Abstract
Mathematical principles of reinforcement (MPR; Killeen, 1994) is a quantitative model of operant behavior that contains 3 parameters representing motor capacity (δ), motivation (a), and short term memory (λ). The present study applied MPR to characterize the effects of bilateral infusions of 6-OHDA into the substantia nigra pars compacta in the rat, a model of Parkinson’s disease. Rats were trained to lever press under a 5-component fixed ratio (5, 15, 30, 60, and 100) schedule of food reinforcement. Rats were tested for 15 days prior to dopamine lesions and again for 15 days post-lesion. To characterize functional loss relative to lesion size, rats were grouped according to the extent and the degree of lateralization of their dopamine loss. Response rates decreased as a function of dopamine depletion, primarily at intermediate ratios. MPR accounted for 98% of variance in pre- and post-lesion response rates. Consistent with reported disruptions in motor behavior induced by dopaminergic lesions, estimates of δ increased when dopamine was severely depleted. There was no support for different estimates of a based on pre- and post-lesion performance of any lesion group, suggesting that dopamine loss has negligible effects on incentive motivation. The present study demonstrates the usefulness of combining operant techniques with a theoretical model to better understand the effects of a neurochemical manipulation.
Keywords: Dopamine, Parkinson’s Disease, motivation, motor, memory, operant behavior, fixed-ratio schedule, MPR
1. Introduction
Parkinson’s disease (PD) is characterized by the loss of mesotelencephalic dopamine (DA) that produces motor deficits including akinesia, bradykinesia, aphagia, adipsia, abnormal gait, and bradyphrenia [18,19,59,60]. Rats with nigrostriatal denervation of DA display deficits analogous to human PD patients, so this animal model has played a crucial role in elucidating unique relationships between motor deficits and neurochemical mediators [15,21,34,49,62].
In addition to motor deficits, deficits in learning and motivation have also been detected following neostriatal lesions [40]. The rat model of PD has been tested in a cued version of the water maze task and results showed that the nigrostriatal pathway plays an important role in associative learning [17]. Unfortunately, there is a paucity of data investigating the role of the nigrostriatal pathway in learning and motivation. Recently, the idea that the nigrostriatal system, specifically, serves a role in attribution of incentive salience and motivation in operant behavior was further bolstered by evidence in which DA signaling was restored using viral-mediated gene transfer of the Th gene delivered into the neostriatum of DA-depleted mice [47].
More generally, the hypothesis that compromising DA systems disrupts associative and motivational factors has gained support [3]. Pharmacological studies have shown that the speed of acquisition of lever-pressing for food is attenuated by pimozide, a DA antagonist [69], and extinction of an acquired response is enhanced by pimozide and haloperidol, another DA antagonist [37,70]. Results from knockout studies also suggest a linkage between DA and learning and motivational processes. DARPP-32 knockout mice trained to nose-poke under a simultaneous discrimination task demonstrated impairment in discrimination learning following a contingency reversal. Specifically, the knockout mice took longer than the wild-type controls to learn the new discrimination when the active and inactive holes were switched [23]. Also, DA-deficient mice performing a licking choice task had a smaller number of licks (consistent with a decreased motor ability) but their lick lengths were longer in duration, which was interpreted as an increase in reinforcer value [13,14].
Recently, Schultz and colleagues have shown that DA neurons are sensitive to deviations from reinforcer expectation when contingencies exist between a stimulus and the presentation (or omission) of reinforcement [24,61,66], suggesting a role for DA neurons in coding for predictive errors in associative learning [68]. The results from these studies support the idea that DA plays more than just a role in motor function. Unfortunately, the role of DA in associative learning is not always clear cut.
Studies have shown that haloperidol [2] or selective blockade of D1 or D2 receptors [12] in the nucleus accumbens does not interfere with tracking of the changes in reinforcer distribution and predictability. Specifically, the discriminability of reinforcer magnitude does not appear to be compromised by receptor blockade or DA depletion in the nucleus accumbens [35,52]. In light of this evidence, it appears that reinforcement efficacy, but not the ability to track its occurrence, is dependent on DA activation. To further complicate matters, accumbens DA depletion [36,54,55] and blockade of D1 or D2 receptors [46] does not generate extinction-like effects. If the activity of DA were necessary for reinforcement, then we would expect such extinction when it is depleted or its uptake blocked.
Much of what is known about motor and motivational functions of DA derives from the work of Salamone and his colleagues on the mesolimbic pathway rather than the nigrostriatal pathway. These investigators [50,51] have contended that the effects of accumbens DA manipulations on operant behavior are symptomatic of changes in the capacity to emit an effortful response and not of decreases in reinforcement efficacy. In other words, responding is attenuated by DA depletion not because of motivation-dampening processes but because of the heightened cost of effort required to emit the response. For instance, the pharmacological blockade of D1 and D2 receptors in the core or shell of nucleus accumbens reduces locomotion toward food but not amount of food ingested [4]. According to this explanation, the effects of accumbens DA depletion on operant behavior should be highly dependent on the schedule of reinforcement. Consistent with this hypothesis, Salamone and associates have shown that DA depletion causes a greater reduction in responding in larger ratio schedules than in smaller ones [1,57]. However, whereas DA depletion disrupts responding under ratio schedules, it apparently does not disrupt responding under increased time or force requirements [25,38].
The hypothesis that DA depletion induces effort-related but not motivational deficits is supported by evidence from concurrent lever pressing/chow feeding procedures [31,56]. In this procedure, rats are exposed to a choice between lever pressing for a preferred food (Bioserve pellets) or eating a freely available but nonpreferred food (chow). When no lever pressing was required to obtain the pellets, both control and DA-depleted rats preferred the pellets over chow. When lever pressing was required, control rats chose lever pressing for pellets more often than chow, whereas rats with low to moderate depletions of accumbens DA chose the freely available chow rather than work for their (preferred) pellets. The change in food preference in DA-depleted rats when lever pressing is required suggests an effort-related impairment caused by DA depletion. In conclusion, the research by Salamone and colleagues suggests that the primary mechanism responsible for the reductions in operant behavior in DA-depleted animals is a compromised “activational” system [51] and not a compromised motivational system.
For the purposes of the current study, Salamone’s work on mesolimbic contributions to motivated behavior can guide some of the interpretations from the data collected and reported herein, since there is a dearth of literature investigating learning and motivational factors based on nigrostriatal DA lesions. We were interested how a mathematical model might be sensitive to disruptions of nigrostriatal DA in reward-seeking behavior as it codes for learning, motivational, and motor effects. Mathematical Principles of Reinforcement (MPR) [28,29,44] has attempted to achieve a formal approach that explicates the relation between these processes. Such a theoretical trajectory is becoming critically important insofar as the role of DA in the acquisition and extinction of conditioned responses is becoming more recognized. For example, Schultz [61] has identified DA activation as the neural basis of the error term of the Rescorla-Wagner and temporal difference reinforcement models [45,64]. From a foraging-model perspective (e.g., [63]), Aparicio [2] suggested that DA modulates the cost of traveling between patches of food, which is detectable in rapidly-changing choice environments by changes in the bias term of the Generalized Matching Law [5]. Mapping DA manipulations to changes in model components is important not only because it identifies what changes in DA activation do, but because it also identifies what they do not do: Presumably they do not affect the salience of conditioned or unconditioned stimuli (Rescorla-Wagner model) and do not modulate sensitivity of choice to rate of reinforcement (Generalized Matching Law).
