Abstract
Migratory birds are assumed to be under stronger sexual selection pressure than sedentary populations, and the fact that their song is more complex has been taken as confirmation of this fact. However, this assumes that sexual selection pressure due to both male competition and female choice increase together. A further issue is that, in many species, songs become less complex during competitive encounters; in contrast, female choice selects for more complex song, so the two selection pressures may drive song evolution in different directions. We analysed song in two sedentary and two migratory populations of blackcaps (Sylvia atricapilla), a species in which different song parts are directed to males and females. We found that migratory populations produce longer, female-directed warbles, indicating sexual selection through female choice is the strongest in these populations. However, the part of the song directed towards males is shorter and more repetitive (as observed in individual competitive encounters between males) in non-migratory populations, indicating sedentary populations, are under stronger selection due to male competition. We show for the first time that the intensity of selection pressure from male competition and female choice varies independently between populations with different migratory behaviours. Rapid alterations in the migration patterns of species are thus likely to lead to unexpected consequences for the costs and benefits of sexual signals.
Keywords: blackcap, Sylvia atricapilla, song complexity, sexual selection, migration, warbler
1. Introduction
It has long been hypothesized that migratory birds are under stronger sexual selection pressure than sedentary birds, because migrants spend less time on their breeding grounds and consequently have less time for territory establishment, pairing and breeding (Catchpole 1982). Comparative analysis has shown that song repertoire size, a sexually selected trait, is larger in migratory species compared with non-migratory species (Read & Weary 1992); more complex songs are found in species (e.g. Vireos; Mountjoy & Leger 2001) or populations (e.g. greenish warblers, Phylloscopus trochiloides; Irwin 2001; Irwin et al. 2008) that migrate greater distances. However, whether the variation in complexity in any of the above cases is due to differences in selection pressure from choosing females or competing males is not clear.
There are two main issues concerning the above hypothesis. First, it is not clear whether both intrasexual selection and intersexual selection are more intense in migratory populations. Second, intersexual and intrasexual selection may not drive the evolution of song characteristics in the same direction. In all the species studied so far, inter-sexual selection leads to more complex songs (Catchpole 1980; Catchpole & Leisler 1996), while intrasexual selection often leads to short stereotyped songs (Catchpole 1982; Collins 2004). These issues can only be resolved if it is possible to investigate the effects of intersexual and intrasexual selection on a trait independently (Moore & Moore 1999; Irwin 2001). Teasing apart the effects of male competition and female choice upon the evolution of any one trait is a complex process, particularly if the optimal outcome from each process separately is in conflict (e.g. Andersson et al. 2002; Bonduriansky & Rowe 2003; Candolin 2004, 2005). Investigating both the strength and effect of intersexual and intrasexual selection on song variation between birds with different migratory behaviours requires a suitable model system.
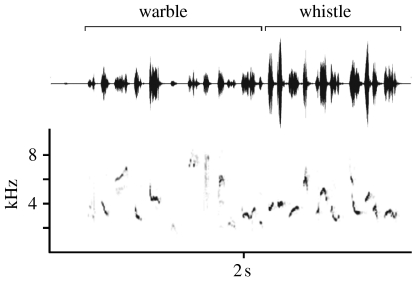
The blackcap provides such a model system because blackcaps show well-studied differences in migratory behaviour between populations, which are likely to result in differences in sexual selection pressures (Telleria & Carbonell 1999; Pérez-Tris & Telleria 2002b; Bearhop et al. 2005). In addition, blackcap song consists of two distinct parts (Bergmann & Helb 1982), a complex warble of low amplitude with a broad frequency range, followed by a loud whistle consisting of tonal, flute-like notes with a narrow frequency range (figure 1). Each part is directed to a receiver of a particular sex, allowing us to determine the effects of intersexual and intrasexual selection on song evolution independently. The warble is important in female attraction, being longer when females are fertile (Cramp 1992; Collins 2004) and used more prior to extra-pair copulations (Johannessen 1998). The whistle is more important in male competition, becoming shorter and more stereotyped during aggressive male–male interactions (Sauer 1955), although it probably also serves to alert females to the presence of a male (Balsby 2000). Also, the whistle is heard throughout the breeding season, i.e. after males have paired (Cramp 1992). It is possible that selection via both male and female receivers acts on whistle characteristics; however, evidence suggests that the primary function is male competition. As mentioned earlier, short stereotyped male-directed song types (Catchpole & Leisler 1996; Forstmeier & Balsby 2002), or a reduction in complexity of songs directed to males (Catchpole 1983), are quite common, particularly in warbler species (see review in Collins 2004).
