Abstract
Visual fields were determined in two species of shorebirds (Charadriiformes) whose foraging is guided primarily by different sources of information: red knots (Calidris canutus, tactile foragers) and European golden plovers (Pluvialis apricaria, visual foragers). The visual fields of both species showed features that are found in a wide range of birds whose foraging involves precision pecking or lunging at food items. Surprisingly, red knots did not show comprehensive panoramic vision as found in some other tactile feeders; they have a binocular field surrounding the bill and a substantial blind area behind the head. We argue that this is because knots switch to more visually guided foraging on their breeding grounds. However, this visual field topography leaves them vulnerable to predation, especially when using tactile foraging in non-breeding locations where predation by falcons is an important selection factor. Golden plovers use visually guided foraging throughout the year, and so it is not surprising that they have precision-pecking frontal visual fields. However, they often feed at night and this is associated with relatively large eyes. These are anchored in the skull by a wing of bone extending from the dorsal perimeter of each orbit; a skeletal structure previously unreported in birds and which we have named ‘supraorbital aliform bone’, Os supraorbitale aliforme. The larger eyes and their associated supraorbital wings result in a wide blind area above the head, which may leave these plovers particularly vulnerable to predation. Thus, in these two shorebirds, we see clear examples of the trade-off between the two key functions of visual fields: (i) the detection of predators remote from the animal and (ii) the control of accurate behaviours, such as the procurement of food items, at close quarters.
Keywords: visual field, binocular field, blind area, tactile cues, shorebirds
1. Introduction
The visual field of an animal determines what part of its surrounding environment can influence its behaviour at any one instant (Martin 2007). Visual fields need to serve two key functions: (i) the detection of predators, conspecifics, potential prey and obstacles, which are remote from the animal, and (ii) the control of accurate behaviours, such as the procurement of food items, at close quarters. Both functions are potent sources of natural selection but they are potentially antagonistic (Fernández-Juricic et al. 2004, 2008). This antagonism is well illustrated in birds.
In species that employ visual information for the guidance of the bill position when taking food items, the projection of the bill falls approximately centrally within the frontal binocular section of the visual field (Martin 2007). This arrangement facilitates the accurate determination of direction of travel towards, and time to contact, an object by the bill. This information is derived primarily from the radially symmetrical linear optic flow field that is generated during forward motion (Gibson 1986; Davies & Green 1994; Martin & Katzir 1999). However, this more forward position of the eyes that is necessary to achieve a binocular field surrounding the projection of the bill always results in a blind area behind the head, rendering the animal more vulnerable to predation (Guillemain et al. 2002; Fernández-Juricic et al. 2004; Martin 2007).
The importance of reducing vulnerability to predator attack is indicated by examples of birds in which non-visual information is used to guide foraging. These birds no longer need to derive accurate information about the direction and speed of travel of the bill relative to a target, and in these species the bill falls at the very periphery or entirely outside the visual field. Often, the eyes are placed high in the skull giving comprehensive panoramic vision around and above the head (Martin 2007). Such visual field topography is found, for example, in the tactile-feeding Eurasian woodcocks Scolopax rusticola (Martin 1994) and a number of filter-feeding duck species: mallards, Anas platyrhynchos; northern shovelers, Anas clypeata; and pink-eared ducks, Malacorhynchus membranaceus (Martin 1986; Guillemain et al. 2002; Martin et al. 2007a,b). Thus, among birds, when accurate visual guidance of the bill position is not necessary, natural selection seems to have favoured comprehensive visual coverage of the hemisphere above and around the head to aid the detection of predators (Guillemain et al. 2002; Martin 2007). However, tactile or filter feeding does not necessarily lead to the evolution of panoramic vision. In black skimmers (Rynchops niger), tactile feeding requires the visual inspection of prey items after they have been caught while ‘blind trawling’ (Martin et al. 2007a,b), and, in filter-feeding lesser flamingos (Phoeniconaias minor), accurate bill positioning under the control of visual cues is required for the feeding of young with oesophageal milk (Martin et al. 2005). Neither species has comprehensive panoramic vision and the projections of their bills fall centrally within a frontal binocular field.
The general principles outlined above have been based upon comparisons between relatively few species that have quite separate evolutionary origins. Here, we describe differences in visual field topography between species with similar ecologies and within a single avian order. We show that differences in visual fields can be attributed to subtle differences in foraging ecology, indicating an evolutionary trade-off between the demands of using vision for accurate guidance during foraging and the detection of predators.
Among the Charadriiformes are two taxa of shorebirds that forage in similar open habitats but are divided into short-billed forms (plovers, Charadriidae), which primarily take surface-living or shallow-dwelling invertebrates, and a separate radiation of longer billed forms (sandpipers and their allies, Scolopacidae), which take invertebrate prey, often buried in soft substrates (Piersma & Wiersma 1996; Piersma et al. 1996). The foraging of plovers is regarded as guided primarily by visual, and possibly auditory (Fallet 1962), cues, while foraging sandpipers are guided primarily by tactile information derived from receptors (Herbst and Grandry corpuscles) located within sensory pits in the bone around the bill tips (Bolze 1968; Piersma et al. 1998; Nebel et al. 2005). Species of both families have precocial chicks, and therefore do not need to employ visual cues for the accurate placement of the bill when provisioning chicks.
