Abstract
Immune function declines with age in Drosophila and humans, and autophagy is implicated in immune function. In addition, autophagy genes are required for life span extension caused by reduced insulin/IGF1-like signaling and dietary restriction in C. elegans. To test if the autophagy pathway might be limiting for immunity and/or life span in adult Drosophila, the Geneswitch system was used to cause conditional inactivation of the autophagy genes Atg5, Atg7 and Atg12 by RNAi. Conditional inhibition of Atg genes in adult flies reduced lysotracker staining of adult tissues, and reduced resistance to injected E. coli, as evidenced by increased bacterial titers and reduced fly survival. However, survival of uninjected flies was unaffected by Atg gene inactivation. The data indicate that Atg gene activity is required for normal immune function in adult flies, and suggest that neither autophagy nor immune function are limiting for adult life span under typical laboratory conditions.
Keywords: innate immunity, life span, bacteria, aging
1. Introduction
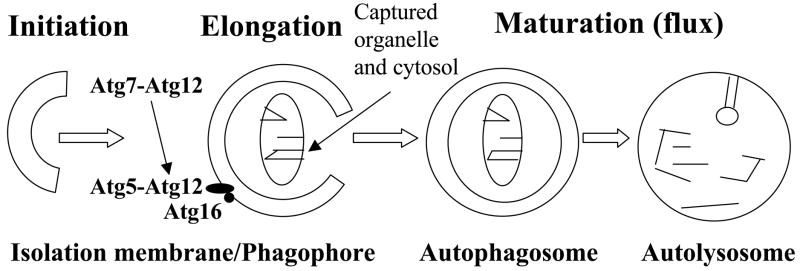
Macroautophagy (hereafter referred to as autophagy) is a fundamental mechanism in which cells autodigest portions of their cytoplasm for recycling or removal. During autophagy a double-membranous structure called the isolation membrane (or phagophore) is extended to surround discrete portions of the cytosol (diagrammed in Figure 1; adapted from (Deretic, 2005)). The phagophore seals to form a double-membrane bound vacuole called the autophagosome. The autophagosome fuses with lysosomes to create the autolysosome, where the trapped material is then degraded. The autophagy pathway is conserved from yeasts to Drosophila to humans, and involves more than 20 proteins (e.g., Atg1 through Atg27 in yeast)(Baehrecke, 2003; Deretic, 2005; Mizushima et al., 2008). Several of the conserved factors show similarities to the ubiquitin system. For example, Atg12 is a small, ubiquitin-like protein, and Atg7 and Atg10 are E1/E2-like enzymes that catalyze cross-linking of the C-terminal glycine of Atg12 to a lysine residue of Atg5. The Atg5-Atg12 conjugate localizes to the concave, cytofacial side of the phagophore, where it is required for elongation.
Fig. 1.
Outline of autophagy pathway and role of Atg5, Atg7 and Atg12.
Recent studies have revealed that autophagy not only functions in the recycling of cytosolic constituents, but also targets intracellular pathogens for destruction during innate immunity (Nakagawa et al., 2004; Ogawa et al., 2005; Schmid and Munz, 2007). For example, Group A Streptococci will proliferate in atg5−/− mouse embryonic fibroblasts, whereas in wild-type cells the bacteria are enveloped by autophagosomes and destroyed (Nakagawa et al., 2004). Similarly, after Listeria monocytogenes bacteria have been inhibited by chloramphenicol treatment they can be efficiently cleared from cells by autophagy (Rich et al., 2003). So far, most studies implicating autophagy in immunity have been performed in cultured cells. However, evidence for an in vivo role for autophagy in immunity was recently reported for mice, where it was found that neurovirulence of HSV-1 is inhibited by autophagy (Orvedahl et al., 2007).
Aging is a multifactorial process with several mechanisms contributing to functional decline. At least three pathways have been found to extend life span in model organisms: reduced insulin/IGF1-like signaling (IIS), dietary restriction (DR), and decreased mitochondrial gene function (Wolff and Dillin, 2006). Autophagy’s relation to aging and longevity is complex and not yet clear (Cuervo et al., 2005; Mizushima et al., 2008). In C. elegans, autophagy has been reported to be required for life-span extension in response to each of the three life span pathways: reduced IIS (Melendez et al., 2003), DR (Jia and Levine, 2007), and reduced mitochondrial gene function (Toth et al., 2008b). Drosophila is a powerful model for the study of immune function and aging (Libert et al., 2006; Libert et al., 2008; Ramsden et al., 2008; Ren et al., 2007; Zerofsky et al., 2005), and most recently the role of autophagy. Previous work has shown that when autophagy gene activity is reduced during both Drosophila development and adulthood, the flies are short-lived, sometimes associated with reduced protein aggregate clearance and degeneration in the adult nervous system (Juhasz et al., 2007; Simonsen et al., 2008; Toth et al., 2008a); however it is not clear if the reduced life span resulted from defects in development or from effects in the adult flies.
Here we test if the autophagy pathway might be limiting for immunity and/or life span specifically in adult-stage Drosophila. The Geneswitch system was used to cause conditional expression of transgenes designed to inactivate the Atg5, Atg7 and Atg12 genes by RNA interference (RNAi). RNAi of Atg genes in adult flies was found to reduce resistance to injected E. coli, as evidence by increased bacterial titers and reduced survival. However, survival of uninjected flies was unaffected by Atg gene inactivation. The data confirm the expected requirement for Atg gene activity for normal immune function in adult flies, but suggest that neither autophagy nor an intact immune response are limiting for adult fly life span under typical laboratory conditions.