The empirical evidence, however, is outpacing theoretical treatments. Our purpose in the present study is to evaluate MPR by explicitly differentiating mnemonic (learning), motivational, and motor processes in instrumental conditioning in a rodent model of PD. The core assumptions of MPR are: (1) activity is elicited by reinforcers; (2) there are time constraints on responding; (3) stimuli and responses are encoded in working memory as they happen; as working memory becomes saturated, older items are displaced by newer items. Reinforcement operates on activities contemporaneously represented in working memory; the temporal roll-off of older items in memory constitutes the delay of reinforcement gradient. MPR specifies three parameters, each associated with one of these different principles, to describe operant performance: The amount of activation elicited by a reinforcer a (specific activation); the minimum duration of the target response δ (delta); and the rate of saturation of working memory λ (lambda). Changes in a, δ, and λ correspond to changes in incentive motivation, motor ability, and working memory saturation, respectively. When the general MPR model is applied to fixed-ratio (FR) schedules, response rates (B) are a function of the ratio requirement N in the form:
| (1) |
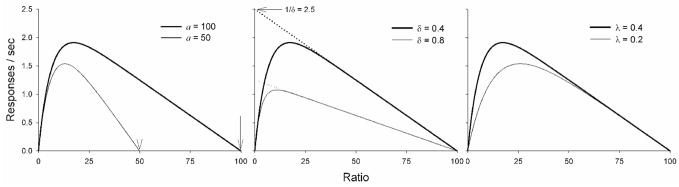
The theory is outlined by Sanabria et al. [58], and described in mathematical detail by Killeen and Sitomer [29]. An important feature of MPR is that changes in parameter values can be detected in a function that relates response rates to ratio requirements (Figure 1).
Figure 1.
Theoretical response rate functions for ratio schedules specified by MPR (Equation 1). Left Panel: The descending limb intercepts the x-axis at a (signaled by arrows), thus providing a measure comparable to the breakpoint in a progressive-ratio procedure. Middle Panel: Extrapolation to the left (dotted lines) intercepts the y-axis at the maximum response rate, 1/δ. Right Panel: The increase in rate over small to moderate ratios is due to the increasing saturation of memory with the target response, with the rate of saturation given by λ; the mean capacity is 1/λ seconds of responding. If the rate is fast, perhaps because of a small memory capacity or complicated response, the function will quickly rise into the decreasing asymptote of the right limb. Larger capacities, associated with smaller values of λ, will rise more slowly.
All identifications of parameters with motivational or motor effects are, of course working hypotheses. They are not, however, ad hoc. They have been erected on simple models of the constituent processes, and the framework has been modified to keep the parameters orthogonal (e.g., by Killeen and Sitomer [29]). Even if this effort has been successful, it is likely that physiological insults will often affect more than one system. We rely on parsimony, as affected by the index of merit described below, to parse real effects from noise.
Validation studies of MPR have demonstrated that changes in the quality and quantity of food reinforcers is described by changes in a for rats (e.g. [6]) and pigeons [7], whereas changes in the force required for a response is described by changes in δ alone [7,43]. That is, a and δ account for incentive-motivational and motor factors in performance, respectively. Prior applications of the model to account for the behavioral effects of neuroleptics (clozapine, haloperidol, and chlorprozamine) indicated divergent effects on a but a systematic increase in δ [39,71,72]. These results are consistent with the assertion, proposed by Salamone and his colleagues [51], that DA depletion produces primarily effort-related deficits.
Whereas a (motivation) sets a ceiling for response-sustaining ratios, and 1/δ (motor ability) sets a ceiling for response rate the role of λ (rate of memory saturation) is less obvious. To illustrate λ, consider an animal whose working memory is quickly saturated (λ = 0.4) such that half of its contents were lost after 2.5 s. Reinforcement following a short ratio would occur when memory is likely to be filled only with lever presses, and thus lever pressing would be more effectively reinforced. If memory was more slowly saturated (λ = 0.2) such that half of its contents were lost after 5 s, reinforcement following a short ratio would occur when memory is likely to include pre-ratio activity, and thus lever pressing would be less effectively reinforced. This difference in the effectiveness of reinforcement following short ratios is illustrated in the right panel of Figure 1.
The present study was designed to complement the pharmacological studies conducted thus far on mesotelencephalic DA systems by using bilateral, intranigral infusions of 6-hydroxydopamine (6-OHDA) to produce a chronically-depleted state. In order to evaluate the impact of dopaminergic lesions on motor ability and motivation, rats must be exposed to larger ratio values beyond FR 30 used in Linder et al. [33]. This is because the parameter a in MPR is equal to the x-intercept or the point at which responding ceases. Thus sufficiently large ratios must be employed to generate data points that will determine the slope of the descending limb and ultimately increase our confidence in the estimation of a. We are also interested on how performance varies with extent of bilateral lesion size. Consistent with prior research, we predicted that decreases in operant behavior are due to motor deficits and not due to reduced memory capacity or motivation. Specifically, these effects would be described by MPR as increases in δ but no changes in a or λ (middle panel in Figure 1).
2. Methods
2.1 Subjects
Male Sprague Dawley rats initially weighing between 300–350 gm were randomly assigned to two groups: lesion (n = 25) and control (n = 15). Rats were food restricted and maintained at 85% of their free-feeding body weights (ffw) during training and testing. The rats were given free access to food two days prior to surgery and food restricted twenty-four hours prior to surgery. Water was freely available ad libitum in the home cage. Rats were maintained on a reverse 12:12 hour light dark cycle (lights on at 1800 hours) and were tested during the dark cycle. The protocol for this experiment was approved by the Arizona State University Institutional Animal Care and Use Committee (Protocol #00-549R), and experiments were carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80-23) revised 1996.
2.2 Intranigral 6-OHDA Microinfusions
Rats were anesthetized with 50 mg/kg sodium pentobarbital (i.p.). All rats were pretreated with 25 mg/kg desipramine HCl (i.p.), a noradrenergic reuptake blocker, 30 min prior to the infusion of the neurotoxin 6-OHDA to protect norepinephrine cells. All drugs were dissolved in distilled water. Standard stereotaxic procedures were used to target the substantia nigra. With bregma and lambda horizontal, the coordinates from skull surface were: AP −5.8 mm, ML +2.5 mm, and DV −8.0 mm. Bilateral infusions of 6-OHDA were made via 30 gauge stainless steel injector cannulae. One minute later, after placement of injectors, 6-OHDA HBr (2.4 μg/μl in deoxygenated saline with 0.01% ascorbate) was infused via a syringe pump at a rate of 0.2 μl/min over 12.5 min via a syringe pump (total 6 μg/2.5 μl). Each needle was connected to polyethylene tubing that was attached to a 25 μl Hamilton gastight syringe. The injector remained lowered for two minutes post infusion to allow for diffusion from the tips. During a two week postoperative recovery period, lesion rats were supplemented with cookie (Chip’s Ahoy, Nabisco) and lab chow mash mixed with liquid vitamins (children’s liquid vitamins, Enfamil). For animals with severe aphagia, peanut butter was also added to the mash. Baseline weights were re-established and each rat’s 85% ffw weight was re-calculated based on post-lesion weights. The rats in the control group were treated identically to their lesion counterparts except the infusion cannulae were not lowered into the brain and no infusions were given. DA loss was determined by post-mortem assay of the neostriatum and nucleus accumbens. Animals were then assigned to three subgroups based on the notion that the extent of DA depletion produces a different profile of loss and recovery of function [74,75]. Animals with a small DA lesion display sparing of motor ability although they posses cognitive deficits [21]. Animals with DA depletions between 60–80% display aphagia and adipsia but recover over time. In contrast, animals with DA depletions greater than 90% display a permanent loss of ingestive abilities. Based on this literature (e.g., [15]), animals in the present study were grouped according to DA lesion size: severe unilateral, moderate bilateral, and severe bilateral.