Figure 1.
The waveform and spectrogram of a blackcap song. The warble part has a relatively low amplitude (broad frequency range), while the whistle part has a high amplitude (narrow frequency range).
We measured the warble and whistle song characteristics of blackcaps from four Iberian populations, two sedentary and two migratory, in order to test the hypothesis that migratory populations are under stronger intra- and inter-selection pressure than sedentary populations. Previous work suggests that in migratory populations the warble will be longer as a response to increased pressure, due to time constraints, to attract females (Catchpole 1982). We can make a clear prediction that in blackcaps more intense male–male competition will result in short, more stereotyped (i.e. small syllable repertoire) whistles. However, which type of population is under stronger selection due to intrasexual competition is not certain. Although both migratory and sedentary males need to acquire and defend a territory, there are different indications as to which population should experience more intense selection through male–male competition (Irwin & Irwin 2005). While migratory blackcaps are under a stronger time pressure to obtain a territory during the breeding season than sedentary blackcaps, the latter defend territories for longer (Perez-Tris & Telleria 2002a,b). Migratory and sedentary populations have rapidly diverged from each other after the last glaciation and show no consistent neutral genetic structuring (Pérez-Tris et al. 2004). Therefore, any song differences are unlikely to be due to drift, and are more easily explained as a result of selection, through variation in benefits and/or costs.
2. Material and methods
(a) Populations
Four distinct Iberian populations, two sedentary and two migratory, were recorded:
Álava (42°55′ N 2°29′ W)—a migratory population that moves to southern Spain in winter. The habitat consists of mixed oak and maple forest (Quercus faginea, Quercus robur and Acer campestre); mean annual rainfall is 1000–1500 mm; mean annual temperature is 12°C at an elevation of 620 m; blackcap spring density=17/10 ha, winter density=1/10 ha.
Guadarrama (40°54′ N 3°53′ W)—a migratory population that moves to southern Spain in winter. The habitat consists of mixed ash (Fraxinus excelsior), alder (Alnus glutinosa) and oak (Quercus pyrenaica) forests; mean annual rainfall is 700–1000 mm; mean annual temperature is 10°C at an elevation of 1100 m; blackcap spring density=5/10 ha, winter density=0.
Tarifa (36°01′ N 5°36′ W)—a sedentary population that has an influx of migratory immigrants in winter. The habitat consists of mixed oak (Quercus suber and Quercus canariensis) forests; mean annual rainfall is 1000–1500 mm; mean annual temperature is 16°C at an elevation of 250 m; blackcap spring density=8/10 ha, winter density=10/10 ha (due to arrival of northern migrants).
Quinta da Saõ Pedro Field Station, Sobreda di Caparica, near Lisbon, Portugal (38°39′ N 9°11′ W)—a sedentary population with migratory immigrants in winter. The habitat consists of mixed eucalyptus (Eucalyptus globules), acacia mimosa (Acacia dealbata), oak (Quercus suber) and various fruit trees; mean annual rainfall is 700–900 mm; mean temperature is 17°C at an elevation of 56 m; blackcap spring density is 11/10 ha; the winter density is not known.
