Abstract
Execution of a response that has been primed by a backward-masked stimulus is inhibited (negative compatibility effect; NCE). Three experiments investigated the locus of this inhibition. Masked primes (left- or right-pointing arrows) were followed either by an arrow or a circle target. Arrow targets always required a left- or right-hand response, but the experiments differed in the response required to circles: press neither, either or both response keys (i.e. nogo, free choice and bimanual, respectively). Arrow targets showed the usual NCEs. Circle targets showed NCEs in the form of a response bias away from the primed response in the nogo and free-choice tasks; primes and targets differed on these trials, ruling out a perceptual explanation of the NCE. The bimanual task showed no such bias, suggesting that the NCE is located at a level of abstract response codes rather than specific muscle commands.
Keywords: inhibition, masked motor priming, nogo, free-choice, bimanual responses
1. Introduction
Stimuli that have not been consciously perceived can nevertheless affect our responses to other, clearly visible stimuli. Although the existence of such subliminal priming effects is well established (e.g. Dehaene et al. 1998; Eimer & Schlaghecken 1998), their precise nature is as yet unknown. A traditional assumption was that subliminal stimuli would only passively increase corresponding neural activation levels, without being susceptible to inhibitory cognitive control. However, Schlaghecken & Eimer (1997) and Eimer & Schlaghecken (1998) presented initial evidence of a low-level inhibitory control mechanism. When a backward-masked prime is followed immediately by an unmasked target, responses are facilitated when both stimuli are associated with the same response, and hindered when they are associated with different responses (positive compatibility effect, PCE), but when the interval between masked prime and target is prolonged, this pattern reverses (negative compatibility effect, NCE), indicating inhibition of the initial prime-related response and disinhibition of the opposite response.
Schlaghecken & Eimer (1997) and Eimer & Schlaghecken (1998) suggested that this was achieved by a self-inhibitory mechanism that rapidly shuts down motor activations that are no longer supported by sensory evidence. By contrast, others have claimed that the NCE is driven by the perceptual properties of primes, mask and targets. One version of this hypothesis is that the NCE reflects an inhibition process actively triggered by the mask (e.g. Jaśkowski & Przekoracka-Krawczyk 2005; Jaśkowski et al. 2008). Another, more extreme version holds that the NCE does not involve any inhibition, as perceptual interactions cause target-like elements in the mask to actively trigger the opposite response (e.g. Lleras & Enns 2004, 2006; Verleger et al. 2004). This mask-triggered activation hypothesis has largely been refuted by evidence of NCEs with masks that do not contain such features (Klapp 2005; Schlaghecken & Eimer 2006; Schlaghecken et al. 2007; Sumner 2008). Similarly, the finding that individuals with inhibitory deficits—both healthy older participants (Schlaghecken & Maylor 2005) and patients with micro-lesions of the supplementary motor cortex (Sumner et al. 2007)—produce robust PCEs, but no NCEs, can not be reconciled with the notion that the NCE merely reflects a second activation process of opposite direction to the one induced by the prime.
It is therefore generally agreed that the NCE does indeed reflect inhibition, either in the form of low-level self-inhibition (e.g. Schlaghecken & Eimer 2000), or in the form of mask-triggered inhibition, as proposed by Jaśkowski and colleagues. Although these accounts differ with respect to the mechanism underlying the inhibition process, they agree that the relevant process is one of motor inhibition. There is, however, a different possibility: as yet, it is not entirely clear as to what extent perceptual interactions between prime and target (irrespective of the mask) contribute to NCEs. In particular, one might argue that repetition blindness (e.g. Kanwisher 1987) or similar phenomena could selectively impair the perception of prime-compatible targets (i.e. targets that are a repetition of the prime). Such mechanisms would result in impaired performance on compatible trials even in the absence of any motor inhibition.
