Abstract
This study uses a neonatal guinea pig model to compare the effects of in utero methadone or morphine exposure upon breathing control. We hypothesize that in utero methadone exposure will result in similar respiratory disturbances to those seen in morphine exposed neonates, but that the onset will be slower and the duration longer, due to methadone’s longer elimination half-life. Pregnant Dunkin-Hartley guinea pigs received once-daily injections of methadone, morphine, or vehicle (saline) during the last half of gestation and pups were studied 3, 7, or 14 days after birth. In utero methadone or morphine exposure resulted in decreased birth weight compared to vehicle, and pups experienced a withdrawal syndrome which included increased locomotor activity and respiratory disturbances but no change in rectal temperature. Both opioid exposures increased inspiratory minute ventilation during CO2 challenge at 3 days after birth, but only in morphine exposed pups was this withdrawal effect still present on day 7. Surprisingly, only morphine exposure increased inspiratory minute ventilation during room air breathing. We conclude that in utero methadone exposure is not equivalent to in utero morphine exposure. With respect to neonatal respiratory control, methadone-induced changes in respiration are only apparent during hypercapnia.
Keywords: In utero methadone and morphine, respiratory control, neonatal abstinence syndrome, development, opioids, substance abuse
1. Introduction
Methadone is the standard of care for opioid addicted pregnant women. The benefits of methadone treatment over continued heroin use include increased prenatal care, reduced fetal mortality, and increased birth weight [18]. However, infants born to women on methadone maintenance experience withdrawal symptoms of significantly greater severity than heroin exposed infants [46]. In human infants, the methadone-induced neonatal abstinence syndrome (NAS) is characterized by hyperexcitability [46], abnormal REM sleep [17], increased sleep apnea [44], respiratory alkalosis [9], decreased sensitivity to hypercapnia [43], diarrhea, fever and central nervous system irritability, which in severe cases progresses to seizures [13]. In addition, opioid exposed infants are smaller at birth and have an increased incidence of sudden infant death syndrome (SIDS), which may in part be due to abnormal respiratory control [20,21].
The developmental and respiratory effects of in utero methadone exposure have been studied in infants of methadone maintenance patients [19,32]. However, these studies are often confounded by the use of alcohol, tobacco, cocaine, and/or illicit supplemental opioids; any one or combination of which can affect perinatal development [1,3,4,34]. In addition, poor maternal nutrition and disease state impact fetal development, and could account for low birth weight [11].
We use an animal model, free of confounding factors associated with drug abuse, to compare in utero methadone exposure with in utero morphine exposure, the main metabolite of heroin. The guinea pig was chosen because, like humans, guinea pigs have a hemomonochorial placenta and unlike rat or mouse pups, guinea pig pups metabolize morphine to both its major metabolites, morphine-3-glucuronide (M3G) and morphine-6-glucuronide (M6G) in similar quantities to human infants [23,40]. Moreover, we have previously shown that acute methadone treatment is more potent, with regard to respiratory disturbances, than morphine in the newborn guinea pig [30] and that chronic in utero morphine exposure increases locomotor activity and causes hyperventilation during the first week of life [14]. Pregnant guinea pigs were treated once daily with methadone, morphine, or a saline control and a non-invasive plethysmographic method was used to measure respiratory parameters in their pups. We selected doses for both methadone and morphine that were the maximum daily dose tolerated without maternal or fetal mortality. We hypothesize that in utero methadone exposure will result in similar locomotor and respiratory disturbances. However, due to its long elimination half-life in pregnant guinea pigs [37], we expect methadone-induced changes to be of slower onset and longer duration than morphine whose elimination half-life is about one tenth as long, while both values are comparable to pregnant human subjects [16,36,40,41].
2. Materials and Methods
2.1. Animals
Dunkin-Hartley female guinea pigs (6 weeks old, 300 to 400 grams) were bred with male guinea pigs that were at least 5 months old at Oregon Health & Science University (OHSU). All animals were purchased from Charles River (Wilmington, MA). Dams and pups were housed together with 12 hr controlled light/dark cycles and ad libitum food and water. Studies were conducted in accordance with guidelines set forth by the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All experiments were approved by the OHSU Institutional Animal Care and Use Committee.
2.2. Treatment groups
A total of 30 pregnant female guinea pigs and 54 pups (27 males and 27 females) were included in the study. Pregnant guinea pigs were randomly assigned to one of three treatment groups: 1ml/kg vehicle (0.9% saline), 12 mg/kg of methadone in saline or 15 mg/kg of morphine in saline. The methadone dose was chosen based on pilot studies in which pregnant females received 10, 12, or 15 mg/kg methadone. In the pilot study, 10 or 12 mg/kg of methadone resulted in no drug associated maternal or neonatal mortality, however, 15 mg/kg methadone treatment resulted in an unacceptable mortality level among dams (67%) and their pups (100%). The morphine dose was chosen based on a previous study in our laboratory [14]. Even though this represents a higher molar dose of morphine (35%), these doses were used because they represented the maximum daily dose tolerated by the maternal-fetal unit. The lower dose of methadone tolerated by the guinea pigs was most likely related to its ten times longer half-life compared to morphine and the propensity to accumulate upon repeated dosing. Pregnant females received once daily, subcutaneous injections from day 35 of pregnancy to parturition which was at about day 68. Day 35 was chosen because it precedes a critical period of neurological development in the fetal guinea pig and because at that time pregnancy could easily be confirmed by palpation. To reduce litter effects, only 1 female and 1 male pup per litter were included in the study. In the case of an all male or all female litter, only one pup was chosen and was later matched with a pup of opposite sex from a different litter. Each litter was randomly assigned to be studied on day 3, 7, or 14 post-partum with day 1 defined as the first day pups were observed in the tub.
2.3. Drug preparation
Methadone·HCl or morphine·SO4 (Research Triangle Institute, Research Triangle Park, NC) was diluted in sterile 0.9% saline (Abbott Laboratories, Chicago, IL). Injection volumes were standardized to 12 mg of methadone base per ml or 15 mg of morphine base per ml to get equal injections volumes (1ml/kg) for each treatment group. Dilutions were sterile filtered and stored at −80 °C in 1 ml aliquots until needed.