We hypothesized that the visual fields and eye positions within the skull of birds from these two families would reflect their differential use of visual and tactile information in the guidance of their foraging. Here, we have compared two species: red knots (Calidris canutus), which can locate buried prey using exclusively tactile cues (Piersma et al. 1998), and European golden plovers (Pluvialis apricaria), which are regarded as visually guided foragers (Barnard & Thompson 1985). We predicted that red knots would have their eyes located high in the skull, resulting in visual fields with similar topography to that of the tactile-foraging Eurasian woodcocks, i.e. a narrow binocular field (less than 10° maximum width) extending throughout the median sagittal plane above the head providing total panoramic vision of the celestial hemisphere, and the projection of their bill falling outside the visual field (Martin 1994). By contrast, golden plovers were predicted to have visual field topography similar to those of other visually guided foraging birds in which the bill projects centrally within a broader binocular field (15–25° wide), and the more frontal placement of the eyes results in a blind area above and to the rear of the head.
2. Material and methods
Four long-term captive adult red knots held at the Royal Netherlands Institute for Sea Research (NIOZ) Texel, The Netherlands, and four adult golden plovers captured in April 2007 as part of a long-term ringing programme at sites close to Gaast (53°01′ N, 05°24′ E), province of Friesland, The Netherlands, were used. This work was conducted at NIOZ and at a field station close to Gaast. The procedure used is non-invasive and has been used in excess of 20 years on more than 30 different bird species. The procedure involves restraint of the birds for between 30 and 45 min. Following a recent review of the procedure by a UK Home Office Inspector, it was not considered to fall within the regulations that govern licensed procedures with animals, which apply in the UK. However, the ethical guidelines with respect to handling and restraining birds required for licensed procedures in the UK (UK Animals (Scientific Procedures) Act 1986) were followed. After measurements, the knots were returned to their aviary and the plovers released close to their capture site. Calibrated macrophotographs of the head of each bird when held in the hand and the apparatus were taken. These were used to determine the eye positions within the skull from the dorsal and lateral views, the relationship between the horizontal and the eye–bill tip angle, and the corneal diameter.
Visual field parameters were determined using an ophthalmoscopic reflex technique. This has been used in a range of birds of different phylogeny, ecology and feeding techniques and readily permits interspecific comparisons (Martin 2007). For a detailed description of the apparatus and methods, see Martin et al. (2007a,b). Briefly, each bird was hand held, with the breast resting in a foam rubber cradle and the legs held out behind the body. The head was held in position at the centre of a visual perimeter (a device that permits the eyes to be examined from known positions about the head) by a specially manufactured steel and aluminium bill holder. The surfaces of the holder were coated in cured silicone to provide a non-slip cushioned surface. The bill was held in place by Micropore tape. The perimeter's coordinate system followed conventional latitude and longitude, with the equator aligned vertically in the birds' median sagittal plane, and this coordinate system is used for the presentation of visual field data (figure 3a,c). The tip of the mandible projected 15° below the horizontal (see the diagram of the head positions in figure 3). In common with most other birds when held in the hand at various body angles, the head position in the knots and golden plovers was stable. The eye–bill tip angle varied between 15° and 20° with respect to the horizontal (verified from photographs). Inspection of artists' illustrations of these species in handbooks (Cramp & Simmons 1983; Hayman et al. 1986) showed bill angles of approximately 20° to the horizontal in birds depicted standing upright. Thus, the head position adopted during measurements, and used in the presentation of the data, matches those commonly adopted by these birds when standing, presumably vigilant for predators and conspecifics, in their natural environment.
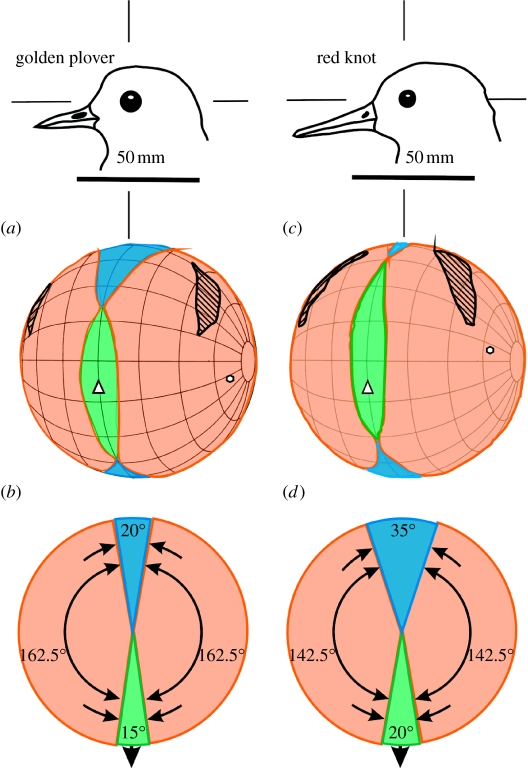
Figure 3.