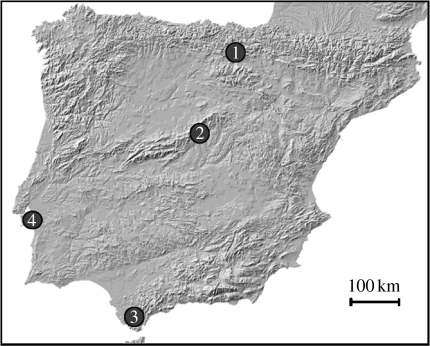
See figure 2 for map showing locations of populations.
Figure 2.
Map showing the location of the study sites. (1) Álava (migratory); (2) Guadarrama (migratory); (3) Tarifa (sedentary) and (4) Lisbon (sedentary).
(b) Song recording
Songs in populations 1–3 were recorded with a Marantz CP430 stereo three-head portable cassette recorder from 16 June to 7 July 1998 by J.P.-T. For population 4 songs were recorded with a Sennheiser MKH70 microphone and Sony TCD-D8 digital tape recorder between 12 and 20 April 2002 by SAC. In each population individuals were recorded at several locations a few km apart within each study area. Within each location recordings of individuals were always separated by at least 500 m. In total 62 individuals (12 Álava, 13 Guadarrama, 15 from Tarifa and 22 from Lisbon) singing a total of 694 songs (an average of 11.2 songs per individual). All the recordings were made after pairing and egg laying (for birds in Lisbon we checked the nest to confirm the presence of eggs). Although we expect that the warble will be relatively shorter within all populations at this point (the warble declines immediately after pairing; Cramp 1992), it allows us to compare populations at comparable points, i.e. post-pairing. The population in Lisbon was recorded earlier in the breeding season (post-laying, but pre-fledging) than the other three populations, due to logistical constraints on the two teams recording the birds. For this reason all statistical analyses were conducted both including and omitting the data from the Lisbon population.
(c) Song measurement and analysis
Acoustic analyses were conducted using Avisoft SASlab Pro. (FFT size 512 Hz, frequency bandwidth 23 Hz, time resolution 43 ms). For each song we measured the warble and whistle duration, and the ratio of the warble to the song duration (warble/total song length). We did not calculate warble repertoire size (due to the complexity of the warble in some populations), but used the length of the warble as an indicator of the importance of intersexual communication (Collins 2004).
We also counted the number of different notes in five subsequent whistles per individual, and calculated the relative complexity of the whistle as the number of different notes to total number of notes in the whistle (i.e. different notes/number of notes). In some cases we did not have five consecutive songs, so these individuals were excluded from the analysis.
(d) Statistical analysis
Differences in song-part duration between populations were tested with mixed-model ANOVA, with population and individual (nested within population) as fixed factors. For relative whistle complexity, means were calculated for each individual bird, and differences among population were analysed using GLM Univariate ANOVA. All statistics were conducted using SPSS 14.0.
3. Results
(a) Warble
The two sedentary populations (Lisbon and Tarifa) have shorter female-directed warbles than the two migratory populations (Álava and Guadarrama; F3,641=12.2, p<0.001; post hoc analysis Bonferroni p<0.05; figure 3a). The ratio of the warble to the song duration also differs between populations (F3,641=23.9, p<0.001, figure 3a). Post hoc analysis shows that the two migratory populations have relatively longer warbles than the two sedentary populations (Bonferroni p<0.05). For both parameters there were also individual differences (warble length: F53,641=9.86, p<0.001; relative warble: F53,641=11.3, p<0.001).
Figure 3.
Song characteristics of migratory and sedentary populations of blackcaps. (a) Mean±s.e. duration of warble (black bars) and whistle (white bars). The numbers on the graphs represent the mean of the warble : whistle ratio in each population. (b) Mean±s.e. whistle characteristics (total number of notes in white; number of different notes in black). The numbers on the graphs represent mean whistle relative to repertoire size (no. of different notes/total no. of notes).
