Abstract
Migratory animals such as seabirds, salmon and whales can transport large quantities of nutrients across ecosystem boundaries, greatly enriching recipient food webs. As many of these animals biomagnify contaminants, they can also focus pollutants at toxic levels. Seabirds arguably represent the most significant biovectors of nutrients and contaminants from the ocean to the land, given their sheer numbers and global distribution. However, long-term census data on seabirds are rare. Using palaeolimnological proxies, we show that a colony of Arctic seabirds has experienced climate-induced population increases in recent decades. We then document increasing concentrations of contaminants, including polychlorinated biphenyls and cadmium, in pond sediments that are linked to biotransport by seabirds. Our findings suggest that climate-related shifts in global seabird populations will have the unexpected consequence of restructuring coastal ecosystems.
Keywords: biotransport, seabirds, palaeolimnology, Arctic ponds, contaminants, climate
1. Introduction
The transport of nutrients by migratory animals across ecosystem boundaries can markedly fertilize recipient food webs. For example, transfer pathways of marine nutrients to terrestrial systems have been documented in seabirds, salmon, sea turtles, sea lions and whales (Fariña et al. 2003; Blais et al. 2007; Hannan et al. 2007). The nutrient subsidies delivered by such biovectors shape the ecosystem structure and function of many coastal and inland sites (Polis et al. 1997). As most biovector organisms feed high on the marine food web, they also biomagnify contaminants, and recent evidence has shown that these ‘biological pumps’ can shunt bio-accumulated contaminants into specific receptor sites at concentrations far beyond those transported by abiotic processes (Blais et al. 2007). Still, the role of biological vectors in the global cycling of nutrients and contaminants is generally ignored.
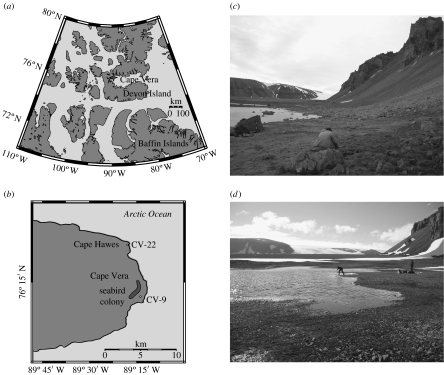
Seabirds may be the most globally relevant biovectors (Sun et al. 2000; Evenset et al. 2007) because they are keystone species that represent the dominant form of wildlife along coastlines worldwide and form dense nesting colonies that can number in the millions of individuals. In the Canadian Arctic alone, there are over 10 million seabirds (Mallory & Fontaine 2004). Our study site at Cape Vera (76°15′ N, 89°15′ W) on Devon Island, Nunavut (High Arctic Canada; figure 1a,b), is an important breeding ground for a colony of 20 000+ northern fulmars (Fulmarus glacialis), a medium-sized petrel common to the North Atlantic (Gaston et al. 2006). At Cape Vera, the fulmars nest on steep cliffs, which rise above a small coastal foreland containing numerous freshwater ponds. Each breeding season the fulmars, which far outnumber all other bird species at Cape Vera, release large amounts of guano and other wastes (e.g. regurgitated stomach contents, carcasses) to the ponds beneath their nesting sites, such that a strong gradient of decreasing aquatic production extends away from the colony (Blais et al. 2005). Although ornithogenic inputs like those at Cape Vera create oases of freshwater and terrestrial production in an otherwise barren Arctic landscape, they also focus contaminants, sometimes at levels exceeding Canadian guidelines for protecting wildlife (Blais et al. 2005). The seabird-delivered nutrients and contaminants are derived solely from marine sources as fulmars feed exclusively in the ocean on zooplankton, squid, fish and carrion (Mallory 2006).
Figure 1.
Maps showing the location of (a) Cape Vera on Devon Island and (b) the study ponds near Cape Vera. Photographs of (c) CV-9 and (d) CV-22.