Visual fields in red knots and European golden plovers. (a,c) Perspective views of orthographic projection of the boundaries of the retinal fields of the two eyes, and the projection of the pectens, optic axes and the line of the bills. The diagrams use a conventional latitude and longitude coordinate system with the equator aligned vertically in the median sagittal plane of the bird (grid at 20° intervals), and values in the sagittal plane correspond with those shown in figure 2. It should be imagined that the bird's head is positioned at the centre of a transparent sphere with the bill tips and field boundaries projected onto the surface of the sphere. The drawings show the heads in the same orientation as when the measurements were made and the bill projected 15° below the horizontal, as indicated in the diagram. (b,d) Horizontal sections through the visual fields in a horizontal plane defined by elevations 270° and 90° in figure 2. Black hatched areas, projections of the pectens; white hexagons, directions of the optic axes; green areas, binocular sector; blue areas, blind sectors; pink areas, monocular sectors; white triangles, projections of the bill tip; black arrows, direction of the bill.
The eyes were examined using an ophthalmoscope mounted against the perimeter arm and its position read to ±0.5°. Alignment of the birds' heads in the perimeter was such that the ophthalmoscope viewing aperture was, in effect, moved over the surface of a virtual sphere (radius 320 mm) centred on the mid-point of the line joining the centres of the pupils. This point was defined as the cyclopean projection centre, and positions of the visual field features are described by reference to it. The measured values of visual field features were corrected to those that would be determined at a hypothetical infinite viewing distance (Martin 1984). Attempts to induce eye movements by moving a point source of light in the lateral visual field or by making sharp noises close to the birds (methods that induce eye movements of large amplitude in other birds; Martin & Katzir 1993) failed to produce eye movements of any noticeable amplitude as determined by direct observation of the eyes and by repeated measurements of the position of the retinal margins at elevations close to the horizontal. The projections of the edges of the pecten in each eye were also recorded. The pecten produces a relatively large blind area within the visual field of each eye. It is a highly pigmented black structure that extends from the retinal surface into the posterior chamber of the eye. It provides nutrition for the retina and is situated above the point where the optic nerve exits and extends ventrally across the retina (Martin 1985). The projection of the pecten provides a significant landmark within the visual field of each eye.
From these combined data (corrected for viewing from a hypothetical viewing point placed at infinity), a topographical map of the visual field and its principal features was constructed for each species. These features are as follows: monocular fields, the visual field of a single eye; binocular field, the area where monocular fields overlap; cyclopean field, the total visual field produced by the combination of both monocular fields; and projections of the pectens. It was possible to measure limits of the visual field at 10° intervals of elevation in an arc from 20° below the horizontal directly behind the head, to above the head and then down to 60° below the horizontal in front of the head. However, at the elevation 30° below the horizontal, the bill holder intruded into the view of the eyes. Therefore, it was not possible to record visual field data at this elevation, and the binocular field width at this elevation was estimated as the mean value of the binocular field widths above and below these elevations. The direction of the optic axis of each eye was determined by recording the positions at which the first and second Purkinje images (reflections from the cornea and the lens anterior surface) of a discrete source of light held close to the line of sight on the perimeter arm were most closely aligned.
Four prepared skulls of each species from the collections of the Natural History Museum (Tring, UK) were examined and calibrated photographs were taken.
3. Results
Photographs of the frontal and dorsal views of the head of a red knot and a golden plover are shown in figure 1. The mean angular separations of the retinal field margins as a function of elevation in the median sagittal plane in each species are shown in figure 2. Maps, based upon these data, show the visual fields in the frontal sector (figure 3a,c). Horizontal sections through these fields in an approximately horizontal plane relative to the head when it is held with the bill projecting 15° below the horizontal (as depicted in the drawings) are shown in figure 3b,d. Figure 4 shows views of the skulls and of distal sections of the bill for each species.
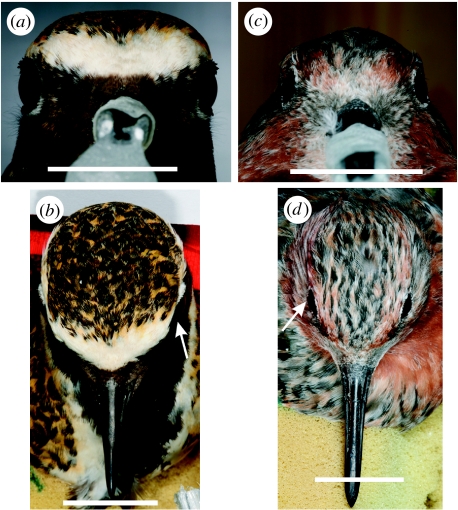
Figure 1.
Anterior and dorsal photographs of the heads of (a,b) European golden plovers and (c,d) red knots showing the positions of the eyes (corneas indicated by arrows). The central blurred section in (a) and (c) is the bill and holder. Scale bars, 20 mm.
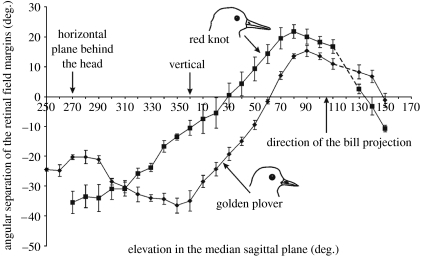
Figure 2.