2.4. Activity measurement
Locomotor activity was measured for a 15 minute period prior to respiratory measurements. On day 3, 7 or 14 after birth, each individual pup was placed in a Plexiglas cage located between the optical sensors of the Opto-Varimax mini activity monitor (Columbus Instruments, Columbus, OH). The monitor measured both total and ambulatory movements. Total activity recorded one count for every broken beam, and measured all animal movements including scratching, grooming, digging, and any stereotypical non-horizontal movements. Ambulatory activity (horizontal movements) was recorded only when two beams were broken consecutively.
2.5. Temperature measurement
Rectal body temperature was measured for each pup following respiratory measurements in order to document withdrawal associated hyper- or hypothermia, which could increase or decrease VO2 and VI, respectively. A TM-200D Bi-Temp Monitor (Respiratory Support Products, Irvine, CA) with a flexible small animal thermistor (Model 402; Yellow Springs Instrument, OH) was used.
2.6. Ventilatory measurements
Ventilatory data were collected using a non-invasive two-chamber plethysmograph (Buxco Electronics, Sharon, CT). Detailed methods have been previously described [14,29,30,35]. Two interchangeable head chambers allow separate inspiration of each study gas; “Room Air”, (RA; 21% O2, balance N2) or “5% CO2” (5% CO2, 30% O2, balance N2). Using BioSystem XA software version 2.7.9 (Buxco Electronics, Sharon, CT), animal breathing movements from the body chamber were averaged over 1 min periods. Measured parameters were: breathing frequency (fR; breaths·min−1), inspiratory time (TI; sec), expiratory time (TE; sec), and respiratory air flow (ml·sec−1) from which were derived, tidal volume (VT; ml·100g−1), inspiratory minute ventilation (VI; ml·min− 1·100g−1), and inspiratory effort (VT/TI; ml·sec−1·100g−1).
2.7. Metabolic measurements
During RA flow, the head chamber was sampled over 30 sec periods. Oxygen consumption (VO2; ml·min−1·100g−1) and CO2 production (VCO2; ml·min−1·100g−1) were measured and the respiratory exchange ratio (RER, VCO2/VO2) was calculated using Oxymax software version 2.4.2 (Columbus Instruments, Columbus, OH).
2.8. Experimental setup
Hypercapnic ventilatory response was measured by exposing the pups to increased CO2. In addition, depression of the ventilatory response to CO2 is characteristic of opioids and thus served as a control to rule out residual effects of opioids on breathing. The plethysmograph chamber was used to study unanesthesized, unintubated and only slightly restrained animals. Therefore we only minimally interfere with the physiological state of the animal and don’t affect the respiratory system [2].
2.9. Experimental procedure
A pup was weighed then placed in the activity monitor for a period of 15 min during which both total and ambulatory movements were measured. Next the pup was placed in the plethysmograph with RA flow (1 L·min−1) for a 10 min acclimation period prior to the start of measurement. Ventilatory data were collected with a steady flow of RA for an additional 10 min, after which the head chamber was changed and 5% CO2 was administered for 5 min. The pup was then removed from the chamber and returned to housing. When ventilatory measurements of all pups were complete, rectal temperatures were measured.
2.10. Data analysis
Gestational length was defined as the number of days from the date of vaginal opening to the date pups were observed in the tub, during which pregnant dams were weighed daily. The weight recorded on the day before pups were born is reported as maternal weight at parturition.
Neonatal respiratory data were collected for 10 min while breathing RA and for 5 min during 5% CO2 challenge. During RA breathing, metabolic parameters were measured for the first 6 min and breathing movements for the remaining 4 min. During CO2 challenge, only breathing movements were measured. In order to ensure a steady-state had been reached, the data reported are the average of minutes 5 and 6 for metabolic parameters and the average of minutes 9 and 10 for breathing movements while breathing RA. During 5% CO2 challenge the average of minutes 4 and 5 was reported, since steady-state had been reached by this time. On occasion a preceding minute may have been substituted due to disruptive animal movements within the chamber. All values are reported as the mean ± standard error (SE) unless otherwise specified.
2.11. Statistical analysis
Statistical significance for gestational length, maternal weights, and maternal weight gain was determined by one-way analysis of variance (ANOVA) with drug treatment as the group factor (SigmaStat version 3.11, SysStat Software Inc., Richmond, CA). Neonate data were analyzed initially by three-way ANOVA with factors of sex, drug, and age. However, since there were no sex effects within any parameter, all data were collapsed with respect to sex and analyzed by two-way ANOVA with factors of age and drug. An overall “p” value less than 0.05 was considered significant and all multiple comparisons were performed using the Holm-Sidak method.
3. Results
3.1. Gestational length, litter size
The length of gestation was not different among methadone, morphine or vehicle treated dams. The average length of gestation was 68.4±0.3 days. Likewise, average litter size was similar among the treatment groups with an average of 3.1±0.1 pups per litter and a range of 2 to 5 pups per litter.
3.2. Maternal weight, neonatal weight
Maternal weight was not different among groups at the start of treatment (686±10 g). However, there were significant differences between groups for weight at parturition (p<0.01). Methadone and morphine treated females gained a significantly lower percentage of weight over the treatment period compared to weight at the start of treatment (p<0.01 and p<0.05, respectively, Table 1). Therefore, opioid treated females had a significantly lower weight at parturition compared to vehicle (p<0.01, Table 1). Weights of guinea pig pups are shown in Table 2. There were main effects of drug (p<0.001) and age (p<0.001) for pup weight. In utero methadone or morphine exposed pups were significantly smaller than vehicle pups from birth to day 7. However, by day 14 morphine exposed pups caught up to vehicle pups, while methadone pups remained significantly smaller than vehicle and morphine exposed pups (Table 2).
Table 1.
Weight of pregnant guinea pigs treated once daily with vehicle (saline, 1 ml/kg), methadone (12 mg/kg), or morphine (15 mg/kg) starting at day 35 of gestation
| Vehicle | Methadone | Morphine | |
|---|---|---|---|
| Maternal wt. at parturition (g) | 1040 ± 39 | 869 ± 21*** | 913 ± 28** |
| Wt. gain (% of treatment start wt) | 50 ± 6 | 30 ± 2** | 34 ± 2* |
| N (dams/treatment) | 9 | 11 | 10 |
p<0.05 vs. vehicle
p<0.01 vs. vehicle
p<0.001 vs. vehicle; all values reported as mean ± S.E.
Table 2.