Conducting the analyses without including the sedentary Lisbon population gives similar results. The migratory populations have longer warbles than the sedentary population (F2,382=17.29 p<0.001; post hoc Bonferroni p<0.05). However, the relative warble length is different among all three Spanish populations (F2,382=31.24, p<0.00; post hoc Bonferroni p<0.05), with the migratory Guadarrama population having the relatively longest warble, and the sedentary Tarifa population the shortest (see figure 3a).
(b) Whistle
We found a significant difference in whistle length with the migratory population in Álava having longer whistles than the other three populations (F3,641=4.63, p=0.003; Bonferroni p<0.05; figure 3a). However, there was no difference between the whistle length of the Guadarrama (central migratory) and the two sedentary populations (Tarifa and Lisbon).
The result is the same if the Lisbon population is excluded; the Álava population has significantly longer whistles compared with the other two populations (F3,382=7.25, p=0.001; Bonferroni p<0.05).
Males from both sedentary populations have fewer different notes in the whistle compared with the two migratory populations (whistle repertoire over five songs: F3,47=12.21, p<0.001; Bonferroni p<0.05; figure 3b). After correcting for the total number of notes in the whistle, we found differences in relative complexity between Álava and the two sedentary populations (Tarifa and Lisbon), but the Guadarrama migratory population was not significantly different from any of the other populations (F3,470=3.6, p=0.006; Bonferroni p<0.05; figure 3b).
Excluding the Lisbon population gives the same results as above: the number of different notes is lower in the sedentary Tarifa population compared with the two migratory populations; the relative complexity is greatest in the Álava population compared with the Tarifa population; and the migratory Guadarrama population does not differ significantly from either.
4. Discussion
Our results show that migratory blackcaps are under stronger sexual selection pressure due to female choice, and suggest that sedentary blackcaps are under strongest selection pressure due to male–male competition, as evidenced by differences in song characteristics. Migratory blackcaps have longer warbles, even after pairing, compared with blackcaps in sedentary populations. This is consistent with previous work (Catchpole 1982; Read & Weary 1992; Irwin 2001; Mountjoy & Leger 2001; Irwin et al. 2008), showing that migratory birds tend to have more complex songs. Interestingly, the differences in song complexity that we found in blackcap populations correspond with differences in the time available for breeding among different Iberian breeding regions (Pérez-Tris & Telleria 2002b), again supporting the hypothesis that sexual selection through female choice is stronger in migratory populations due to time constraints for breeding (Catchpole 1982). Since all studies on female song preferences have found that females prefer a more complex song (for review see Collins 2004), the story appears clear: in migratory populations the strong pressure to find a mate, breed quickly and make the most of the short breeding season (e.g. extra pair copulations; Johannessen 1998) drives the evolution of a more complex song than in sedentary populations.
As we predicted, the results concerning the part of the song mainly involved in male competition are more complex. Males in the most northern population (Álava) of migratory blackcaps have relatively long, complex whistles; those in the central migratory population (Guadarrama) have short, but intermediately complex, whistles; those in the two sedentary populations have short, repetitive whistles. Given that blackcap whistles become short and stereotyped during competitive encounters (Sauer 1955; Cramp 1992; S. A. Collins 2000–2003, personal observation), the results are not consistent with the idea that migratory populations are under stronger selection pressure due to male competition, but suggest that male competition is stronger in sedentary populations. However, the migratory central population in Guadarrama does have short whistles, although the whistle tends to have more different notes than the two sedentary populations, so the picture is somewhat unclear. As noted earlier, it is likely that although the primary function of the whistle is male competition, females are probably attracted to a territory via the whistle (Balsby 2000). Strong selection from female choice in the Guadarrama population may have maintained some variability in the whistle, despite male competition selecting for a reduction in the length and complexity.