It has been shown that this explanation can not account for priming effects with the usual two-alternative forced-choice responses, which show NCEs even when targets are perceptually dissimilar to the primes and appear at a different location (e.g. Schlaghecken & Eimer 2000). However, the same has not yet been shown for nogo- and free-choice responses. Such responses differ from the usual ‘standard’ responses in that they are not tied by instruction to a particular target. In the case of nogo responses, they are, in fact, errors (‘false alarms’), committed in response to a target that required participants to withhold any overt response. In the case of free-choice responses, they are subjectively free and randomly chosen responses to a target that does not require a specific response. As with standard responses, however, both false alarms and free choices exhibit a systematic bias away from the compatible and towards the incompatible response (i.e. fewer and slower primed than unprimed false alarms and ‘free’ response choices, respectively; Eimer & Schlaghecken 1998; Klapp & Hinkley 2002; Schlaghecken & Eimer 2004; Klapp & Haas 2005). However, in those experiments the non-standard (nogo and free-choice) targets had features in common with standard targets and primes (e.g. the latter were unidirectional arrows, such as ‘<<’, and the former bi-directional arrows, ‘<>’). Consequently, effects akin to repetition blindness might have ‘blinded’ participants to the prime-compatible component of the target, leaving the prime-incompatible component more salient and consequently more likely to elicit a corresponding response. The observed effects could therefore be interpreted as being perceptually induced rather than reflecting a genuine, subliminally induced response bias.
The notion that subliminal stimuli might directly affect even seemingly voluntary decisions to select or withhold a particular response is clearly more controversial than the alternative perceptual explanation, and requires strong supporting evidence. Already, the use of different locations for primes and targets by Schlaghecken & Eimer (2004) rules out simple perceptual interactions of this type. A more definitive test though would be the use of targets that do not possess any elements in common with the prime. The present experiments therefore employed arrow primes and targets in combination with circle targets (on nogo and free-choice trials in experiments 1 and 2, respectively), again with different prime and target locations. If, as hypothesized, NCEs reflect low-level motor activation and inhibition processes instead of perceptually driven processes, then the response bias observed in the earlier experiments should be replicated under these conditions.
However, confirming that NCEs originate in the motor rather than in the perceptual system leaves open the issue of precisely where within the motor system they are generated. Eimer et al. (2002) demonstrated that when, for example, the left hand is primed, no priming of the left foot can be obtained, and vice versa. This was taken as evidence that priming effects are effector specific rather than being generated at the level of an abstract directional code. On the other hand, Schlaghecken et al. (2003) found that priming effects are not altered by repetitive transcranial magnetic stimulation (rTMS) of the primary motor cortex, suggesting that masked primes affect processing stages that are upstream from M1 and are thus more abstract than individual muscle commands.
The present study aimed to investigate whether the locus of the NCE within the motor system could be determined with greater precision. Participants in experiment 3 had to give the usual left or right unimanual response to arrow targets, but a bimanual left-and-right response to circle targets. Behavioural evidence (e.g. Klapp 1979; Jagacinski et al. 1988; Klapp et al. 1998), supported by monkey single-cell data (e.g. Donchin et al. 1998) and human imaging studies (for a review, see Swinnen & Wenderoth 2004), suggests that bimanual movements are represented as integrated response codes rather than as coordinated but separate effector-specific codes. Nevertheless, stimuli causing low-level automatic motor activations can speed up one effector relative to another (e.g. in a Simon task, when unilaterally presented targets require bimanual responses, the hand ipsilateral to the target location responds more quickly; Miller & Franz 2005). Thus if masked priming similarly affected individual muscle commands, then on bimanual trials (as with false-alarm and free-choice responses), the hand opposite to the prime direction should be favoured relative to the primed hand, and thus lead the response (as it is nearly impossible to achieve perfect simultaneity of key presses). However, if masked priming (in contrast to stimulus location in the Simon task) selectively operates on levels upstream from M1, then no such bias should be observed.
2. Material and methods
Except for the instructions pertaining to circle targets (see below), the three experiments were identical. Each contained two-thirds of standard masked prime trials, where left- and right-pointing arrow targets required a speeded left- or right-hand response, respectively, and one-third of circle-target trials. All targets were preceded by masked arrow primes, and all trial types were randomly intermixed.
(a) Participants
Fifty-four volunteers participated in experiment 1. Because the main objective of this experiment was to investigate priming effects on false alarms (responses to nogo circle targets), only those 18 participants who produced more than the overall average of 4.9 false alarms were included in the analysis (average numbers of false alarms were 1.5 for the excluded and 11.6 for the included participants). They were aged 19–25 years (M=20.4), and eight of them were male. Twenty volunteers (8 male), aged 18–28 years (M=20.7), participated in experiment 2, and 20 volunteers (11 male), aged 18–46 years (M=23.6), in experiment 3. All participants received either payment of £5 or course credit. According to self-report, all but four participants in experiment 1, and three in experiments 2 and 3, were right-handed, and all had normal or corrected-to-normal vision.