Weights (g) of in utero opioid exposed neonatal guinea pigs compared to vehicle
| Age | Vehicle | Methadone | Morphine |
|---|---|---|---|
| Birth | 102 ± 4 | 81 ± 4** | 82 ± 3** |
| 3 days | 102 ± 4 | 82 ± 5** | 84 ± 3** |
| 7 days | 132 ± 6 | 116 ± 8* | 112 ± 5** |
| 14 days | 189 ± 12 | 147 ± 10*^^ | 178 ± 9 |
p<0.05 vs. vehicle
p<0.01 vs. vehicle
p<0.001 vs. vehicle
p<0.01 vs. morphine, all values reported as mean ± S.E. of all pups in each treatment group, n (per treatment): Birth and day 3, 18 pups; day 7, 12 pups; day 14, 6 pups.
3.3. Pup activity and rectal temperature
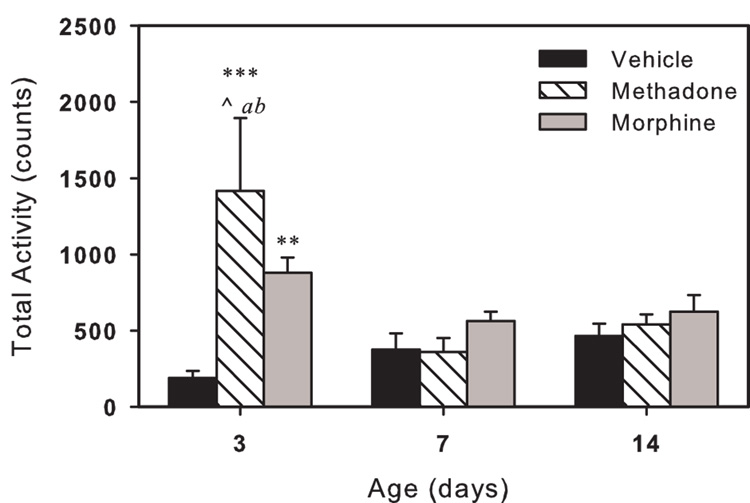
Total locomotor activity, a measure of both ambulatory activity and grooming movements, is shown in Fig. 1. There were main effects of age (p<0.05) and drug (p<0.05), and an age-drug interaction for total activity (p<0.01). On day 3, methadone or morphine exposed pups were more active than control pups and methadone pups were more active than morphine pups. On day 7 and 14 there were no differences between the treatment groups. Methadone exposed pups were significantly more active on day 3 than on day 7 or 14.
Fig. 1.
Total activity of in utero drug exposed neonatal guinea pigs. Activity is the total count of photo-beam breaks recorded in a 15 minute period. Methadone (striped bars) or morphine (gray bars) exposed pups showed signs of withdrawal related hyperactivity on day 3; by day 7 and 14 counts were not different from control (black bars). Values are average of 6 pups per treatment. Vertical bars represent standard error (S.E). ** p<0.01, *** p<0.001 vs. vehicle; ^ p<0.05 vs. morphine; a p<0.05 vs. 7-day same treatment; b p<0.05 vs. 14-day same treatment.
There was a main effect of drug (p<0.01) when the ratio of ambulatory to total activity was analyzed. Ambulatory activity was a greater portion of total activity for methadone or morphine exposed pups compared to control pups (p<0.01, both). For methadone or morphine exposed pups the ratio was 65±3 % and 64±3 %, respectively. For vehicle exposed pups ambulatory activity was 53±3 % of total activity.
Rectal body temperature was not different among pups at any age or in any treatment group.
3.4. Respiratory measurements
3.4.1. Inspiratory and tidal volume
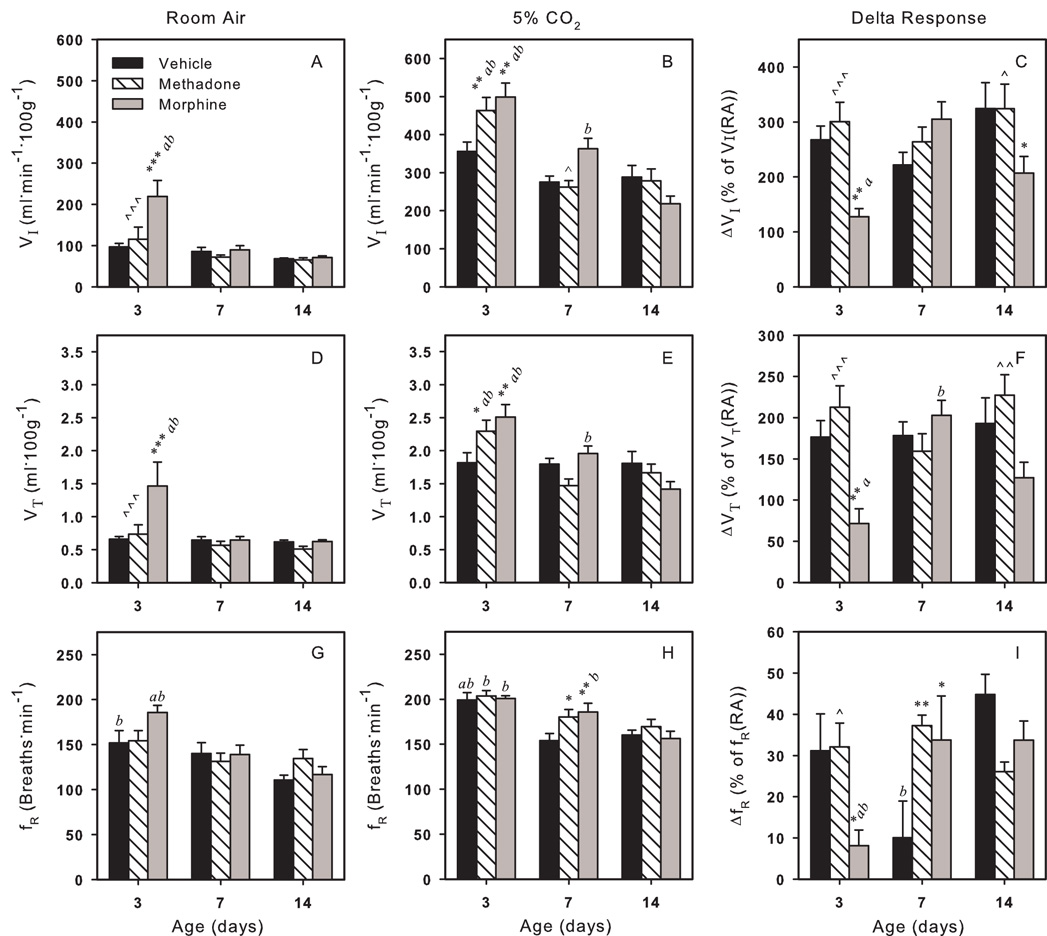
For VI there were main effects of drug (p<0.01) and age (p<0.001), and a drug-age interaction (p<0.01) during RA breathing. VI of vehicle and methadone exposed pups did not change with age and VI of methadone pups was not different from control at any age (Fig. 2A). However, VI for morphine exposed neonates on day 3 was significantly greater than VI of control or methadone pups at this age as well as morphine exposed pups on day 7 and 14. The increased VI on day 3 for morphine exposed pups was due to an increased VT.