It is possible that male competition selected for shorter, more repetitive whistles in both the sedentary (Lisbon and Tarifa) and the central (Guadarrama) populations, but for different reasons. Sedentary blackcaps remain on their breeding territory all year round and defend their territories for a larger part of the year (Perez-Tris & Telleria 2002a). Therefore, territory defence causes more continuous selection on sedentary males, while such selection is seasonal for migratory populations (Irwin & Irwin 2005). The migratory Guadarrama blackcaps, on the other hand, breed in a montane, highly seasonal environment that can be exploited during comparatively short periods (Pérez-Tris & Telleria 2002b); that is, there is a very short breeding season. Competition is therefore likely to be particularly intense in this population, which lives in a somewhat marginal habitat (Pérez-Tris & Telleria 2002b). Overall the results suggest that the Álava (migratory) population is under strong female-directed but weak male-directed selection pressure; the Guadarrama population is under strong female-directed and strong male-directed selection pressure; both sedentary populations are under weak female-directed but strong male-directed selection pressures.
Variation in the duration of the whistle among blackcap populations could possibly have other causes. It has been predicted that greater population density should lead to relatively short, stereotyped songs (Slater 1981; Catchpole 1982; Wiley & Richards 1982), but the population with the highest density is the northern population (Carbonell & Telleria 1998), which has long variable whistles. Perhaps, owing to the lower population density in the central population, songs need to be transmitted over longer distances and selection for long-distance transmission favours shorter, more stereotyped signals (Wiley & Richards 1982). However, the two sedentary blackcap populations have equally short whistles as the central population, but a higher population density. Furthermore, the results from the greenish warbler populations do not support the above hypothesis; greenish warblers found in lower population densities had a more complex, less stereotypical song (Irwin 2001). None of the other environmental variables (e.g. annual rainfall, average temperature or elevation; Carbonell et al. 2003) covaried with whistle characteristics; therefore, a purely environmental explanation for the difference in whistle characteristics is unlikely. This is consistent with the results for the greenish warbler, where environmental and morphological differences did not map onto song differences (Irwin 2001; Irwin et al. 2008).
Our results have implications for the study of differences in song structure between populations. If the effects of both processes of sexual selection lead to an increase in song complexity, and a particular population has more complex song, we cannot conclude whether either one, or both, sexual selection processes are responsible for the increase in complexity without detailed observations of behaviour. However, if, as in the blackcap, female choice drives the evolution of complexity and male competition drives the evolution of stereotypy in a song, there are a number of possible outcomes with important evolutionary implications. If the species in question cannot respond to the two evolutionary processes independently (i.e. via separate song components for the two receivers), then the optimal outcome in song structure will be a trade-off between the selection due to male and female receivers (Bonduriansky & Rowe 2003). Again this will make it difficult to make conclusions about the relative strength of intrasexual and intersexual selections in different populations as any one optimal outcome in song structure could be due to different combinations of values for the strength of intrasexual and intersexual selections. This issue has not been addressed in previous studies, as it was assumed implicitly that the two processes had similar effects on song evolution and varied in concert.
Our study shows for the first time that the relative strength of selection pressure from male competition and female choice can vary independently within different populations of the same species. In addition, we show for the first time, in contrast to what was generally believed, that sexual selection exerted through female choice is more intense for migratory than sedentary populations, but that sedentary populations may be under stronger selection than migratory populations from male competition. It is generally agreed that more species will change their migratory behaviour in response to climate change (Visser & Both 2005). How this will impact upon population fitness is a matter of great importance. In particular, conflicting selection processes, such as the fact that two processes of sexual selection may drive a character trait in different directions, could lead to suboptimal outcomes in trait characteristics. Whether birds can adapt their behaviour to the new requirements will depend upon the genetic composition and phenotypic plasticity of the population.
Acknowledgments
We would like to thank Dr H. Slabbekoorn, Prof. C. J. Barnard (sadly no longer with us) and two anonymous referees for comments on an earlier version of the paper. We would also like to thank Daniel Bermejo for his assistance on the project. The research was funded by the Spanish Ministry of Education and Science (projects RYC-2005-000206 to J.P.-T. and CGL2004-02744/BOS to J.L.T.).