2.3 Operant Testing
2.3.1 Apparatus
Five rodent chambers (32 L × 33 H × 25.3 W cm, Med Associates, St. Albans, VT, USA) were contained in sound-attenuating cubicles each with a house light and exhaust fan that helped to mask external noise. Chambers were equipped with one retractable lever that required a force of approximately 0.17 N to activate. The lever was 6.4 cm from the floor and 3.7 cm to the left of the food hopper where pellets (Noyes® Precision pellets for rodents, 45 mg) were dispensed. The food hopper was 2.7 cm from the floor and centered on the work panel. The operant chambers were controlled by Med-PC software. A house light provided general illumination, and three LEDs lined up horizontally (left-colored red, middle-yellow, and right-green) 4.7 cm above the lever served as cues.
2.3.2 Procedure
Rats were autoshaped to lever press [9,10]. Next, the rats were trained to lever press under increasing ratio requirements until they reliably earned 60 food pellets on an FR 30 schedule (approximately 8 days). Rats were then exposed to a five-component multiple schedule for 15 days. A 5-min acclimation period, during which no stimuli were present, preceded each session. During each session, five ratio schedules (FR 5, 15, 30, 60 and 100) were presented in an ascending order, with each ratio in effect for 10 reinforcers or until 20 min elapsed. The ratio schedules were differentially signaled by the position of the LEDs: FR 5: left position, FR 15: middle position, FR 30: right position, FR 60: middle/right position, and FR 100: middle/left position. Each component was separated by a 30-s inter-component interval. Sessions took place at the same time each day, 6 days a week for 15 days. Subsequently, about two thirds of the rats were given bilateral DA lesions and the other third received only burr-holes. After 14-days post lesion, the rats were re-exposed to the five-component multiple schedule for another 15 days.
2.4 Biochemical Analysis
Immediately following the completion of behavioral assessment, which took place 4 weeks after 6-OHDA infusions, the rats were decapitated and their brains were immediately removed to determine the extent of DA depletion. Decapitation was necessary to avoid drug-induced changes in brain neurotransmitter content that occurs with anesthetics [22,26,48]. Brain dissections of the neostriatum and nucleus accumbens were obtained by the method established by Heffner, Hartman and Seiden (1980). Briefly, a 2 mm coronal section through the neostriatum was sliced in a tissue dissection block, and the neostriatum and nucleus accumbens were removed by using a 2-mm and 1-mm tissue punch, respectively. Tissue from each hemisphere was assayed separately. Each piece of tissue was weighed and then placed in microcentrifuge tubes containing 400 μl of 0.05 N perchloric acid and dihydroxybenzylamine (1 ng/μl) as an internal standard. Samples were homogenized and centrifuged (4000 rpm at 4° C for 25 min) and the supernatant was filtered (Nalgene 4 mm syringe filters 0.45 and 0.2 μm pore size) before storing at −20° C.
High performance liquid chromatography coupled with electro-chemical detection (HPLC-EC) was used to determine the concentrations of the monoamines in the neostriatum and nucleus accumbens tissue [65]. The supernatant was injected into a 4.6 mm × 5 cm Varian Microsorb-MV 3 μm, 100 μm pore size C18 column, (Varian, Walnut Creek, CA). The mobile phase (60mM NaH2PO4, 30 mM citric acid, 0.1 mM EDTA, 37 mg/L sodium dodecyl sulfate, 20% MeOH, and pH 3.75 with the latter three components adjusted to optimize separation as required) was pumped at a rate of 0.5–1.5 ml/min. A conditioning cell (ESA Model 5021) was placed after the analytical column and set to oxidize at +100 mV. A dual electrode model 5011 High Sensitivity Analytical Cell and Model 5200 Coulochem II detector (ESA) was used to collect chromatographic data. The first analytical cell was set to +340 mV and 200 nA sensitivity to measure the oxidation of DA, dihydroxyphenyl acetic acid (DOPAC), homovanillic acid (HVA), 5-hydroxytryptamine (5-HT, serotonin), 5-hydroxyindoleacetic acid (5-HIAA), norepinephrine (NE) and the internal standard dihydroxybenzylamine. When DA and 5-HT were not measurable on the oxidation signal, reduction at the second electrode, set to −350 mV and 50 nA sensitivity, provided a high sensitivity signal of these depleted monoamines. A Linear Model 1200 dual pen recorder was used to record peak heights (Alltech, Deerfield, IL). Peaks from eight serially diluted standards were used to calculate the concentration of the monoamines based on linear regression analysis.
2.5 Statistical Analysis
Response rates were calculated by dividing the number of responses in each multiple-schedule component by the duration of that component, including the post-reinforcement pause (PRP) and excluding the duration of reinforcement. Next, response rates were averaged for the last ten sessions before the lesion (pre-lesion) and the last ten sessions after the lesion (post-lesion) for each rat. Average response rates were pooled into three groups defined by the extent of striatal DA depletion.
Parameters from MPR were estimated by minimizing the squared deviations between Equation 1 and the mean response rates of individual rats during pre-lesion and post-lesion sessions. We wanted to know whether parameters would change between control and experimental conditions. However, some change due to sampling error is inevitable. To restrict attention to those changes that were meaningful, we utilized a model-comparison approach [11]. We evaluated the improvement in goodness-of-fit in relation to the number of free parameters in a model, and used the corrected Akaike Information Criterion (AICc) for admitting additional parameters [11]. This is analogous to a test of significance. However, traditional significance tests are unable to say which parameters need to be allowed to change, and which are relatively constant. The model comparison approach does this by taking into account both the goodness of fit and the number of data points, to set a criterion (AIC) for the minimal improvement conferred by introduction or variation in a parameter. Improvements below that level are just “fitting noise”. Whenever we cite the need for changing a parameter, it is because it has crossed this threshold for paying its way. The newest version of AIC, AICc, has an additional correction for situations with a relatively small number of data [11]; it is this that we employed. Thus, to claim that a parameter was affected required more than a nominal increase in goodness of fit when it was allowed to vary: It crossed a threshold akin to α = .05.
We compared models that allowed various combinations of parameters (a, δ, λ) to vary across experimental conditions (lesion size, pre-lesion versus post-lesion testing). Smaller values of AICc are indicative of better account of the data given the degrees of freedom in the model. In addition to requiring that parameters cross the threshold of AICc for entry as factors that were affected by the manipulation, a more intuitive mode of statistical presentation is possible. The strength of evidence for each model was represented as Akaike weights, which indicate the probability that the model is the best (i.e., with replication, will continue to have the lowest AICc) among those compared. Details on the computation of weights are provided in the appendix; their formal justification is provided by Burnham and Anderson [11]. The model with the highest Akaike probability indicated which parameters varied between pre- and post-lesion testing for each lesion group; models within about 5 points of the highest Akaike weight were also viable contenders for the “best” model. In this analysis, the “null hypothesis”—the absence of change in any parameter across experimental conditions—is just one model among many combinations of changed and preserved parameters. The assignment of the highest Akaike weight to the “no change” model is akin to the finding of “no significant change” in null-hypothesis tests.