2. Materials and methods
2.1 Drosophila strains
The Atg5-RNAi strain (UAS-Atg5-IR8-1, insert on third chromosome), Atg7-RNAi strain (UAS-Atg7-IR17-6/CyO, insert on second chromosome) and Atg12-RNAi strain (UAS-Atg12-IR26-2, insert on third chromosome) were provided by Thomas P. Neufeld, and were described previously (Scott et al., 2004). The GFP-RNAi transgenic strain (Roignant et al., 2003) was genotype w1118; P{UAS-Avic\GFP.dsRNA.R}142, and was obtained from Bloomington Drosophila Stock Center. The Geneswitch driver line GS-Act-255B contains a P element construct in which the Geneswitch cDNA is cloned downstream of the tissue-general actin5C promoter, and was described previously (Ford et al., 2007). The flies used in this paper were the male progeny from the Geneswitch driver crossed to the Atg RNAi strains, and the progeny of Geneswitch driver crossed to the Oregon-R wild-type and w[1118] control strains (referred to in the Figures as “Oregon-R controls” and “w[1118] controls”).
2.2 Drosophila culture
Drosophila culture and life span assays were performed as described previously (Ford et al., 2007; Ren et al., 2007). Stocks were maintained on a standard cornmeal agar medium that contains the antifungal agent tegosept (Sigma) at a final concentration of 11mM. Food recipe: 105g dextrose, 7.5g agar, 26g yeast, 50g cornmeal, 1 liter purified H20, boil for 30 minutes with constant agitation, then add 1.7g tegosept dissolved in 8.5ml 95% ethanol and 1.9ml propionic acid (99%, Mallinckrodt Baker). Life span measurements were conducted with ~25 male flies per vial, and a total 5 vials for each cohort. For survival assays performed at 25°C, flies were transferred to fresh vials ever other day. For adult survival assays preformed at 29°C, flies were transferred to fresh vials every other day during the first 30–40 days, and then every day for the remainder of the life span. Culture of adults at 29°C results in shorter life span and therefore allows for more rapid experiments; no significant difference in effect of Atg gene inhibition was observed for experiments conducted at 25°C versus 29°C. For the Geneswitch system experiments, food vials were adjusted to a final concentration of ~160ug/ml of RU486 (Mifepristone, Sigma) by applying 50ul of an ethanol stock solution to the surface of fresh food vials, while the control food vials were treated with ethanol solvent alone; vials were air-dried for 48 hours to allow the ethanol to evaporate (detailed protocols for food preparation are provided online at http://towerlab.usc.edu/). Flies were injected with E. coli at 3 days of age (as described below), and immediately transferred to food vials with and without drug. Recording of deaths for life span assays of E. coli-injected flies was initiated 2 days after injection to eliminate any deaths due to the trauma of injection (always < 5% of total flies). Females were excluded from these experiments to reduce the life span assay workload and expense by half. In addition males were chosen over females because in general female life span appears more affected by subtleties in the food source (Magwere et al., 2004).
2.3 Real-time RT-PCR
Total RNA was isolated from 30 male flies at 1 week of age using TRIzol reagent (Invitrogen). RNA concentration was measured using a spectrophotometer (NanoDrop) and the RNA was converted to cDNA using the QuantiTect Reverse Transcription kit (Qiagen) according to the manufacturer’s instructions. The primers used to amplify the Atg7 and Atg12 genes were as previously described (Juhasz et al., 2007; Scott et al., 2004). The primers used to amplify Atg5 gene were as follows: Atg5F (gcactacatgtcctgcctga) and Atg5R (agattcgcaggggaatgttt). The Atg5, Atg7 and Atg12 gene sequences were amplified from fly cDNA, quantitated by spectrophotometry, and used as the standard. Varying amounts of standard cDNA (102, 103, 104, 105, 106, 107, 108 molecules) was amplified in parallel reactions alongside the experimental cDNA samples. Real-time PCR was performed using the Bio-RAD DNA Engine Opticon 2 Real-time PCR detector, and SYBR green dye. The threshold cycle (Ct) is the point where each kinetic curve reaches a common arbitrary fluorescence level (AFL), placed to intersect each curve in the region of exponential increase (Kang et al., 2000). The Ct number was plotted vs. input DNA, and experimental sample concentrations were derived from the standard curves. Values are plotted as mean ± SD of triplicate assays.
2.4 Lysotracker Staining and Quantification
Flies were cultured on standard media (“fed”) or fasted overnight (18hrs; “fasted”) before dissection. Fasting was performed in a culture vial containing a folded tissue (Kimwipe, Kimberly-Clark) that was wetted with 1% dextrose in deionized water. Three flies from each treatment were dissected in PBS, and the midguts were then incubated in 0.5μM Lysotracker Red DND-99 (Invitrogen) for 3 minutes. Midguts were transferred to PBS on glass dissection plates and immediately photographed using a Leica MZ FLIII fluorescence stereomicroscope. Three images were taken of each midgut under fluorescent light (DsRED) with the gain selected as 4 and exposure time 8 sec. The lysotracker staining level was determined from the images using ImagePro-Plus Software (Media Cybernetics): First, the whole midgut was circled as the area for analysis and counted as a subject, and then the red density was measured. We note that the midgut tissue outline was more readily discernable in the monitor than is apparent in the images presented in the Figures. Finally, the measurements were viewed in Excel format and the means and SD were calculated. The data is presented in bar graphs where each bar represents the average and SD of three images of each of three midgets, for a total of nine images per bar. To visualize lysotracker staining at subcellular resolution, the lysotracker-stained midguts were transferred to 70% glycerol on glass slides under a coverslip, and photographed using a DeltaVision Spectris restoration microscope (Applied Precision, Issaquah WA), with an Olympus 60x NA 1.4 PlanApo objective and 12-bit Roper CoolSnap HQ CCD camera. The system x-y pixel size was 0.1092 μm. Acquisition and image processing was done in softWoRx v3.3.