Mean (±s.e.) angular separation of the retinal field margins as a function of elevation in the median sagittal plane in red knots and European golden plovers. Positive values indicate the overlap of the field margins (binocular vision); negative values indicate the width of the blind areas. The coordinate system is such that the horizontal plane is defined by the elevations 270° (behind the head), 90° (in front of the head) and 0° (directly above the head), the same coordinates are used in figure 3. The direction of the projection of the line of the bill is indicated.
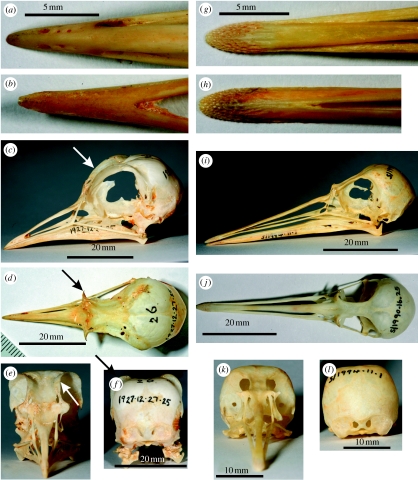
Figure 4.
Photographs of intact prepared skulls of (a–f) European golden plovers and (g–l) red knots. The photographs are paired to aid comparison between the species. (a,g) Dorsal views of the premaxillae (Os premaxillare); (b,h) ventral views of the dental bones (Os dentale). Views of the complete skulls: (c,i) lateral; (d,j) dorsal; (e,k) anterior; and (f,l) posterior. Arrows in (c–f) indicate the wing-like lateral extensions of the bones (Os supraorbitale aliforme) around the anterior and dorsal margins of the orbits in golden plovers.
(a) Visual fields
Figures 2 and 3 indicate that the overall topography of the visual fields of these two species is surprisingly similar, and in neither species is there total panoramic vision about the head. However, the visual fields differ significantly in certain dimensions. For example, the width and vertical extent of the frontal binocular field are larger in the knots than in the plovers (figure 3a,c). Also, the width and vertical extent of the blind area above and to the rear of the head are larger in the plovers than in the knots (figure 2). One result of these differences is that the region about the head from which visual information can be extracted at any one instant is smaller in golden plovers than in red knots; the blind sector in the hemisphere above the horizontal plane and centred upon the head is approximately 1.5 times larger in plovers than in knots.
(i) Binocular fields
In both species, there is a frontal region of binocular overlap, which is vertically long and relatively narrow (figure 3a,c). In knots, the binocular field has a maximum width of approximately 22° and a vertical extent of 120°, while, in plovers, the dimensions are 15° and 75°. In both species, the projection of the bill lies within the lower half of the binocular field, but it is more centrally placed in the binocular field in the plovers.
(ii) Monocular fields
The monocular retinal fields equalled 177° in the plovers and 162° in knots in an approximately horizontal plane (figure 3b,d), resulting in wide areas of monocular visual coverage (more than 90% of the total visual field in the horizontal plane) in both species.
(iii) Cyclopean fields and blind areas
Lateral placement of the eyes in the skull, coupled with the wide monocular fields and the relatively small binocular overlap provide both species with extensive cyclopean fields of 325° and 340° in an approximately horizontal plane in the plovers and the knots, respectively (figure 3b,d). However, the blind areas above and to the rear of the head differed between the species (figure 2). Of particular note is the way in which the blind area in golden plovers is of maximum width above the head and then narrows considerably towards the horizontal. In knots, however, the blind area increases steadily in width from just behind the head down to the horizontal, resulting in golden plovers having a narrower blind area behind the head in the horizontal plane compared with knots (figures 2 and 3b,d).
(b) Optic axes
In both species, the eyes project laterally, but while they are directed slightly downwards relative to the horizontal in plovers they project slightly upwards in knots; 22° below and 17° above the horizontal, respectively, in our coordinate system (figure 3a,c).
(c) Skull structure
Inspection of prepared skulls of the two species shows a number of differences in their size and structure (figure 4). The most notable differences are (i) the high number of pits surrounding the bill tips, which house clusters of Herbst and Grandry corpuscles in the knots (figure 4g,h), and their virtual absence in the plovers (figure 4a,b), and (ii) the presence of an extended rim of bone surrounding the anterior and dorsal margins of the orbits in golden plovers (figure 4c–f). These wings of bone are not present in red knots (figure 4i–l).
4. Discussion
We hypothesized that the general topography of visual fields and the eye positions within the skull of golden plovers and red knots would differ, reflecting their differential use of visual and tactile information in the guidance of their foraging.