Pre-lesion Performance
To test for differences in pre-lesion response rates across groups, AICc obtained from fitting a single value for each free parameter of Equation 1 (a, δ, λ) to all pre-lesion response rates (i.e., collapsed across all groups) was compared to the AICc obtained from varying each free parameter across lesion groups.
Post-lesion Performance
To test the effect of lesion size on model parameters, each lesion group was analyzed separately. For each lesion group, each possible combination of parameters was allowed to vary between pre- and post-lesion conditions, while the remaining parameters were held constant. The lowest AICc across parameter combinations identified the parameters that were affected by the lesion.
3. Results
3.1 Postmortem DA levels
Five weeks following lesion, neostriatum and nucleus accumbens samples were assayed bilaterally. Table 1 shows the DA levels (ng/mg), as a range and mean (SEM), for the sham-lesion/controls and rats treated with 6-OHDA. For animals with similarly moderate or severe bilateral lesions, the two hemispheres were averaged together. Animals with DA depletions were stratified into groups defined by their percent loss of neostriatal DA based upon control group levels: unilateral severe lesion (Unilateral Severe, N=8), 80–90% loss of DA unilaterally; bilateral moderate lesion (Moderate, N=11), 50–79% loss of DA for each hemisphere; and bilateral severe lesion (Severe, N=6), 80–99% loss of DA for each hemisphere. Analysis of all behavioral data was based on this classification. Animals with a mild DA depletion (<50% bilaterally) were not different than the control animals in their distribution of postmortem tissue DA levels or in their behavioral measures and therefore not reported.
Table 1.
Dopamine levels (ng/mg protein) in the neostriatum: range, mean (SEM) for the intact and lesion hemisphere.
| Group | Sample Size | Neostriatum (ng/mg) | Sample Size | Nucleus Accumbens** (ng/mg) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intact Hemisphere* | Lesion Hemisphere | Intact Hemisphere* | Lesion Hemisphere | |||||||
| Range | Mean (SEM) | Range | Mean (SEM) | Range | Mean (SEM) | Range | Mean (SEM) | |||
| Control | 15 | 7.53–18.39 | 13.30 (0.56) | 7 | 3.10–12.6 | 7.7 (0.87) | ||||
| Unilateral Severe | 8 | 3.06–17.15 | 10.60 (1.86) | 0.05–2.19 | 1.50 (0.3) | 4 | 7.92–12.09 | 10.6 (0.7) | 0.2–9.95 | 6.1 (1.97) |
| Moderate | 11 | 3.03–17.19 | 7.80 (1.01) | 6 | 4.9–10.6 | 7.8 (0.5) | ||||
| Severe | 6 | 0.08–2.26 | 0.93 (0.2) | 3 | 1.2–7.3 | 5.2 (0.84) | ||||
All lesion animals received bilateral infusions of 6-OHDA. Values listed under the intact hemisphere column for the unilateral severe lesion group represent the less affected hemisphere. Only 2 animals had less than 7.53 ng/mg in the neostriatum (lowest range for the control animals).
Nucleus accumbens samples were not collected from all animals.
Nucleus accumbens samples were collected midway through the study because we were initially focused on neostriatal lesions as a result of intranigral 6-OHDA infusions. Table 1 demonstrates the few animals we assayed nucleus accumbens DA levels, the lesion sizes were always relatively smaller than the neostriatal lesions as expected (13).
3.2 Pre-lesion Performance
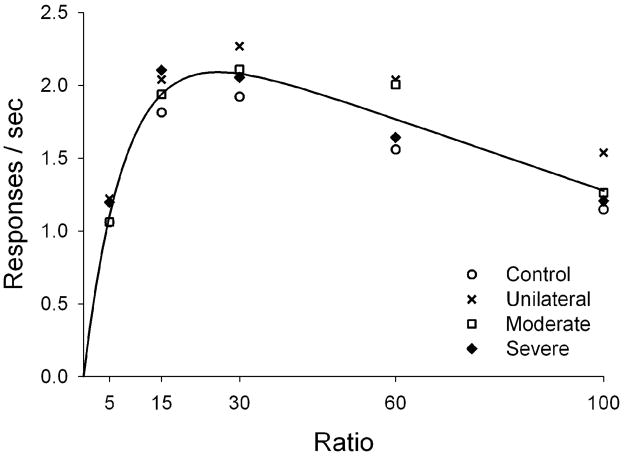
After grouping the rats by lesion size, we examined pre-lesion performance in the operant task; we expected similar performance across all groups at this stage. Figure 2 shows that response rates of all groups were a bitonic function of the ratio schedule. In all cases, intermediate ratios (15 and 30) generated maximal responding, whereas extreme ratios (5 and 100) generated minimal responding. This is a standard finding.
Figure 2.
Mean response rates under the multiple FR schedule during the last 10 sessions of baseline. The curve is drawn by Equation 1 using a single set of parameters, as dictated by the parsimony intrinsic to AICc. Lesion groups are represented by different symbols.
The single set of free parameters from Equation 1 that best described pre-lesion performance was a = 204, δ = 0.40, λ = 0.31 (solid line in Figure 2). These values yielded a smaller AICc (−82.4) than fitting each parameter to each lesion group (AICc = −68.6). Absolute values of AIC are not meaningful, but the difference between them—here 13.8 points—indicates that the chosen model is about e14 better than the next contender. Naturally, fitting was improved when parameters were allowed to vary freely across groups. AICc indicates, however, that this improvement is not justified, given the cost in parsimony lost to additional free parameters. Baseline performance is thus well described by a single set of parameters, suggesting that behavior was not significantly different between groups prior to lesion.
3.3 Post-lesion Performance
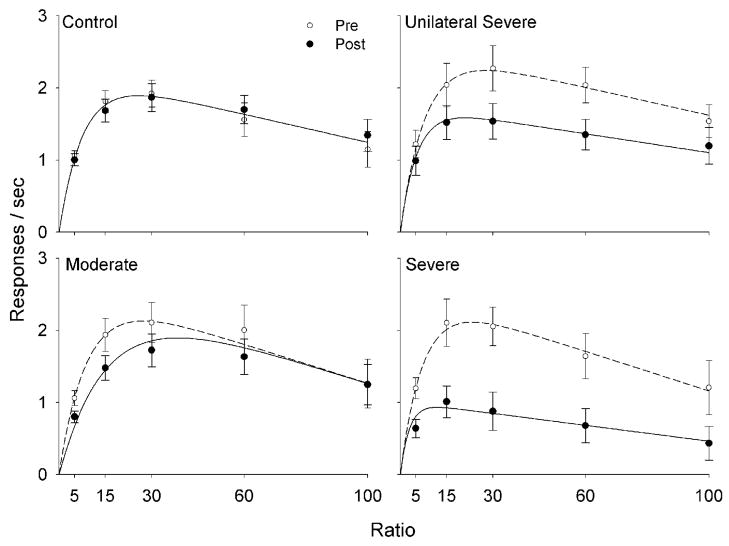
Post-lesion performance was also well characterized by bitonic response rate functions, regardless of lesion size (Figure 3). In the control group, pre- and post-lesion response rates were similar. Response rates in all 6-OHDA-treated groups, however, peaked at lower levels than before lesion; larger lesions were related to larger reductions in response rate.
Figure 3.
Mean response rates under the multiple FR schedule during the last 10 sessions of baseline and post-lesion. The mean response rates are represented as solid circles for measures recorded before 6-OHDA lesion (Pre) and by open circles for measures recorded after the lesion (Post). The curves were drawn by Equation 1, using parameters from Table 2; for the Moderate group, parameters correspond to the model that assumes a change in minimum response duration, δ.