We reconstructed long-term fluctuations in northern fulmar populations using dated sediment cores recovered from the ponds beneath the nesting sites at Cape Vera. Specifically, we contrasted two ponds with disparate histories of seabird influence. Pond CV-9 is located immediately beneath the present-day fulmar nesting sites (figure 1c), whereas pond CV-22 is located at Cape Hawes, over 10 km away from the nearest seabird colony (figure 1d). We tracked the influence of seabirds on aquatic production, contaminant transport and species composition using multiple proxies, including stable isotope ratios of nitrogen (δ15N), chlorophyll a determinations, concentrations of polychlorinated biphenyls (PCBs) and cadmium (Cd), and the subfossil remains of diatom (single-celled algae) and chironomid (non-biting midges) taxa in dated sediment cores (Michelutti et al. 2008).
2. Material and methods
High-resolution sediment cores, using equipment specifically designed to collect surface sediments (Glew et al. 2001), were recovered during July 2005 using plexiglass tubes that were pushed directly into the sediments. Cores were sectioned on-site in continuous 0.5 cm intervals using a close-interval extruder (Glew et al. 2001). Geochronology is based primarily on 137Cs dating (Michelutti et al. 2008). Stable isotopes of nitrogen and PCB analyses were performed at the University of Ottawa according to the methods described by Yamamuro & Kayanne (1995) and Krümmel et al. (2005), respectively. Sediments were submitted to an aqua regia leach and trace element concentrations were determined by ICP-MS at SGS laboratories, Toronto, Canada. Sediment chlorophyll a was inferred using visible reflectance spectroscopy using a FOSS NIRSystems Model 6500 series Rapid Content Analyzer (Tidestone Technologies, Inc.), following the methods described by Michelutti et al. (2005). Diatom and chironomid preparation for microscopic analyses followed standard procedures described by Battarbee et al. (2001) and Walker (2001), respectively. At CV-22, chlorophyll a and chironomid analyses were performed on a secondary core, immediately adjacent to the primary coring site. Ordination of diatom and chironomid data by principal components analysis (PCA) was used to determine the primary direction of variation in the biological communities, and regressed against δ15N with the objective of assessing the importance of seabird activity on structuring the composition of botanical and zoological communities. All PCA analyses were conducted using Canoco v. 4.0 and all linear regression analyses were performed in SigmaPlot v. 10.0.
3. Results and discussion
The tissues and waste products of northern fulmars are enriched in δ15N relative to terrestrial and freshwater sources. The breast muscle tissue of fulmars at Cape Vera record δ15N values that range from 12.5 to 16‰ (M. L. Mallory 2008, unpublished data). This reflects their high position on the marine pelagic food web, as δ15N is enriched by approximately 3–5‰ per trophic level (Hobson et al. 2002). Modern δ15N values in our seabird-affected site (CV-9) were nearly six times greater than in our control site (CV-22; figure 2). Surface sediments from other seabird-affected sites at Cape Vera have δ15N values ranging from 7 to 16‰ (Blais et al. 2005). This is markedly higher than the δ15N values recorded in Arctic lake sediments that are not impacted by marine-derived nutrients, which typically range from 1 to 5‰ (Douglas et al. 2004; Wolfe et al. 2006).
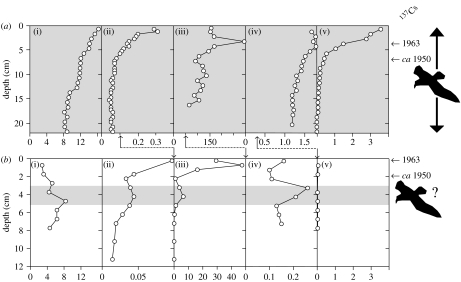
Figure 2.
Profiles of (i) δ15N (‰), (ii) inferred chlorophyll a (mg g−1 dry mass), (iii) chironomid head capsule abundance (per g dry mass), (iv) Cd (μg g−1 dry mass) and (v) (ng g−1 dry mass) for the (a) CV-9 and (b) CV-22 sediment cores. Note the scale changes between study ponds for inferred chlorophyll a, chironomid head capsule abundance and Cd concentrations. The years 1963 and 1950 based on 137Cs dating are reported on the right-hand side (Michelutti et al. 2008). Grey shading denotes the period of inferred seabird activity at the study sites.