References
- Andersson S., Pryke S.R., Ornborg J., Lawes M.J., Andersson M. Multiple receivers, multiple ornaments, and a trade-off between agonistic and epigamic signaling in a widowbird. Am. Nat. 2002;160:683–691. doi: 10.1086/342817. doi:10.1086/342817 [DOI] [PubMed] [Google Scholar]
- Balsby T.J.S. The function of song in Whitethroats Sylvia communis. Bioacoustics. 2000;11:17–30. [Google Scholar]
- Bearhop S., Fiedler W., Furness R.W., Votier S.C., Waldron S., Newton J., Bowen G.J., Berthold P., Farnsworth K. Assortative mating as a mechanism for rapid evolution of a migratory divide. Science. 2005;310:502–504. doi: 10.1126/science.1115661. doi:10.1126/science.1115661 [DOI] [PubMed] [Google Scholar]
- Bergmann H.H., Helb H.W. BLV Verlagsgesellschaft; Munich, Germany: 1982. Stimmen der Vogel Europas. [Google Scholar]
- Bonduriansky R., Rowe L. Interactions among mechanisms of sexual selection on male body size and head shape in a sexually dimorphic fly. Evolution. 2003;57:2046–2053. doi: 10.1111/j.0014-3820.2003.tb00384.x. doi:10.1554/03-047 [DOI] [PubMed] [Google Scholar]
- Candolin U. Opposing selection on a sexually dimorphic trait through female choice and male competition in a water boatman. Evolution. 2004;58:1861–1864. doi: 10.1111/j.0014-3820.2004.tb00470.x. doi:10.1554/04-058 [DOI] [PubMed] [Google Scholar]
- Candolin U. Why do multiple traits determine mating success? Differential use in female choice and male competition in a water boatman. Proc. R. Soc. B. 2005;272:47–52. doi: 10.1098/rspb.2004.2932. doi:10.1098/rspb.2004.2932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonell R., Telleria J.L. Selection and habitat use by five Iberian blackcap (Sylvia atricapilla) populations. Ardeola. 1998;45:1–10. [Google Scholar]
- Carbonell R., Perez-Tris J., Telleria J.L. Effects of habitat heterogeneity and local adaptation on the body condition of a forest passerine at the edge of its distributional range. Biol. J. Linn. Soc. 2003;78:479–488. doi:10.1046/j.0024-4066.2002.00156.x [Google Scholar]
- Catchpole C.K. Sexual selection and the evolution of complex songs among European warblers of the genus Acrocephalus. Behaviour. 1980;74:149–166. doi:10.1163/156853980X00366 [Google Scholar]
- Catchpole C.K. The evolution of bird sounds in relation to mating and spacing behavior. In: Kroodsma D.E., Miller E.H., editors. Acoustic communication in birds. vol. 1. Academic Press; London, UK: 1982. pp. 297–319. [Google Scholar]
- Catchpole C.K. Variation in the song of the great reed warbler Acrocephalus arundinaceus in relation to mate attraction and territorial defence. Anim. Behav. 1983;31:1217–1225. doi:10.1016/S0003-3472(83)80028-1 [Google Scholar]
- Catchpole C.K., Leisler B. Female aquatic warblers (Acrocephalus paludicola) are attracted by playback of longer and more complicated songs. Behaviour. 1996;133:1153–1164. doi:10.1163/156853996X00341 [Google Scholar]
- Collins S.A. Vocal fighting and flirting: the functions of birdsong. In: Marler P., Slabbekoorn H., editors. Nature's music. Elsevier; San Diego, CA: 2004. pp. 39–79. [Google Scholar]
- Cramp S. In: The birds of the western Palearctic. Cramp S., editor. vol. IV. Oxford University Press; Oxford, UK: 1992. pp. 496–515. [Google Scholar]