Table 2 shows the Akaike weights obtained from the model comparison analysis. The most likely model to be best for the Control group indicates no change in MPR parameters between pre- and post-lesion performance (wNone = .35). For the Moderate group, the model most likely to be best assumed only a decrease in memory saturation (wλ = .24). Nonetheless, it is also likely that only the minimum response duration increased (wδ = .20); the latter effect is shown in Figure 3. In both Severe lesion groups it is more certain that only δ changed between pre- and post-lesion performance, with Akaike weights around .5.
Table 2.
Akaike weights for models within each lesion group.
| Group | Model |
|||||||
|---|---|---|---|---|---|---|---|---|
| None | a | δ | λ | a, δ | a, λ | δ, λ | a, δ, λ | |
| Control | .35 | .17 | .13 | .14 | .07 | .07 | .06 | .02 |
| Unilateral Severe | .01 | .04 | .50 | .01 | .18 | .03 | .17 | .06 |
| Moderate | .13 | .07 | .20 | .24 | .10 | .09 | .12 | .04 |
| Severe | .00 | .00 | .53 | .02 | .16 | .04 | .19 | .06 |
Note. Models are labeled with the parameters that could vary freely between pre-and post-lesion conditions (column headers). The Akaike weight is indicative of the relative strength of a model being best among those compared within each lesion group (rows add to 1). The best model for each lesion group is underlined.
Table 3 shows the parameter values that best describe pre- and post-lesion performance in each lesion group. For parameters that appear to be affected by DA depletion, the values for pre- and post-lesion performance are reported separately (refer to Note in Table 3). For the Control group, the absence of significant changes in parameter values indicates that, as expected, their pre-and post-lesion performances were not systematically different. For the Moderate lesion group, changes in performance due to DA depletion may be described by a 43% reduction in λ or by a 24% increase in δ. For the Unilateral Severe and Severe groups, changes in performance due to DA depletion are captured by 46% and 154% increases in the minimal time required for a response, δ. The overall pattern of the data suggest that the results may be summarized in the following way: The parameter a remained approximately constant at a value of over 200; λ remained approximately constant at around 0.39; and δ increased systematically with the degree of dopamine depletion.
Table 3.
Quantitative indices of performance on multiple FR schedules as a function of lesion severity.
| Group | Model | a (resps) | δ (s) | λ (s−1) |
|---|---|---|---|---|
| Control | No Change (w = .35) | 228 | 0.45 | 0.29 |
| Unilateral Severe | δ (w = .50) | 268 | 0.39 → 0.57 | 0.33 |
| Moderate | λ (w = .24) | 194 | 0.38 | 0.30 → 0.17 |
| δ (w = .20) | 213 | 0.37 → 0.46 | 0.25 | |
| Severe | δ (w = .53) | 185 | 0.39 0.99 | 0.35 |
Note. The arrows indicate changes in parameter (Pre → Post); no arrows indicate that parameter estimates were constant across pre- and post-lesion conditions. Two models are shown for the Moderate lesion group, because their likelihood of being best is similar. The units of the parameters are given in parentheses; resps stands for number of responses.
4. Discussion
Response rates were generally reduced as a function of DA depletion, while maintaining the bitonic relation with fixed ratio values predicted by MPR. With the parameters identified in Table 3, MPR accounted for 98% of variance in mean pre- and post-lesion group performance. No meaningful changes in response rates were observed in comparisons between the pre-lesion and post-lesion values within the Control group, permitting us to infer that the DA depletions contributed to the observed decrements in 6-OHDA-treated rats.
Response rates of the rats with moderate lesions decreased across all ratios except the largest (FR 100); those with severe lesions decreased across all ratios. These results replicate those reported by Salamone and colleagues [1,57], who provided an account of these effects in terms of the effort that an animal would exert to obtain a reinforcer. Inferences based on MPR reinforce that parsimonious account: 6-OHDA lesions increased the minimum time to complete a response, indicated by δ. This conclusion does not specify why animals take longer to complete each response, and thus increased effortfullness is a plausible hypothesis. It may require additional motivation to make up for that handicap, and absent that, rates will be depressed. But the absence of changes in a suggests that motor deficit was the primary cause of the rate changes.
A noteworthy outcome in the present study was thus the finding that no changes in a, specific activation, could be inferred from differences in performance before and after lesion, supporting the conclusion that there were no systematic changes in motivation for food as a function of lesion size. The present findings complement previous evidence that decreases in mesotelencephalic DA function interferes with motor function but does not impair a, rat’s motivation for reinforcers [1,20,53,56,57].
The effect of moderate lesions is somewhat ambiguous. Although it is possible that rate of memory saturation (λ) decreased for this group, as indicated by the AIC analysis, it is also possible that instead the minimum response duration (δ) increased, as indicated by the runner-up model. The latter scenario is consistent with the motor effect of more severe lesions. The former scenario implies that moderate lesions do not affect motor ability, but facilitate the reinforcement of non-target behavior that interferes with instrumental conditioning at small and intermediate ratios. This effect is not altogether surprising, because it is well established that locomotion is spared by partial lesions [73], and partial lesions of the substantia nigra pars compacta are known to cause learning and memory deficits without sensorimotor deficits [16]. As Da Cunha and associates point out, these partial lesions “modeled the early phases of PD when cognitive impairments are observed but the motor symptoms of the disease are barely present” (16, p. 227). Schallert has previously demonstrated that cognitive deficits exist well before motor impairments are manifest [21]. It is plausible that the comparator function of limbic striosomal-motor matrisomal interactions are more sensitive to DA disruption than are the permissive functions of basal ganglia motor function [8,41,42]. The lesion-induced reduction in rate of memory saturation, if replicable, has intriguing implications that requires further testing to delineate more clearly the functional roles of mesotelencephalic DA systems in motor, motivational and associative aspects within the operant paradigm. It remains peculiar that in the present study memory was apparently unaffected with the most severe lesions.
The present results demonstrate how MPR successfully detects motor deficits in the rodent model of PD, in agreement with a well-documented literature involving nigrostriatal DA depletions. What remains elusive is a clear-cut demonstration of the ability of MPR to detect changes in mnemonic or motivational aspects related to the loss of mesotelencephalic DA. This problem is reminiscent of the binding problem [32], which suggests that performance may not be dissociable from deficits in either motor, mnemonic or motivational capacities (e.g.,[67]). Fortunately, Killeen and his colleagues have demonstrated the dissociability of MPR’s components utilizing simple manipulations with normal animals. It will be important to test MPR in future studies involving the rodent model of PD by experimentally manipulating, for example, workload by changing the resistance of the manipulandum [7,39,43,44,71,72], incentive value of the reinforcer by increasing reinforcer palatability [6,7,27,30,39,44,58,71,72], or memory demand by changing the reinforcer duration [6,7,44]. The availability of an explicit quantitative model of behavior increases the power and precision of experiments. Whereas traditional statistical inference generally used instantiations of the general linear model (e.g., ANOVA) and tested for nonnegligible differences between groups, MPR may be mapped to data using the Akaike criterion (AIC). This permits inferences to which processes, not groups, are affected by a manipulation. Because it is a novel metric, we deployed a more intuitive relative measure, the Aikaike weights, to better display the relative merits of the more-or-less restricted models. Together, MPR and AIC may be a more efficient and economical approach to infer whether experimental manipulations alter motor, mnemonic or motivational components of behavior.