Previously, Blais et al. (2005) used the spatial pattern of δ15N in 11 Cape Vera ponds to determine the effect of distance from the nesting cliffs on biotransported inputs. They showed that δ15N (and other geochemical variables related to seabird inputs) in surficial pond sediments could be related to differing amounts of ornithogenic inputs from the fulmar colony. The sediment core data presented here provide for a temporal dimension, and indicate that δ15N also tracks seabird activity through time, as values in CV-9 were consistently higher than in CV-22 (figure 2). We record remarkable consistency in the trajectories of δ15N with independent proxies of seabird influence over the past several hundred years.
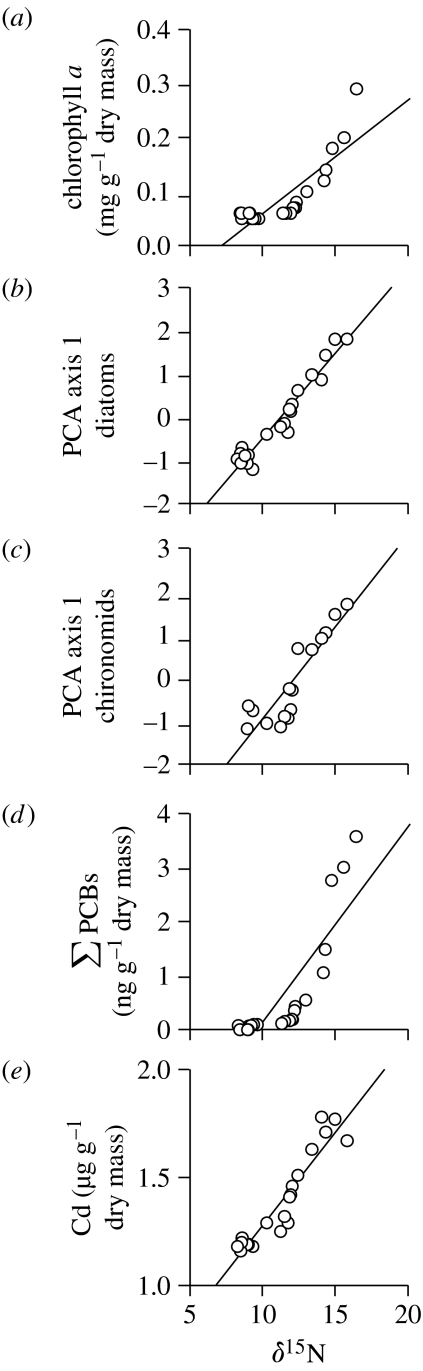
Sedimentary chlorophyll a determinations provide an indication of overall aquatic primary production. As expected, chlorophyll a concentrations throughout the CV-9 core were markedly higher than in CV-22, reflecting the nutrient amendments from the fulmars (figure 2). Both study ponds show increased chlorophyll a concentrations beginning ca 1950, which may be related, in part, to extended growing seasons associated with recent warming, as documented throughout most of the circumpolar Arctic (Michelutti et al. 2005; Smol et al. 2005). However, the increase recorded in the seabird-free site (CV-22) was relatively muted compared with our seabird-affected site (CV-9), suggesting that the nutrient inputs from fulmars create a greater opportunity for change in seasonal temperature to support enhanced production. The significant correlation (p<0.05) between chlorophyll a and δ15N in CV-9 (figure 3a) indicates that aquatic primary production at this site is driven largely by seabird-derived nutrients.
Figure 3.
Relationships between sedimentary δ15N and (a) inferred chlorophyll a concentrations (r=0.84), PCA axis 1 sample scores of (b) diatom (r=0.97) and (c) chironomid assemblages (r=0.89), (d) (r=0.85) and (e) Cd concentrations (r=0.94) in the CV-9 core.