However, our hypothesis is rejected. The visual fields of both species exhibit the same four general characteristics which in other birds are associated with visually guided pecking or lunging at prey (Martin 2007): (i) the bill tip projection falls centrally or within the lower half of the binocular area, (ii) the binocular field is relatively long and narrow, (iii) maximum binocularity occurs at or above the projection of the bill tip, and (iv) there is a blind area to the rear of the head. All of these features are found in a wide range of bird species that differ in their ecology and phylogeny but have in common precision pecking or lunging at food items. While we expected to find such an arrangement in the visually guided golden plovers, we did not expect this in red knots, with their sophisticated system of ‘remote touch’ mediated by clusters of Herbst and Grandry corpuscles around the bill tips (figure 4g,h) capable of detecting prey buried in soft substrates (Piersma et al. 1998). Golden plovers lack these clusters of tactile receptors at their bill tips (figure 4a,b). While the sizes of the key parameters that are used to characterize visual fields (binocular field maximum width and height, monocular field width and blind sector width above the head) differ between these species, their sizes fall well within the range of values found in a wide range of bird species (including herons (Ardeidae), pigeons (Columbidae), petrels (Procellariidae), hornbills (Bucorvidae), skimmers (Rhynchopidae) and flamingos (Phoenicopteridae)), which differ in their ecology and phylogeny but have in common the use of vision for the accurate placement of the bill when foraging (see Martin 2007 for a review).
We now examine reasons why the visual fields of red knots show general features associated with visually guided foraging, and also examine the possible bases for the differences in visual field topography between the two species.
(a) Vision, foraging and predation in red knots
Based upon comparisons with other tactile-feeding species (woodcocks and various species of ducks) investigated to date (Martin 2007), we expected to find that the tactile-feeding knots had visual fields that provide comprehensive panoramic vision. We predicted that selection would favour the evolution of comprehensive vision in knots for three principal reasons: (i) tactile-feeding birds do not require visual information to guide their foraging, (ii) knot chicks are precocial self-feeders; hence, adult birds do not require vision to guide the bill position for chick provisioning, and (iii) knots are known to be especially vulnerable to attack by aerial predators within the open habitats in which they feed (Cresswell & Whitfield 1994; Lind & Cresswell 2005; Van den Hout et al. 2007). Comprehensive vision allows birds to remain vigilant without the need to periodically interrupt feeding and scan for predators (Guillemain et al. 2002; Fernández-Juricic et al. 2004).
This absence of comprehensive visual coverage in a tactile feeder has been found previously in black skimmers. However, in these birds, the forward positioning of the eyes is associated with the visual inspection of items held in the bill after being caught during blind trawling (Martin et al. 2007a,b). Such an explanation cannot apply to red knots since they have rhynchokinetic bills that allow buried prey items to be seized by the bill tips and then ingested using tongue movements without the need to bring them to the surface (Burton 1974). It is also known that knots can determine the palatability of buried prey using taste cues (Gerritsen et al. 1983).
Of crucial importance may be the fact that, although red knots can use tactile cues to locate prey buried within the substrates of intertidal mud and sand flats, such substrates are exploited only when knots are within their non-breeding range, which they typically occupy for 40 weeks each year. Individual birds occupy their high-arctic breeding grounds for only 12 weeks each year, and we hypothesis that during that time the birds must switch to visually guided foraging. Thus, when breeding, knots are known to take prey items that are not buried in a substrate and some of these items may be highly mobile. Diet during the breeding period includes a variety of insects, predominantly larval and adult Diptera, but also Lepidoptera, beetles and bees, spiders, small crustaceans, snails and worms (Cramp & Simmons 1983; Piersma et al. 1996; Tulp et al. 1998). Thus, during their annual life cycle, red knots need to switch between exclusively tactile information to guide their foraging on the non-breeding habitats and visual information when breeding. We hypothesize that the need for accurate visually guided bill placement when taking prey on the breeding grounds has resulted in the eyes being more frontally placed than would be otherwise if foraging was guided by tactile information at all times. Crucially, this results in a blind area to the rear of the head that renders the birds vulnerable to predation when employing tactile feeding in their non-breeding locations where predation by falcons accounts for much of the behavioural routines and an estimated 6.2 per cent of juvenile and 0.8 per cent of adult annual mortality (Van den Hout et al. 2007).
(b) Interspecific differences in the visual fields of red knots and golden plovers
Although the frontal visual fields of red knots and golden plovers embody the same general features that are associated with the visual guidance of the bill position towards prey, they do differ in certain key features. Most notable of these are: (i) the extent of the blind areas above and behind the head, which is larger in golden plovers (figure 2), (ii) the orientations of the optic axes of the eyes, which are more downward facing in golden plovers (figure 3a,c), and (iii) the size of the binocular fields, which are both narrower and vertically shorter in golden plovers (figure 3a,c). We suggest that the key to these visual field differences lies in the difference in eye size between the two species and that this size difference is functionally related to the ranges of light levels under which these birds employ visual information to guide their foraging (Martin 1990).
The larger eyes of the plovers are readily seen in figure 1 with respect to the diameter of the corneas, approximately 7.2 and 5.2 mm in the golden plovers and knots, respectively (determined from the calibrated photographs taken to produce figure 1). Overall eye size is best estimated by the axial length of the eyes, and this can be estimated from the photographs of figure 1 and the known orientation of the optic axes. Using the assumptions that the posterior sections of the birds' eyes have a spherical radius and that the two eyes almost meet at the median sagittal plane of the head (Martin & Osorio 2008), eye axial lengths in red knots and golden plovers equal approximately 10.8 and 14.9 mm, respectively. This larger eye size in the plovers is unlikely to be simply a result of allometry since the allometric scaling relationship of eye mass on body mass in birds (Brooke et al. 1999) predicts that eye size (mass) in golden plovers (mean body mass 210 g) should be 1.3 times that in red knots (mean body mass 140 g). Assuming that eye mass is equivalent to eye volume, and that the eye axial length is twice the radius of the eye, then the eyes with axial lengths of 14.9 mm (golden plovers) and 10.8 mm (knot) differ in their size by a factor of 2.6. Thus, the difference in eye size between golden plovers and knots is twice that predicted by allometric scaling. It is likely therefore that the larger eyes of the plovers are the result of selection concerned with a specific aspect of visual function.