Fig. 2.
Respiratory effects of in utero methadone (striped bars) or morphine (gray bars) exposures compared to vehicle (black bars) during room air (RA) breathing (left column) and during 5% CO2 challenge (middle column). CO2 challenge induced delta response is shown as percent of baseline (RA breathing) (right column). Three major respiratory parameters, inspiratory volume (VI) (A–C), tidal volume (VT) (D–F) and breathing frequency (fR) (G–I) are shown. Several differences exist between methadone and morphine exposed pups. Vertical bars represent standard error (S.E). * p<0.05, ** p<0.01, *** p<0.001 vs. vehicle; ^^^ p<0.001 vs. morphine; a p<0.05 vs. 7-day same treatment; b p<0.05 vs. 14-day same treatment.
There were main effects for VT (Fig. 2D) of drug (p<0.05) and age (p<0.01), and a drug-age interaction (p<0.05). On day 3, VT of morphine exposed pups was greater than VT of control and methadone pups, as well as VT of older morphine pups (days 7 and 14). VT of vehicle and methadone treated pups did not change with age.
During 5% CO2 challenge, there were main effects of age (p<0.001) and drug-age interactions (p<0.01) for both VI (Fig. 2B) and VT (Fig. 2E). VI and VT of vehicle exposed pups did not change with age, however there was a change with age for morphine and methadone exposed pups. For methadone and morphine exposed pups, VI and VT were greater than control only on day 3. On day 7, VI of morphine exposed pups was significantly greater than methadone.
An increase in inspired CO2 is a powerful stimulus to breathing. For the relative increase (delta response) in VI and VT from RA breathing during 5% CO2 challenge there were main effects of drug (p≤0.01) and drug-age interactions (p<0.01) (Fig. 2C,F). On day 3 and 14, ΔVI and ΔVT for morphine pups was lower than for control (ΔVI only on day 14) or methadone pups. Morphine pups also showed an age dependent change in the delta response for both VI and VT.
3.4.2. Breathing frequency
For fR there was a main effect of age (p<0.001) (Fig. 2G), but no main drug effect during RA breathing. Control pups had a greater fR on day 3 than on day 14, while fR of methadone exposed pups did not change with age. For morphine fR was elevated on day 3 compared to day 7 and day 14 pups of the same treatment.
There was only a main effect of age (p<0.001) during 5% CO2 challenge for fR (Fig. 2H). Compared to 3 day old pups fR of vehicle treated pups decreased on day 7 and 14 compared and for methadone or morphine exposed pups fR decreased on day 14. Only on day 7, fR of methadone and morphine exposed pups was significantly greater than control.
For the relative increase in fR from RA to 5% CO2 breathing there were main effects of drug (p<0.05) and drug-age interaction (p=0.001), but no effect of age (Fig. 2I). On day 3, the percent increase in frequency (ΔfR) for morphine pups was lower compared to both vehicle or methadone exposed pups as well as compared to older morphine exposed pups due to the increased fR of morphine pups during RA breathing. Both methadone and morphine pups had an increased delta response compared to vehicle on day 7.
3.4.3. Inspiratory and expiratory time
There was only a main age effect for both TI and TE (p<0.01) (Table 3). There were no age related changes in TI or TE for vehicle or methadone exposed pups, and no difference between any treatment groups on any particular day. However, TI and TE were lower on day 3 than on day 14 for morphine exposed pups.
Table 3.
Respiratory measurements of in utero opioid exposed neonatal guinea pigs
| Age | Room Air | 5% CO2 | |||||
|---|---|---|---|---|---|---|---|
| Vehicle | Methadone | Morphine | Vehicle | Methadone | Morphine | ||
| TI [sec] | 3 days | 0.18 ± 0.02 | 0.16 ± 0.01 | 0.14 ± 0.01b | 0.14 ± 0.01ab | 0.13 ± 0.00b | 0.13 ± 0.00b |
| 7 days | 0.19 ± 0.02 | 0.20 ± 0.02 | 0.18 ± 0.01 | 0.17 ± 0.01 | 0.13 ± 0.01*** | 0.13 ± 0.00***b | |
| 14 days | 0.23 ± 0.01 | 0.20 ± 0.02 | 0.23 ± 0.02 | 0.16 ± 0.00 | 0.15 ± 0.01 | 0.16 ± 0.01 | |
| TE [sec] | 3 days | 0.25 ± 0.03 | 0.25 ± 0.03 | 0.21 ± 0.01b | 0.17 ± 0.01ab | 0.17 ± 0.01ab | 0.17 ± 0.01ab |
| 7 days | 0.27 ± 0.03 | 0.27 ± 0.02 | 0.28 ± 0.02 | 0.23 ± 0.01 | 0.21 ± 0.01 | 0.21 ± 0.01 | |
| 14 days | 0.32 ± 0.02 | 0.27 ± 0.02 | 0.33 ± 0.03 | 0.22 ± 0.01 | 0.22 ± 0.01 | 0.22 ± 0.01 | |
| VT/TI | 3 days | 3.8 ± 0.3 | 4.7 ± 1.1^^^ | 10.4 ± 2.3***ab | 13.2 ± 0.9 | 18.1 ± 1.2**ab | 18.9 ± 1.2***ab |
| [ml.sec−1.100g−1] | 7 days | 3.7 ± 0.6 | 2.8 ± 0.2 | 3.7 ± 0.4 | 10.8 ± 0.5 | 11.2 ± 0.6^^ | 15.3 ± 0.6**b |
| 14 days | 2.7 ± 0.1 | 2.6 ± 0.1 | 2.8 ± 0.2 | 11.2 ± 1.1 | 11.4 ± 1.1 | 8.8 ± 0.7 | |
p<0.01 vs. vehicle
p<0.001 vs. vehicle
p<0.01 vs. morphine
p<0.001 vs. morphine
p<0.05 vs. 7 day same treatment
p<0.05 vs. 14 day same treatment, all values reported as mean ± S.E.