Chironomids play a key role in the transference of nutrients and contaminants to higher trophic levels. The feeding habits of chironomid larvae vary considerably and include detritivores, filter feeders and those that graze on algae or bacteria. A few species are omnivorous and will eat other midge larvae or invertebrates (Walker 2001). Their chitinous, and thus well-preserved, head capsules from their larval stage represent the most abundant insect remains in lake sediments (Walker 2001), therefore providing an indication of secondary (or higher) production, and serve here as an additional, independent measure of aquatic production. The chironomid abundance data corroborate the chlorophyll a profiles (figure 2), indicating greater overall production at CV-9 compared with CV-22. The increases in head capsule abundance in the near-surface sediments match the timing of the chlorophyll a increases in both study ponds and, although part of this increase may be climate-related, the head capsule numbers in CV-22 are markedly lower than the overall abundances recorded in CV-9. Thus, the chironomid abundance data suggest that the seabird-delivered nutrients play a dominant role in driving secondary production at the Cape Vera ponds.
The diatom and chironomid assemblage data from CV-9 are summarized here as PCA axis 1 scores. PCA is a commonly used ordination technique designed to capture the variance in a species dataset in terms of principal components, such that the primary axis is the direction of the most important variation in species assemblages. In CV-9, PCA axes 1 and 2 explained 68 per cent of the total diatom variation with eigenvalues λ1=0.56 and λ2=0.12, and 63 per cent of the total chironomid variation with eigenvalues λ1=0.50 and λ2=0.13. The large disparity in eigenvalues between PCA axes 1 and 2 in both diatom and chironomid ordinations implies that the environmental variables that characterize axis 1 drive most of the variation in the species data. The significant correlation (p<0.05) between δ15N and PCA axis 1 sample scores from both diatom (figure 3b) and chironomid (figure 3c) assemblages strongly suggests that the seabird-derived nutrients structure the biological communities at CV-9.
PCB concentrations in CV-9 were considerably higher than in CV-22 (figure 2), suggesting that the contributions of contaminants from atmospheric transport pathways are minimal compared with the effects of biomagnification and biotransport from the fulmars. The widespread industrial usage of PCBs that began in the early 1950s is clearly reflected in the sediments of CV-9, but not in CV-22. In CV-9, PCB concentrations were greatest in the most recent sediments despite a large-scale global ban of these compounds in 1976. These data demonstrate that the ‘biological pump’ driven by the fulmars greatly overrides atmospheric input with respect to contaminant recruitment to the sediments. The significant correlation (p<0.05) between δ15N and PCB concentrations in CV-9 (figure 3d) further highlights the tight coupling between fulmar activity and the input of marine-derived nutrients and contaminants.
Sedimentary Cd determinations in CV-9 and CV-22 serve as additional, independent geochemical markers of seabird activity. Cadmium, a highly toxic metal, is known to bioaccumulate in marine organisms, from natural and anthropogenic sources, and has been shown to reach elevated levels in terrestrial ecosystems due to biotransport from seabirds (Bargagli et al. 1988; Evenset et al. 2007). Our data indicate that seabirds are focusing Cd into the ponds at Cape Vera, as Cd concentrations are up to 16 times greater in CV-9 than in CV-22, where levels are similar to those measured in a lake nearby on Devon Island that is not impacted by seabirds (Outridge et al. 2005). Moreover, the Cd profiles in our sediment cores show a temporal pattern similar to the other independent indicators of seabird activity (figure 2), and in CV-9 there is a strong positive correlation between Cd and δ15N (figure 3e). Heavy metals in lake sediments can be subject to redox mobilization. However, the effect of a fluctuating redox boundary on the distribution of Cd is a downward migration to the lower limit of the redox excursion; there is no upward transport of Cd in response to redox shifts (Gobeil et al. 1997). Thus, although the mechanism by which Cd ultimately becomes incorporated into the sediments may differ from that of PCBs, the nature of the Cd profiles and strong similarities to other proxies of seabird influence indicate that sedimentary Cd in our study sites is not subject to significant post-burial remobilization and is controlled by the supply of Cd to the pond through drainage.