2.5 E. coli injection into flies
The E. coli K-12 bacterial strain ZK1142 (Palchevskiy and Finkel, 2006) is resistant to the antibiotic nalidixic acid. Bacteria were grown in Luria-Betaini (LB) broth for 12–18h and the density of bacterial culture was estimated by spectrophotometry using a standard curve. The bacterial culture was diluted to approximately 8×108 cells/ml in PBS. Green food coloring (Kroger brand) was added to the bacterial solution to aid in liquid handling and scoring of injections. Microneedles were made using the PN-30 puller (Narishige) and Brosil glass capillary tubing (1.0mm OD × 0.75mm ID; FHC Inc.). The needles were graduated before use with a scale of 1/32 inch, and ~3ul of bacterial solution was added to the needle. The needles with bacterial solution were then assembled into the FemtoJet express microinjector (Eppendorf). The flies were anaesthetized using CO2 and positioned on the pad with the abdomen oriented towards the needle using brushes. The colored bacterial solution was then injected into the abdomen of the adult fly using the microinjector; the volume injected was ~ 0.05ul per fly, based on the gradation markings on the needle.
2.6 Bacterial counts from E. coli injected flies
At the indicated time points after E. coli injection, three males were removed from each of five different vials (each vial contained ~20 flies). The first time point (3 days) was generated with flies at 3 hours after injection. Each fly was individually homogenized in 100μl sterile PBS using a small pestle for about 1 minute, until pieces of tissue were no longer visible. The homogenates were diluted as necessary and plated on LB agar with 20 ug/ml nalidixic acid. The values obtained for the 15 flies were averaged, and standard deviations are indicated in the figures by error bars.
2.7 Statistical analyses
Median life spans were compared using two-sided log-rank tests (Crawly, 2005). The Cox Proportional Hazards Regression Model was used with Breslow method as a default. All analyses were performed in the R 2.4.1 statistical environment (RDevelopmentCoreTeam, 2006). Survival statistics results are presented in the Figures. Unpaired, two-sided t-tests were used to determine the significance of the difference of bacterial load between the RU486-treated and control flies, and statistically significant differences (p < 0.05) are indicated in the bar graphs by asterisks. Unpaired, two-sided t-tests were used to determine the significance of the difference in lysotracker staining between control and RU486-treated flies’ midgut tissue, and p values are presented in the Figures for the various pair-wise comparisons.
3. Results
3.1 Conditional inactivation of Atg genes using Geneswitch system
The Atg5, Atg7, and Atg12 genes were chosen for study because (i) they are conserved across species with regard to their sequence and function in the autophagy pathway, (ii) well-characterized RNAi lines are available for these genes that have been shown (in larvae) to effectively inhibit the autophagy pathway (Scott et al., 2004), and (iii) these genes act at the early membrane elongation/engulfment stage of the pathway (Fig. 1), which is likely to be critical for turnover of cellular constituents and organelles as well as for engulfment and destruction of bacteria. The Atg5, Atg7 and Atg12 RNAi strains contain constructs where UAS-promoters drive expression of inverted-repeat sequences derived from the corresponding Atg genes (Scott et al., 2004). These RNAi strains have previously been demonstrated to mediate inactivation of Atg genes and the autophagy pathway in transgenic Drosophila using the constitutively active transcription factor GAL4, which binds to UAS sites and activates transcription. Here the conditional gene expression system Geneswitch was utilized, where the engineered transcriptional activator protein Geneswitch becomes active only upon interaction with the drug Mifepristone (RU486), and then binds to the UAS sites and drives high-level transcription (Osterwalder et al., 2001; Roman et al., 2001). The Geneswitch driver line “GS-Actin-255B” was used, in which the powerful and tissue-general Actin5C cytoplasmic actin gene promoter drives expression of Geneswitch protein in all somatic tissues of the fly. GS-Actin-255B yields robust, RU486-dependent expression of target transgenes in all the tissues of larvae and adult flies, including abundant expression in nervous system, gut, fat body, muscle, gonads and other tissues (Ford et al., 2007)(J. Shen and J.T., manuscript in preparation). The GS-Actin-255B line was crossed to each RNAi strain, and the male progeny containing both constructs were cultured as adults in the presence and absence of the RU486 drug in the food. Control flies were progeny of the GS-Actin-255B driver crossed to Oregon-R wild-type and w[1118] strain flies. Real-time RT-PCR assays confirmed that Atg gene expression was partially reduced (Supplemental Figure S1), consistent with the previous characterization of these RNAi strains, where partial inhibition of autophagy was scored in larvae using lysotracker dye as an assay (Scott et al., 2004).