In contrast, there was a main effect of age for TI and TE (p<0.001) and a main effect of drug for TI (p<0.001) with a drug-age interaction (p<0.05) during 5% CO2 challenge (Table 3). For control pups, TI increased between day 3 and day 7, while TI of methadone or morphine exposed pups did not and was significantly lower than control on day 7. On day 14, TI of all treatment groups was not different from each other, but increased compared to day 3. TE of methadone or morphine treated pups was not different from control at any age. The age effect was present because TE increased on day 7 and remained at this level on day 14 for all treatment groups.
3.4.4. Inspiratory effort
For inspiratory effort (VT/TI), there were main effects of age (p<0.001) and drug (p<0.01), with a drug-age interaction (p<0.01) (Table 3). VT/TI of methadone exposed pups was not different from control pups at any age. Morphine exposed pups had a significantly greater VT/TI on day 3 than on day 7 or 14 and a significantly greater VT/TI than vehicle or methadone exposed pups on day 3.
There were main effects of age (p<0.001) and drug (p<0.01) and a drug-age interaction (p<0.001) during 5% CO2 challenge (Table 3). On day 3, VT/TI for methadone and morphine exposed pups was greater than control. For vehicle treated pups VT/TI did not change with age. On day 7, VT/TI of morphine exposed pups remained above control levels but pups exposed to methadone did not. On day 14 there was no difference between treatment groups.
3.5. Oxygen consumption and CO2 production
3.5.1. Oxygen consumption VO2
There was a main effect of age (p<0.001) for VO2. For vehicle exposed pups, VO2 was equivalent on day 3 and 7 and decreased on day 14 (Table 4). VO2 of both methadone and morphine exposed pups decreased with age. Methadone and morphine exposed pups had a greater VO2 on day 3 than control but were not different from control on day 7 and 14.
Table 4.
Ventilatory measurements of in utero opioid exposed neonatal guinea pigs during RA breathing
| Age | VO2 [ml.min−1.100g−1] | VCO2 [ml.min−1.100g−1] | VI/VO2 | VI/VCO2 | RER | |
|---|---|---|---|---|---|---|
| Vehicle | 3 days | 3.5 ± 0.5b | 2.7 ± 0.2b | 30 ± 4 | 37 ± 3 | 0.82 ± 0.06 |
| 7 days | 3.3 ± 0.1b | 2.5 ± 0.1 | 25 ± 3 | 34 ± 4 | 0.75 ± 0.01 | |
| 14 days | 2.5 ± 0.1 | 2.1 ± 0.1 | 27 ± 1 | 32 ± 1 | 0.86 ± 0.03 | |
| Methadone | 3 days | 4.2 ± 0.1*ab | 3.0 ± 0.1b | 27 ± 6^^ | 38 ± 9^^^ | 0.75 ± 0.02b |
| 7 days | 3.3 ± 0.2b | 2.7 ± 0.1 | 22 ± 2 | 27 ± 2 | 0.81 ± 0.02 | |
| 14 days | 2.7 ± 0.3 | 2.4 ± 0.1 | 25 ± 2 | 28 ± 2 | 0.92 ± 0.06 | |
| Morphine | 3 days | 4.4 ± 0.2**ab | 3.3 ± 0.1**ab | 51 ± 9**ab | 68 ± 12***ab | 0.74 ± 0.02b |
| 7 days | 3.3 ± 0.2b | 2.6 ± 0.1 | 28 ± 4 | 36 ± 5 | 0.78 ± 0.04b | |
| 14 days | 2.3 ± 0.1 | 2.1 ± 0.1 | 32 ± 2 | 34 ± 2 | 0.94 ± 0.06 | |
p<0.05 vs. vehicle
p<0.01 vs. vehicle
p<0.001 vs. vehicle
p<0.01 vs. morphine
p<0.001 vs. morphine
p<0.05 vs. 7 day same treatment
p<0.05 vs. 14 day same treatment, all values reported as mean ± S.E.
3.5.2. Carbon dioxide production VCO2
For VCO2, there was a main effect of drug (p<0.05) and age (p<0.001), but no drug-age interaction. VCO2 for methadone was not different from control for all ages (Table 4). Morphine exposed pups had a greater VCO2 on day 3, but showed a decrease in VCO2 back to control levels between day 3 and day 7. VCO2 of all pups decreased on day 14.
3.5.3. VI/VO2 and VI/VCO2
There were main effects of age (p<0.05) and drug (p<0.01) for VI/VO2 and VI/VCO2, two parameters commonly used to indicate hypo- or hyperventilation (Table 4). Both did not change with age for control or methadone pups, but were drastically elevated on day 3 in morphine exposed pups, indicating withdrawal induced hyperventilation.
3.5.4. Respiratory exchange rate
There was a main effect of age (p<0.001), but no drug effect for the respiratory exchange ratio. RER did not change with age for vehicle treated pups and was not different among treatment groups at any particular age (Table 4). However, RER of methadone treated pups was greater on day 14 than on day 3. RER of morphine treated pups was equivalent on day 3 and 7 but increased on day 14.
4. Discussion
Methadone, a long acting synthetic opioid, is the standard of care for opioid addiction during pregnancy. Human studies of the effects of in utero methadone exposure are often confounded by maternal alcohol, tobacco, and/or supplemental opioid use [1,3,4,34]. The present study compares the effects of in utero methadone to in utero morphine (the predominant active metabolite of heroin) exposure in an animal model free of the confounding factors related to maternal polypharmacy, nutritional deficiencies, or disease states.
The neonatal guinea pig was chosen for several important reasons. Guinea pigs [12] and humans [25,38] hyperventilate during pregnancy, which is suppressed by opioids, and both species have a hemomonochorial placenta with similar permeability characteristics [6,31]. Importantly, the elimination half-life of methadone is 15 hrs in the pregnant guinea pig [37] which is comparable to that in humans [16,36,41]. In contrast, the elimination half-life of morphine in the pregnant guinea pig is 1.3 hr [40]. Consequently, daily administration of methadone would be more prone to drug accumulation than morphine requiring different doses for the two drugs in a model of once daily drug administration over a long period of time. Finally, human infants and guinea pig pups, unlike rat or mouse pups, metabolize morphine to both pharmacologically active metabolites, M3G and M6G [23,40], making the guinea pig an excellent model for the present study.