(b) Stimuli and apparatus
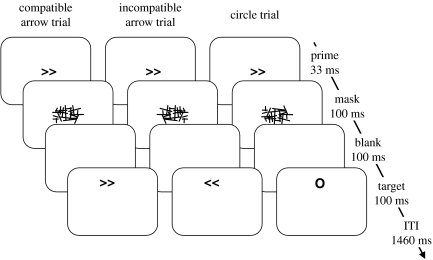
Left- and right-pointing double arrows (< < and >>) served as prime and target stimuli, subtending a visual angle of approximately 0.9°×0.5°. Masks were constructed from a 9×9 matrix, randomly filled with overlapping horizontal, vertical and oblique lines of different length, resulting in a roughly rectangular array of approximately 2.5°×1.0°. A circle (approximately 0.8° diameter) served as an additional target stimulus. All stimuli were presented in black on a white background on a 17-inch computer screen (see figure 1).
Figure 1.
Schematic representation of stimulus material and trial structure in all three experiments. Note that targets appeared randomly and with equal probability above (as depicted) or below fixation.
(c) Procedure
Participants were seated in a dimly lit, sound attenuated chamber in front of a computer screen (viewing distance=1.2 m), with response buttons under their left and right index fingers.
Experiments comprised 10 blocks of 60 trials each, preceded by one 20-trial practice block. Each trial began with a 33-ms prime immediately followed by a 100-ms mask, both presented at fixation. A new random mask was constructed on each trial in order to avoid perceptual learning of the mask and correspondingly increased prime identification (Schubö et al. 2001; Schlaghecken et al. 2008). In a staircase procedure, prime identification performance under these particular stimulus and timing conditions was at chance level (Schlaghecken et al. submitted). After a 100-ms blank screen,1 a 100-ms target was displayed randomly and with equal probability 3.0° above or below fixation. ITI was 1460 ms.
The participants were instructed to respond as quickly and accurately as possible to the direction of the target arrows (i.e. left-hand key presses to left-pointing arrows, right-hand key-presses to right-pointing arrows), maintaining central eye fixation. Experiments differed only with respect to the instruction regarding circle targets. Participants in experiment 1 (nogo) were instructed to withhold any response to circle targets. Participants in experiment 2 (free choice) were instructed to respond to them randomly with either a left or a right key press (it was stressed that they should respond with whatever key they ‘felt like’ pressing at the time, without producing any pre-planned patterns, e.g. without always giving a left-hand response to circles appearing below fixation). Participants in experiment 3 (bimanual) were instructed to respond with a simultaneous left and right key press to circle targets.
All six conditions (two primes x three targets) were presented randomly and with equal probability throughout each block.
(d) Data analysis
Error rates and mean reaction times (RTs) on correct-response trials were calculated for compatible (prime and target pointing in the same direction) and incompatible (prime and target pointing in different directions) arrow-target trials. For circle-target trials, mean RTs were calculated separately for prime-compatible and prime-incompatible responses (nogo task: false alarm executed with the primed or the unprimed hand; free-choice task: freely chosen response with the primed or the unprimed hand; bimanual task: primed or unprimed hand leading in the bimanual response). Response bias was calculated as the difference between the relative proportion of prime-incompatible responses and chance level (i.e. 50%, bias free). RTs and error rates on compatible and incompatible arrow trials and RTs on prime-compatible and prime-incompatible circle trials were analysed using paired t-tests. Response bias was analysed using one-sample t-tests. Finally, data from the bimanual and the free-choice experiments were compared directly (note that a meaningful comparison can not be made with the nogo experiment as here, responses to circle targets are errors, whereas in the other two experiments, they are correct). RTs were analysed using a repeated measures ANOVA with the within-subjects factors target type (arrow/circle) and prime (compatible/incompatible relative to the leading response hand), and the between-subjects factor experiment (bimanual/free choice).
3. Results
Mean RTs, error rates and response frequencies, together with the relevant t-tests, are displayed in table 1. Figure 2 shows the corresponding priming effects. For arrow trials, all experiments produced significant NCEs, with faster responses and fewer errors on incompatible than on compatible trials.
Table 1.