Acknowledgments
This work was supported in part by Arizona State University-Office for Vice Provost for Research and the ASU Hispanic Research Center (Edward Castañeda) and NIDA-KO1 DA00485 (Mark P. Reilly). Irene Avila was supported by a predoctoral fellowship NIH-5 T32 MH18882 from the American Psychological Association Diversity Fellowship Program in Neuroscience; Diana Posadas-Sánchez was supported by NIMH R01MH066860; Claudia L. Chavez was supported in part by a grant from the National Institutes of Health through the Minority Access to Research Careers program NIH-5 T34 GM08491-10; Nikhil Banerjee was supported in part by a grant from the Howard Hughes Medical Institute through the ASU Undergraduate Biology Enrichment Program.
Appendix
The corrected Akaike Information Criterion (AICc) was computed for each model i, within each lesion group, as
| (A1) |
where k is the number of free parameters, n is the number of observations, and RSS is the residual sum of squares, which is minimized when fitting the model to data. When the number of observations is large relative to the number of parameters, the bracketed term reduces to 1, the simpler AIC.
The number of free parameters in our study, k, was 2v + c + 1, where v is the number of parameters that were varied across pre- and post-lesion conditions, and c is the number parameters that were held constant (the added unit corresponds to the estimate of error variance, de facto an additional parameter for all models). Three parameters might—or might not—have been significantly affected by the manipulation: a, δ, and λ. Rearranging yields k = v + 4. The number of observations, n, was the number of subjects in the lesion group times the number of multiple-schedule components (5), times 2 (the pre- and post-lesion conditions).
Aikaike criteria are logarithmic; to convert them to relative probability, exponentiate each and divide by the scores for all eight competitors:
| (A2) |
An Akaike weight may be interpreted as the probability that a model is the “best” among those compared, given the data [11].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aberman JE, Salamone JD. Nucleus accumbens dopamine depletions make rats more sensitive to high ratio requirements but do not impair primary food reinforcement. Neuroscience. 1999;92:545–552. doi: 10.1016/s0306-4522(99)00004-4. [DOI] [PubMed] [Google Scholar]
- 2.Aparicio CF. Haloperidol, dynamics of choice, and the parameters of the matching law. Behav Proces. 2007;75:206–212. doi: 10.1016/j.beproc.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 3.Baldo BA, Kelley AE. Discrete neurochemical coding of distinguishable motivational processes: Insights from nucleus accumbens control of feeding. Psychopharmacol. 2007;191:439–459. doi: 10.1007/s00213-007-0741-z. [DOI] [PubMed] [Google Scholar]
- 4.Baldo BA, Sadeghian K, Basso AM, Kelley AE. Effects of selective dopamine D1 or D2 receptor blockade within nucleus accumbens subregions on ingestive behavior and associated motor activity. Behav Brain Res. 2002;137:165–177. doi: 10.1016/s0166-4328(02)00293-0. [DOI] [PubMed] [Google Scholar]
- 5.Baum WM. On two types of deviation from the matching law: Bias and undermatching. J Exp Anal Behav. 1974;22:231–242. doi: 10.1901/jeab.1974.22-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bizo LA, Kettle LC, Killeen PR. Rats don’t always respond faster for more food: The paradoxical incentive effect. Anim Learn Behav. 2001;29:66–78. [Google Scholar]
- 7.Bizo LA, Killeen PR. Models of ratio schedule performance. J Exp Psychol: Anim Behav Proces. 1997;23:351–367. doi: 10.1037//0097-7403.23.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brooks VB. How does the limbic system assist motor learning? A limbic comparator hypothesis. Brain Behav Evol. 1986;29:29–53. doi: 10.1159/000118670. [DOI] [PubMed] [Google Scholar]
- 9.Brown PL, Jenkins HM. Conditioned inhibition and excitation in operant discrimination learning. J Exp Psychol. 1967;75:255–266. doi: 10.1037/h0024985. [DOI] [PubMed] [Google Scholar]
- 10.Brown PL, Jenkins HM. Auto-shaping of the pigeon’s key-peck. J Exp Analy Behav. 1968;11:1–8. doi: 10.1901/jeab.1968.11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burnham KP, Anderson DR. Model selection and multimodel inference: A practical information-theoretic approach. 2. Springer-Verlag; New York: 2002. p. 488. [Google Scholar]
- 12.Calaminus C, Hauber W. Intact discrimination reversal learning but slowed responding to reward-predictive cues after dopamine D1 and D2 receptor blockade in the nucleus accumbens of rats. Psychopharmacol. 2007;3:551–566. doi: 10.1007/s00213-006-0532-y. [DOI] [PubMed] [Google Scholar]
- 13.Cannon CM, Bseikri MR. Is dopamine required for natural reward? Physiology and Behavior. 2004;81:741–748. doi: 10.1016/j.physbeh.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 14.Cannon CM, Palmiter RD. Reward without dopamine. J Neurosci. 2003;23:10827–10831. doi: 10.1523/JNEUROSCI.23-34-10827.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castañeda E, Whishaw IQ, Robinson TE. Changes in striatal dopamine neurotransmission assessed with microdialysis following recovery from a bilateral 6-OHDA lesion: Variation as a function of lesion size. J Neurosci. 1990;10:1847–1854. doi: 10.1523/JNEUROSCI.10-06-01847.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Da Cunha C, Angelucci MEM, Canteras NS, Wonnacott S, Takahashi RN. The lesion of the rat substantia nigra pars compacta dopaminergic neurons as a model for Parkinson’s disease memory disabilities. Cell Mol Neurobiol. 2002;22:227–237. doi: 10.1023/A:1020736131907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Da Cunha C, Wietzikoski S, Wietzikoski EC, Miyoshi E, Ferro MM, Anselmo-Franci JA, Canteras NS. Evidence for the substantia nigra pars compacta as an essential component of a memory system independent of the hippocampal memory system. Neurobiol Learn Mem. 2003;79:236–242. doi: 10.1016/s1074-7427(03)00008-x. [DOI] [PubMed] [Google Scholar]
- 18.Fahn S. The history of Parkinsonism. Move Disord. 1989;4:S2–S10. doi: 10.1002/mds.870040502. [DOI] [PubMed] [Google Scholar]
- 19.Fahn S. Description of Parkinson’s disease as a clinical syndrome. Ann NY Acad Sci. 2003;991:1–14. doi: 10.1111/j.1749-6632.2003.tb07458.x. [DOI] [PubMed] [Google Scholar]
- 20.Farrar AM, Pereira M, Velasco F, Hockemeyer J, Muller CE, Salamone JD. Adenosine A(2a) receptor antagonism reverses the effects of dopamine receptor antagonism on instrumental output and effort-related choice in the rat: Implications for studies of psychomotor slowing. Psychopharmacol. 2007;191:579–586. doi: 10.1007/s00213-006-0554-5. [DOI] [PubMed] [Google Scholar]