Birth weights of pups exposed in utero to methadone or morphine were lower than control pups. However, by day 14, morphine exposed pups had achieved weights equivalent to control pups, while methadone pups remained below expected weights.
This is contrary to human studies where methadone infants were reported to have higher birth weights than heroin and presumably morphine exposed infants. This discrepancy may be the result of social factors as opposed to a true drug effect because women in methadone maintenance programs are more likely to receive adequate prenatal care and have improved nutrition, thus their infants are likely to have higher birth weights than the infants of heroin abusing women [18]. In addition, methadone treatment in humans often reduces the abuse of other drugs and reduces the risk of I.V. drug related diseases (i.e. HIV and hepatitis) [7]. The explanation for the difference in the weight gain between methadone and morphine exposed pups on day 14 is unclear. This effect could be due to lingering methadone in the body, but this has not been documented in the literature.
In addition to the difference in weight gain, there was also a difference in total locomotor activity between treatment groups. On day 3, in utero methadone exposed pups had a greater total activity count than morphine or control pups. The greater total activity of methadone exposed pups may reflect a change in N-methyl-D-aspartate (NMDA) receptors, since methadone acts as a non-competitive NMDA antagonist [10]. In infant rats, co-treatment with the non-competitive NMDA antagonist MK-801 and the mu opioid receptor agonist morphine increased the incidence of certain withdrawal behaviors [45] including walking behaviors. The developmental maturity of the NMDA receptor is likely more advanced in the 3 day old guinea pig compared to the young rat; nonetheless, the study of Zhu and Barr [45] does demonstrate the involvement of the NMDA receptor in opioid withdrawal and emphasizes an important difference between methadone and morphine. Increased locomotor activity may indicate in utero alterations or adaptation of the NMDA receptor system due to the presence of methadone, which has both NMDA antagonist activity [10] and mu opioid agonist activity [42].
In regard to breathing control, the results were contrary to our hypothesis. Methadone did not have a slower onset of withdrawal symptoms nor did symptoms last longer than in morphine induced withdrawal. Most surprising, methadone exposed pups only showed signs of respiratory disturbances during the stress of CO2 challenge while morphine induced changes were seen during both RA and CO2 breathing.
During RA breathing, methadone exposed pups showed no withdrawal-related respiratory changes, while morphine exposed pups had an elevated VI. Although this could be a result of central nervous system stimulation by M3G, a morphine metabolite that reportedly has an excitatory effect on the central nervous system [22,33], in a previous study, M3G did not affect breathing when administered postnatally to neonatal guinea pigs [28]. Besides, it is unlikely that M3G would remain in the blood by day 3. Instead the respiratory changes may indicate a down-regulation of the opioid system due to chronic in utero morphine exposure or a change in receptor efficiency. The literature remains controversial with regard to chronic opioid induced up- or downregulation of opioid receptors, but previous studies from our laboratory support a change in receptor efficiency. For example, after chronic in utero morphine exposure guinea pig pups showed decreased DAMGO-stimulated 35S-GTPγS binding in respiratory control areas of the brainstem [26] with no difference in brainstem mu opioid receptor mRNA expression or protein levels [39], which suggests a change in receptor efficiency rather than a decrease in density. Unfortunately similar studies have not been done in the guinea pig with methadone.
In addition, the inspiratory effort of morphine exposed pups was increased while breathing RA during the withdrawal period, which corresponded to a period of hyperventilation. This is consistent with previous work from our laboratory in which in utero morphine exposed pups hyperventilated during withdrawal [14] and studies in human infants withdrawing from heroin [9]. This hyperventilation would increase oxygen needs for the newborn and in that sense would be deleterious. It should be noted that Glass and colleagues [9] excluded acid-base disturbances as the cause of hyperventilation. In contrast, the methadone exposed guinea pig pups did not exhibit increased inspiratory effort and hyperventilation, similar to a previous study of human neonates of methadone maintenance patients [32]. The mechanism for this difference is not apparent but may be due in part to differences in pharmacokinetics [15] or different agonist properties of methadone and morphine at the kappa and delta opioid receptors [24]. However, the latter seems unlikely, as mu opioid receptor knock-out studies have shown that morphine had no effect on the hypercapnic response in mu knock-out mice [5].
During 5% CO2 challenge both methadone and morphine caused a withdrawal related increase in VI on day 3. However, this effect remained in morphine pups through day 7. This is interesting because we expected the respiratory effects of methadone to be of longer duration and slower onset than that of morphine. One possible explanation is the effect of lipid solubility which is two orders of magnitude greater for methadone than morphine [27]. It is possible that the long elimination half-life of methadone, combined with its high lipid solubility, results in a constant release of drug from fat stores to the bloodstream that prevents or reduces respiratory withdrawal effects.
Abnormal respiratory control caused by chronic drug use has long been presumed to be the leading cause of an increased risk of sudden infant death syndrome [20,21]. Recent evidence shows that SIDS may result from a combination of prenatal vulnerability, postnatal stress and a critical developmental stage and may be caused by defiencies in breathing, autonomic and sleep-wake control [8]. Our data clearly show an altered response to hypercapnic challenge in the very young caused by in utero drug exposure. Therefore this could significantly increase the likelihood of all three causes to occur simultaneously and result in an increased SIDS incidence in infants of substance abusing mothers [20,21].
There is no doubt that illicit drug abuse during pregnancy has fetal and neonatal consequences. This study has demonstrated that in utero methadone or morphine exposure is not equivalent with respect to neonatal withdrawal. In fact, overall in utero methadone exposure had fewer detrimental effects than in utero morphine and presumably heroin exposure when ventilatory effects are taken into account. This could be related to the lower dose of methadone used, but more likely reflects the difference in elimination half-lives and the different effects of the two drugs on other receptors, such as the NMDA receptor. With the exception of birth weight, where both methadone and morphine exposure reduced pup weight at birth, morphine had fewer disadvantageous outcomes for postnatal weight gain and central nervous system stimulation (as measured by locomotor activity during withdrawal), however, methadone clearly had fewer ventilatory effects, and does not alter hypercapnic ventilatory response.
Acknowledgements
The authors wish to thank Ty A. Ransom and Cole S. Nelson for their contribution to pilot studies, and Daniel N. Silverman for careful review of the manuscript. This research was funded by The National Institute on Drug Abuse (Grant 07912, G.D.O). The National Institute on Drug Abuse also provided the opioids used in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors and author’s institution have no financial or other relationships with other people or organizations that would constitute a conflict of interest.