Mean RTs (ms) and error rates (%) on compatible and incompatible arrow-target trials for each experiment; and for circle targets, mean RT on compatible and incompatible false alarms (experiment 1), compatible and incompatible free choices (experiment 2), and compatible and incompatible leading hand on bimanual responses (experiment 3), and the corresponding frequency (%) of the incompatible false alarm, free-choice and leading-hand responses, respectively, together with the results of the corresponding statistical analyses (mean difference, 95% confidence interval [CI], t- and p-value). (RTs and error rates on compatible and incompatible trials were compared using paired t-tests; response frequencies were tested against chance level (50%) using one-sample t-tests.)
| experiment 1 (nogo) | experiment 2 (free choice) | experiment 3 (bimanual) | ||||||
|---|---|---|---|---|---|---|---|---|
| target | measure | condition/statistics | M | SE | M | SE | M | SE |
| arrow | RT (ms) | compatible | 364.0 | 7.7 | 336.0 | 8.7 | 376.9 | 8.1 |
| incompatible | 340.9 | 7.5 | 322.6 | 6.9 | 362.2 | 6.9 | ||
| difference (95% CI) | −23.1 | (−15.2 to −31.1) | −13.4 | (−5.9 to −20.8) | −14.7 | (−8.0 to −21.4) | ||
| t (p) | −6.14 | (<0.001) | −3.76 | (<0.01) | −4.61 | (<0.001) | ||
| error rate (%) | compatible | 17.5 | 1.6 | 22.5 | 2.4 | 5.2 | 0.9 | |
| incompatible | 7.7 | 1.0 | 14.4 | 2.4 | 2.9 | 0.9 | ||
| difference (95% CI) | −9.7 | (−6.5 to −13.0) | −8.1 | (−5.4 to −10.7) | −2.3 | (−0.6 to 4.1) | ||
| t (p) | −6.31 | (<0.001) | −6.25 | (<0.001) | −2.87 | (<0.01) | ||
| circle | RT (ms) | compatible | 304.4 | 17.8 | 352.7 | 11.7 | 383.6 | 9.3 |
| incompatible | 301.7 | 17.8 | 327.3 | 12.4 | 382.3 | 9.5 | ||
| difference (95% CI) | −2.7 | (−42.9 to 37.5) | −25.4 | (−6.0 to −44.8) | −1.3 | (−7.0 to 4.4) | ||
| t (p) | −0.14 | (>0.8) | −2.74 | (<0.05) | −0.48 | (>0.6) | ||
| response frequency (%) | incompatible (95% CI) | 62.7 | (54.4–70.9) | 57.6 | (55.0–60.1) | 48.4 | (46.5–50.3) | |
| t (p) | −3.24 | (<0.01) | −6.26 | (<0.001) | 1.78 | (>0.09) | ||
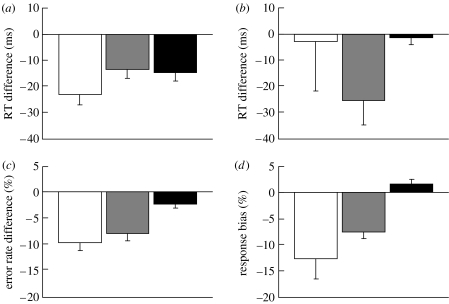
Figure 2.
Priming effects in all three experiments (experiment 1, nogo: white bar; experiment 2, free choice: grey bar; experiment 3, bimanual: black bar). (a) RT difference between compatible and incompatible arrow-target trials and (b) between prime-compatible and -incompatible responses on circle-target trials. (c) Error rate difference between compatible and incompatible arrow-target trials and (d) response selection bias on circle-target trials, expressed as chance level (50%) minus percentage of prime-incompatible response choices. Error bars indicate one standard error of the mean difference.
For circle trials, however, the results differed markedly between experiments. NCEs were obtained for nogo and free-choice trials, but not for bimanual responses: false alarms and freely chosen responses were significantly more likely to be executed with the unprimed than with the primed hand, and freely chosen prime-incompatible RTs were significantly shorter than freely chosen prime-compatible ones.2 In marked contrast to these results, no bias towards the unprimed hand occurred for bimanual responses: the unprimed (incompatible) response was not more likely to be the earlier of the two key presses (numerically, it was less likely to be the earlier one), and prime-incompatible leading key-presses were not faster than prime-compatible leading key-presses.
Direct comparison of the free choice and the bimanual experiments confirmed these results. For RTs, there was no interaction between target and prime or between target and experiment (both Fs<1), but the three-way interaction was significant, F1,38=6.88, MSE=235.39, p=0.012. As expected, follow-up ANOVAs showed that between-experiment differences in priming effects were not significant for arrow targets, F<1, but were significant for circle targets, F1,38=6.23, MSE=466.41, p=0.017. Similarly, the difference in circle-target response bias between experiments was highly significant, t(38)=6.08, p<0.001.