Larger eye size may be associated with increased visual sensitivity or with increased visual resolution. Increased sensitivity is achieved by an increase in the absolute area of the cornea and a relative increase in pupil aperture with respect to the focal length of the eye; increased resolution is achieved by an increased retinal image size with respect to the retinal grain (density of photoreceptors and ganglion cells; Land & Nilsson 2002). We do not have any data on the retinal structure of these species. However, since corneal area in plovers is twice that in knots, it seems likely that increased eye size in plovers is associated with increased visual sensitivity. Furthermore, increased visual sensitivity may be more important in golden plovers than in red knots. This is because knots use visual guidance for their foraging only when on the breeding grounds at very high latitudes in the summer when daylight is continuous (June–August; 70–80°N). When knots move south from the breeding grounds (non-breeding sites lie between latitudes 0° and 55° in both hemispheres) and experience the reduced light levels of night-time, the birds are no longer visually guided in their foraging. They can forage at night following the tidal cycle of food availability, guided by tactile information. Golden plovers, on the other hand, breed at lower latitudes (50–70°N) and winter at mid-latitudes (30–60°N), and therefore experience night-time for much of their annual cycle. They are known to feed at night or at dusk and dawn, as well as by day within their non-breeding range (Jukema et al. 2001; Gillings et al. 2005; Gillings & Sutherland 2007). Since this feeding is guided primarily by vision, high visual sensitivity is likely to have greater adaptive value in golden plovers compared with the knots.
That the eyes of golden plovers are exceptionally large relative to the skull may be correlated with the presence of the extended rim of bone surrounding the anterior and dorsal margins of the orbits (figure 4c–f). It is likely that these wings of bone serve to provide additional anchorage for the eyes which literally bulge from the skull (figure 1a), rather than being enclosed within the orbit. These wings of bone surrounding the orbit are clearly absent from red knot (figure 4i–l). In golden plovers, they give rise to a flat topped/square-shaped head (figure 1a) compared with the more typically avian dome-shaped head of the knots (figure 1c).
The occurrence of a wing of bone around the dorsal margin of the orbit does not appear to have been described previously in any species of bird (Baumel 1993; G. Mayr 2008, personal communication), and we propose the name supraorbital aliform bone, Os supraorbitale aliforme. They may be restricted in their occurrence to larger eyed species among the Charadriidae (they are also present in grey plovers (Pluvialis squatarola), but appear to be absent from Scolopacidae, G. R. Martin 2008, personal observation), but more extensive interspecific comparisons of skull structure will be required to establish this.
An additional difference between the two species is that, in golden plovers, the eyes are orientated more downwards (figure 3a,c) and that the upwardly projecting visual field margins are constrained by the supraorbital wings of bone. This produces a wide blind area above the head (in plovers the blind area is 38° wide compared with 11° in knots, figures 2 and 3a), while the narrowing of the blind area to the rear of the head (figure 2) may be attributed to the absence of the orbital wings along the posterior margins of the orbits (figure 4d,f). It would seem that, in golden plovers, vision to the rear of the head is maximized within the constraints imposed by the anchoring of these larger eyes within the skull and the requirement for binocular vision about the direction of the bill.
(c) Conclusion: trade-offs in the evolution of visual fields
We had expected to find that knots would have comprehensive vision about the head, as reported previously in woodcocks, another Charadriiform bird species whose foraging is guided by tactile cues. However, a simple relationship between the use of tactile foraging and visual field topography was not found. The comparisons presented here reveal clear examples of a set of trade-offs between the two key functions of visual fields: (i) the detection of predators remote from the animal and (ii) the control of accurate behaviours, such as the procurement of food items, at close quarters. In both knots and plovers, visual fields are of the type associated with the use of visual information for the detection and capture of prey. This requires the binocular field to encompass the projection of the bill tip and provides accurate information on the direction of travel towards, and time to contact, an object by the bill (Martin & Katzir 1999). Thus, vision in both species is concerned with the control of accurate bill placement at close quarters when foraging; this is despite the fact that red knots can forage for buried prey using exclusively tactile cues. However, both species are always found in open habitats where they are vulnerable to predation by raptors, an evolutionary pressure that would favour the evolution of comprehensive visual coverage of the space around the bird. Clearly, there is an evolutionary trade-off between having binocular vision about the direction of the bill and panoramic vision about the head. In golden plovers, we see evidence of a further factor that has been traded in the evolution of visual field topography: increased eye size. Increased eye size in golden plovers has probably evolved to gain enhanced sensitivity to guide feeding at night. It has resulted not only in a relatively smaller binocular field surrounding the direction of the bill but also in a wider blind area above the head compared with knots. We would predict that this will entail increased vulnerability to predation in golden plovers with consequent differences in behaviours associated with predator detection and/or avoidance in these two species. Further analyses of the trade-offs between behavioural and ecological aspects of foraging and predator detection are clearly required to understand all of the factors that can contribute to the evolution of the eye position and visual fields in birds.