References
- 1.Bhat R, Chari G, Rao R. Effects of prenatal cocaine, morphine, or both on postnatal opioid (mu) receptor development. Life Sci. 2006;78:1478–1482. doi: 10.1016/j.lfs.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 2.Blake CI, Banchero N. Ventilation and oxygen consumption in the guinea pig. Respir. Physiol. 1985;61:347–355. doi: 10.1016/0034-5687(85)90077-5. [DOI] [PubMed] [Google Scholar]
- 3.Chiriboga CA. Fetal alcohol and drug effects. Neurologist. 2003;9:267–279. doi: 10.1097/01.nrl.0000094941.96358.d1. [DOI] [PubMed] [Google Scholar]
- 4.Choo RE, Huestis MA, Schroeder JR, Shin AS, Jones HE. Neonatal abstinence syndrome in methadone-exposed infants is altered by level of prenatal tobacco exposure. Drug Alcohol Depend. 2004;75:253–260. doi: 10.1016/j.drugalcdep.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 5.Dahan A, Sarton E, Teppema L, Olievier C, Nieuwenhuijs D, Matthes HW, Kieffer BL. Anesthetic potency and influence of morphine and sevoflurane on respiration in mu-opioid receptor knockout mice. Anesthesiology. 2001;94:824–832. doi: 10.1097/00000542-200105000-00021. [DOI] [PubMed] [Google Scholar]
- 6.Faber JJ, Thornburg KL. Placental Physiology. New York, NY: Raven Press; 1983. pp. 17,27,44,58,59,83. [Google Scholar]
- 7.Fischer G. Treatment of opioid dependence in pregnant women. Addiction. 2000;95:1141–1144. doi: 10.1046/j.1360-0443.2000.95811411.x. [DOI] [PubMed] [Google Scholar]
- 8.Franco P, Kugener B, Dijoud F, Scaillet S, Groswasser J, Kato I, Montemitro E, Lin J-S, Kahn A. Sudden Infant Death Syndrome from Epidemiology to Pathophysiology. Current Pediatric Reviews. 2007;3:177–189. [Google Scholar]
- 9.Glass L, Rajegowda BK, Kahn EJ, Floyd MV. Effect of heroin withdrawal on respiratory rate and acid-base status in the newborn. N. Engl. J. Med. 1972;286:746–748. doi: 10.1056/NEJM197204062861403. [DOI] [PubMed] [Google Scholar]
- 10.Gorman AL, Elliott KJ, Inturrisi CE. The d- and l-isomers of methadone bind to the non-competitive site on the N-methyl-D-aspartate (NMDA) receptor in rat forebrain and spinal cord. Neurosci. Lett. 1997;223:5–8. doi: 10.1016/s0304-3940(97)13391-2. [DOI] [PubMed] [Google Scholar]
- 11.Harding J. Nutritional causes of impaired fetal growth and their treatment. J. R. Soc. Med. 1999;92:612–615. doi: 10.1177/014107689909201202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hosenpud JD, Hart MV, Morton MJ, Hohimer AR, Resko JA. Progesterone-induced hyperventilation in the guinea pig. Respir. Physiol. 1983;52:259–264. doi: 10.1016/0034-5687(83)90010-5. [DOI] [PubMed] [Google Scholar]
- 13.Huestis MA, Choo RE. Drug abuse's smallest victims: in utero drug exposure. Forensic Sci. Int. 2002;128:20–30. doi: 10.1016/s0379-0738(02)00160-3. [DOI] [PubMed] [Google Scholar]
- 14.Hunter MA, Vangelisti GR, Olsen GD. Chronic intermittent in utero exposure to morphine: effects on respiratory control in the neonatal guinea pig. Biol. Neonate. 1997;72:293–304. doi: 10.1159/000244496. [DOI] [PubMed] [Google Scholar]
- 15.Inturrisi CE. Clinical pharmacology of opioids for pain. Clin. J. Pain. 2002;18:S3–S13. doi: 10.1097/00002508-200207001-00002. [DOI] [PubMed] [Google Scholar]
- 16.Jarvis MA, Wu-Pong S, Kniseley JS, Schnoll SH. Alterations in methadone metabolism during late pregnancy. J. Addict. Dis. 1999;18:51–61. doi: 10.1300/J069v18n04_05. [DOI] [PubMed] [Google Scholar]
- 17.Kandall SR. Late complications in passively addicted infants. In: Rementeria JL, editor. Drug abuse in pregnancy and neonatal effects. Saint Louis, Mo: The C. V. Mosby Company; 1977. pp. 117–128. [Google Scholar]
- 18.Kandall SR, Albin S, Lowinson J, Berle B, Eidelman AI, Gartner LM. Differential effects of maternal heroin and methadone use on birthweight. Pediatrics. 1976;58:681–685. [PubMed] [Google Scholar]
- 19.Kandall SR, Doberczak TM, Jantunen M, Stein J. The methadone-maintained pregnancy. Clin. Perinatol. 1999;26:173–183. [PubMed] [Google Scholar]
- 20.Kandall SR, Gaines J. Maternal substance use and subsequent sudden infant death syndrome (SIDS) in offspring. Neurotoxicol. Teratol. 1991;13:235–240. doi: 10.1016/0892-0362(91)90016-p. [DOI] [PubMed] [Google Scholar]
- 21.Kandall SR, Gaines J, Habel L, Davidson G, Jessop D. Relationship of maternal substance abuse to subsequent sudden infant death syndrome in offspring. J. Pediatr. 1993;123:120–126. doi: 10.1016/s0022-3476(05)81554-9. [DOI] [PubMed] [Google Scholar]
- 22.LaBella FS, Pinsky C, Havlicek V. Morphine derivatives with diminished opiate receptor potency show enhanced central excitatory activity. Brain Res. 1979;174:263–271. doi: 10.1016/0006-8993(79)90849-7. [DOI] [PubMed] [Google Scholar]
- 23.Lawrence AJ, Michalkiewicz A, Morley JS, MacKinnon K, Billington D. Differential inhibition of hepatic morphine UDP-glucuronosyltransferases by metal ions. Biochem. Pharmacol. 1992;43:2335–2340. doi: 10.1016/0006-2952(92)90311-6. [DOI] [PubMed] [Google Scholar]
- 24.Liu JG, Liao XP, Gong ZH, Qin BY. Methadone-induced desensitization of the delta-opioid receptor is mediated by uncoupling of receptor from G protein. Eur. J. Pharmacol. 1999;374:301–308. doi: 10.1016/s0014-2999(99)00322-2. [DOI] [PubMed] [Google Scholar]