4. Discussion
Results of experiments 1 and 2 replicate and extend earlier findings (Eimer & Schlaghecken 1998; Klapp & Hinkley 2002; Schlaghecken & Eimer 2004; Klapp & Haas 2005): In nogo and free-choice masked prime tasks, a systematic response bias away from the primed response occurred, resulting in a preference for (and faster execution of) responses with the unprimed hand. Because this bias was observed in responses to targets that did not share features with primes or masks, it cannot be explained in terms of perceptual interactions between these stimuli, and thus strongly supports the motor interpretation of masked priming effects.3
Importantly, no such bias was found for bimanual responses, where the primed hand was as likely to be the one leading the response as the unprimed hand. This indicates that masked primes—in contrast to, for example, stimulus location in a Simon task (Miller & Franz 2005)—do not affect individual muscle commands, but rather more abstract response representations upstream from M1.
However, possible alternative explanations need to be considered. First, it might be that participants simply favoured their preferred hand, which would have been primed on half of the circle-target trials, and unprimed on the other half. This seems unlikely for two reasons: (i) even though most participants were right-handed, there was a small overall tendency to respond with the left hand first, irrespective of prime direction; and (ii) participants without any pronounced left- or right-hand bias (n=12) still failed to show any prime-induced response bias (frequency of responses with leading unprimed response hand=49.2%).
A second alternative explanation for the lack of response bias in bimanual responses is that participants actively withheld the faster response in order to comply with the explicit task instruction to respond with both hands simultaneously. Several factors speak against this interpretation. First, the average delay between the two key presses was 28 ms (range: 12–86 ms), approximately twice the size of the arrow-target NCE in this experiment. Furthermore, if strategic delays played a role, then participants who synchronized their hands more successfully should show less response bias, and participants who failed to synchronize should show more bias. However, there was no correlation between size of between-hand delay and size of response bias, r=0.28, n=20, p>0.2, and the marginally significant correlation between size of between-hand delay and that of RT effect (i.e. difference between primed and unprimed leading-hand RTs), r=0.43, n=20, p=0.059, was in fact opposite to the expected direction: participants with larger delays tended to show smaller NCEs than participants with shorter delays.4
Finally, it has to be noted that overall RTs were substantially longer in the bimanual experiment than in the other two experiments. Specifically, they were on average 48 ms longer than in the free-choice experiment. This is in line with the claim that there were three separate response alternatives in the bimanual experiment (left, right and bimanual), but only two (left and right) in the free-choice experiment. Therefore, the possibility that the increase in the number of response alternatives itself caused the lack of response bias for bimanual responses needs to be considered.
However, this notion is difficult to reconcile with the fact that there is no corresponding reduction in NCE size for unimanual (arrow-target) responses—if anything, the arrow-target NCE is larger (by 1 ms) in the bimanual than in the free-choice experiment. Furthermore, in an earlier study (Klapp & Hinkley 2002; exp. 3), increasing the number of response alternatives from two to three only caused a slight decrease in NCE size (by approximately 11% rather than 100%).
On the other hand, the self-inhibition model does predict a reduction in NCE size with increasing number of response alternatives, and increasing the number of alternatives from two to four has been found to reduce the NCE by approximately 50 per cent (Schlaghecken et al. 2006). Therefore, an additional analysis was conducted to investigate whether a similar reduction could be found in the present data. The size of the circle-target NCE with two response alternatives (i.e. in the free-choice experiment) was 25.4 ms. Comparing bimanual circle-target NCEs against 50 per cent of this value (12.7 ms) using an independent-sample t-test confirmed that the bimanual NCE was significantly smaller than this estimate, t(19)=4.20, p<0.001.
It thus has to be concluded that the absence of NCEs for bimanual responses indicates that masked arrow primes do not selectively activate muscle commands specifying one or the other hand; consequently, there is no subsequent inhibition of any such partial response activation. This in turn can be explained by assuming that primes affect motor representations at a relatively abstract level, that is, upstream from M1,5 where bimanual responses are represented as fully integrated codes. A possible neural substrate might be reverberating activity in supplementary motor-anterior striatal loops, which are assumed to mediate the preparation of instructed movements (e.g. Romo & Schultz 1992), as these structures have been implicated in masked prime-task performance on the basis of fMRI results (Aron et al. 2003) and patient studies (Sumner et al. 2007).