3.2 Lysotracker staining of adult midgut tissue
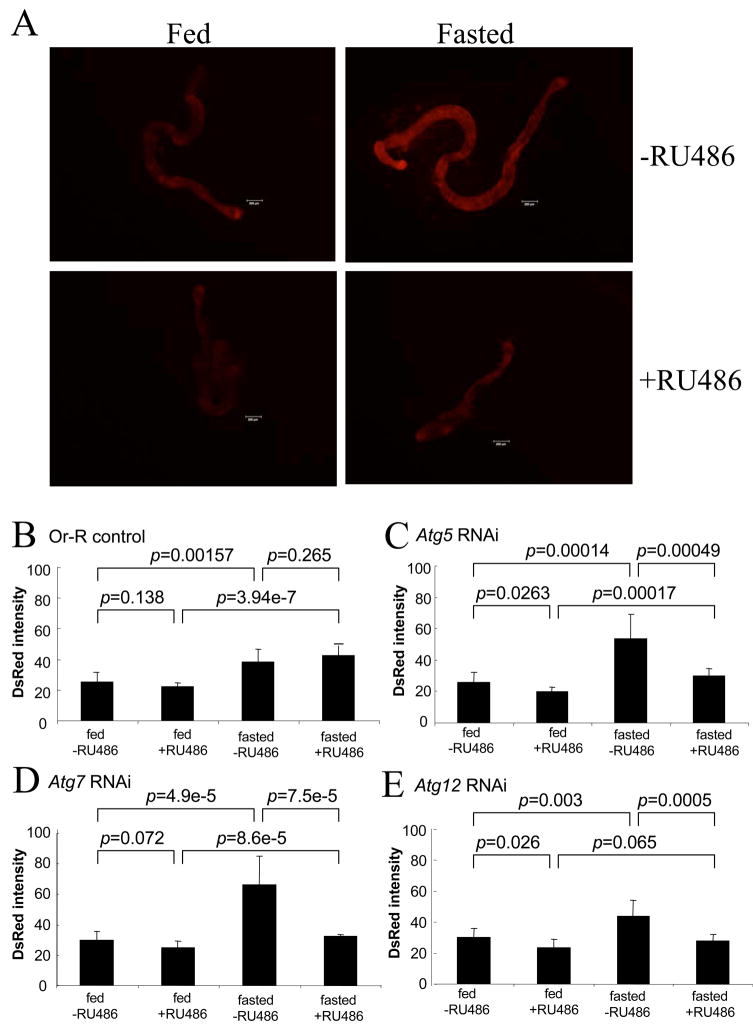
Lysotracker red is a red-fluorescent dye that stains acidic compartments in live cells, including lysosomes and autophagosomes. Previously lysotracker red staining has been used to assay autophagy pathway activation in Drosophila larval tissues including the hepatic/adipose tissue called the fat body, as well as gut and salivary gland tissue (Juhasz and Neufeld, 2008). Lysotracker red staining in Drosophila larvae has been used to document basal levels of autophagy, induction of autophagy in response to fasting/starvation, and the inhibition of the autophagy pathway by the Atg5, Atg7 and Atg12 RNAi constructs (Scott et al., 2004). Here lysotracker red staining was quantified in midgut tissue isolated from adult flies. The adult midgut was chosen because it is easy to isolate and was expected to show robust autophagy in response to fasting; midgut is also a critical immune tissue (Ha et al., 2005), contains actively dividing stem cells (Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006), and undergoes characteristic ultrastructural and molecular changes during aging (Anton-Erxleben et al., 1983; Choi et al., 2008). Lysotracker red staining of adult midgut from Oregon R wild type flies was readily visualized, was increased by fasting, and exhibited the punctate cytoplasmic pattern characteristic of autophagic vacuoles (Supplemental Figure S2). To quantify the lysotracker staining in adult midguts, a fluorescence stereomicroscope and image-capture assay was employed (Figure 2). In Or-R control flies, the lysotracker red staining was significantly induced by fasting, consistent with staining of fasting-induced autophagic vacuoles (Fig. 2B). Moreover, the lysotracker red staining was unaffected by administration of RU486 drug to control flies, demonstrating that the Geneswitch system itself does not interfere with the assay. In Atg5 RNAi flies, the lysotracker staining was significantly reduced when Atg5 gene expression was inhibited by RU486 feeding, both in fed and fasted flies (Figure 2A, C). Similar results were obtained upon inhibition of Atg7 and Atg12 gene expression (Figure 2D,E). These results indicate that inhibition of Atg gene expression by RNAi reduced both basal and induced levels of autophagy in adult flies.
Fig. 2.
Effect of Atg5, Atg7 and Atg12 RNAi on adult midgut lysotracker staining. Adult flies were cultured under control conditions (“Fed”), or fasted overnight (“Fasted”), prior to dissection and staining of midguts with lysotracker red. The flies contained the indicated RNAi construct and the rtTA(3)E2 driver, and had been cultured in the absence (−RU486) and presence (+RU486) of drug for one week prior to dissection, as indicated. Or-R control flies contained the rtTA(3)E2 driver but no target transgene. (A) Representative fluorescence images of Atg5 RNAi fly midguts stained with lysotracker red. Scale bars represent 200μm. (B–E) Quantification of lysotracker staining in adult fly midguts. (B) Oregon R control flies. (C) Atg5 RNAi flies. (D) Atg7 RNAi flies. (E) Atg12 RNAi flies. The p values for the various indicated comparisons were calculated using unpaired, two-sided t-tests.