Acknowledgments
Ethical guidelines with respect to handling and restraining birds required for licensed procedures in the UK (UK Animals (Scientific Procedures) Act 1986) were followed.
We thank the wilsternetters of Fryslân for making available freshly captured golden plovers used in the visual field measurements, and the Bird Group, Department of Zoology, The Natural History Museum at Tring, UK, for access to the skeleton collection and permission to take measurements and photographs of the skulls. Dr Gerald Mayr, Forschungsinstitut Senckenberg, Frankfurt am Main, Germany, provided valuable advice on avian skull anatomy. Kevin Down provided advice about Latin used in the naming of Os supraorbitale aliforme.
References
- Barnard C.J., Thompson D.B.A. Croom-Helm; London, UK: 1985. Gulls and plovers. The ecology and behaviour of mixed-species feeding groups. [Google Scholar]
- Baumel J.J. 2nd edn. Nuttall Ornithological Club; Cambridge, MA: 1993. Handbook of avian anatomy: Nomina Anatomica Avium. [Google Scholar]
- Bolze G. Anordnung und Bau der Herbstschen Korperchen in Limicolenschnabeln im Zusammenhang mit Nahrungsfindung. Zool. Anz. 1968;181:313–355. [Google Scholar]
- Brooke M. de L., Hanley S., Laughlin S.B. The scaling of eye size with body mass in birds. Proc. R. Soc. B. 1999;266:405–412. doi:10.1098/rspb.1999.0652 [Google Scholar]
- Burton P.J.K. British Museum (Natural History); London, UK: 1974. Feeding and the feeding apparatus in waders. [Google Scholar]
- Cramp S., Simmons K.E.L. Waders to gulls. vol. 3. Oxford University Press; Oxford, UK: 1983. Handbook of the birds of Europe, the Middle East and North Africa; the birds of the western Palearctic. [Google Scholar]
- Cresswell W., Whitfield D.P. The effects of raptor predation on wintering wader populations at the Tyninghame estuary, southeast Scotland. Ibis. 1994;136:223–232. doi:10.1111/j.1474-919X.1994.tb01088.x [Google Scholar]
- Davies M.N.O., Green P.R. Multiple sources of depth information: an ecological approach. In: Davies M.N.O., Green P.R., editors. Perception and motor control in birds: an ecological approach. Springer; Berlin, Germany: 1994. pp. 339–356. [Google Scholar]
- Fallet M. Über Bodenvogel und ihre terricolen Beutetiere: Technik der Nahrungssuche-Populationsdynamik. Zool. Anz. 1962;168:187–212. [Google Scholar]
- Fernández-Juricic E., Erichsen J.T., Kacelnik A. Visual perception and social foraging in birds. Trends Ecol. Evol. 2004;19:25–31. doi: 10.1016/j.tree.2003.10.003. doi:10.1016/j.tree.2003.10.003 [DOI] [PubMed] [Google Scholar]
- Fernández-Juricic, E., Gall, M. D., Dolan, T., Tisdale, V. & Martin, G. R. 2008 The visual fields of two ground-foraging birds, house finches and house sparrows, allow for simultaneous foraging and anti predator vigilance. Ibis150, 779–787. (doi:10.1111/j.1474-919X.2008.00860.x)
- Gerritsen A.F.C., Van Heezik Y.M., Swennen C. Chemoreception in two further Calidris species (C. Maritima and C. Canutus) Neth. J Zool. 1983;33:485–496. doi:10.1163/002829683X00219 [Google Scholar]
- Gibson J.J. Erlbaum; Hove, UK: 1986. The ecological approach to visual perception. [Google Scholar]
- Gillings S., Sutherland W.J. Comparative diurnal and nocturnal diet and foraging in Eurasian golden plovers Pluvialis apricaria and northern lapwings Vanellus vanellus wintering on arable farmland. Ardea. 2007;95:243–257. [Google Scholar]
- Gillings S., Fuller R.J., Sutherland W.J. Diurnal studies do not predict nocturnal habitat choice and site selection of European golden-plovers (Pluvialis apricaria) and northern lapwings (Vanellus vanellus) Auk. 2005;122:1249–1260. doi:10.1642/0004-8038(2005)122[1249:DSDNPN]2.0.CO;2 [Google Scholar]
- Guillemain M., Martin G.R., Fritz H. Feeding methods, visual fields and vigilance in dabbling ducks (Anatidae) Funct. Ecol. 2002;16:522–529. doi:10.1046/j.1365-2435.2002.00652.x [Google Scholar]
- Hayman P., Marchant J., Prater A.J. Croom Helm; Beckenham, UK: 1986. Shorebirds: an identification guide to waders of the world. [Google Scholar]
- Jukema J., Piersma T., Hulscher J.B., Bunskoeke E.J., Koolhaas A.K., Veenstra A. KNNV Uitgeverij; Utrecht, The Netherlands: 2001. Goudplevieren en wilsterflappers: eeuwenoude fascinatie voor trekvogels. [Golden plovers and wilsternetters: a deeply rooted fascination with migrating birds] [Google Scholar]
- Land M.F., Nilsson D.-E. Oxford University Press; Oxford, UK: 2002. Animal eyes. [Google Scholar]
- Lind J., Cresswell W. Determining the fitness consequences of antipredation behaviour. Behav. Ecol. 2005;16:945–956. doi:10.1093/beheco/ari075 [Google Scholar]
- Martin G.R. The visual fields of the tawny owl Strix aluco. Vision Res. 1984;24:1739–1751. doi: 10.1016/0042-6989(84)90005-1. doi:10.1016/0042-6989(84)90005-1 [DOI] [PubMed] [Google Scholar]
- Martin, G. R. 1985 Eye. In Form and function in birds vol. 3 (eds A. S. King & J. McLelland), pp. 311–373. London, UK: Academic Press.