Taken together, the present results suggest the following scenario: at least in relatively simple choice RT tasks, participants prepare a number of direct perceptuo-motor links (e.g. Neumann & Klotz 1994), such that the perceptuo-motor system becomes particularly sensitive to specific stimuli and particularly likely to activate specific responses in their presence. In line with hierarchical models of motor control (e.g. Tresilian 1999), these links are formed between perceptual representations and relatively abstract response codes (as opposed to low-level muscle commands), as evidenced by the fact that primes associated with a specific response hand affect unimanual responses, but fail to affect the same effectors in a bimanual response. Perceiving a response-relevant stimulus in this context (even if only subliminally) will cause activation of the corresponding response code, which under appropriate conditions will be subsequently inhibited. Response-code activational levels will in turn affect M1 activation, but are themselves unaffected by any influence (e.g. rTMS, Schlaghecken et al. 2003) that directly impacts on M1. Thus in the present experiments, an arrow prime will activate an abstract representation of the corresponding unimanual motor code, thereby producing NCEs when responses are also unimanual (arrow-target responses, nogo responses [false alarms] and free-choice responses). However, an arrow prime will not activate the abstract representation of a bimanual response, which therefore is not subsequently inhibited.
Acknowledgements
This research was supported by a grant from the Economic and Social Research Council of Great Britain (grant no. RES-000-22-1841). We thank Malik Refaat, Daniella McNulty and Jasmin Chahal for running the experiments, and James Tresilian for helpful discussions.
Endnotes
In earlier experiments, this interval was usually 50 ms. However, pilot tests indicated that NCEs might be slightly larger and more robust with a 100-ms interval.
Numerically, though not statistically, the same was true for false alarms; however, the average number of false alarms was very low—4.3 and 7.3 for compatible and incompatible responses, respectively, resulting in a total of approximately 5.5 per cent of all nogo trials—thus false-alarm RT values are unlikely to be reliable.
Note that results of the nogo experiment were the same when all the participants were included in the analysis: RTs were 19 ms shorter and error rates 7.2 per cent lower on incompatible arrow trials compared with compatible arrow trials, and false alarms on circle trials were more likely with the unprimed hand than with the primed one (59.2% of all false alarms). All effects were statistically significant, all ts>2.5, all ps<0.02.
We conducted an additional experiment where the instruction to press both keys simultaneously was less emphasized. Again, a normal NCE (14 ms) was obtained for arrow trials, but no response bias was found in the circle trials responses (proportion of responses with unprimed hand leading: 49.7%). However, the lag between leading and trailing hands was only 5 ms larger in this than that in the original experiment, and the range of lags was actually smaller (13–66 ms). It thus seems safe to assume that despite the difference in instruction, the participants aimed just as much to produce a synchronous response to circle targets as in the original experiment.
This interpretation also fits with the results of Eimer et al. (2002), which had been taken to indicate that priming effects are effector specific: if in that study hand and foot responses had separate response codes, then no transfer from a primed hand response to an unprimed foot response should occur.
References
- Aron A., Schlaghecken F., Fletcher P., Bullmore E., Eimer M., Barker R., Sahakian B., Robbins T. Inhibition of subliminally primed responses is mediated by the caudate and thalamus: evidence from fMRI and Huntington's disease. Brain. 2003;126:713–723. doi: 10.1093/brain/awg067. doi:10.1093/brain/awg067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S., Naccache L., Le Clec H.G., Koechlin E., Mueller M., Dehaene-Lambertz G., van de Moortele P.F., Le Bihan D. Imaging unconscious semantic priming. Nature. 1998;395:597–600. doi: 10.1038/26967. doi:10.1038/26967 [DOI] [PubMed] [Google Scholar]