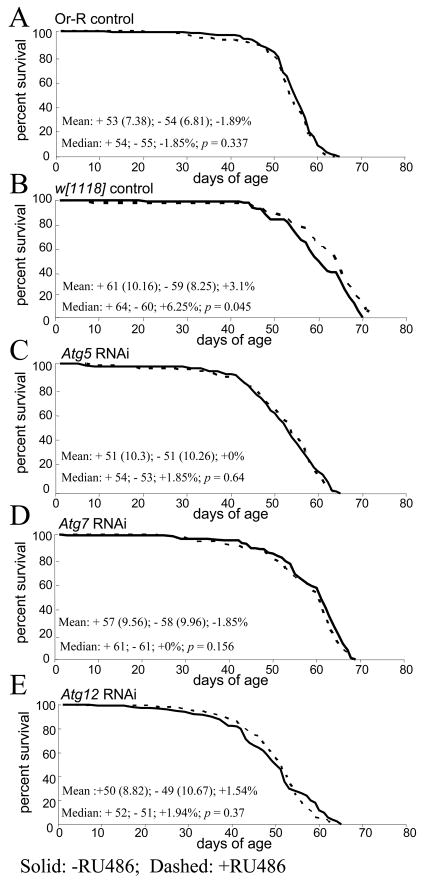
3.2 Life span is not altered by adult-specific inhibition of Atg5, Atg7 and Atg12 genes
Adult male flies were cultured in the presence and absence of RU486 and life span was quantified. Experimental flies contained both the GS-Actin-255B driver and the Atg gene RNAi construct, while control flies contained only the driver. Assays were performed at 29°C (Figure 3) and at 25°C (Supplemental Figure S3), and similar results were obtained at both culture temperatures with respect to the effect of Atg gene inhibition. Assay of control flies demonstrated that life span is not reduced by the drug itself (Figure 3A, B). Strikingly, life span was not altered by adult-specific expression of RNAi constructs specific for Atg5 (Figure 3C), Atg7 (Figure 3D) or Atg12 (Figure 3E). The only possible exceptions to this were a 5.6% decrease in one out of two experiments with Atg5 RNAi (Figure S3A), and a 6.3% increase in one out of two experiments with Atg12 RNAi (Figure S3E). Because these changes were small in magnitude, in opposite direction, and were not replicated, we conclude that adult-specific inhibition of the Atg genes has no effect on adult life span.
Fig. 3.
Effect of autophagy gene inhibition on survival of adult flies. (A–E) Survival of control and autophagy-inhibited flies. Survival is plotted as a function of adult age in days. Each curve represents >125 flies, cultured as adults at 29°C. Solid lines indicate minus-drug, dashed lines indicate plus-drug. Mean life span was calculated for plus-drug (+) and minus-drug (−) cohorts, with SD in parentheses, followed by the percent change. Median is presented for each cohort, followed by percent change and p value generated using log-rank tests. (A) Oregon-R controls. (B) w[1118] controls. (C) Atg5 RNAi. (D) Atg7 RNAi. (E) Atg12 RNAi.
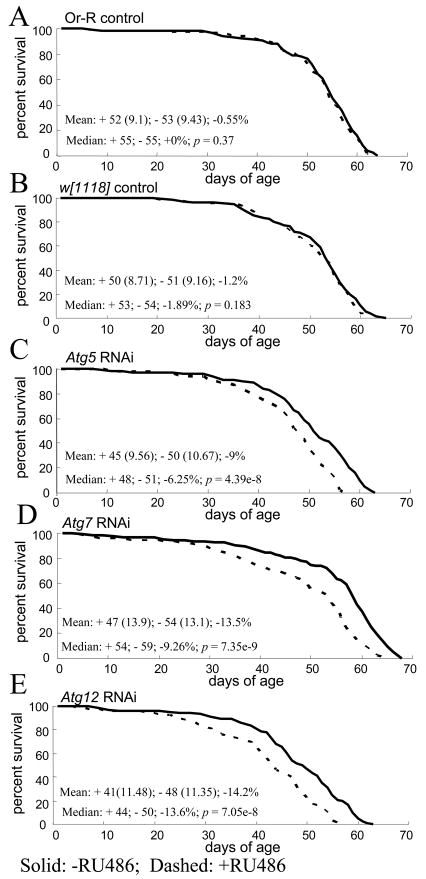
3.3 Atg gene inhibition reduces survival after E. coli injection
To assay for effects of Atg gene inhibition on immune function, flies were assayed for survival after injection of the abdomen with approximately 40,000 E. coli bacteria. E. coli was chosen not only because it is convenient to manipulate, but also because it has been found to be a target for cellular immunity in Drosophila (Kocks et al., 2005; Ramsden et al., 2008) and for the autophagy pathway in macrophages (Amer et al., 2005). Injection of PBS buffer alone did not have a detectable effect on fly life span (Supplemental Figure S4A). Moreover, injection of E. coli did not have a significant effect on life span in control flies (Figure 4A, B; Supplemental Figure S4B). However, the life span of flies injected with E. coli was significantly and consistently reduced by ~8–15% upon inhibition of Atg gene expression (Figure 4C–E; Supplemental Figure S4C–E), indicating that autophagy is required for resistance to E. coli toxicity.
Fig. 4.
Effect of autophagy gene inhibition on survival of flies after E. coli injection. Approximately 40,000 bacterial cells were injected into each fly at three days after eclosion. Survival is plotted as a function of adult age in days. Each curve represents >125 flies, cultured as adults at 29°C. Solid lines indicate minus-drug, dashed lines indicate plus-drug. Mean life span was calculated for plus-drug (+) and minus-drug (−) cohorts, with SD in parentheses, followed by the percent change. Median is presented for each cohort, followed by percent change and p value generated using log-rank tests. (A) Oregon-R controls. (B) w[1118] controls. (C) Atg5 RNAi. (D) Atg7 RNAi. (E) Atg12 RNAi.