The high δ15N values and elevated measures of aquatic production at CV-9 relative to CV-22 suggest that seabirds have been present at Cape Vera for at least the time period captured by our sediment core record. Although seabirds are not currently nesting near CV-22, the cliffs at this region contain atypically high abundances of the orange jewel lichen (Xanthoria elegans), which flourishes on nutrients from guano. This suggests that a seabird colony probably existed at Cape Hawes, a notion that is supported by a subsurface maximum in δ15N, as well as corresponding peaks in cadmium, chlorophyll a and chironomid head capsule abundances in the CV-22 core (figure 2). The δ15N maximum of 8.4‰ in the CV-22 core is equivalent to the values in the lower portion of the CV-9 sediment core, a site that always had seabird inputs during the period of record. The considerably lower δ15N levels (2.8–5.2‰) in the surface sediments of the CV-22 core reflect the absence of a modern seabird colony, and provide evidence against diagenetic N loss in these ponds, further corroborating the usefulness of using sedimentary δ15N as a tracer of past seabird abundance. These data illustrate the potential of using palaeolimnological approaches to track the timing and duration of seabird colonization at different nesting sites.
There is strong evidence to suggest that the northern fulmar populations in the North Atlantic have been increasing over the last two centuries (Fisher 1952; Stenhouse & Montevecchi 1999). Salomonsen (1965) argued that climate-driven changes in the marine environment probably explained much of the fulmar expansion since the eighteenth century. He noted that, although there was not an expansion in the number of new fulmar colonies in the Arctic, changes in the number of birds at existing colonies could not be ruled out, especially as fulmars are notoriously difficult to census (Gaston et al. 2006). The increases in sedimentary δ15N, as well as other productivity-related variables, PCBs and Cd recorded in CV-9 (figure 2), would be expected given greater seabird inputs, which would reflect an increase in the local numbers of fulmars breeding on the cliffs. The time period captured in the CV-9 core also corresponds to a number of changes the Arctic is currently experiencing including warming (Kuzmina et al. 2008) and declining sea-ice cover (Stroeve et al. 2008). Thus, we posit that the pond sediments at Cape Vera have been recording climate-induced increases in local fulmar populations.
The clear signal of seabird activities archived in our sedimentary records has direct implications for reconstructing histories of seabird colonies, as well as past population dynamics, at Cape Vera and elsewhere. Such data are critical to setting realistic management goals and identifying important long-term bird habitats. This work also has linkages to global change research, as many seabird species are sentinels of environmental change, often responding sensitively to changes in their food supplies that are attributable to variations in sea surface temperature, sea-ice extent and anthropogenic harvest activities (Croxall et al. 2002). Our data show that contaminants such as PCBs have been steadily increasing at Cape Vera, despite decreasing atmospheric concentrations, which we interpret to be a result of increased fulmar populations at this site. Seabird populations are expected to fluctuate markedly in response to climatic warming; an unexpected consequence of these changes will be the restructuring of coastal ecosystems.
Acknowledgments
This work was funded by Natural Science and Engineering Research Council (NSERC) awards to J.M.B., J.P.S. and M.S.V.D. We are grateful to the Indian and Northern Affairs Canada (NSTP), Natural Resources Canada (PCSP) and Environment Canada (CWS) for financial and logistical support pertaining to field work. This project is PCSP/EPCP no. 03508.
References
- Bargagli R., Sanchez-Hernandez J.C., Martella L., Monaci F. Mercury, cadmium and lead accumulation in Antarctic mosses growing along nutrient and moisture gradients. Polar Biol. 1988;19:316–322. doi:10.1007/s003000050252 [Google Scholar]
- Battarbee, R. W., Jones, V. J., Flower, R. J., Cameron, N. G., Bennion, H., Carvalho, L. & Juggins, S. 2001 Diatoms. In Terrestrial, algal, and siliceous indicators (eds J. P. Smol, H. J. B. Birks & W. M. Last). Tracking Environmental Change Using Lake Sediments, vol. 3, pp. 155–202. Dordrecht, The Netherlands: Kluwer Academic Publishers.