- Donchin O., Gribova A., Steinberg O., Bergman H., Vaadia E. Primary motor cortex is involved in bimanual coordination. Nature. 1998;395:17–274. doi: 10.1038/26220. doi:10.1038/25604 [DOI] [PubMed] [Google Scholar]
- Eimer M., Schlaghecken F. Effects of masked stimuli on motor activation: behavioral and electrophysiological evidence. J. Exp. Psychol. Hum. 1998;24:1737–1747. doi: 10.1037//0096-1523.24.6.1737. doi:10.1037/0096-1523.24.6.1737 [DOI] [PubMed] [Google Scholar]
- Eimer M., Schubö A., Schlaghecken F. The locus of inhibition in the masked priming of response alternatives. J. Motor. Behav. 2002;34:3–10. doi: 10.1080/00222890209601926. [DOI] [PubMed] [Google Scholar]
- Jagacinski R.J., Marshburn E.A., Klapp S.T., Jones M.R. Tests of streamed vs. integrated structure in polyrhythmic tapping. J. Motor. Behav. 1988;20:416–442. doi: 10.1080/00222895.1988.10735455. [DOI] [PubMed] [Google Scholar]
- Jaśkowski P., Przekoracka-Krawczyk A. On the role of mask structure in subliminal priming. Acta Neurobiol. Exp. 2005;65:409–317. doi: 10.55782/ane-2005-1569. [DOI] [PubMed] [Google Scholar]
- Jaśkowski P., Białuńska A., Tomanek M., Verleger R. Mask- and distractor-triggered inhibitory processes in the priming of motor responses: an EEG study. Psychophysiology. 2008;45:70–85. doi: 10.1111/j.1469-8986.2007.00595.x. [DOI] [PubMed] [Google Scholar]
- Kanwisher N.G. Repetition blindness: type recognition without token individuation. Cognition. 1987;27:117–143. doi: 10.1016/0010-0277(87)90016-3. doi:10.1016/0010-0277(87)90016-3 [DOI] [PubMed] [Google Scholar]
- Klapp S.T. Doing two things at once: the role of temporal compatibility. Mem. Cogn. 1979;7:375–381. [Google Scholar]
- Klapp S.T. Two versions of negative compatibility have different properties: a reply to Lleras and Enns (2004) J. Exp. Psychol. Gen. 2005;134:431–435. doi: 10.1037/0096-3445.134.3.431. doi:10.1037/0096-3445.134.3.431 [DOI] [PubMed] [Google Scholar]
- Klapp S.T., Haas B.W. The non-conscious influence of masked stimuli on response selection is limited to concrete stimulus-response associations. J. Exp. Psychol. Hum. 2005;31:193–209. doi: 10.1037/0096-1523.31.1.193. [DOI] [PubMed] [Google Scholar]
- Klapp S.T., Hinkley L.B. The negative compatibility effect: unconscious inhibition influences reaction time and response selection. J. Exp. Psychol. Gen. 2002;131:255–269. doi: 10.1037//0096-3445.131.2.255. doi:10.1037/0096-3445.131.2.255 [DOI] [PubMed] [Google Scholar]
- Klapp S.T., Nelson J.M., Jagacinski R.J. Can people tap concurrent bimanual rhythms independently? J. Motor. Behav. 1998;30:301–322. doi: 10.1080/00222899809601346. [DOI] [PubMed] [Google Scholar]
- Lleras A., Enns J.T. Negative compatibility or object updating? A cautionary tale of mask-dependent priming. J. Exp. Psychol. Gen. 2004;133:475–493. doi: 10.1037/0096-3445.133.4.475. doi:10.1037/0096-3445.133.4.475 [DOI] [PubMed] [Google Scholar]
- Lleras A., Enns J.T. How much like a target can a mask be? Geometric, spatial, and temporal similarity in priming—a reply to Schlaghecken and Eimer. J. Exp. Psychol. Gen. 2006;135:495–500. doi: 10.1037/0096-3445.135.3.495. doi:10.1037/0096-3445.135.3.495 [DOI] [PubMed] [Google Scholar]
- Miller J.O., Franz E.A. Dissociation of bimanual responses with the Simon effect: on the nonunitization of bimanual responses. J. Motor. Behav. 2005;37:146–156. doi: 10.3200/JMBR.37.2.146-156. doi:10.3200/JMBR.37.2.146-156 [DOI] [PubMed] [Google Scholar]
- Neumann O., Klotz W. Motor responses to nonreportable, masked stimuli: where is the limit of direct parameter specification? In: Umiltà C., Moscovitch M., editors. Attention and performance XV: conscious and nonconscious information processing. MIT Press; Cambridge, MA: 1994. pp. 123–150. [Google Scholar]
