bielawska A, Obeid LM, Hannun YA. Role of ceramide-activated protein phosphatase in ceramide-mediated signal transduction. J Biol Chem. 1994;269:19605–9. [PubMed] [Google Scholar]
- 27.Cazzolli R, Carpenter L, Biden TJ, Schmitz-Peiffer C. A role for protein phosphatase 2A-like activity, but not atypical protein kinase Czeta, in the inhibition of protein kinase B/Akt and glycogen synthesis by palmitate. Diabetes. 2001;50:2210–8. doi: 10.2337/diabetes.50.10.2210. [DOI] [PubMed] [Google Scholar]
- 28.Chalfant CE, Ogretmen B, Galadari S, Kroesen BJ, Pettus BJ, Hannun YA. FAS activation induces dephosphorylation of SR proteins; dependence on the de novo generation of ceramide and activation of protein phosphatase 1. J Biol Chem. 2001;276:44848–55. doi: 10.1074/jbc.M106291200. [DOI] [PubMed] [Google Scholar]
- 29.Misteli T, Spector DL. Serine/threonine phosphatase 1 modulates the subnuclear distribution of pre-mRNA splicing factors. Mol Biol Cell. 1996;7:1559–72. doi: 10.1091/mbc.7.10.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen PT. Protein phosphatase 1--targeted in many directions. J Cell Sci. 2002;115:241–56. doi: 10.1242/jcs.115.2.241. [DOI] [PubMed] [Google Scholar]
- 31.Cortright RN, Azevedo JL, Jr, Zhou Q, Sinha M, Pories WJ, Itani SI, Dohm GL. Protein kinase C modulates insulin action in human skeletal muscle. Am J Physiol Endocrinol Metab. 2000;278:E553–62. doi: 10.1152/ajpendo.2000.278.3.E553. [DOI] [PubMed] [Google Scholar]
- 32.Itani SI, Zhou Q, Pories WJ, MacDonald KG, Dohm GL. Involvement of protein kinase C in human skeletal muscle insulin resistance and obesity. Diabetes. 2000;49:1353–8. doi: 10.2337/diabetes.49.8.1353. [DOI] [PubMed] [Google Scholar]
- 33.Sampson SR, Cooper DR. Specific protein kinase C isoforms as transducers and modulators of insulin signaling. Mol Genet Metab. 2006;89:32–47. doi: 10.1016/j.ymgme.2006.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chalfant CE, Watson JE, Bisnauth LD, Kang JB, Patel N, Obeid LM, Eichler DC, Cooper DR. Insulin regulates protein kinase CbetaII expression through enhanced exon inclusion in L6 skeletal muscle cells. A novel mechanism of insulin- and insulin-like growth factor-i-induced 5′ splice site selection. J Biol Chem. 1998;273:910–6. doi: 10.1074/jbc.273.2.910. [DOI] [PubMed] [Google Scholar]
- 35.Patel NA, Apostolatos HS, Mebert K, Chalfant CE, Watson JE, Pillay TS, Sparks J, Cooper DR. Insulin regulates protein kinase CbetaII alternative splicing in multiple target tissues: development of a hormonally responsive heterologous minigene. Mol Endocrinol. 2004;18:899–911. doi: 10.1210/me.2003-0391. [DOI] [PubMed] [Google Scholar]
- 36.Patel NA, Chalfant CE, Watson JE, Wyatt JR, Dean NM, Eichler DC, Cooper DR. Insulin regulates alternative splicing of protein kinase C beta II through a phosphatidylinositol 3-kinase-dependent pathway involving the nuclear serine/arginine-rich splicing factor, SRp40, in skeletal muscle cells. J Biol Chem. 2001;276:22648–54. doi: 10.1074/jbc.M101260200. [DOI] [PubMed] [Google Scholar]
- 37.Patel NA, Kaneko S, Apostolatos HS, Bae SS, Watson JE, Davidowitz K, Chappell DS, Birnbaum MJ, Cheng JQ, Cooper DR. Molecular and genetic studies imply Akt-mediated signaling promotes protein kinase CbetaII alternative splicing via phosphorylation of serine/arginine-rich splicing factor SRp40. J Biol Chem. 2005;280:14302–9. doi: 10.1074/jbc.M411485200. [DOI] [PubMed] [Google Scholar]
- 38.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 39.Bielawski J, Szulc ZM, Hannun YA, Bielawska A. Simultaneous quantitative analysis of bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Methods. 2006;39:82–91. doi: 10.1016/j.ymeth.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 40.Jiang G, Dallas-Yang Q, Liu F, Moller DE, Zhang BB. Salicylic acid reverses phorbol 12-myristate-13-acetate (PMA)- and tumor necrosis factor alpha (TNFalpha)-induced insulin receptor substrate 1 (IRS1) serine 307 phosphorylation and insulin resistance in human embryonic kidney 293 (HEK293) cells. J Biol Chem. 2003;278:180–6. doi: 10.1074/jbc.M205565200. [DOI] [PubMed] [Google Scholar]
- 41.Perry DK, Carton J, Shah AK, Meredith F, Uhlinger DJ, Hannun YA. Serine palmitoyltransferase regulates de novo ceramide generation during etoposide-induced apoptosis. J Biol Chem. 2000;275:9078–84. doi: 10.1074/jbc.275.12.9078. [DOI] [PubMed] [Google Scholar]
- 42.Bouzakri K, Karlsson HK, Vestergaard H, Madsbad S, Christiansen E, Zierath JR. IRS-1 serine phosphorylation and insulin resistance in skeletal muscle from pancreas transplant recipients. Diabetes. 2006;55:785–91. doi: 10.2337/diabetes.55.03.06.db05-0796. [DOI] [PubMed] [Google Scholar]