3.4 Atg gene inhibition enables E. coli proliferation in injected flies
To further characterize the role of autophagy in Drosophila immune function, flies were injected with E. coli and bacterial load was assayed with and without inhibition of autophagy genes by RNAi. Bacterial load was quantified on the same day as injection, and at various time points throughout the adult life span, by plating whole-fly extracts and counting E. coli colonies. Since the injected E. coli strain was resistant to the antibiotic nalidixic acid, E. coli could be unambiguously identified by growth on plates containing the antibiotic. In control flies the E. coli load was unaffected by the RU486 drug itself, and rapidly fell to low levels that were maintained throughout the fly life span (Figure 5A, B; Supplemental Figure S5A, B). Moreover, expression of a control inverted-repeat construct (GFP-RNAi) did not affect E. coli load (Figure 5C; Supplemental Figure S5C). In contrast, inhibition of Atg genes resulted in a significant increase in E. coli load (Figure 5D–F; Supplemental Figure S5D–F). The data suggest that normal autophagy pathway function is required to suppress the growth of injected E. coli, particularly in older flies.
Fig. 5.
Effect of autophagy gene inhibition on bacterial titers in E. coli-injected flies. Approximately 40,000 bacterial cells were injected to each fly at 3 days after eclosion. Bacterial load was assayed the same day, and at 25 days and 35 days after injection, as indicated (day 28 and day 38, respectively); flies were cultured as adults at 29°C. Solid bars indicate minus-drug, open bars indicate plus-drug. (A) Oregon-R controls. (B) w[1118] controls. (C) GFP RNAi controls. (D) Atg5 RNAi. (E) Atg7 RNAi. (F) Atg12 RNAi. Statistical significance was determined using unpaired, two-sided t-tests and all significant differences (p < 0.05) are indicated by asterisk.
4. Discussion
The goal of this study was to determine if autophagy gene function might be limiting in adult Drosophila flies for immune function and/or life span. Conditional expression of transgenes designed to inactivate the Atg5, Atg7 and Atg12 genes resulted in reduced expression of the endogenous genes, and caused inhibition of the autophagy pathway in adults as evidenced by reduced lysotracker red staining of midgut tissue isolated from control and fasted flies. The conditional inhibition of the Atg genes produced flies that were more susceptible to toxicity from injected E. coli, as evidenced by decreased fly survival. These Atg-inhibited flies were also less able to control the proliferation of injected E. coli. This indicates that the function of these genes and the autophagy pathway is required in adult flies for optimal immune function. It could be argued that the Atg5, Atg7 and Atg12 genes could be required for immunity through a function unrelated to their role in autophagy, but no such possible alternative function for these genes has yet been reported. Quantification of the injected E. coli as a function of time in the control flies shows that the levels drop rapidly and then persist at that low level throughout the adult life span, with some increases observed in the oldest flies (Figure S5A, B). In the current experimental design, flies were first injected with E. coli, and then subsequently the autophagy pathway was inhibited. This means we are primarily assaying the effect of Atg gene inhibition on the flies’ ability to suppress an existing infection, as opposed to the ability to combat the initial stages of infection. Larger negative effects on survival of flies with injected E. coli might be expected if the flies were pre-treated with RU486 for several days to inhibit autophagy prior to the first stages of infection, and such an experimental design may be useful in future studies of autophagy as it relates specifically to immune function. This system should be useful in the future for more extensive studies of the in vivo role of autophagy for resistance to bacteria, and genetic manipulation of the E. coli is promising for analysis of virulence factors.
Strikingly, in flies without the challenge of injected E. coli, life span was not affected by expression of Atg RNAi constructs. This suggests that under our typical laboratory conditions autophagic immune function is not limiting for adult fly life span. This data is consistent with our previous observations that flies cultured under bacteria-free conditions have no alteration in life span (Ren et al., 2007), and therefore provides further support for the conclusion that in the laboratory assay, fly life span is not limited by bacteria, but must be limited by some other factor(s) not directly related to immune function or pathogens.
It is somewhat surprising that our experiments suggest that the autophagy pathway is not limiting for adult fly life span. Recent studies have reported that mutations in the Drosophila autophagy genes Atg7 (Juhasz et al., 2007) and Atg8a (Simonsen et al., 2008) resulted in flies with reduced life span and neurodegeneration in the adult. One possible explanation for these differing results is that in the previous studies the function of the Atg genes was reduced throughout the life span of the flies, and therefore reduced autophagy during development might have caused defects that resulted in reduced subsequent adult life span. Consistent with this idea, when we used the Geneswitch system to express the Atg7 RNAi construct during Drosophila development, we did observe reductions in subsequent adult life span (Supplemental Figure S5C), however this experiment was complicated by a smaller, but significant, negative effect of the RU486 drug itself during development. Another possibility, and the one that we favor, is that in experiments where autophagy gene function was reduced by mutation, autophagy was reduced below some threshold required for normal adult life span, while in the present experiments the partial inhibition of autophagy genes produced by RNAi did not reduce function below such a critical threshold. Based on their observations that morphological development in Atg mutant flies appeared largely normal, and the fact that adult flies had significant indicators of neurodegeneration as well as reduced life span, it was previously concluded that autophagy may be required in the adult for normal life span (Juhasz et al., 2007; Simonsen et al., 2008). The fact that we did not observe reduced life span upon partial reduction of autophagy specifically in adults may be because autophagy is not limiting for adult life span, but severe reductions can cause a novel pathology resulting in both neurodegeneration and reduced life span. Previously, over-expression of Atg8a in Drosophila using a non-conditional system and a nervous system-specific driver (APPL-Gal4) was found to yield increased life span, while another nervous-system-specific driver (Elav-Gal4) did not (Simonsen et al., 2008), and those results were also interpreted to suggest that autophagy gene function might be limiting for adult life span. However, that approach yields over-expression during development as well as in adults, and therefore the longevity effect may have been due to developmental changes. Another possibility is that Atg8a over-expression might affect life span through effects other than on autophagy. Finally, it is relevant that in the present study the Geneswitch conditional system was utilized, and therefore control and experimental flies have identical genetic backgrounds, and differ only in the presence or absence of the triggering drug RU486. In contrast, in previous studies using mutants and non-conditional expression systems, the control and experimental flies necessarily have slight differences in genetic background that could potentially affect life span.