- Romo R., Schultz W. Role of primate basal ganglia and frontal cortex in the internal generation of movements III. Neuronal activity in the supplementary motor area. Exp. Brain Res. 1992;91:396–407. doi: 10.1007/BF00227836. [DOI] [PubMed] [Google Scholar]
- Schlaghecken F., Eimer M. The influence of subliminally presented primes on response preparation. Sprache Kognit. 1997;16:166–175. [Google Scholar]
- Schlaghecken F., Eimer M. A central/peripheral asymmetry in subliminal priming. Percept. Psychophys. 2000;62:1367–1382. doi: 10.3758/bf03212139. [DOI] [PubMed] [Google Scholar]
- Schlaghecken F., Eimer M. Masked prime stimuli can bias ‘free’ choices between response alternatives. Psychon. Bull. Rev. 2004;11:463–468. doi: 10.3758/bf03196596. [DOI] [PubMed] [Google Scholar]
- Schlaghecken F., Eimer M. Active masks and active inhibition: a comment on Lleras and Enns (2004) and on Verleger, Jaskowski, Aydemir, van der Lubbe, and Groen. J. Exp. Psychol. Gen. 2006;135:484–494. doi: 10.1037/0096-3445.135.3.484. doi:10.1037/0096-3445.135.3.484 [DOI] [PubMed] [Google Scholar]
- Schlaghecken F., Maylor E.A. Motor control in old age: evidence of impaired low-level inhibition. J. Gerontol. B Psychol. Sci. Soc. Sci. 2005;60:158–161. doi: 10.1093/geronb/60.3.p158. [DOI] [PubMed] [Google Scholar]
- Schlaghecken F., Münchau A., Bloem B., Rothwell J.C., Eimer M. Slow frequency repetitive transcranial magnetic stimulation affects reaction times, but not priming effects, in a masked prime task. Clin. Neurophysiol. 2003;114:1272–1277. doi: 10.1016/s1388-2457(03)00118-4. doi:10.1016/S1388-2457(03)00118-4 [DOI] [PubMed] [Google Scholar]
- Schlaghecken F., Bowman H., Eimer M. Dissociating activation, inhibition, and disinhibition in low-level motor control. J. Exp. Psychol. Hum. 2006;32:618–632. doi: 10.1037/0096-1523.32.3.618. [DOI] [PubMed] [Google Scholar]
- Schlaghecken F., Rowley L., Sembi S., Simmons R., Whitcomb D. The negative compatibility effect: a case for inhibition. Adv. Cognit. Psychol. 2007;3:227–240. doi: 10.2478/v10053-008-0027-y. doi:10.2478/v10053-008-0027-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaghecken F., Blagrove E., Maylor E.A. No difference between conscious and nonconscious visuomotor control: evidence from perceptual learning in the masked prime task. Consc. Cogn. 2008;17:84–93. doi: 10.1016/j.concog.2006.11.004. doi:10.1016/j.concog.2006.11.004 [DOI] [PubMed] [Google Scholar]
- Schlaghecken, F., Refaat, M. & Maylor, E. A. Submitted. Conflict and control: differences between masked and unmasked priming as revealed by a hybrid Simon task.
- Schubö A., Schlaghecken F., Meinecke C. Learning to ignore the mask in texture segmentation tasks. J. Exp. Psychol. Hum. 2001;27:919–931. doi: 10.1037//0096-1523.27.4.919. doi:10.1037/0096-1523.27.4.919 [DOI] [PubMed] [Google Scholar]
- Sumner P. Mask-induced priming and the negative compatibility effect. Exp. Psychol. 2008;55:133–141. doi: 10.1027/1618-3169.55.2.133. doi:10.1027/1618-3169.55.2.133 [DOI] [PubMed] [Google Scholar]
- Sumner P., Nachev P., Morris P., Peters A.M., Jackson S.R., Kennard C., Husain M. Human medial frontal cortex mediates unconscious inhibition of voluntary action. Neuron. 2007;54:697–711. doi: 10.1016/j.neuron.2007.05.016. doi:10.1016/j.neuron.2007.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinnen S.P., Wenderoth N. Two hands, one brain: cognitive neuroscience of bimanual skill. Trends Cogn. Sci. 2004;8:18–25. doi: 10.1016/j.tics.2003.10.017. doi:10.1016/j.tics.2003.10.017 [DOI] [PubMed] [Google Scholar]
- Tresilian J.R. Abstract levels of motor control in prehension: normal and pathological performance. Hum. Mov. Sci. 1999;18:219–239. doi:10.1016/S0167-9457(99)00009-3 [Google Scholar]
- Verleger R., Jaśkowski P., Aydemir A., van der Lubbe R.H.J., Groen M. Qualitative differences between conscious and non-conscious processing? On inverse priming induced by masked arrows. J. Exp. Psychol. Gen. 2004;133:494–515. doi: 10.1037/0096-3445.133.4.494. doi:10.1037/0096-3445.133.4.494 [DOI] [PubMed] [Google Scholar]