- 43.Gual P, Le Marchand-Brustel Y, Tanti JF. Positive and negative regulation of insulin signaling through IRS-1 phosphorylation. Biochimie. 2005;87:99–109. doi: 10.1016/j.biochi.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 44.Csehi SB, Mathieu S, Seifert U, Lange A, Zweyer M, Wernig A, Adam D. Tumor necrosis factor (TNF) interferes with insulin signaling through the p55 TNF receptor death domain. Biochem Biophys Res Commun. 2005;329:397–405. doi: 10.1016/j.bbrc.2005.01.140. [DOI] [PubMed] [Google Scholar]
- 45.Russell AP. Lipotoxicity: the obese and endurance-trained paradox. Int J Obes Relat Metab Disord. 2004;28 Suppl 4:S66–71. doi: 10.1038/sj.ijo.0802859. [DOI] [PubMed] [Google Scholar]
- 46.Begum N, Ragolia L. Effect of tumor necrosis factor-alpha on insulin action in cultured rat skeletal muscle cells. Endocrinology. 1996;137:2441–6. doi: 10.1210/endo.137.6.8641197. [DOI] [PubMed] [Google Scholar]
- 47.Begum N, Ragolia L, Srinivasan M. Effect of tumor necrosis factor-alpha on insulin-stimulated mitogen-activated protein kinase cascade in cultured rat skeletal muscle cells. Eur J Biochem. 1996;238:214–20. doi: 10.1111/j.1432-1033.1996.0214q.x. [DOI] [PubMed] [Google Scholar]
- 48.Teruel T, Hernandez R, Lorenzo M. Ceramide mediates insulin resistance by tumor necrosis factor-alpha in brown adipocytes by maintaining Akt in an inactive dephosphorylated state. Diabetes. 2001;50:2563–71. doi: 10.2337/diabetes.50.11.2563. [DOI] [PubMed] [Google Scholar]
- 49.Roth MB, Murphy C, Gall JG. A monoclonal antibody that recognizes a phosphorylated epitope stains lampbrush chromosome loops and small granules in the amphibian germinal vesicle. J Cell Biol. 1990;111:2217–23. doi: 10.1083/jcb.111.6.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saghizadeh M, Ong JM, Garvey WT, Henry RR, Kern PA. The expression of TNF alpha by human muscle. Relationship to insulin resistance. J Clin Invest. 1996;97:1111–6. doi: 10.1172/JCI118504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ugi S, Imamura T, Maegawa H, Egawa K, Yoshizaki T, Shi K, Obata T, Ebina Y, Kashiwagi A, Olefsky JM. Protein phosphatase 2A negatively regulates insulin’s metabolic signaling pathway by inhibiting Akt (protein kinase B) activity in 3T3-L1 adipocytes. Mol Cell Biol. 2004;24:8778–89. doi: 10.1128/MCB.24.19.8778-8789.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chalfant CE, Szulc Z, Roddy P, Bielawska A, Hannun YA. The structural requirements for ceramide activation of serine-threonine protein phosphatases. J Lipid Res. 2004;45:496–506. doi: 10.1194/jlr.M300347-JLR200. [DOI] [PubMed] [Google Scholar]
- 53.Trinkle-Mulcahy L, Sleeman JE, Lamond AI. Dynamic targeting of protein phosphatase 1 within the nuclei of living mammalian cells. J Cell Sci. 2001;114:4219–28. doi: 10.1242/jcs.114.23.4219. [DOI] [PubMed] [Google Scholar]
- 54.Jagiello I, Van Eynde A, Vulsteke V, Beullens M, Boudrez A, Keppens S, Stalmans W, Bollen M. Nuclear and subnuclear targeting sequences of the protein phosphatase-1 regulator NIPP1. J Cell Sci 113 Pt. 2000;21:3761–8. doi: 10.1242/jcs.113.21.3761. [DOI] [PubMed] [Google Scholar]
- 55.Murphy LI, Jones PM. Phospho-serine/threonine phosphatases in rat islets of Langerhans: identification and effect on insulin secretion. Mol Cell Endocrinol. 1996;117:195–202. doi: 10.1016/0303-7207(95)03747-0. [DOI] [PubMed] [Google Scholar]
- 56.Chavez JA, Summers SA. Characterizing the effects of saturated fatty acids on insulin signaling and ceramide and diacylglycerol accumulation in 3T3-L1 adipocytes and C2C12 myotubes. Arch Biochem Biophys. 2003;419:101–9. doi: 10.1016/j.abb.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 57.Ragolia L, Begum N. Protein phosphatase-1 and insulin action. Mol Cell Biochem. 1998;182:49–58. [PubMed] [Google Scholar]
- 58.Begum N. Stimulation of protein phosphatase-1 activity by insulin in rat adipocytes. Evaluation of the role of mitogen-activated protein kinase pathway. J Biol Chem. 1995;270:709–14. doi: 10.1074/jbc.270.2.709. [DOI] [PubMed] [Google Scholar]
- 59.Moeschel K, Beck A, Weigert C, Lammers R, Kalbacher H, Voelter W, Schleicher ED, Haring HU, Lehmann R. Protein kinase C-zeta-induced phosphorylation of Ser318 in insulin receptor substrate-1 (IRS-1) attenuates the interaction with the insulin receptor and the tyrosine phosphorylation of IRS-1. J Biol Chem. 2004;279:25157–63. doi: 10.1074/jbc.M402477200. [DOI] [PubMed] [Google Scholar]