- Martin G.R. Total panoramic vision in the mallard duck, Anas platyrhynchos. Vision Res. 1986;26:1303–1306. doi: 10.1016/0042-6989(86)90112-4. doi:10.1016/0042-6989(86)90112-4 [DOI] [PubMed] [Google Scholar]
- Martin G.R. T & A D Poyser; London, UK: 1990. Birds by night. [Google Scholar]
- Martin G.R. Visual fields in woodcocks Scolopax rusticola (Scolopacidae; Charadriiformes) J. Comp. Physiol. A. 1994;174:787–793. doi:10.1007/BF00192728 [Google Scholar]
- Martin G.R. Visual fields and their functions in birds. J. Ornithol. 2007;148:S547–S562. doi:10.1007/s10336-007-0213-6 [Google Scholar]
- Martin G.R., Katzir G. Visual fields and eye movements in herons (Ardeidae) Brain Behav. Evol. 1993;44:74–85. doi: 10.1159/000113571. doi:10.1159/000113571 [DOI] [PubMed] [Google Scholar]
- Martin G.R., Katzir G. Visual field in short-toed eagles Circaetus gallicus and the function of binocularity in birds. Brain Behav. Evol. 1999;53:55–66. doi: 10.1159/000006582. doi:10.1159/000006582 [DOI] [PubMed] [Google Scholar]
- Martin, G. R. & Osorio, D. 2008 Vision in birds. In The senses: a comprehensive reference. Vision 1 (eds A. I. Basbaum, A. Kaneko, G. M. Shepherd & G. Westheimer), pp. 25–52. San Diego, CA: Academic Press.
- Martin G.R., Jarrett N., Tovey P., White C.R. Visual fields in flamingos: chick-feeding versus filter-feeding. Naturwissenschaften. 2005;92:351–354. doi: 10.1007/s00114-005-0010-0. doi:10.1007/s00114-005-0010-0 [DOI] [PubMed] [Google Scholar]
- Martin G.R., Jarrett N., Williams M. Visual fields in blue ducks and pink-eared ducks: visual and tactile foraging. Ibis. 2007a;149:112–120. doi:10.1111/j.1474-919X.2007.00641.x [Google Scholar]
- Martin G.R., McNeil R., Rojas L.M. Vision and the foraging technique of skimmers (Rynchopidae) Ibis. 2007b;149:750–759. doi:10.1111/j.1474-919X.2007.00706.x [Google Scholar]
- Nebel S., Jackson D.L., Elner R.W. Functional association of bill morphology and foraging behaviour in calidrid sandpipers. Anim. Biol. 2005;55:235–243. doi:10.1163/1570756054472818 [Google Scholar]
- Piersma T., Wiersma P. Family Charadriidae (plovers) In: del Hoyo J., Elliott A., Sargatal J., editors. Handbook of the birds of the world. Hoatzin to auks. Lynx Edicions; Barcelona, Spdain: 1996. pp. 384–442. [Google Scholar]
- Piersma, T., Van Gils, J. & Wiersma, P. 1996 Family Scolopacidae (sandpipers, snipes and phalaropes) In Handbook of the birds of the world. Hoatzin to auks (eds J. del Hoyo, A. Elliott & J. Sargatal), pp. 444–533. Barcelona, Spain: Lynx Edicions.
- Piersma T., Van Aelst R., Kurk K., Berkhoudt H., Maas L.R.M. A new pressure sensory mechanism for prey detection in birds: the use of principles of seabed dynamics? Proc. R. Soc. B. 1998;265:1377–1383. doi:10.1098/rspb.1998.0445 [Google Scholar]
- Tulp, I., Schekkerman, H., Piersma, T., Jukema, J., de Goeij, P. & Van de Kam, J. 1998 Breeding waders at Cape Sterlegova, northern Taimyr, in 1994. WIWO-report 61. Zeist, The Netherlands: Working Group International Wetland and Waterbird Research.
- Van den Hout P.J., Spaans B., Piersma T. Differential mortality of wintering shorebirds on the Banc d'Arguin, Mauritania, due to predation by large falcons. Ibis. 2007;15:219–230. doi:10.1111/j.1474-919x.2007.00785.x [Google Scholar]