- 21.Hall S, Schallert T. Striatal dopamine and the interface between orienting and ingestive functions. Physiol Behav. 1988;44:469–471. doi: 10.1016/0031-9384(88)90307-1. [DOI] [PubMed] [Google Scholar]
- 22.Hamilton ME, Mele A, Pert A. Striatal extracellular dopamine in conscious vs. anesthetized rats: Effects of chloral hydrate anesthetic on responses to drugs of different classes. Brain Res. 1992;597:1–7. doi: 10.1016/0006-8993(92)91498-4. [DOI] [PubMed] [Google Scholar]
- 23.Heyser CJ, Fienberg AA, Greengard P, Gold LH. DARPP-32 knockout mice exhibit impaired reversal learning in a discriminated operant task. Brain Res. 2000;867:122–130. doi: 10.1016/s0006-8993(00)02272-1. [DOI] [PubMed] [Google Scholar]
- 24.Hollerman JR, Schultz W. Dopamine neurons report an error in the temporal prediction of reward during learning. Nature Neurosci. 1998;1:304–309. doi: 10.1038/1124. [DOI] [PubMed] [Google Scholar]
- 25.Ishiwari K, Weber SM, Mingote S, Correa M, Salamone JD. Accumbens dopamine and the regulation of effort in food-seeking behavior: Modulation of work output by different ratio or force requirement. Behav Brain Res. 2004;151:83–91. doi: 10.1016/j.bbr.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 26.Kelland MD, Chiodo LA, Freeman AS. Anesthetic influences on the basal activity and pharmacological responsiveness of nigrostriatal dopamine neurons. Synapse. 1990;6:207–209. doi: 10.1002/syn.890060213. [DOI] [PubMed] [Google Scholar]
- 27.Kheramin S, Body S, Herrera FM, Bradshaw CM, Szabadi E, Deakin JF, Anderson IM. The effect of orbital prefrontal cortex lesions on performance on a progressive ratio schedule: Implications for models of inter-temporal choice. Behav Brain Res. 2005;156:145–152. doi: 10.1016/j.bbr.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 28.Killeen PR. Mathematical principles of reinforcement. Behav Brain Sci. 1994;17:105–172. [Google Scholar]
- 29.Killeen PR, Sitomer MT. MPR. Behav Proces. 2003;62:49–64. doi: 10.1016/S0376-6357(03)00017-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kleen JK, Sitomer MT, Killeen PR, Conrad CD. Chronic stress impairs spatial memory and motivation for reward without disrupting motor ability and motivation to explore. Behav Neurosci. 2006;120:842–851. doi: 10.1037/0735-7044.120.4.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koch M, Schmid A, Schnitzler HU. Role of nucleus accumbens dopamine D1 and D2 receptors in instrumental and Pavlovian paradigms of conditioned reward. Psychopharmacol. 2000;152:67–73. doi: 10.1007/s002130000505. [DOI] [PubMed] [Google Scholar]
- 32.Kolb B, Whishaw IQ. Fundamentals of Human Neurophysiology. 4. W.H. Freeman and Company; New York: 1996. p. 691. [Google Scholar]
- 33.Lindner MD, Plone MA, Francis JM, Blaney TJ, Salamone JD, Emerich DF. Rats with partial striatal dopamine depletions exhibit robust and long-lasting behavioral deficits in a simple fixed-ratio bar-pressing task. Behav Brain Res. 1997;86:25–40. doi: 10.1016/s0166-4328(96)02240-1. [DOI] [PubMed] [Google Scholar]
- 34.Lundblad M, Andersson M, Winkler C, Kirik D, Wierup N, Cenci MA. Pharmacological validation of behavioural measures of akinesia and dyskinesia in a rat model of Parkinson’s disease. Eur J Neurosci. 2002;15:120–132. doi: 10.1046/j.0953-816x.2001.01843.x. [DOI] [PubMed] [Google Scholar]
- 35.Martin-Iverson MT, Wilkie D, Fibiger HC. Effects of haloperidol and d-amphetamine on perceived quantity of food and tones. Psychopharmacol. 1987;93:374–381. doi: 10.1007/BF00187260. [DOI] [PubMed] [Google Scholar]
- 36.McCullough LD, Salamone JD. Involvement of nucleus accumbens dopamine in the motor activity induced by periodic food presentation: A microdialysis and behavioral study. Brain Res. 1992;592:29–36. doi: 10.1016/0006-8993(92)91654-w. [DOI] [PubMed] [Google Scholar]
- 37.McFarland K, Ettenberg A. Haloperidol differentially affects reinforcement and motivational processes in rats running an alley for intravenous heroin. Psychopharmacol. 1995;122:346–350. doi: 10.1007/BF02246264. [DOI] [PubMed] [Google Scholar]
- 38.Mingote S, Weber SM, Ishiwari K, Correa M, Salamone JD. Ratio and time requirements on operant schedules: Effort-related effects of nucleus accumbens dopamine depletions. Eur J Neurosci. 2005;21:1749–1757. doi: 10.1111/j.1460-9568.2005.03972.x. [DOI] [PubMed] [Google Scholar]
- 39.Mobini S, Chiang TJ, Ho MY, Bradshaw CM, Szabadi E. Comparison of the effects of clozapine, haloperidol, chlorpromazine, and d-amphetamine on performance on a time-constrained progressive ratio schedule and on locomotor behaviour in the rat. Psychopharmacol. 2000;152:47–54. doi: 10.1007/s002130000486. [DOI] [PubMed] [Google Scholar]
- 40.Nieoullon A, Coquerel A. Dopamine: A key regulator to adapt action, emotion, motivation and cognition. Curr Opin Neurol. 2003;16:S3–S9. [PubMed] [Google Scholar]
- 41.Nishino H, Hattori S, Muramoto K, Ono T. Basal ganglia neural activity during operant feeding behavior in the monkey: Relation to sensory integration and motor execution. Brain Res Bull. 1991;27:463–468. doi: 10.1016/0361-9230(91)90143-8. [DOI] [PubMed] [Google Scholar]
- 42.Ono T, Nishijo H, Nishino H. Functional role of the limbic system and basal ganglia in motivated behaviors. J Neurol. 2000;247:V23–32. doi: 10.1007/pl00007780. [DOI] [PubMed] [Google Scholar]
- 43.Posadas-Sánchez D. Unpublished Dissertation. Arizona State University; Tempe: 2004. Evaluating models of motivation: Role of reinforcer quality, lever force, and drug effects. [Google Scholar]
- 44.Reilly MP. Extending mathematical principles of reinforcement into the domain of behavioral pharmacology. Behav Proces. 2003;62:75–88. doi: 10.1016/s0376-6357(03)00027-5. [DOI] [PubMed] [Google Scholar]
- 45.Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. In: Black AH, Prokasy WF, editors. Classical conditioning II: Current research and theory. Appleton Century Crofts; New York: 1972. pp. 64–99. [Google Scholar]
- 46.Rick JH, Horvitz JC, Balsam PD. Dopamine receptor blockade and extinction differentially affect behavioral variability. Behav Neurosci. 2006;120:488–492. doi: 10.1037/0735-7044.120.2.488. [DOI] [PubMed] [Google Scholar]
- 47.Robinson S, Rainwater A, Hnasko T, Palmiter RD. Viral restoration of dopamine signaling to the dorsal striatum restores instrumental conditioning to dopamine-deficient mice. Psychopharmacol. 2007;191:567–578. doi: 10.1007/s00213-006-0579-9. [DOI] [PubMed] [Google Scholar]