- Forstmeier W., Balsby T.J.S. Why mated dusky warblers sing so much: territory guarding and male quality announcement. Behaviour. 2002;139:89–111. doi:10.1163/15685390252902300 [Google Scholar]
- Irwin D.E. Speciation in an avian ring species. Evolution. 2001;54:998–1010. doi: 10.1111/j.0014-3820.2000.tb00099.x. [DOI] [PubMed] [Google Scholar]
- Irwin D.E., Irwin J.H. Siberian migratory divides: the role of seasonal migration in speciation. In: Greenberg R., Marra P.P., editors. Birds of two worlds: the ecology and evolution of migration. Johns Hopkins University Press; Baltimore, MD: 2005. pp. 27–40. [Google Scholar]
- Irwin D.E., Thimgan M.P., Irwin J.H. Call divergence is correlated with geographic and genetic distance in greenish warblers (Phylloscopus trochiloides): a strong role for stochasticity in signal evolution? J. Evol. Biol. 2008;21:435–448. doi: 10.1111/j.1420-9101.2007.01499.x. doi:10.1111/j.1420-9101.2007.01499.x [DOI] [PubMed] [Google Scholar]
- Johannessen K. Territorial behaviour and sperm competition in male blackcaps Sylvia atricapilla. Fauna Norv. Ser. C Cinclus. 1998;21:17–36. [Google Scholar]
- Moore A.J., Moore P.J. Balancing sexual selection through opposing mate choice and male competition. Proc. R. Soc. B. 1999;266:711–716. doi:10.1098/rspb.1999.0694 [Google Scholar]
- Mountjoy J., Leger D.W. Vireo song repertoires and migratory distance: three sexual selection hypotheses fail to explain the correlation. Behav. Ecol. 2001;12:98–102. [Google Scholar]
- Perez-Tris J., Telleria J.L. Migratory and sedentary blackcaps in sympatric non-breeding grounds: implications for the evolution of avian migration. J. Anim. Ecol. 2002a;71:211–224. doi:10.1046/j.1365-2656.2002.00590.x [Google Scholar]
- Pérez-Tris J., Telleria J.L. Regional variation in seasonality affects migratory behaviour and life-history traits of two Mediterranean passerines. Acta Oecol. Int. J. Ecol. 2002b;23:13–21. doi:10.1016/S1146-609X(01)01129-8 [Google Scholar]
- Pérez-Tris J., Bensch S., Carbonell R., Helbig A.J., Tellería J.L. Historical diversification of migration patterns in a passerine bird. Evolution. 2004;58:1819–1832. doi: 10.1554/03-731. doi:10.1554/03-731 [DOI] [PubMed] [Google Scholar]
- Read A.F., Weary D.M. The evolution of bird song—comparative analyses. Phil. Trans. R. Soc. B. 1992;338:165–187. doi:10.1098/rstb.1992.0137 [Google Scholar]
- Sauer F. Uber variationen der Artgesange bei Grasmucken. Ein beitrag zur Frage des ‘Leierens’ des Monchsgrasmucke. J. Ornithol. 1955;96:129–146. doi:10.1007/BF01975008 [Google Scholar]
- Slater P.J.B. Chaffinch song repertoires—observations, experiments and a discussion of their significance. Zeitschrift Fur Tierpsychologie-J. Comp. Ethol. 1981;56:1–24. [Google Scholar]
- Telleria J.L., Carbonell R. Morphometric variation of five Iberian Blackcap Sylvia atricapilla populations. J. Avian Biol. 1999;30:63–71. doi:10.2307/3677244 [Google Scholar]
- Visser M.E., Both C. Shifts in phenology due to global climate change: the need for a yardstick. Proc. R. Soc. B. 2005;272:2561–2569. doi: 10.1098/rspb.2005.3356. doi:10.1098/rspb.2005.3356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley R.H., Richards D.G. Adaptations for acoustic communication in birds: sound transmission and signal detection. In: Kroodsma D.E., Miller E.H., editors. Acoustic communication in birds. Academic Press; New York, NY: 1982. pp. 131–181. [Google Scholar]