- Blais J.M., Kimpe L.E., McMahon D., Keatley B.E., Mallory M.L., Douglas M.S.V., Smol J.P. Arctic seabirds transport marine-derived contaminants. Science. 2005;309:445. doi: 10.1126/science.1112658. doi:10.1126/science.1112658 [DOI] [PubMed] [Google Scholar]
- Blais J.M., Macdonald R.W., Mackey D., Webster E., Harvey C., Smol J.P. Biologically mediated transport of contaminants to aquatic systems. Environ. Sci. Technol. 2007;41:1075–1084. doi: 10.1021/es061314a. doi:10.1021/es061314a [DOI] [PubMed] [Google Scholar]
- Croxall J.P., Trathan P.N., Murphy E.J. Environmental change and Antarctic seabird populations. Science. 2002;297:1510–1514. doi: 10.1126/science.1071987. doi:10.1126/science.1071987 [DOI] [PubMed] [Google Scholar]
- Douglas M.S.V., Smol J.P., Savelle J.M., Blais J.M. Prehistoric Inuit whalers affected Arctic freshwater ecosystems. Proc. Natl Acad. Sci. USA. 2004;101:1613–1617. doi: 10.1073/pnas.0307570100. doi:10.1073/pnas.0307570100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenset A., Christensen G.N., Caroll J., Zaborska A., Berger U., Herzke D., Gregor D. Historical trends in persistent organic pollutants and metals recorded in sediment from Lake Ellasjøen, Bjornoya, Norwegian Arctic. Environ. Pollut. 2007;146:196–205. doi: 10.1016/j.envpol.2006.04.038. doi:10.1016/j.envpol.2006.04.038 [DOI] [PubMed] [Google Scholar]
- Fariña J.M., Salazar S., Wallem K.P., Witman J.D., Ellis J.C. Nutrient exchanges between marine and terrestrial ecosystems: the case of the Galapagos sea lion Zalophus wollebaecki. J. Anim. Ecol. 2003;72:873–887. doi:10.1046/j.1365-2656.2003.00760.x [Google Scholar]
- Fisher J. Collins; London, UK: 1952. The fulmar. pp. 496. [Google Scholar]
- Gaston A.J., Mallory M.L., Gilchrist H.G., O'Donovan K. Status, trends and attendance patterns of the Northern Fulmar Fulmarus glacialis in Nunavut, Canada. Arctic. 2006;59:165–178. [Google Scholar]
- Glew, J. R., Smol, J. P. & Last, W. M. 2001 Sediment core collection and extrusion. In Basin analysis, coring, and chronological techniques (eds W. M. Last & J. P. Smol). Tracking Environmental Change Using Lake Sediments, vol. 1, pp. 73–105. Dordrecht, The Netherlands: Kluwer Academic Publishers.
- Gobeil C., Macdonald R.W., Sundby B. Diagenetic separation of cadmium and manganese in suboxic continental margin sediments. Geochim. Cosmochim. Acta. 1997;61:4647–4654. doi:10.1016/S0016-7037(97)00255-X [Google Scholar]
- Hannan L.B., James J.D., Ehrhardt L.M., Weishampel J.F. Dune vegetation fertilization by nesting sea turtles. Ecology. 2007;88:1053–1058. doi: 10.1890/06-0629. doi:10.1890/06-0629 [DOI] [PubMed] [Google Scholar]
- Hobson K.A., Fisk A., Karnovsky N., Holst M., Gagnon J.-M., Fortier M. A stable isotope (δ13C, δ15N) model for the North Water food web: implications for evaluating trophodynamics and the flow of energy and contaminants. Deep Sea Res. II. 2002;49:5131–5150. doi:10.1016/S0967-0645(02)00182-0 [Google Scholar]
- Krümmel E.M., Gregory-Eaves I., Macdonald R.W., Kimpe L.E., Demers M.J., Smol J.P., Finney B., Blais J.M. Concentrations and fluxes of salmon-derived polychlorinated biphenyls (PCBs) in lake sediments. Environ. Sci. Technol. 2005;39:7020–7026. doi: 10.1021/es050657q. doi:10.1021/es050657q [DOI] [PubMed] [Google Scholar]
- Kuzmina S.I., Johannessen O.M., Bengtsson L., Aniskina O.G., Bobylev L.P. High northern latitude surface air temperature: comparison of existing data and creation of a new gridded data set 1900–2000. Tellus. 2008;60:289–304. doi:10.1111/j.1600-0870.2008.00303.x [Google Scholar]
- Mallory M.L. The northern fulmar (Fulmarus glacialis) in Arctic Canada: ecology, threats, and what it tells us about marine environmental conditions. Environ. Rev. 2006;14:187–216. doi:10.1139/A06-003 [Google Scholar]
- Mallory, M. L. & Fontaine, A. J. 2004 Key marine habitat sites for migratory birds in Nunavut and the Northwest Territories. Canadian Wildlife Service occasional paper, no. 109. Ottawa, Canada: Canadian Wildlife Service.