- 48.Roizen MF, Kopin IJ, Zivin J, Muth EA, Jacobowitz DM. The effect of two anesthetic agents on norepinephrine and dopamine in discrete brain nuclei, fiber tracts, and terminal regions of the rat. Brain Res. 1976;110:515–522. doi: 10.1016/0006-8993(76)90862-3. [DOI] [PubMed] [Google Scholar]
- 49.Sakai K, Gash DM. Effect of bilateral 6-OHDA lesions of the substantia nigra on locomotor activity in the rat. Brain Res. 1994;633:144–150. doi: 10.1016/0006-8993(94)91533-4. [DOI] [PubMed] [Google Scholar]
- 50.Salamone JD, Correa M. Motivational views of reinforcement: Implications for understanding the behavioral functions of nucleus accumbens dopamine. Behav Brain Res. 2002;137:3–25. doi: 10.1016/s0166-4328(02)00282-6. [DOI] [PubMed] [Google Scholar]
- 51.Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacol. 2007;191:461–482. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- 52.Salamone JD, Cousins MS, Bucher S. Anhedonia or anergia? Effects of haloperidol and nucleus-accumbens dopamine depletion on instrumental response selection in a t-maze cost/benefit procedure. Behav Brain Res. 1994;65:221–229. doi: 10.1016/0166-4328(94)90108-2. [DOI] [PubMed] [Google Scholar]
- 53.Salamone JD, Cousins MS, Maio C, Champion M, Turski T, Kovach J. Different behavioral effects of haloperidol, clozapine, and thioridazine in a concurrent lever pressing and feeding procedure. Psychopharmacol. 1996;125:105–112. doi: 10.1007/BF02249408. [DOI] [PubMed] [Google Scholar]
- 54.Salamone JD, Kurth P, McCullough LD, Sokolowski JD. The effects of nucleus accumbens dopamine depletions on continuously reinforced operant responding: Contrasts with the effects of extinction. Pharmacol Biochem Behav. 1995;50:437–443. doi: 10.1016/0091-3057(94)00294-s. [DOI] [PubMed] [Google Scholar]
- 55.Salamone JD, Kurth P, McCullough LD, Sokolowski JD, Cousins MS. The role of brain dopamine in response initiation: Effects of haloperidol and regionally specific dopamine depletions on the local rate of instrumental responding. Brain Res. 1993;628:218–226. doi: 10.1016/0006-8993(93)90958-p. [DOI] [PubMed] [Google Scholar]
- 56.Salamone JD, Steinpreis RE, McCullough LD, Smith P, Grebel D, Mahan K. Haloperidol and nucleus accumbens dopamine depletion suppress lever pressing for food but increase free food consumption in a novel food choice procedure. Psychopharmacol. 1991;104:515–521. doi: 10.1007/BF02245659. [DOI] [PubMed] [Google Scholar]
- 57.Salamone JD, Wisniecki A, Carlson BB, Correa M. Nucleus accumbens dopamine depletions make animals highly sensitive to high fixed ratio requirements but do not impair primary food reinforcement. Neuroscience. 2001;105:863–870. doi: 10.1016/s0306-4522(01)00249-4. [DOI] [PubMed] [Google Scholar]
- 58.Sanabria F, Acosta JI, Killeen PR, Neisewander JL, Bizo LA. Modeling the effects of fluoxetine on food-reinforced behavior. Behav Pharmacol. 2008;19:61–70. doi: 10.1097/FBP.0b013e3282f3df9b. [DOI] [PubMed] [Google Scholar]
- 59.Schallert T, Hall S. ‘Disengage’ sensorimotor deficit following apparent recovery from unilateral dopamine depletion. Behav Brain Res. 1988;30:15–24. doi: 10.1016/0166-4328(88)90003-4. [DOI] [PubMed] [Google Scholar]
- 60.Schallert T, Whishaw IQ, Ramirez VD, Teitelbaum P. Compulsive, abnormal walking caused by anticholinergics in akinetic, 6-hydroxydopamine-treated rats. Science. 1978;199:1461–1463. doi: 10.1126/science.564552. [DOI] [PubMed] [Google Scholar]
- 61.Schultz W. Getting formal with dopamine reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- 62.Schwarting RK, Huston JP. The unilateral 6-hydroxydopamine lesion model in behavioral brain research. Analysis of functional deficits, recovery and treatments. Prog Neurobiol. 1996;50:275–331. doi: 10.1016/s0301-0082(96)00040-8. [DOI] [PubMed] [Google Scholar]
- 63.Stephens DW, Krebs JR. Foraging Theory. Princeton University Press; Princeton, NJ: 1986. [Google Scholar]
- 64.Sutton RS, Barto AG. Toward a modern theory of adaptive networks: Expectation and prediction. Psychol Rev. 1981;88:135–170. [PubMed] [Google Scholar]
- 65.Tran-Nguyen LT, Castañeda E, MacBeth T. Changes in behavior and monoamine levels in microdialysate from dorsal striatum after 6-OHDA infusions into ventral striatum. Pharmacol Biochem Behav. 1996;55:141–150. doi: 10.1016/0091-3057(96)00068-8. [DOI] [PubMed] [Google Scholar]
- 66.Waelti P, Dickinson A, Schultz W. Dopamine responses comply with basic assumptions of formal learning theory. Nature. 2001;412:43–48. doi: 10.1038/35083500. [DOI] [PubMed] [Google Scholar]
- 67.White NM, McDonald RJ. Multiple parallel memory systems in the brain of the rat. Neurobiol Learn Mem. 2002;77:125–184. doi: 10.1006/nlme.2001.4008. [DOI] [PubMed] [Google Scholar]
- 68.Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 69.Wise RA, Schwartz HV. Pimozide attenuates acquisition of lever-pressing for food in rats. Pharmacol Biochem Behav. 1981;15:655–656. doi: 10.1016/0091-3057(81)90225-2. [DOI] [PubMed] [Google Scholar]
- 70.Wise RA, Spindler J, deWit H, Gerber GJ. Neuroleptic-induced “anhedonia” in rats: Pimozide blocks reward quality of food. Science. 1978;201:262–264. doi: 10.1126/science.566469. [DOI] [PubMed] [Google Scholar]
- 71.Zhang Z, Rickard JF, Asgari K, Body S, Bradshaw CM, Szabadi E. Quantitative analysis of the effects of some “atypical” and “conventional” antipsychotics on progressive ratio schedule performance. Psychopharmacol. 2005a;179:489–497. doi: 10.1007/s00213-004-2049-6. [DOI] [PubMed] [Google Scholar]
- 72.Zhang Z, Rickard JF, Body S, Asgari K, Bradshaw CM, Szabadi E. Comparison of the effects of clozapine and 8-hydroxy-2-(di-n-propylamino) tetralin (8-OH-DPAT) on progressive ratio schedule performance: Evidence against the involvement of 5-HT1A receptors in the behavioural effects of clozapine. Psychopharmacol. 2005b;181:381–391. doi: 10.1007/s00213-005-2258-7. [DOI] [PubMed] [Google Scholar]
- 73.Zigmond MJ, Acheson AL, Stachowiak MK, Stricker EM. Neurochemical compensation after nigrostriatal bundle injury in an animal model of preclinical Parkinsonism. Arch Neurol. 1984;41:856–861. doi: 10.1001/archneur.1984.04050190062015. [DOI] [PubMed] [Google Scholar]
- 74.Zigmond MJ, Stricker EM. Deficits in feeding behavior after intraventricular injection of 6-hydroxydopamine in rats. Science. 1972;177:1211–1214. doi: 10.1126/science.177.4055.1211. [DOI] [PubMed] [Google Scholar]
- 75.Zigmond MJ, Stricker EM. Recovery of feeding and drinking by rats after intraventricular 6-hydroxydopamine or lateral hypothalamic lesions. Science. 1973;182:717–720. doi: 10.1126/science.182.4113.717. [DOI] [PubMed] [Google Scholar]