- 25.Lucius H, Gahlenbeck H, Kleine HO, Fabel H, Bartels H. Respiratory functions, buffer system, and electrolyte concentrations of blood during human pregnancy. Respir. Physiol. 1970;9:311–317. doi: 10.1016/0034-5687(70)90088-5. [DOI] [PubMed] [Google Scholar]
- 26.Matsuda AY, Olsen GD. Chronic in utero morphine exposure alters mu-agonist-stimulated [35S]-GTPgammaS binding in neonatal and juvenile guinea pig brainstem regions associated with breathing control. Neurotoxicol. Teratol. 2001;23:413–419. doi: 10.1016/s0892-0362(01)00162-3. [DOI] [PubMed] [Google Scholar]
- 27.Medzihradsky F, Emmerson PJ, Mousigian CA. Lipophilicity of opioids determined by a novel micromethod. J. Pharmacol. Toxicol. Methods. 1992;27:67–69. doi: 10.1016/1056-8719(92)90023-t. [DOI] [PubMed] [Google Scholar]
- 28.Murphey LJ, Olsen GD. Diffusion of morphine-6-beta-D-glucuronide into the neonatal guinea pig brain during drug-induced respiratory depression. J. Pharmacol. Exp. Ther. 1994;271:118–124. [PubMed] [Google Scholar]
- 29.Murphey LJ, Olsen GD. Morphine-6-beta-D-glucuronide respiratory pharmacodynamics in the neonatal guinea pig. J. Pharmacol. Exp. Ther. 1994;268:110–116. [PubMed] [Google Scholar]
- 30.Nettleton RT, Ransom TA, Abraham SL, Nelson CS, Olsen GD. Methadone-induced respiratory depression in the neonatal guinea pig. Pediatr. Pulmonol. 2007;42:1134–1143. doi: 10.1002/ppul.20707. [DOI] [PubMed] [Google Scholar]
- 31.Olsen GD. Placental permeability for drugs of abuse and their metabolites. NIDA Res. Monogr. 1995;154:152–162. [PubMed] [Google Scholar]
- 32.Olsen GD, Lees MH. Ventilatory response to carbon dioxide of infants following chronic prenatal methadone exposure. J Pediatr. 1980;96:983–989. doi: 10.1016/s0022-3476(80)80622-6. [DOI] [PubMed] [Google Scholar]
- 33.Olsen GD, Sommer KM, Wheeler PL, Boyea SR, Michelson SP, Cheek DB. Accumulation and clearance of morphine-3-beta-D-glucuronide in fetal lambs. J. Pharmacol. Exp. Ther. 1988;247:576–584. [PubMed] [Google Scholar]
- 34.Olsen GD, Weil JA. In utero cocaine exposure: Effect on neonatal breathing in guinea pigs. J Pharmacol Exp Ther. 1992;261:420–428. [PubMed] [Google Scholar]
- 35.Olsen GD, Weil JA. Noninvasive breathing analysis: repetitive chloral hydrate in neonatal guinea pigs. Pediatr. Pulmonol. 1992;13:227–234. doi: 10.1002/ppul.1950130410. [DOI] [PubMed] [Google Scholar]
- 36.Olsen GD, Wilson JE, Robertson GE. Respiratory and ventilatory effects of methadone in healthy women. Clin. Pharmacol. Ther. 1981;29:373–380. doi: 10.1038/clpt.1981.51. [DOI] [PubMed] [Google Scholar]
- 37.Pak RC, Ecobichon DJ. The effect of pregnancy and lactation on the elimination of methadone in guinea pigs. Drug Metab Dispos. 1981;9:170–171. [PubMed] [Google Scholar]
- 38.Pernoll ML, Metcalfe J, Kovach PA, Wachtel R, Dunham MJ. Ventilation during rest and exercise in pregnancy and postpartum. Respir. Physiol. 1975;25:295–310. doi: 10.1016/0034-5687(75)90005-5. [DOI] [PubMed] [Google Scholar]
- 39.Smith SA, Stupfel JT, Ilias NA, Olsen GD. Guinea pig mu opioid receptor: brainstem expression in the morphine-exposed neonate. Neurotoxicol. Teratol. 2004;26:121–129. doi: 10.1016/j.ntt.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 40.Smith SA, Woolsey JB, Olsen GD. Morphine metabolism in the pregnant guinea pig and her pups. Biol. Neonate. 1999;76:362–373. doi: 10.1159/000014180. [DOI] [PubMed] [Google Scholar]
- 41.Swift RM, Dudley M, DePetrillo P, Camara P, Griffiths W. Altered methadone pharmacokinetics in pregnancy: implications for dosing. J. Subst. Abuse. 1989;1:453–460. [PubMed] [Google Scholar]
- 42.Wallisch M, Nelson CS, Mulvaney JM, Hernandez HS, Smith SA, Olsen GD. Effects of chronic opioid exposure on guinea pig mu opioid receptor in Chinese hamster ovary cells: comparison with human and rat receptor. Biochem. Pharmacol. 2007;73:1818–1828. doi: 10.1016/j.bcp.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ward SL, Bautista DB, Woo MS, Chang M, Schuetz S, Wachsman L, Sehgal S, Bean X. Responses to hypoxia and hypercapnia in infants of substance-abusing mothers. J. Pediatr. 1992;121:704–709. doi: 10.1016/s0022-3476(05)81896-7. [DOI] [PubMed] [Google Scholar]
- 44.Ward SL, Schuetz S, Kirshna V, Bean X, Wingert W, Wachsman L, Keens TG. Abnormal sleeping ventilatory pattern in infants of substance-abusing mothers. Am. J. Dis. Child. 1986;140:1015–1020. doi: 10.1001/archpedi.1986.02140240061028. [DOI] [PubMed] [Google Scholar]
- 45.Zhu H, Barr GA. Inhibition of morphine withdrawal by the NMDA receptor antagonist MK-801 in rat is age-dependent. Synapse. 2001;40:282–293. doi: 10.1002/syn.1051. [DOI] [PubMed] [Google Scholar]
- 46.Ziegler M, Poustka F, von Loewenich V, Englert E. Postpartum risk factors in the development of children born to opiate-addicted mothers; comparison between mothers with and without methadone substitution. Nervenarzt. 2000;71:730–736. doi: 10.1007/s001150050657. [DOI] [PubMed] [Google Scholar]