In C. elegans, autophagy gene function was found to be required for life span extension in response to reduced IIS, DR, and reduced mitochondrial gene activity (Jia and Levine, 2007; Melendez et al., 2003; Toth et al., 2008b). However, differing results were obtained as to whether the autophagy pathway limits life span in adult worms under normal conditions. One possibility suggested by the present results with Drosophila is that under the particular experimental conditions where inhibition of autophagy was found to limit life span of wild-type C. elegans, life span was being limited by bacteria and immune function (Garigan et al., 2002).
In summary, the present results indicate that autophagy is required for optimal immune function in adult Drosophila flies, but that neither immune function nor autophagy gene function are limiting for adult life span under laboratory conditions that have been optimized for long life spans. It will be of interest in the future to determine what are the factors that limit adult fly life span under these laboratory conditions.
Acknowledgments
We thank Susan Forsburg and Marc Green for help with confocal microscopy, and thank Michelle Arbeitman and Steven Goodman for comments. This work was supported by a grant from the Department of Health and Human Services to JT (AG11833). SF was supported in part by an NSF CAREER Award (MCB-0237975).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amer AO, Byrne BG, Swanson MS. Macrophages rapidly transfer pathogens from lipid raft vacuoles to autophagosomes. Autophagy. 2005;1:53–8. doi: 10.4161/auto.1.1.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton-Erxleben F, Miquel J, Philpott DE. Fine-structural changes in the midgut of old Drosophila melanogaster. Mech Ageing Dev. 1983;23:265–76. doi: 10.1016/0047-6374(83)90027-1. [DOI] [PubMed] [Google Scholar]
- Baehrecke EH. Autophagic programmed cell death in Drosophila. Cell Death Differ. 2003;10:940–5. doi: 10.1038/sj.cdd.4401280. [DOI] [PubMed] [Google Scholar]
- Choi NH, Kim JG, Yang DJ, Kim YS, Yoo MA. Age-related changes in Drosophila midgut are associated with PVF2, a PDGF/VEGF-like growth factor. Aging Cell. 2008;7:318–34. doi: 10.1111/j.1474-9726.2008.00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawly M. Statistics: An Introduction using R. John Wiley & Sons, Inc.; Hoboken: 2005. [Google Scholar]
- Cuervo AM, Bergamini E, Brunk UT, Droge W, Ffrench M, Terman A. Autophagy and aging: the importance of maintaining “clean” cells. Autophagy. 2005;1:131–40. doi: 10.4161/auto.1.3.2017. [DOI] [PubMed] [Google Scholar]
- Deretic V. Autophagy in innate and adaptive immunity. Trends Immunol. 2005;26:523–8. doi: 10.1016/j.it.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Ford D, Hoe N, Landis GN, Tozer K, Luu A, Bhole D, Badrinath A, Tower J. Alteration of Drosophila life span using conditional, tissue-specific expression of transgenes triggered by doxycyline or RU486/Mifepristone. Exp Gerontol. 2007;42:483–97. doi: 10.1016/j.exger.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garigan D, Hsu AL, Fraser AG, Kamath RS, Ahringer J, Kenyon C. Genetic analysis of tissue aging in Caenorhabditis elegans: a role for heat-shock factor and bacterial proliferation. Genetics. 2002;161:1101–12. doi: 10.1093/genetics/161.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha EM, Oh CT, Ryu JH, Bae YS, Kang SW, Jang IH, Brey PT, Lee WJ. An antioxidant system required for host protection against gut infection in Drosophila. Dev Cell. 2005;8:125–32. doi: 10.1016/j.devcel.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Jia K, Levine B. Autophagy is required for dietary restriction-mediated life span extension in C. elegans. Autophagy. 2007;3:597–9. doi: 10.4161/auto.4989. [DOI] [PubMed] [Google Scholar]
- Juhasz G, Erdi B, Sass M, Neufeld TP. Atg7-dependent autophagy promotes neuronal health, stress tolerance, and longevity but is dispensable for metamorphosis in Drosophila. Genes Dev. 2007;21:3061–6. doi: 10.1101/gad.1600707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhasz G, Neufeld TP. Experimental control and characterization of autophagy in Drosophila. Methods Mol Biol. 2008;445:125–33. doi: 10.1007/978-1-59745-157-4_8. [DOI] [PubMed] [Google Scholar]
- Kang JJ, Watson RM, Fisher ME, Higuchi R, Gelfand DH, Holland MJ. Transcript quantitation in total yeast cellular RNA using kinetic PCR. Nucleic Acids Res. 2000;28:e2. doi: 10.1093/nar/28.2.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocks C, Cho JH, Nehme N, Ulvila J, Pearson AM, Meister M, Strom C, Conto SL, Hetru C, Stuart LM, Stehle T, Hoffmann JA, Reichhart JM, Ferrandon D, Ramet M, Ezekowitz RA. Eater, a transmembrane protein mediating phagocytosis of bacterial pathogens in Drosophila. Cell. 2005;123:335–46. doi: 10.1016/j.cell.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Libert S, Chao Y, Chu X, Pletcher SD. Trade-offs between longevity and pathogen resistance in Drosophila melanogaster are mediated by NFkappaB signaling. Aging Cell. 2006;5:533–43. doi: 10.1111/j.1474-9726.2006.00251.x. [DOI] [PubMed] [Google Scholar]