- Michelutti N., Wolfe A.P., Vinebrooke R.D., Rivard B., Briner J. Recent primary production increases in Arctic lakes. Geophys. Res. Lett. 2005;32:L19715. doi:10.1029/2005GL023693 [Google Scholar]
- Michelutti N., Blais J.M., Liu H., Keatley B.E., Douglas M.S.V., Mallory M.L., Smol J.P. A test of the possible influence of seabird activity on the 210Pb flux in high Arctic ponds at Cape Vera, Devon Island, Nunavut: implications for radiochronology. J. Paleolimnol. 2008;40:783–791. doi:10.1007/s10933-008-9198-2 [Google Scholar]
- Outridge P.M., Stern G.A., Hamilton P.B., Percival J.B., Lockhart W.L. Trace metal profiles in the varved sediment of an Arctic lake. Geochim. Cosmochim. Acta. 2005;69:4881–4894. doi:10.1016/j.gca.2005.06.009 [Google Scholar]
- Polis G.A., Anderson W.B., Holt R.D. Toward an integration of landscape and food web ecology: the dynamics of spatially subsidized food webs. Annu. Rev. Ecol. Syst. 1997;28:289–316. doi:10.1146/annurev.ecolsys.28.1.289 [Google Scholar]
- Salomonsen F. The geographical variation of the fulmar (Fulmarus glacialis) and the zones of the marine environment in the North Atlantic Ocean. Auk. 1965;82:327–355. [Google Scholar]
- Smol J.P., et al. Climate-driven regime shifts in the biological communities of Arctic lakes. Proc. Natl Acad. Sci. USA. 2005;102:4397–4402. doi: 10.1073/pnas.0500245102. doi:10.1073/pnas.0500245102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenhouse I.J., Montevecchi W.A. Increasing and expanding populations of breeding northern fulmars in Atlantic Canada. Waterbirds. 1999;22:382–391. doi:10.2307/1522114 [Google Scholar]
- Stroeve J., Serreze M., Drobot S., Gearheard S., Holland M., Maslanik J., Meier W., Scambos T. Arctic sea ice extent plummets in 2007. EOS. 2008;89:13–14. doi:10.1029/2008EO020001 [Google Scholar]
- Sun L.G., Xie Z.Q., Zhao J.L. A 3,000-year record of penguin populations. Nature. 2000;407:858. doi: 10.1038/35038163. doi:10.1038/35038163 [DOI] [PubMed] [Google Scholar]
- Walker I.R. Midges: Chironomidae and related Diptera. In: Smol J.P., Birks J.B., Last W.M., editors. Tracking Environmental Change Using Lake Sediments:In Zoological indicators. Kluwer Academic Publishers; Dordrecht, The Netherlands: 2001. pp. 43–66. [Google Scholar]
- Wolfe A.P., Cook C.A., Hobbs W.O. Are current rates of atmospheric nitrogen deposition influencing lakes in the eastern Canadian Arctic? Arct. Antarct. Alp. Res. 2006;38:465–476. doi:10.1657/1523-0430(2006)38[465:ACROAN]2.0.CO;2 [Google Scholar]
- Yamamuro M., Kayanne H. Rapid direct determination of organic-carbon and nitrogen in carbonate-bearing sediments with a Yanaco mt-5 chn analyzer. Limnol. Oceanogr. 1995;40:1001–1005. [Google Scholar]