- Libert S, Chao Y, Zwiener J, Pletcher SD. Realized immune response is enhanced in long-lived puc and chico mutants but is unaffected by dietary restriction. Mol Immunol. 2008;45:810–7. doi: 10.1016/j.molimm.2007.06.353. [DOI] [PubMed] [Google Scholar]
- Magwere T, Chapman T, Partridge L. Sex differences in the effect of dietary restriction on life span and mortality rates in female and male Drosophila melanogaster. J Gerontol A Biol Sci Med Sci. 2004;59:3–9. doi: 10.1093/gerona/59.1.b3. [DOI] [PubMed] [Google Scholar]
- Melendez A, Talloczy Z, Seaman M, Eskelinen EL, Hall DH, Levine B. Autophagy genes are essential for dauer development and life-span extension in C. elegans. Science. 2003;301:1387–91. doi: 10.1126/science.1087782. [DOI] [PubMed] [Google Scholar]
- Micchelli CA, Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006;439:475–9. doi: 10.1038/nature04371. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–75. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa I, Amano A, Mizushima N, Yamamoto A, Yamaguchi H, Kamimoto T, Nara A, Funao J, Nakata M, Tsuda K, Hamada S, Yoshimori T. Autophagy defends cells against invading group A Streptococcus. Science. 2004;306:1037–40. doi: 10.1126/science.1103966. [DOI] [PubMed] [Google Scholar]
- Ogawa M, Yoshimori T, Suzuki T, Sagara H, Mizushima N, Sasakawa C. Escape of intracellular Shigella from autophagy. Science. 2005;307:727–31. doi: 10.1126/science.1106036. [DOI] [PubMed] [Google Scholar]
- Ohlstein B, Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439:470–4. doi: 10.1038/nature04333. [DOI] [PubMed] [Google Scholar]
- Orvedahl A, Alexander D, Talloczy Z, Sun Q, Wei Y, Zhang W, Burns D, Leib DA, Levine B. HSV-1 ICP34.5 confers neurovirulence by targeting the Beclin 1 autophagy protein. Cell Host Microbe. 2007;1:23–35. doi: 10.1016/j.chom.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Osterwalder T, Yoon KS, White BH, Keshishian H. A conditional tissue-specific transgene expression system using inducible GAL4. Proc Natl Acad Sci U S A. 2001;98:12596–601. doi: 10.1073/pnas.221303298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palchevskiy V, Finkel SE. Escherichia coli competence gene homologs are essential for competitive fitness and the use of DNA as a nutrient. J Bacteriol. 2006;188:3902–10. doi: 10.1128/JB.01974-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsden S, Cheung YY, Seroude L. Functional analysis of the Drosophila immune response during aging. Aging Cell. 2008;7:225–36. doi: 10.1111/j.1474-9726.2008.00370.x. [DOI] [PubMed] [Google Scholar]
- RDevelopmentCoreTeam. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2006. [Google Scholar]
- Ren C, Webster P, Finkel SE, Tower J. Increased internal and external bacterial load during Drosophila aging without life-span trade-off. Cell Metab. 2007;6:144–52. doi: 10.1016/j.cmet.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Rich KA, Burkett C, Webster P. Cytoplasmic bacteria can be targets for autophagy. Cell Microbiol. 2003;5:455–68. doi: 10.1046/j.1462-5822.2003.00292.x. [DOI] [PubMed] [Google Scholar]
- Roignant JY, Carre C, Mugat B, Szymczak D, Lepesant JA, Antoniewski C. Absence of transitive and systemic pathways allows cell-specific and isoform-specific RNAi in Drosophila. Rna. 2003;9:299–308. doi: 10.1261/rna.2154103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman G, Endo K, Zong L, Davis RL. P[Switch], a system for spatial and temporal control of gene expression in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2001;98:12602–7. doi: 10.1073/pnas.221303998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid D, Munz C. Innate and adaptive immunity through autophagy. Immunity. 2007;27:11–21. doi: 10.1016/j.immuni.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott RC, Schuldiner O, Neufeld TP. Role and regulation of starvation-induced autophagy in the Drosophila fat body. Dev Cell. 2004;7:167–78. doi: 10.1016/j.devcel.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Simonsen A, Cumming RC, Brech A, Isakson P, Schubert DR, Finley KD. Promoting basal levels of autophagy in the nervous system enhances longevity and oxidant resistance in adult Drosophila. Autophagy. 2008;4:176–84. doi: 10.4161/auto.5269. [DOI] [PubMed] [Google Scholar]
- Toth ML, Sigmond T, Borsos E, Barna J, Erdelyi P, Takacs-Vellai K, Orosz L, Kovacs AL, Csikos G, Sass M, Vellai T. Longevity pathways converge on autophagy genes to regulate life span in Caenorhabditis elegans. Autophagy. 2008;4:330–8. doi: 10.4161/auto.5618. [DOI] [PubMed] [Google Scholar]
- Wolff S, Dillin A. The trifecta of aging in Caenorhabditis elegans. Exp Gerontol. 2006;41:894–903. doi: 10.1016/j.exger.2006.06.054. [DOI] [PubMed] [Google Scholar]
- Zerofsky M, Harel E, Silverman N, Tatar M. Aging of the innate immune response in Drosophila melanogaster. Aging Cell. 2005;4:103–8. doi: 10.1111/j.1474-9728.2005.00147.x. [DOI] [PubMed] [Google Scholar]